Side-effects of a number of insecticides on predatory mites in apple orchards
Malagnini, Valeria  1
; Baldessari, Mario
1
; Baldessari, Mario  2
; Duso, Carlo
2
; Duso, Carlo  3
; Pozzebon, Alberto
3
; Pozzebon, Alberto  4
and Angeli, Gino
4
and Angeli, Gino  5
5
1Dipartimento Innovazione nelle Produzioni Vegetali, Centro Trasferimento Tecnologico, Fondazione Edmund Mach, San Michele all’Adige, Trento, Italy.
2Dipartimento Alimenti e Trasformazione, Centro Trasferimento Tecnologico, Fondazione Edmund Mach, San Michele all’Adige, Trento, Italy.
3✉ Department of Agronomy, Food, Natural Resources, Animals and Environment, University of Padova, Legnaro, Padova, Italy.
4Department of Agronomy, Food, Natural Resources, Animals and Environment, University of Padova, Legnaro, Padova, Italy.
5Dipartimento Innovazione nelle Produzioni Vegetali, Centro Trasferimento Tecnologico, Fondazione Edmund Mach, San Michele all’Adige, Trento, Italy.
2023 - Volume: 63 Issue: Suppl pages: 17-28
https://doi.org/10.24349/9tgw-xrc4Proceedings of the 9th Symposium of the EurAAc, Bari, July, 12th–15th 2022
Keywords
Abstract
Introduction
Despite progress in reducing pesticide use in fruit growing areas of Europe and North America, insecticides are still requested to control aphids, scales and stink bugs (Ioriatti et al. 2019; Beers et al. 2019). Insecticide side-effects on beneficials can promote outbreaks of secondary pests since of their impact on natural antagonists. Infestations of phytophagous mites in fruit orchards are a clear example of this syndrome: mite pests are commonly controlled by predators, mainly by phytoseiid mites (Acari, Phytoseiidae) where pesticide use is minimal or selective pesticides are used (Blommers 1994; Croft 1994; Beers et al. 2016a, b; Schmidt-Jeffris et al. 2019). A number of predatory mite species have been considered non-target organisms in trials aimed at evaluating the effects of pesticides on beneficials and various approaches have been adopted in this field of research (Bergeron and Schmidt-Jeffris 2020; Schmidt-Jeffris et al. 2021). The need to conduct field and laboratory trials has been stressed since longtime (e.g., Sterk et al. 1999; Bostanian et al. 2009). The variety of pesticide side-effects, from lethal to sublethal as well from direct to indirect, on beneficials has suggested new experimental models (e.g., Desneux et al. 2007; Stavrinides and Mills 2009; Duso et al. 2020). In addition, it has been recognized that the compatibility of pesticides with conservation biological control tactics based on predatory mites should be developed at a local level (Bostanian et al. 2010; Lefebvre et al. 2011, 2012; Pozzebon et al. 2014).
In the last two decades, reduced risk-insecticides (e.g., neonicotinoids) have been presented as an alternative to broad-spectrum insecticides but their side-effects on beneficials have been matter of discussion (e.g., Calvo-Agudo et al. 2019; Furlan et al. 2021). In this framework the side-effects of neonicotinoids on predatory mites represent an interesting case-study (e.g., Bostanian et al. 2009, 2010; Zanuzo Zanardi et al. 2017). The side-effects of four neonicotinoids on the predatory mite Amblyseius andersoni (Chant) have been investigated in field and laboratory conditions. Amblyseius andersoni is a common predatory mite occurring in fruit orchards in Europe and North America (Ivancich Gambaro 1975; Genini et al. 1991; Messing and Croft 1991; Blommers 1994; Szabó et al. 2014). Strains resistant to conventional pesticides (organophosphates, carbamates, pyrethroids) in European fruit orchards have been reported for this species since the 1970s (Ivancich Gambaro 1975; Anber and Overmeer 1988; Anber and Oppenoorth 1989; Duso et al. 1992; Bonafos et al. 2007). The replacement of broad-spectrum insecticides by reduced risk-insecticides represents an interesting scenario for studies on the compatibility between pesticides and beneficial organisms.
Material and methods
Field studies
The effects of insecticides on A. andersoni populations were evaluated in an apple orchard located at the experimental station of E. Mach Foundation (FEM, S. Michele all′Adige, Trento, Italy) in the 2009 growing season. Five insecticides commonly applied in apple orchards were considered (Table 1). An untreated control was included for comparison.


Insecticides were applied once (12 May), twice (9 June) or three times (8 July) in separate blocks according to codling moth control timing. A completely randomized design was followed with four replicates per treatment; each replicate consisted of 15 plants. Sampling was carried out before and every 5-10 days after insecticide applications (for about one month from the last application). A total of 60 leaves per treatment (15 leaves per replicate) were removed and transferred to the laboratory where predatory and phytophagous mites were counted under a dissecting microscope. Phytoseiid specimens were mounted on slides, in Hoyer's medium, and identified under a phase contrast microscope.
Data were analyzed with a linear mixed repeated measures model with the MIXED procedure of SAS® (ver. 9.4; SAS Institute, 2016). Mite densities were considered as response variables with repeated measures made at different times, i.e., sampling dates. Using an F test (α = 0.05) we evaluated the effect of insecticide application, time and their interaction. Degrees of freedom were estimated using the Kenward–Roger method (Littell et al. 1996). Differences among treatments were evaluated with a t-test (α = 0.05) to the least-square means with the Tukey's adjustment. Slice option of the LSMEANS statement was used for the F-test partition of interactions between insecticide application and time. Data were checked for the analysis' assumptions and square-root transformation was applied.
Laboratory studies
Insecticides applied in field trials were tested at the same concentrations in the laboratory. Apple leaves were treated with insecticides using a Potter Burkard tower (1.7±0.1 mg/cm2 of insecticide solution) and then mated A. andersoni females of same age were transferred onto the leaves to expose them to fresh insecticide residues. To prevent mite escape and leaf desiccation, leaves were placed onto wet cotton prior to mite transfer. Tetranychus urticae Koch eggs and females were provided every day as food for predatory mites. The experimental units were kept in a climatic chamber at 25±2 °C, 70±10% relative humidity and 16L:8D photoperiod. Effect of insecticides on female survival was evaluated after 2 and 72 h. Surviving females were observed daily for additional 4 days to assess pesticide effects on fecundity. Escaped or drowned females were removed from the initial number. In total we assessed 45–50 females per insecticide.
We analyzed the data with a linear model using the GLM procedure of SAS® (ver. 9.4; SAS Institute, 2016). An F test (α = 0.05) was used to evaluate the effect of insecticides on mite survival, fecundity and escaping rate (number of escaped or drowned females/initial number of females). Treatments were compared using Tukey–Kramer test (α = 0.05). The Blümel and Hausdorf (2002) formula was used for fecundity calculation. In order to meet the models' assumptions, data on survival were arcsin-transformed while log x+1 transformation was applied to data on fecundity.
Results
Field studies
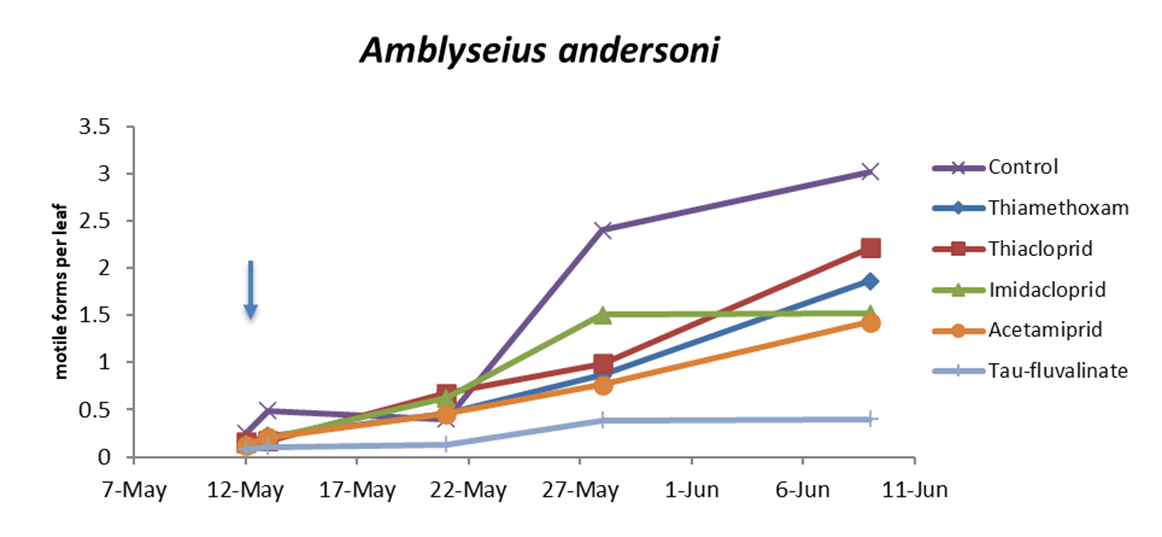
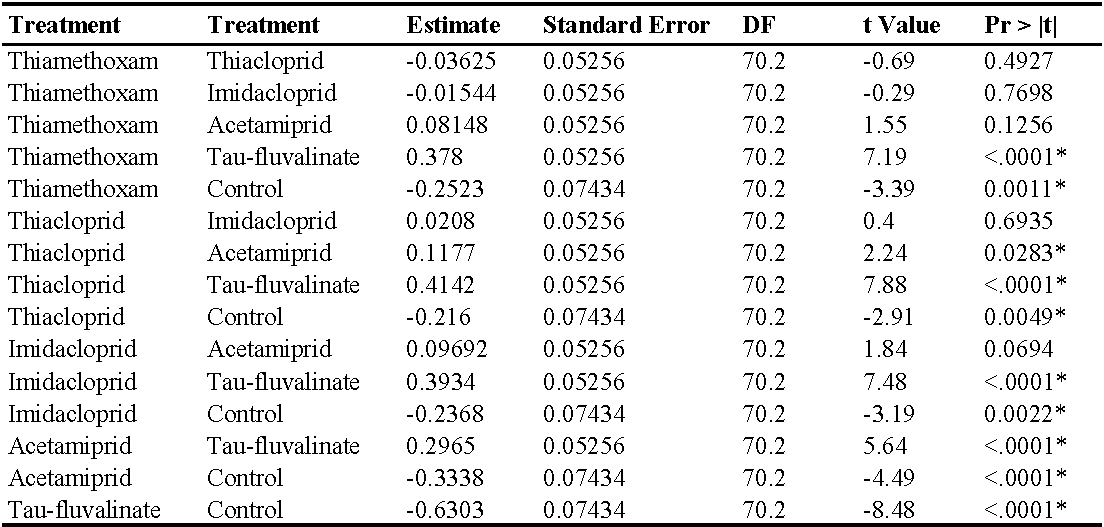
In the first experiment (single insecticide application) there were no differences among treatments prior to insecticide applications (F5, 202 = 0.67; P = 0.648). Later, insecticides affected predatory mite populations compared with the control (F5, 70.2 = 22.1; P < 0.0001; Figure 1; Table 2). The effects of time and interaction treatment * time were also significant (respectively: F4, 160 = 110.64; P < 0.0001; F20, 172 = 4.01; P < 0.0001). Predatory mite populations were lower in tau-fluvalinate than in neonicotinoid plots and among the latter in acetamiprid than in thiacloprid plots (Table 2). In this trial P. ulmi populations were not detected.




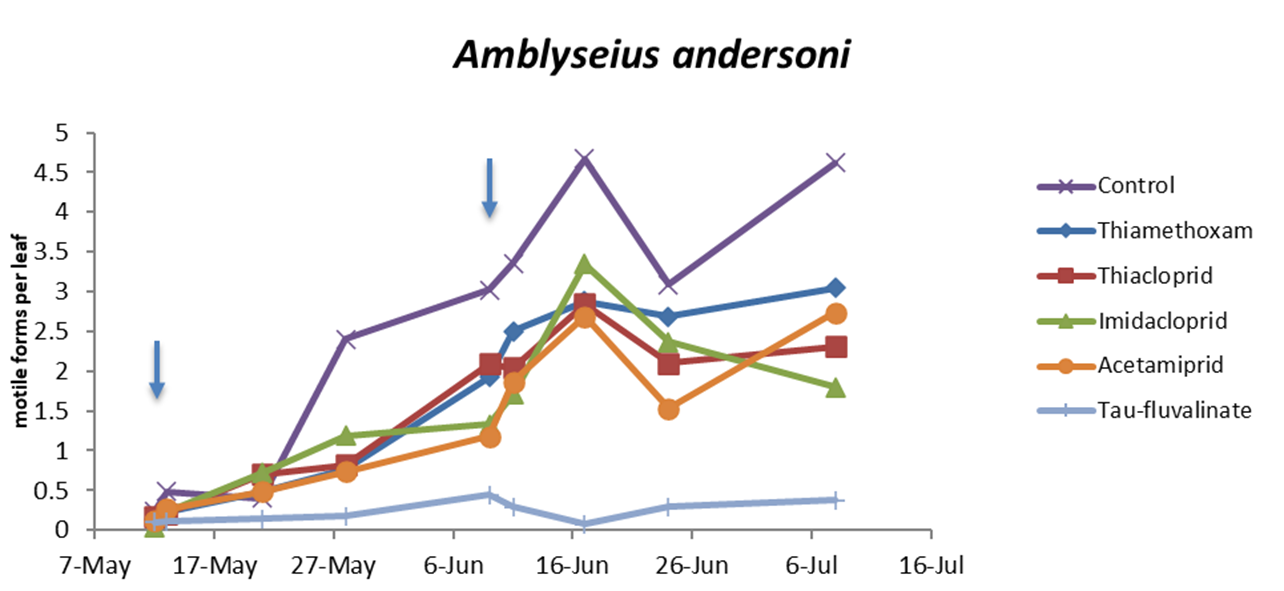
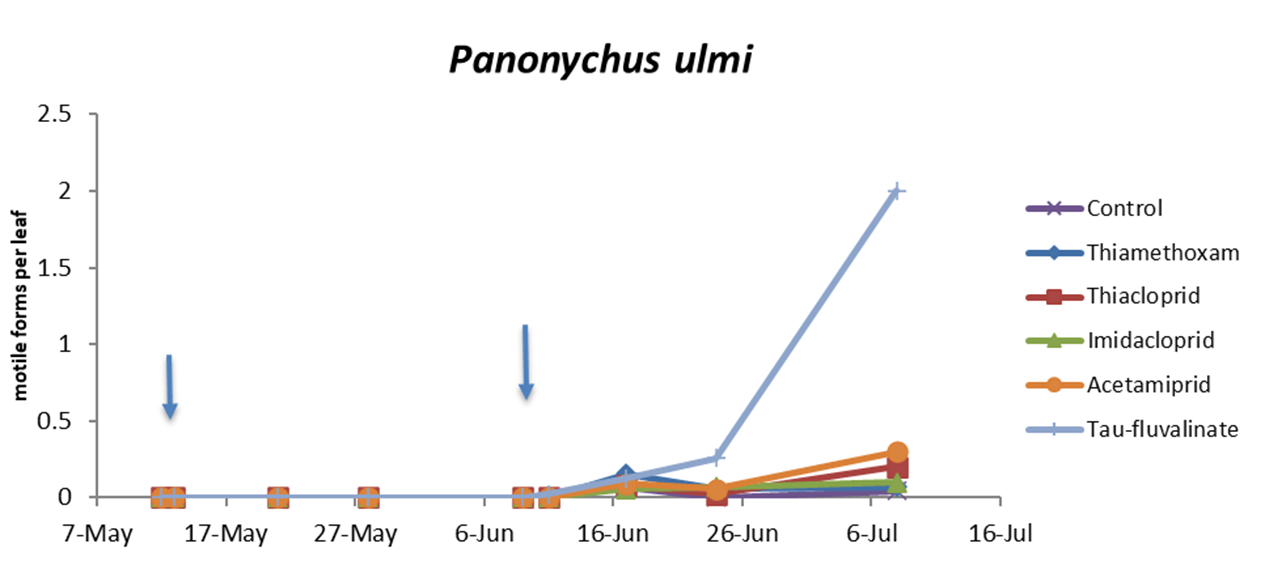
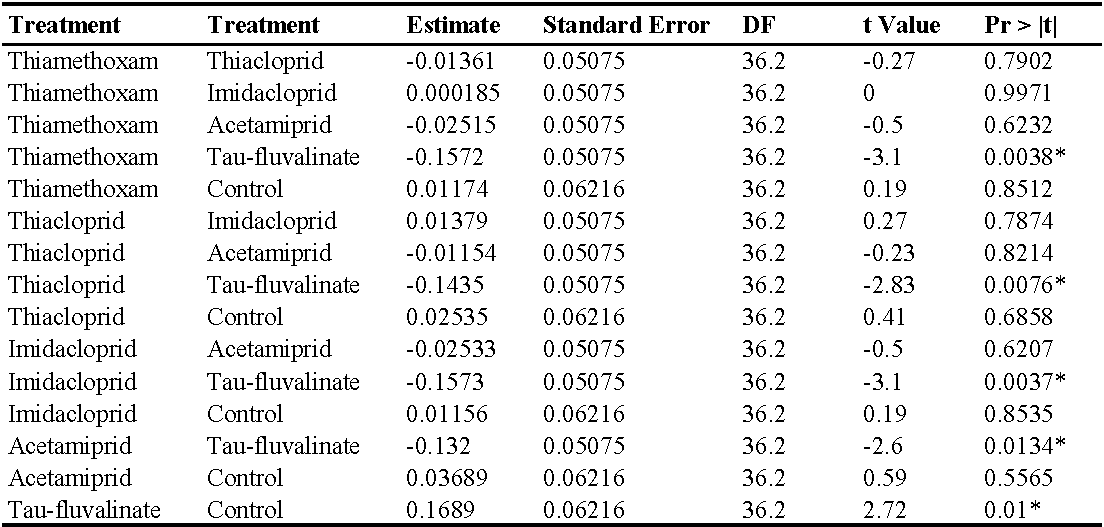
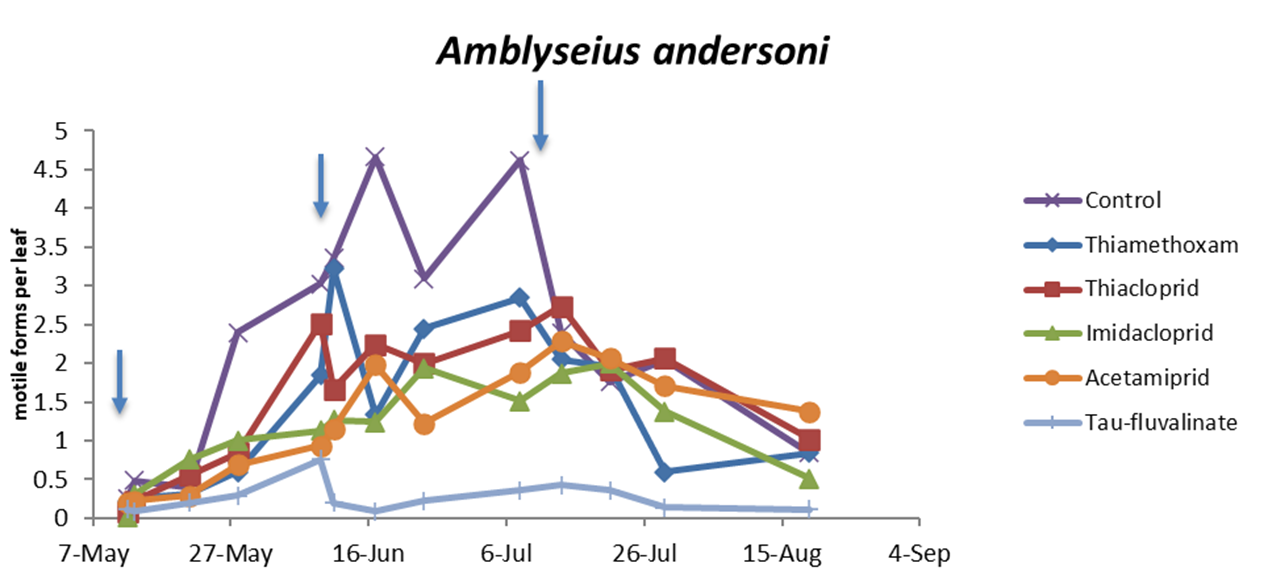
Also in the second experiment (two insecticide applications) there were no differences among treatments prior to the first insecticide application (F5, 228 = 0.76; P = 0.578) while insecticides significantly reduced predatory mite populations compared with the control (F5, 72 = 46.99; P < 0.0001; Figure 2; Table 3). The effects of time and interaction treatment * time were also significant (respectively: F8, 204 = 63; P < 0.0001; F40, 209 = 3.17; P < 0.0001). Predatory mite populations reached the lowest densities in tau-fluvalinate plots. Additional differences emerged among neonicotinoids: predatory mites were less abundant in acetamiprid than in thiamethoxam plots (Table 3). Panonychus ulmi populations were detected from mid-June onwards. There were more spider mites in tau-fluvalinate than in the remaining plots (F5, 36.2 = 2.96; P = 0.025; Figure 3; Table 4). The effects of time and interaction treatment * time were also significant (respectively: F8, 182 = 15.32; P < 0.0001; F40, 193 = 2.67; P < 0.0001).


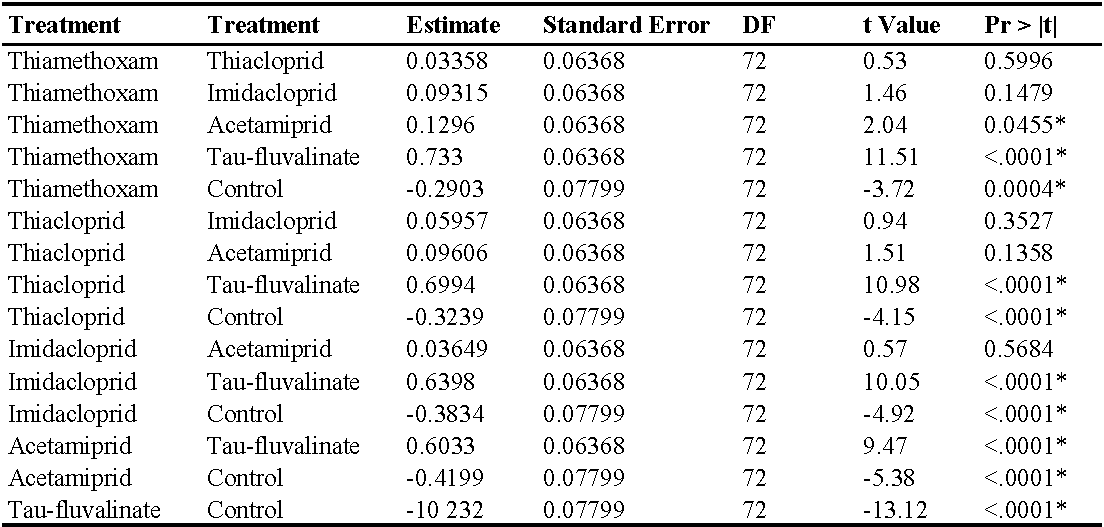






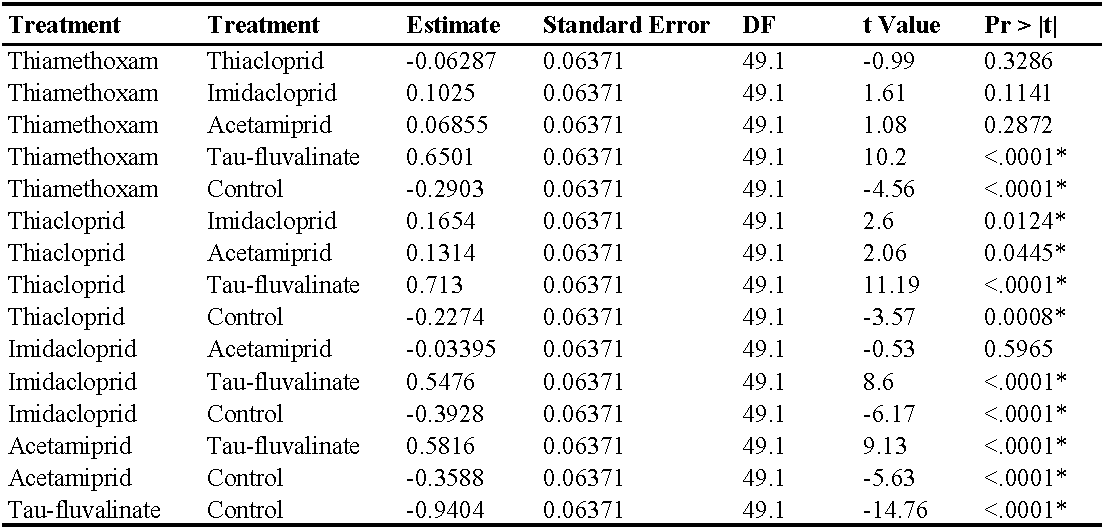
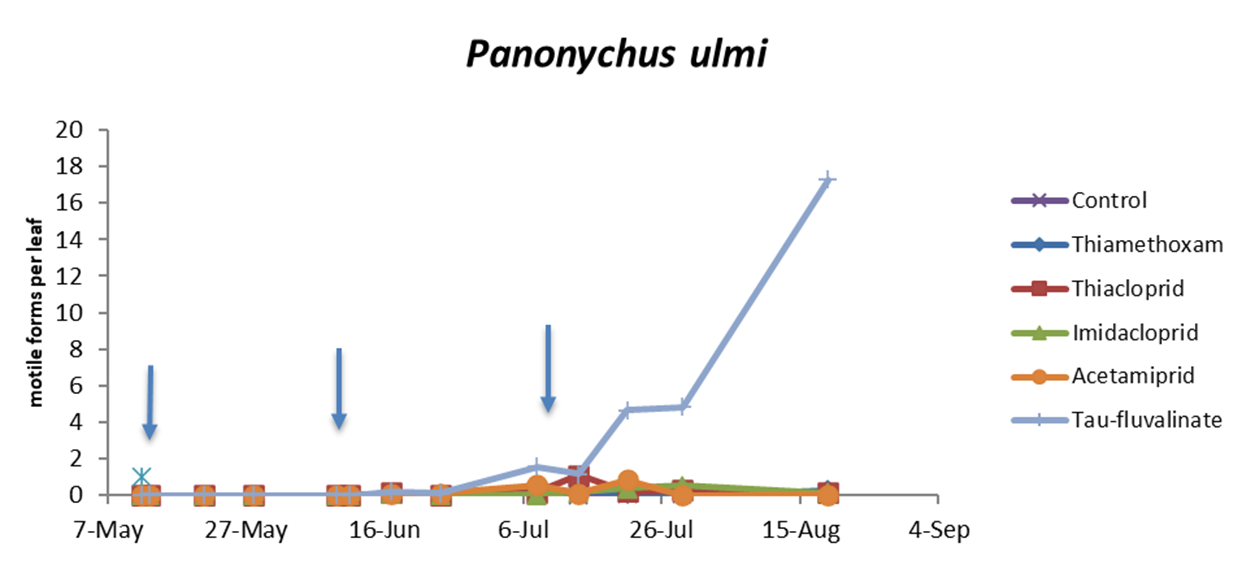
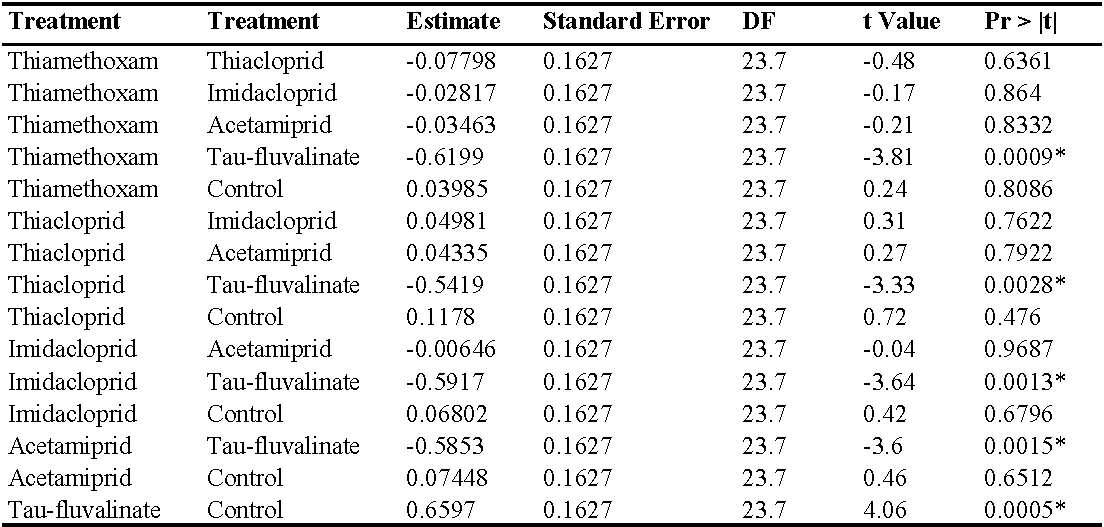
In the third experiment (three insecticide applications) there were no differences among treatments prior to the first insecticide application (F5, 154 = 0.87; P = 0.50) while insecticides significantly reduced predatory mite populations compared with the control (F5, 49.1 = 48.25; P < 0.0001; Figure 4; Table 5). The effects of time and interaction treatment * time were also significant (respectively: F12, 132 = 29.34; P < 0.0001; F60, 120 = 2.49; P < 0.0001). The lowest A. andersoni densities were reached in tau-fluvalinate plots. Moreover, there were less predatory mites in acetamiprid and imidacloprid than in thiacloprid plots (Table 5). Panonychus ulmi numbers increased from mid-June onwards in tau-fluvalinate than in the remaining plots (F5, 23.7 = 4.65; P = 0.004; Figure 5; Table 6). The effects of time and interaction treatment * time were also significant (respectively: F12, 133 = 5.98; P < 0.0001; F60, 119 = 3.1; P < 0.0001).








Laboratory experiments
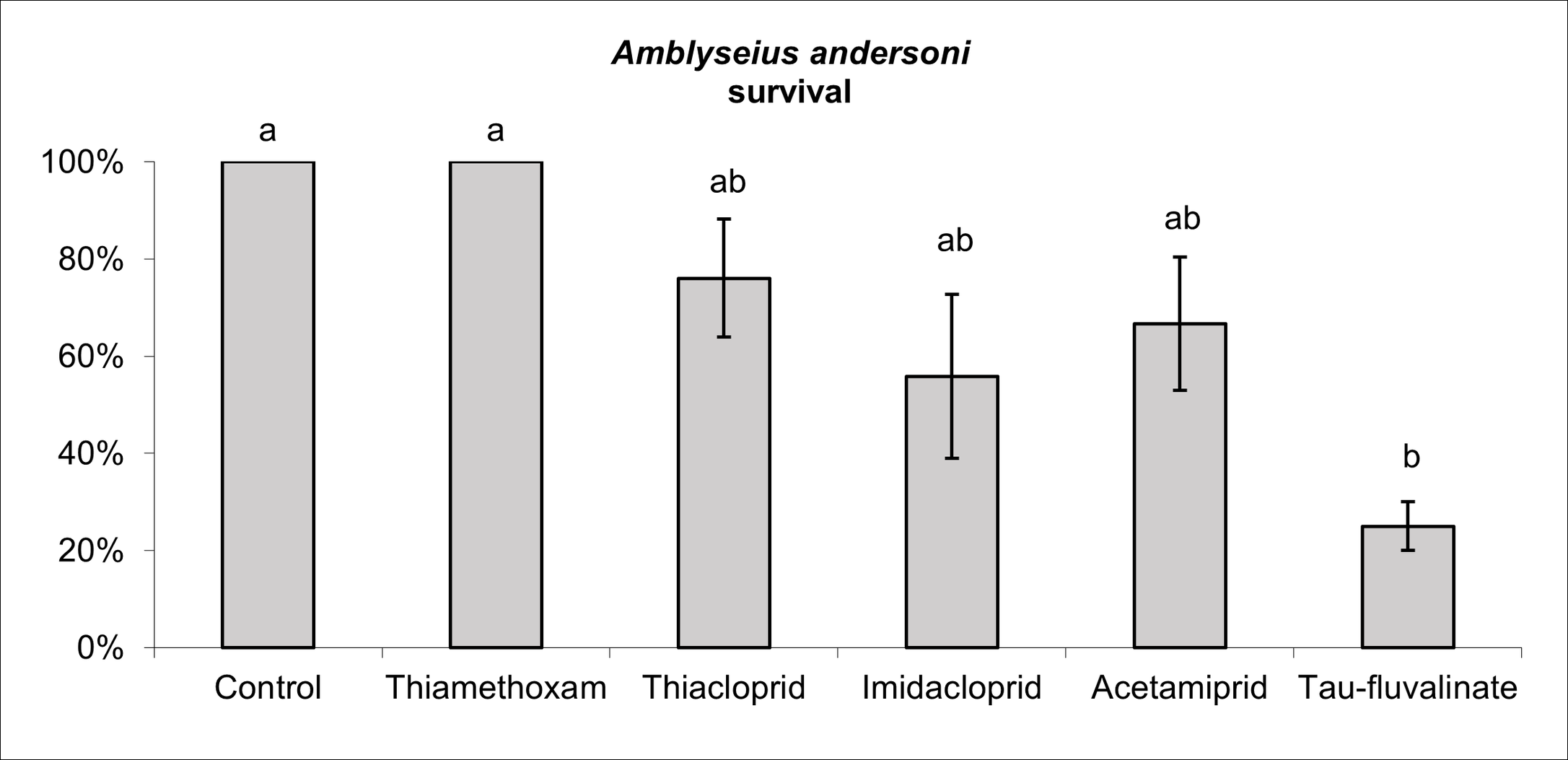
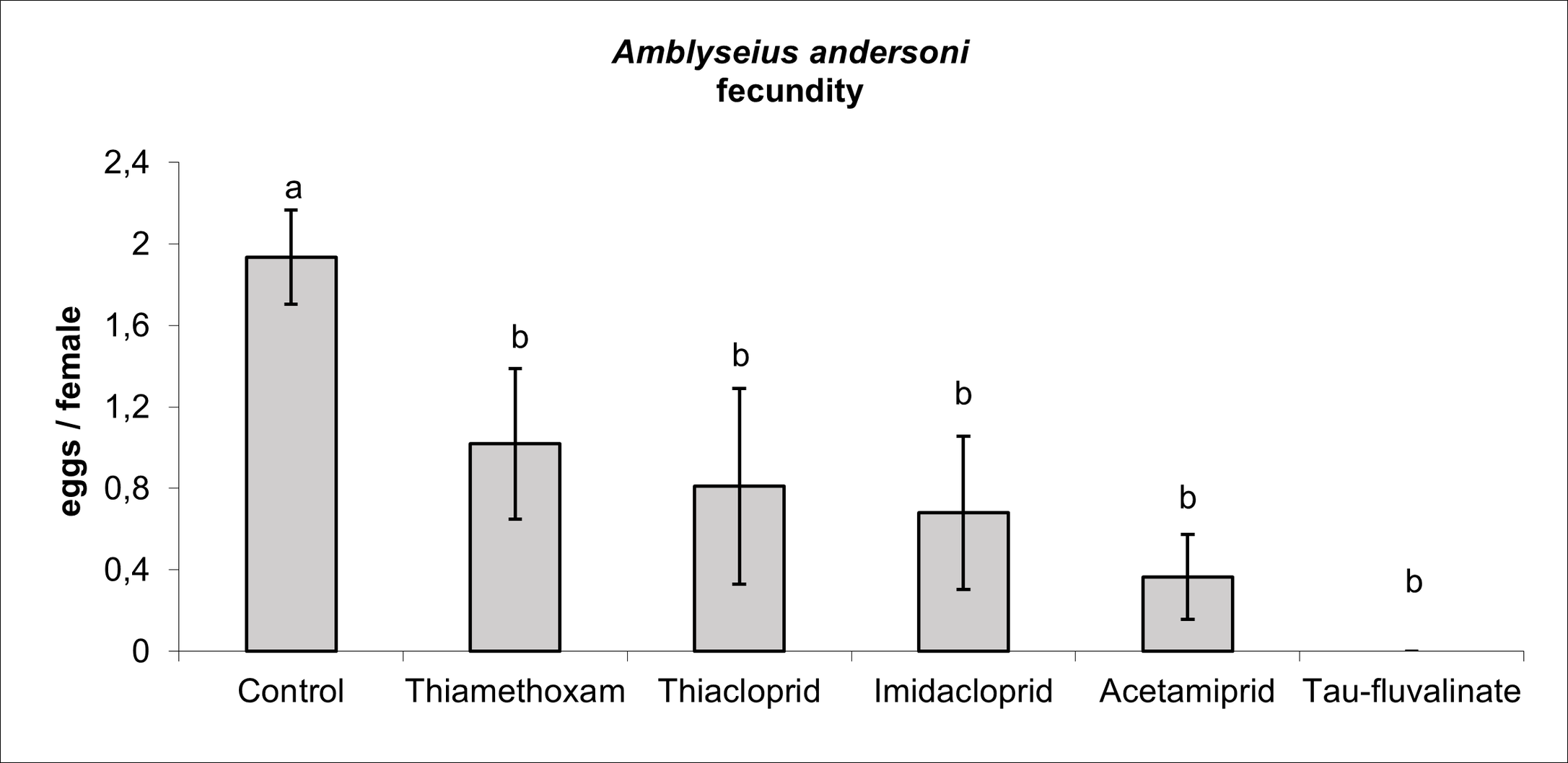
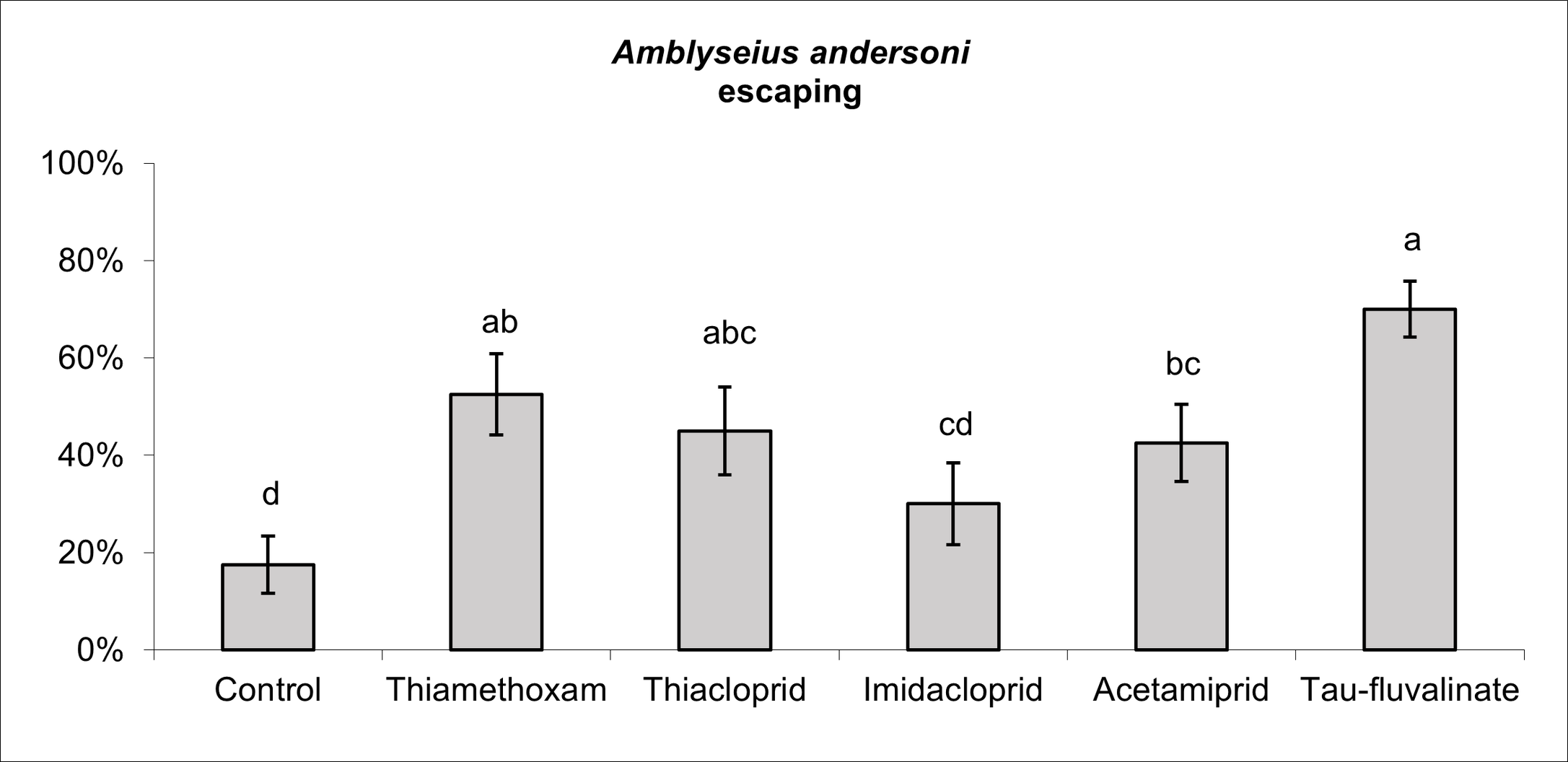
Insecticides affected A. andersoni survival (F5, 38 = 4.04; P = 0.005; Figure 6), in particular tau-fluvalinate reduced female survival by 75%. Among neonicotinoids, there were no differences between thiamethoxam and the control while the remaining active ingredients were associated to intermediate effects (Figure 6). All insecticides reduced A. andersoni fecundity (F5, 30 = 2.96; P = 0.027) compared to the control (Figure 7). Escaping rate was also influenced by insecticides (F5, 38 = 3.92; P = 0.006) and the most relevant effects were caused by tau-fluvalinate (Figure 8). Only imidacloprid was not associated to adverse effects in terms of escaping rate.






Discussion
Field applications of tau-fluvalinate significantly reduced A. andersoni densities especially when repeated two-three times during the growing season. These observations confirm the results obtained on Kampimodromus aberrans (Oudemans) in fruit orchards located in the same region (Duso et al. 2014). In the latter investigation, tau-fluvalinate caused 100% mortality on K. aberrans females in the laboratory. In the present study A. andersoni mortality was of about 75% but surviving females did not lay eggs. The detrimental effects of tau-fluvalinate on predatory mites have been recorded even on other species using different experimental procedures (Petit and Karan 1991; Bellows et al. 1992; Grout et al. 1997; Amin et al. 2009). Regarding sublethal effects, tau-fluvalinate significantly increased A. andersoni escaping rate, a phenomenon likely associated to repellency. It should be mentioned that pyrethroid residues can induce adverse effects (increased locomotory activity or escape) also on spider mites (Holland et al. 1994) with implications for mite outbreaks. Field concentrations of pyrethroids can disrupt predator–prey dynamics in apple orchards (Bostanian et al. 1985; Bowie et al., 2001) and this phenomenon was noticed in two of our field trials. Therefore, the use of tau-fluvalinate in fruit orchards requires a careful evaluation.
Most of insecticides tested in the present study belonged to neonicotinoids. Their application reduced A. andersoni densities in field conditions compared to the control plots, but these effects were less severe than those reported for tau-fluvalinate. The reduction in population size observed in our trials could be caused by the effect of neonicotinoids on predatory mite fecundity. Similar effects have been reported for other predatory mite species (Castagnoli et al. 2005; Villanueva and Walgenbach 2005; Bostanian et al. 2009). There were some differences between neonicotinoids, in particular acetamiprid proved to be less selective than other neonicotinoids and these effects could be related to a more pronounced reduction in fecundity observed in laboratory trials. Bostanian et al. (2009) reported a high toxicity of acetamiprid and imidacloprid to Galendromus occidentalis (Nesbitt) whereas thiamethoxam and thiacloprid showed slight or negligible effects. An increase in escaping rate was noticed for three out of four neonicotinoids suggesting some alterations in predatory mite behavior. Various sublethal and behavioral effects (included irritancy) have been reported for some neonicotinoids even if their implications for spider mite control are not always clear (e.g., Poletti et al. 2007; Beers and Schmidt-Jeffris 2015; Schmidt-Jeffris et al. 2021). Previous research found that irritability and repellency may favor the escape of phytoseiids from contaminated surfaces and seemed associated with the least selective products (Monteiro et al., 2019). In our case the highest escaping rates were associated with thiamethoxam and thiacloprid; implications of this phenomenon should be investigated more in depth.
In this study single or multiple applications of neonicotinoids were not associated with spider mite increases in the experimental season. The limited effect of neonicotinoids on A. andersoni survival in the laboratory confirms the results of experiments conducted on various predatory mites (James 1997; James and Vogele 2001; Poletti et al. 2007; Lefebvre et al. 2011; Duso et al. 2014). The negative effects of neonicotinoids on A. andersoni fecundity represent a serious risk for one of the most important objectives of IPM tactics: to preserve stable populations of predatory mites in fruit orchards. While most of these insecticides have been banned in Europe, their use is still significant in other continents.
It should be stressed that predatory mite species and strains exhibit a variation in their susceptibility to pesticides. In North America, A. andersoni proved to be less susceptible to imidacloprid than Galendromus occidentalis (Nesbitt) and Neoseiulus fallacis (Garman) (James 2003). The use of neonicotinoids could favor the less susceptible species (and strains) in predatory mite communities irrespectively of their adaptation to environmental factors. Climate change is also influencing the composition and structure of predatory mite communities in perennial crops. Experimental studies should be addressed to evaluate the impact of pesticides in different environmental scenarios.
Acknowledgements
The project was supported by the Grant ''CRPV project: SELETTIVITÀ AGROFARMACI VS ORGANISMI UTILI (SAO)'' of University of Bologna to G.A., M.B. and V.M; C.D. and A.P. were supported by DOR funds from the University of Padova.
References
- Amin M.M., Mizell R.F., Flowers R.W. 2009. Response of the predatory mite Phytoseiulus macropilis (Acari: Phytoseiidae) to pesticides and kairomones of three spider mite species (Acari: Tetranychidae), and non-prey food. Flo. Entomol., 92: 554-562. https://doi.org/10.1653/024.092.0404
- Anber H.A.I., Overmeer W.P.J. 1988. Resistance to organophosphates and carbamates in the preda-cious mite Amblyseius potentillae (Garman) due to insensitive acetylcholinesterase. Pestic. Bio-chem. Phys., 31: 91-98.
- Anber H.A.I., Oppenoorth F.J. 1989. A mutant esterase degrading organophosphates in a resistant strain of the predacious mite Amblyseius potentillae (Garman). Pestic. Biochem. Phys., 33: 283-297. https://doi.org/10.1016/0048-3575(89)90127-2
- Beers E.H., Schmidt-Jeffris R.A. 2015. Effects of orchard pesticides on Galendromus occidentalis (Acari: Phytoseiidae): Repellency and irritancy. J. Econ. Entomol, 108: 259-265. https://doi.org/10.1093/jee/tou047
- Beers E.H., Horton D.R., Miliczky E. 2016a. Pesticides used against Cydia pomo nella disrupt bio-logical control of secondary pests of apple. Biol. Control, 102: 35-43. https://doi.org/10.1016/j.biocontrol.2016.05.009
- Beers E.H., Mills N.J., Shearer P.W., Horton D.R., Miliczky E.R., Amarasekare, K.G., Gontijo, L.M. 2016b. Nontarget effects of orchard pesticides on natural enemies: Lessons from the field and la-boratory. Biol. Control, 102: 44-52.
- Beers E.H., Marshall A., Hepler J., Milnes J. 2019. Prospects for integrated pest management of brown marmorated stink bug in Washington tree fruits. Outlooks Pest Manag., 30: 25-32. https://doi.org/10.1564/v30_feb_07
- Bellows T.S., Morse J.G., Gaston L.K. 1992. Residual toxicity of pesticides used for control of lepi-dopteran insects in citrus to the predaceous mite Euseius stipulatus Athias-Henriot (Acarina, Phy-toseiidae). J. Appl. Entomol. 113: 493-501.
- Bergeron P.E., Schmidt-Jeffris R.A. 2020. Not all predators are equal: miticide nontarget effects and differential selectivity. Pest. Manag. Sci., 76: 2170-2179. https://doi.org/10.1002/ps.5754
- Blommers L.H.M. 1994. Integrated pest management in European apple orchards. Ann. Rev. Ento-mol., 39: 213-241. https://doi.org/10.1146/annurev.en.39.010194.001241
- Bowie M.H., Worner S.P., Krips O.E., Penman D.R. 2001. Sublethal effects of esfenvalerate residues on pyrethroid resistant Typhlodromus pyri (Acari: Phytoseiidae) and its prey Panonychus ulmi and Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 25: 311-319. https://doi.org/10.1023/A:1017927502679
- Blumel S., Hausdorf H., 2002. Results of 8th and 9th IOBC joint pesticides testing programme: persis-tence test with Phytoseiulus persimilis Athias-Heriot (Acari: Phytoseiidae). IOBC/wprs Bull., 25: 43-51.
- Bonafos R., Serrano E., Auger P., Kreiter S., 2007. Resistance to deltamethrin, lambda-cyhalothrin and chlorpyriphos-ethyl in some populations of Typhlodromus pyri Scheuten and Amblyseius an-dersoni (Chant) (Acari: Phytoseiidae) from vineyards in the south-west of France. Crop Prot. 26: 169-172. https://doi.org/10.1016/j.cropro.2006.10.001
- Bostanian N.J., Balenger A., Revard I. 1985. Residues of four synthetic pyrethroids and azinphos-methyl onapple foliage and their toxicity to Amblyseius fallacis (Acari: Phytoseiidae). Can. En-tomol. 117: 143-152. https://doi.org/10.4039/Ent117143-2
- Bostanian N.J., Thistlewood H.A., Hardman J.M., Laurin M.C., Racette G. 2009. Effect of seven new orchard pesticides on Galendromus occidentalis in laboratory studies. Pest. Manag. Sci., 65: 635-639. https://doi.org/10.1002/ps.1721
- Bostanian N.J., Hardman J.M., Thistlewood H.A., Racette G. 2010. Effects of six selected orchard in-secticides on Neoseiulus fallacis (Acari: Phytoseiidae) in the laboratory. Pest. Manag. Sci., 66: 1263-1267. https://doi.org/10.1002/ps.2010
- Calvo-Agudo M., Gonzalez-Cabrera J., Pico' Y., Calatayud-Vernich P., Urbaneja A., Dicke M., Tena A. 2019. Neonicotinoids in excretion product of phloem-feeding insects kill beneficial insects. Proc. Natl. Acad. Sci. USA, 116: 16817-16822. https://doi.org/10.1073/pnas.1904298116
- Castagnoli M., Liguori M., Simoni S., Duso C. 2005. Toxicity of some insecticides to Tetranychus ur-ticae, Neoseiulus californicus and Tydeus californicus. Biocontrol, 50: 611-622. https://doi.org/10.1007/s10526-004-8121-7
- Croft B.A. 1994. Biological control of apple mites by a phytoseiid mite complex and Zetzellia mali (Acari: Stigmaeidae): Long-term effects and impact of azinphosmethyl on colonization by Amblyseius andersoni (Acari: Phytoseiidae). Environ. Entomol., 23: 1317-1325. https://doi.org/10.1093/ee/23.5.1317
- Desneux N., Decourtye A., Delpuech J.-M. 2007. The sublethal effects of pesticides on beneficial ar-thropods. Annu. Rev. Entomol., 52: 81-106. https://doi.org/10.1146/annurev.ento.52.110405.091440
- Duso C., Camporese P., Van der Geest L.P.S. 1992. Toxicity of a number of pesticides to strains of Typhlodromus pyri and Amblyseius andersoni (Acari: Phytoseiidae). Entomophaga, 37: 363-372. https://doi.org/10.1007/BF02373110
- Duso C., Ahmad S., Tirello P., Pozzebon A., Klaric V., Baldessari M., Malagnini V., Angeli G., 2014. The impact of insecticides applied in apple orchards on the predatory mite Kampimodromus aber-rans (Acari: Phytoseiidae). Exp. Appl. Acarol., 62: 391-414. https://doi.org/10.1007/s10493-013-9741-3
- Duso C., van Leeuwen T., Pozzebon A. 2020. Improving the compatibility of pesticides and predatory mites: recent findings on physiological and ecological selectivity. Curr. Opin. Insect Sci, 39: 63-68. https://doi.org/10.1016/j.cois.2020.03.005
- Furlan L., Pozzebon A., Duso C., Simon-Delso N., Sánchez-Bayo F., Marchand P.A., Codato F., Bijleveld van Lexmond M., Bonmatin J-M. 2021. An update of the Worldwide Integrated Assess-ment (WIA) on systemic insecticides. Part 3: alternatives to systemic insecticides. Environ. Sci. Poll. Res., 28: 11798-11820. https://doi.org/10.1007/s11356-017-1052-5
- Genini M., Klay A., Baumgärtner J., Delucchi V., Baillod M. 1991. Etudes comparatives de l′influence de la température et de la nourriture sur le développement de Amblyseius andersoni, Neoseiulus fallacis, Galendromus longipilus et Typhlodromus pyri [Acari: Phytoseiidae]. Entomophaga, 36: 139-154. https://doi.org/10.1007/BF02374645
- Grout T.G., Richards G.I., Stephen P.R. 1997. Further non-target effects of citrus pesticides on Euseius addoensis and Euseius citri (Acari: Phytoseiidae). Exp. Appl. Acarol. 21: 171-177. https://doi.org/10.1023/A:1018486602989
- Holland J.M., Chapman R.B., Penman D.R. 1994. Effects of fluvalinate on two-spotted spider mite dispersal, fecundity and feeding. Entomol. Exp. Appl., 71: 145-153. https://doi.org/10.1111/j.1570-7458.1994.tb01780.x
- Ioriatti C., Angeli G., Krawczyk G., Duso C., 2019. Optimizing insecticide use in integrated manage-ment of fruit insect pests. In: Xu, X. and Fountain, M. (Eds), Integrated management of diseases and insect pests of tree fruit, Burleigh Dodds Science Publishing, Cambridge, UK. https://doi.org/10.19103/AS.2019.0046.28
- Ivancich-Gambaro P. 1975. Observations on the biology and behaviour of the predaceous mite Typhlodromus italicus (Acarina: Phytoseiidae) in peach orchards. Entomophaga, 20: 171-177. https://doi.org/10.1007/BF02371657
- James D.G. 1997. Imidacloprid increases egg production in Amblyseius victoriensis (Acari: Phytosei-idae). Exp. Appl. Acarol. 21: 75-82. https://doi.org/10.1023/A:1018493409832
- James D.G. 2003. Toxicity of imidacloprid to Galendromus occidentalis, Neoseiulus fallacis and Amblyseius andersoni (Acari: Phytoseiidae) from hops in Washington State, USA. Exp. Appl. Acarol. 31: 275-281. https://doi.org/10.1023/B:APPA.0000010383.33351.2f
- James D.G., Vogele B. 2001. The effect of imidacloprid on survival of some beneficial arthropods. Plant Prot. Quart. 16: 58-62.
- Lefebvre M., Bostanian N.J., Thistlewood H.M.A., Mauffette Y., Racette G. 2011. A laboratory as-sessment of the toxic attributes of six ′reduced risk insecticides' on Galendromus occidentalis (Acari: Phytoseiidae). Chemosphere, 84: 25-30. https://doi.org/10.1016/j.chemosphere.2011.02.090
- Lefebvre M., Bostanian N.J., Mauffette Y., Racette G., Thistlewood H.A., Hardman J.M. 2012. Labor-atory based toxicological assessments of new insecticides on mortality and fecundity of Neoseiulus fallacis (Acari: Phytoseiidae). J. Econ. Entomol., 105: 866-871. https://doi.org/10.1603/EC11260
- Littell R.C., Milliken G.A., Stroup W.W., Wolfinger R.D. 1996. SAS system for mixed models. SAS Institute, Cary, NC.
- Messing R.H., Croft B.A. 1991. Biosystematics of Amblyseius andersoni and A. potentillae (Aca-rina: Phytoseiidae): implications for biological control. Exp. Appl. Acarol., 10: 267-278. https://doi.org/10.1007/BF01198655
- Monteiro V.B., Lima D.B., Melo J.W.S., Guedes R.N.C., Gondim M.G.C. 2019. Acaricide-Mediated Colonization of Mite-Infested Coconuts by the Predatory Phytoseiid Neoseiulus baraki (Acari: Phytoseiidae). J. Econ. Entomol. 112:213-218. https://doi.org/10.1093/jee/toy291
- Petit F.L., Karan D.J. 1991. Influence of pesticide treatments on consumption of Tetranychus urti-cae (Acarina, Tetranychidae) eggs by Phytoseiulus persimilis (Acarina, Phytoseiidae). Entomophaga, 36: 539-545. https://doi.org/10.1007/BF02374436
- Poletti M., Maia A.H.N., Omoto C. 2007. Toxicity of neonicotinoid insecticides to Neoseiulus cali-fornicus and Phytoseiulus macropilis (Acari: Phytoseiidae) and their impact on functional re-sponse to Tetranychus urticae (Acari: Tetranychidae). Biol. Control, 40: 30-36. https://doi.org/10.1016/j.biocontrol.2006.09.001
- Pozzebon A., Ahmad S., Tirello P., Lorenzon M., Duso C. 2014. Does pollen availability mitigate the impact of pesticides on generalist predatory mites? BioControl, 59: 585-596. https://doi.org/10.1007/s10526-014-9598-3
- Schmidt-Jeffris R., Beers E.H., Duso C. 2019. Integrated management of mite pests of tree fruit. In: Xu, X. and Fountain, M. (Eds), Integrated management of diseases and insect pests of tree fruit, Burleigh Dodds Science Publishing, Cambridge, UK. https://doi.org/10.19103/AS.2019.0046.20
- Schmidt-Jeffris R.A., Beers E.H., Sater C. 2021. Meta-analysis and review of pesticide non-target ef-fects on phytoseiids, key biological control agents. Pest Manag. Sci., 77: 4848-4862. https://doi.org/10.1002/ps.6531
- Stavrinides M.C., Mills N.J. 2009. Demographic effects of pesticides on biological control of Pacific spider mite (Tetranychus pacificus) by the western predatory mite (Galendromus occidentalis). Biol. Control, 48: 267-273.
- Sterk G., Hassan S.A., Baillod M., Bakker F., Bigler F., Blumel S., Bogenschutz H., Boller E., Bro-mand B., Brun J. et al. 1999. Results of the seventh joint pesticide testing programme carried out by the IOBC/WPRS-Working Group′ pesticides and beneficial organisms. BioControl, 44: 99-117. https://doi.org/10.1023/A:1009959009802
- Szabó Á., Pénzes B., Sipos P., Hegyi T., Hajdú Z., Markó V. 2014. Pest management systems affect composition but not abundance of phytoseiid mites (Acari: Phytoseiidae) in apple orchards. Exp. Appl. Acarol., 62: 525-537. https://doi.org/10.1007/s10493-013-9752-0
- Villanueva R.T., Walgenbach J.F. 2005. Development, oviposition, and mortality of Neoseiulus fal-lacis (Acari: Phytoseiidae) in response to reduced-risk insecticides. J. Econ. Entomol., 98: 2114-2120. https://doi.org/10.1093/jee/98.6.2114
- Zanuzo Zanardi O., Pavan Bordini G., Aparecida Franco A., Jacob C.R.O., Takao Yamamoto P. 2017. Sublethal effects of pyrethroid and neonicotinoid insecticides on Iphiseiodes zuluagai Denmark and Muma (Mesostigmata: Phytoseiidae). Ecotoxicology, 26: 1188-1198. https://doi.org/10.1007/s10646-017-1844-x



2023-07-06
Edited by:
Enrico de Lillo, Roberto Nannelli

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Malagnini, Valeria; Baldessari, Mario; Duso, Carlo; Pozzebon, Alberto and Angeli, Gino
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



