Experienced generation-dependent functional and numerical responses of Neoseiulus californicus (Acari: Phytoseiidae) long-term reared on thorn apple pollen
Eini, Narges  1
; Jafari, Shahriar
1
; Jafari, Shahriar  2
; Fathipour, Yaghoub
2
; Fathipour, Yaghoub  3
and Prager, Sean M.
3
and Prager, Sean M.  4
4
1Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khorramabad, Iran.
2✉ Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khorramabad, Iran.
3Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, Tehran, Iran.
4Department of Plant Sciences, College of Agriculture and Bioresources, University of Saskatchewan, Canada.
2023 - Volume: 63 Issue: 2 pages: 539-552
https://doi.org/10.24349/isgo-9oicOriginal research
Keywords
Abstract
Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Trombidiformes: Tetranychidae) is worldwide among the most economically important pests of a variety of crops (Van Leeuwen et al. 2015). This phytophagous mite feeds on over 1,100 host plant species including over 150 economic crops (Pavela 2017). Usually, control of phytophagous mites in agricultural systems occurs by chemical pesticides. However, pesticide use has drawbacks including resistance development in target pests, phytotoxicity, secondary pest outbreaks, increased production costs (labor, materials, and equipment), environment pollution, and risks to human health (Fathipour and Sedaratian 2013). Replacing the use of chemical pesticides with eco-friendly methods, such as biological control is therefore vital. The use of predaceous mites is one of the more reliable biological control strategies for pests including T. urticae (Fathipour and Maleknia 2016; Jafari et al. 2010, 2011).
Phytoseiid mites are the most important natural enemies of phytophagous mites (McMurtry et al. 1970; Jafari 2010). They are classified into two groups; specialists (types I, II) including oligophagous predators of the tetranychid mites and generalists (types III, IV) that can feed on some phytophagous mites, immature stages of some tiny insects and also plant pollen as a supplementary or alternative food source (McMurtry and Croft 1997; Moraes et al. 2004). Neoseiulus californicus (McGregor) is one of the important species of phytoseiid mites that is distributed in habitats with high temperature and low humidity (Ahn et al. 2010) in Asia, Africa, Europe and North and South America (McMurtry 1977; Moraes et al. 2004). It is a type II phytoseiid mite and an effective control agent of T. urticae on many crops (McMurtry et al. 2013).
Studies of the foraging behaviors of predators helps us to predict their performance in the field and their potential impact on population dynamics of their prey (Jervis et al. 1996; Fathipour and Maleknia 2016). A functional response is the number of prey consumed per time unit by predator as a function of prey density (Solomon 1949). Holling (1959) presented three basic types of functional response: linear (I), convex (II), and sigmoid (III). Many predators and parasitoid arthropods have exhibited a type II functional response on their prey, while vertebrate predators which exhibit the type III functional response are regarded as more efficient biocontrol agents (Xiao and Fadamiro 2010; Jafarian et al. 2022). Numerical response of predator, the number of offspring produced per time unit at different prey densities by predator, is another component of foraging behavior that can be used to assess a predator's efficiency (Cédola et al. 2001; Carrillo and Peña 2012). Functional response and numerical responses of predators may be affected by different factors including temperature (Mohaghegh et al., 2001; Jafari et al. 2012), predator's generation (Yazdanpanah et al. 2022), density of preys (Cédola et al. 2001; Carrillo and Peña 2012), predator age (Fathipour et al. 2018; Dalir et al., 2021), host plants of the prey (Madadi et al., 2007; Jafarian et al., 2022), availability of pollen as a supplement to nutritional rescources (Fathipour et al., 2020), the sex of the predator (Parajulee et al. 1994) and long-term rearing effects (Castagnoli and Simoni 1999).
Mass rearing of natural enemies for extended time periods or multiple generations has been shown to affect their foraging efficiency and quality (Sørensen et al. 2012). Long-term rearing under constant conditions may also lead to the loss of some important characters and result in inbreeding depression, resulting in poor performance of the released individuals at the release time (Sadat et al. 2021). Therefore, it is important to be take measures to ensure maintaining biocontrol agent efficacy during the rearing period and before release in the field or in the greenhouse. When the predators are released in the field for biological control, the diet used in the mass-rearing culture influences not only on their predation rate but also their oviposition rates through changes in encounter rate, success ratio, food acquisition, and conversion of food into egg production (Dicke et al. 1986; Sabelis and Janssen 1993). Thus, the success of a predator in a biological control program will depend on its efficiency in converting ingested food (ECI) (in number of prey items) into egg biomass (in number of eggs) at different prey densities (Sabaghi et al. 2011).
The role of pollen grains as an alternative diet component, and in sustaining the population of phytoseiid mites in the event of prey scarcity is well established (McMurtry and Croft, 1997). Our preliminary tests indicated that among 23 tested pollen grains of different plant species for rearing the N. californicus, feeding on Datura stramonium (thorn apple) and pistachio pollen grains lead to the best performance in the second generation (Eini et al. 2022). In comparison to pistachio, cultivation of D. stramonium is relatively easier and from the economic point of view, more pollen grains are obtained from it. Therefore, we studied the life history of N. californicus on D. stramonium pollen for 40 generations and showed that different life table parameters remained almost constant over this period (Eini et al. 2023). In this study, we evaluate the functional and numerical responses of N. californicus to T. urticae deutonymphs after long-term rearing on pollen thorn apple at G0, G10, G20, G30 and G40. The results of this research can be used to predict the efficiency of N. californicus to control T. urticae.
Material and methods
Plants
Bean plants (Phaseolus vulgaris L. var. Nine) were cultivated and grown in plastic pots (diameter 20 cm) containing sand, clay and compost (in the ratio of 1:1:1) in a greenhouse in the Faculty of Agriculture, Lorestan University, Iran, at 25±5 °C and 65±10% RH. The plants were watered every three days.
Pollen source
Thorn apple (Datura stramonium L.) pollen grains were collected from the garden of medicinal plants at the Faculty of Agriculture, Lorestan University, Khorramabad, Iran, during the Summer of 2019. After drying at 36-38 °C for two days, they were held at -20 and 4 °C for long and short-term storage, respectively (Kolokytha et al. 2011).
Stock culture of mites
The initial population of T. urticae was collected from bean leaves infested by T. urticae from the Faculty of Agriculture, Yasouj University, Kohgiluyeh and Boyer-Ahmad Province, Iran. They were reared on bean plants (P. vulgaris) in a greenhouse. Then, the individuals of T. urticae were reared on detached fresh bean leaves in a plastic container under laboratory conditions (25±1 °C, 65±5% RH, 16: 8 L: D h). The initial population of the predatory mite N. californicus was obtained from Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands). Before conducting experiments, the predatory mites were reared for 5 generations on the infested bean leaf discs with different stages of T. urticae as prey under laboratory conditions (25±1 °C, 65±5% RH, 16:8 L:D h). Laboratory colonies of N. californicus were reared in arenas that consisted of a piece of green plastic sheet (6×4 cm) with short cotton villi on a water-saturated sponge in a plastic container (9×7×4 cm) half-filled with water. The edges of the arena were covered with tissue paper submerged in the water to provide moisture and prevent predators from escaping (Walzer and Schausberger 1999).
Experimental setup
The predatory mite, N. californicus was reared on thorn apple pollen for over 40 generations and maintained under laboratory conditions as above. Thorn apple pollen grains were offered ad libitum as the only food source to the predators at every 3 days. The functional and numerical responses of N. californicus reared on thorn apple pollen in G0, G10, G20, G30 and G40 on T. urticae deutonymphs were assessed.
Experimental arenas composed of a 3×3 cm bean leaf disc, were placed on a water-saturated cloth pad in a Petri dish (8 cm diameter). Then, Petri dishes were placed in the middle of a larger Petri dish (10 cm). The edges of the bean leaf disc were covered with wet tissue paper drenched in the water to prevent predators from escaping and to provide a water source. To obtain prey mites of the same age, 150 gravid females of T. urticae were randomly taken from the colony and transferred to a bean leaf disc (4 × 7 cm). After 24 h, T. urticae females were removed from the arenas. Plastic containers with T. urticae eggs were kept and monitored until the emergence of deutonymphs. The newly emerged deutonymphs of T. urticae were transferred onto leaf discs with a fine paintbrush (number 000) at densities of 2, 4, 8, 16, 32, 64, and 128 per experimental unit. To obtain same-aged predatory mites, 100 gravid females of N. californicus reared on thorn apple pollen were randomly taken from the colony and transferred to a new rearing environment containing pollen. After 24 h, N. californicus females were removed from the arenas. Plastic containers with N. californicus eggs were kept and monitored. After emergence of the larva, they were reared on thorn apple pollen to reach adult stage. The newly emerged mated females of the predator (24 h old) after 24 h harvesting, were placed individually in experimental arenas containing different densities of T. urticae deutonymphs. This procedure repeated in G0, G10, G20, G30 and G40. After 24 h, the number of prey consumed by each female predator was counted and then predators transferred to new leaf discs without T. urticae deutonymphs to determine the numerical response. Then, the total number of eggs laid by the predatory mite over 48 hours was recorded to estimate the numerical response. Fifteen replicates were prepared for each prey density. All experiments were conducted in the laboratory at 25±1 °C, 60±10% RH and 16:8 h L: D photoperiod.
Data analyses
Functional responses were assessed in two steps (Juliano 2001): the logistic regression of the proportion of prey eaten (Na /Nt ) as a function of prey density (Nt ) was used to determine the type of functional response (Eq. (1)):
\[\frac{N_a}{N_t} = \frac{exp(P_0 + P_1 N_t + P_2 N_t^2 + P_3 N_t^3)}{1 + exp(P_0 + P_1 N_t + P_2 N_t^2 + P_3 N_t^3)} (1)\]
Where Na /Nt is the proportion of consumed prey, Na is the number of killed prey, Nt is the initial number of prey offered, and P0, P1, P2 and P3 are the intercept, linear, quadratic and cubic coefficients, respectively, estimated using the maximum likelihood method. If the sign of the linear coefficient (P1) being negative (P1 < 0), it implies a type II functional response, whereas If P1 being positive and P2 is negative shows a type III functional response (Juliano 2001).
In next step the handling time (Th ) and the attack rate or searching efficiency (a) were estimated by Rogers's random predator equation (Rogers 1972; Hassell et al. 1977).
\[N_a = N_t [1 - exp (\alpha (T_h N_a - T))] (2)\]
Where Na is the number of killed prey per experimental unit, Nt is the initial number of prey offered, T is total time available for attack (1 day or 24 h), a is the attack rate, Th is the handling time of prey by the predator (hours). Nonlinear least-squares regression was used to estimate the attack rate and the handling time parameters (Proc NLIN, SAS Institute, 2003).
In the numerical response experiment, the following equation was used to the efficiency of conversion of ingested food (ECI) into egg biomass at different prey density treatments (Omkar and Pervez 2004).
\[ECI = \frac{\text{Number of eggs laid} }{\text{Number of preys consumed} } × 100\]
The data on the number of eggs laid by N. californicus female and ECI versus prey densities were fitted using regression analysis to determine the relationship between oviposition and ECI of female predators and prey density.
The effects of prey density and predator generation and their interaction on prey consumption and the number of eggs laid were determined using the SAS general linear model (GLM) procedure (SAS Institute, 2003). Tukey's HSD test was used for subsequent pairwise comparisons. The two-way interaction between generation and density was separated by the slicing method (densities at ages and vice versa). This method is a post-ANOVA procedure where a significant interaction term is present and involves breaking the data set into separate parts (Schabenberger et al. 2000).
The predator's prey consumption data at different densities of T. urticae deutonymphs were regressed linearly against initial prey densities to determine the relationship between the
number of consumed prey and prey density. In addition, to determine the relationship between the number of prey consumed and predator generation, the data on prey attacked by predators at the highest density (128 deutonymphs) versus different predator generations were fitted using both linear and nonlinear regression analyses. The statistical analyses were performed in SAS (version 9.1) and Minitab version 18 (Minitab, 2017).
Results
Functional response
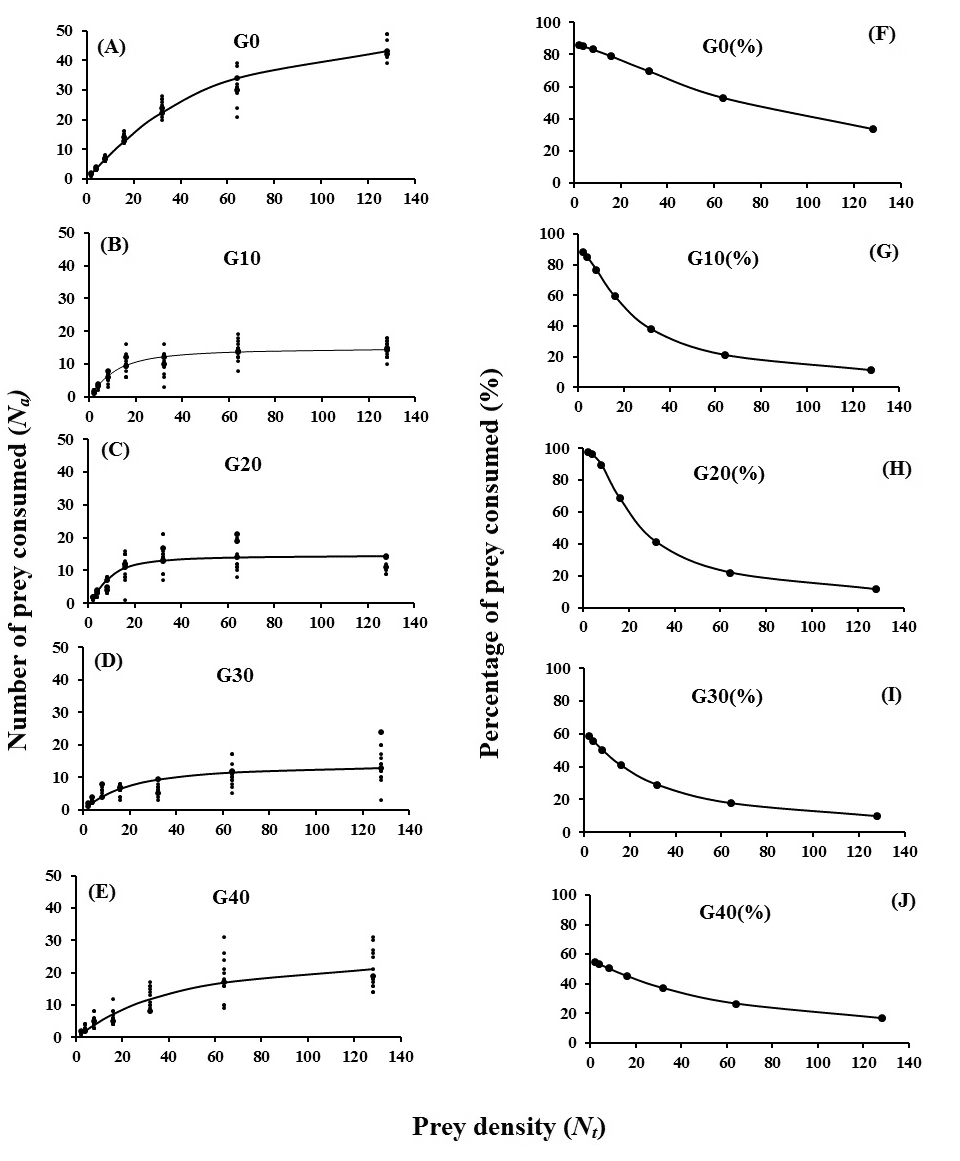


The functional response and percentage of prey consumption of N. californicus reared on the thorn apple pollen to different T. urticae densities in different generations are presented in Fig. 1. Comparison of the functional response curves demonstrated differences among different tested generations of the predator (Fig 1). The number of T. urticae killed per day increased with increasing prey density from 2 to 128. The percentage of consumed T. urticae deutonymphs decreased as the prey density increased in all tested generations (Fig 1). The linear coefficient of equation 1 (P1) in results of logistic regression analysis for predator in all tested generations was negative, indicating a type II functional response (Table 1). With increasing the number of generations from G0 (0.079 h-1) to G20 (0.182 h-1), α values increased, then decreased in G30 (0.040 h-1) and G40 (0.034 h-1) (Table 2). The Th values ranged among generations: decreased from G0 to G10, then increased to G30, and declined again in G40. The longest (1.670 h) and shortest handling time (Th ) (0.890 h) were observed in G0 and G40, respectively. The highest (26.96 prey/day) and lowest (14.371 prey/day) estimated T/Th for a female of N. californicus on T. urticae were observed in G40 and in G0, respectively (Table 2).






Estimating the predation capacity
The effects of prey density and predator generation on the predation rate of N. californicus on T. urticae deutonymph, as well as the interaction between them were significant. There was a significant difference among seven offered prey densities in every generation (Table 3). In almost all generations, the consumption rate increased with increasing the prey density (Table 4). In most generations, the highest mean consumption rate was observed at densities of 64 and 128 (Table 4). The lowest and highest number of prey consumed by N. californicus were observed at density of 2 in G10 (1.50 prey) and at density of 128 in G40 (20.73 prey), respectively (Table 4). There was no significant difference in daily consumption of predator at densities of 2, 4 and 8 among generations, but in other densities the consumed prey among generations was different (Table 4).






Numerical response
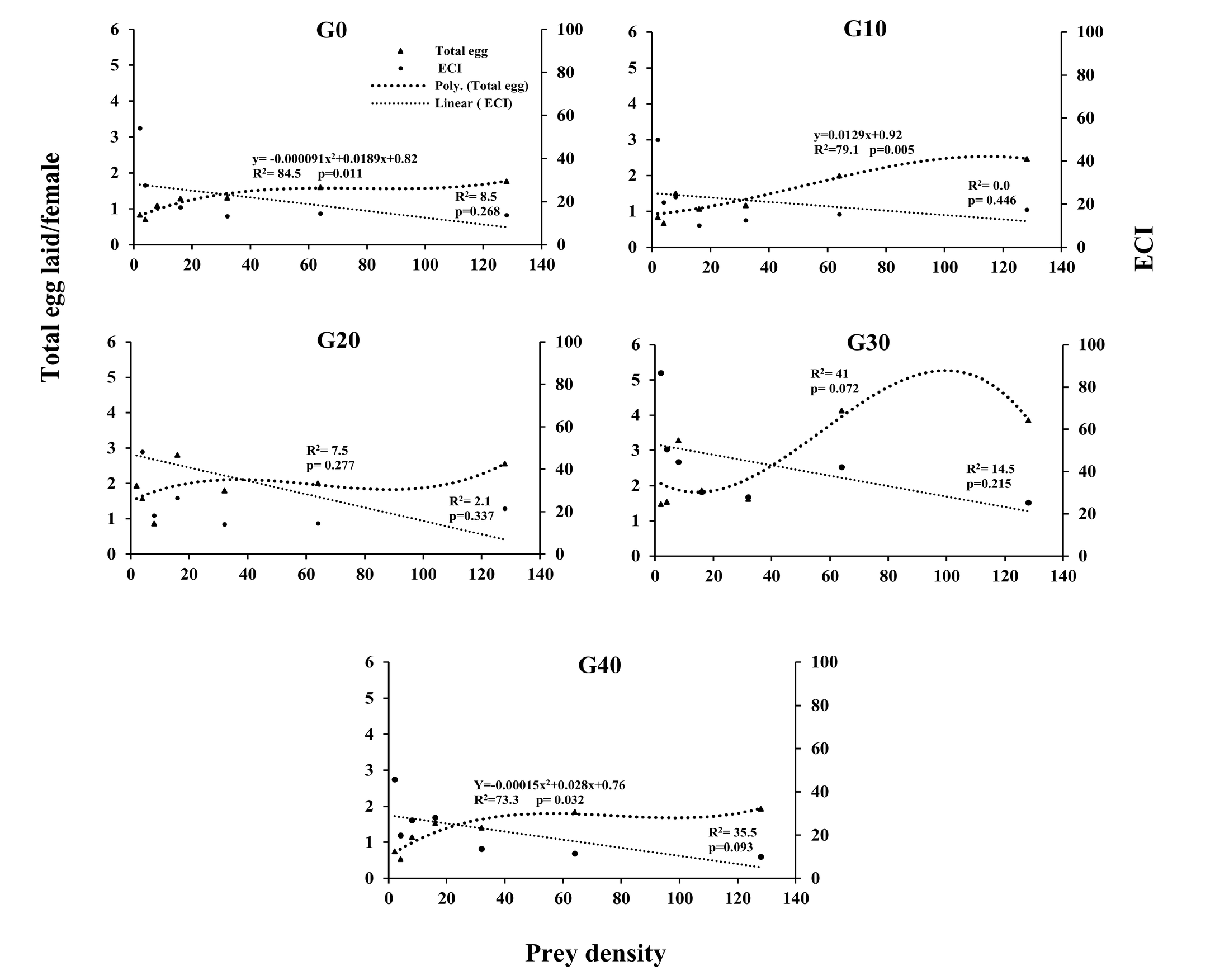


The results of linear and nonlinear regression analyses of the relationship between the prey density and eggs laid and ECI in different generations of N. californicus are shown in Figure 2. The relationship between the prey density and eggs laid in G0, G10 and G40 was significant, and the number of laid eggs increased with increasing the density. The relationship between the number of eggs laid and the prey density was linear in G10, whereas it was nonlinear in G0 and G40. The relationship between the prey density and ECI was not significant in all tested generations (Fig 2).
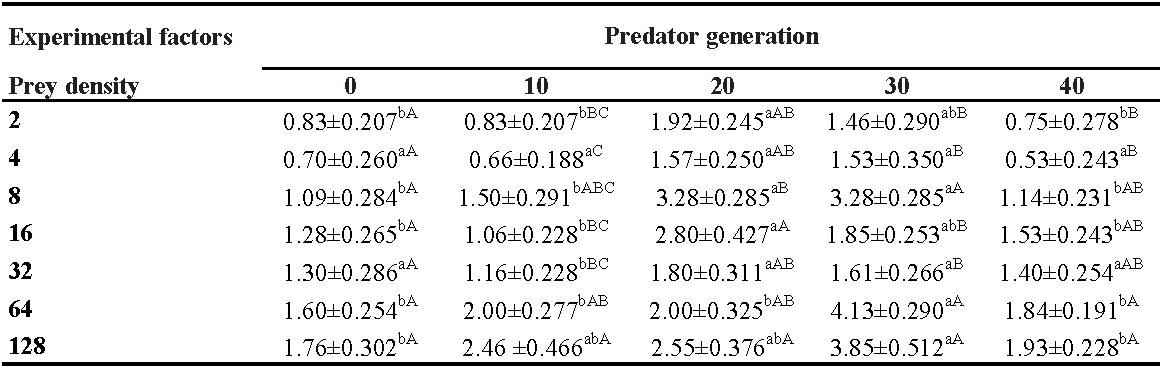
GLM procedure indicated that predator generation, prey density and interaction between them significantly affected the eggs laid (Table 5). The interaction effects between predator generation and prey density on the number of eggs laid was shown in table 6. In all tested generations, a significant difference was observed between different densities in the oviposition rate of N. californicus, except for the G0. In addition, the laid eggs increased with increasing density of the prey in all generations (Table 6). There was no significant difference in densities of 4 and 32 deutonymph density in all generations tested (Table 6). But at other densities, significant differences were observed in different generations. So that the highest number of eggs laid by N. californicus were observed at a density of 64 and 128 per G30 (Table 6).





Discussion
Functional response experiments have been frequently used to characterize and compare the effectiveness of biological control agents. These indicate that a Type II functional response is common in predators belonging to the family Phytoseiidae including: Amblyseius swirskii Athias-Henriot (Fathipour et al. 2020), Neoseiulus cucumeris (Oudemans) (Shipp and Whitfield 1991; Yazdanpanah et al. 2022), Iphiseius degenerans Berlese (Eveleigh and Chant 1981), Neoseiulus barkeri Hughes (Jafari et al. 2011), Galendromus occidentalis (Nesbitt) (Laing and Osborn 1974), Phytoseiulus longipes Evans (Badii and McMurtry 1988) and Phytoseiulus persimilis Athias-Henriot (Laing and Osborn 1974; Everson 1979; Eveleigh and Chant 1981; Skirvin and Fenlon 2001), N. californicus (Pereira de Sousa Neto et al. 2019; Khanamani et al. 2017; Ahn et al. 2010; Cédola et al. 2001), Typhlodromus bagdasarjani Wainstein & Arutunjan (Jafarian et al. 2022). Our results confirmed the type II functional response of N. californicus to deutonymphs of T. urticae in five tested generations. In addition, the results showed mass rearing on thorn apple pollen for 40 generations did not affect the type of functional response in the face of T. urticae. This type of functional response suggests that the percentage of killed prey by the predator is inversely related to prey density and this predator would be more efficient at low or moderate densities of T. urticae deutonymphs. These findings are consistent with those reported by Khanamani et al. (2017), who indicated a type II functional response for N. californicus reared over 20 generations on almond pollen to deutonymphs of T. urticae. Sometimes the functional response of phytoseiid mites may change from type II to type III in response to morphological characters of the host plant (Skirvin and Fenlon 2001), temperature (Skirvin and Fenlon 2003), prey stage (Ganjisaffar and Perring 2015), age of predator (Dalir et al. 2021) and prey species (Escudero and Ferragut 2005).
The attack rate (a) and handling time (Th ) coefficients help us to assess the magnitude of functional responses (Pervez and Omkar 2006). Our results show that with increasing number of generations from G0 to G20 the attack rate (α) increased, then it began to decrease. Handling time provides information about the efficiency of a predator, as well as reflects the cumulative time spent to capture, kill, and digest the prey (Holling 1959). The handling time values were different in tested generations. The longest and shortest handling times were recorded in G0 and in G40 respectively, indicating that N. californicus spent less time resting or doing other activities such as hunting after mass rearing on pollen in G40, which suggest that predators could consume more prey in each time interval. According to our results, by continuing the mass-rearing process the predator recovered its efficiency, as shown by a decrease in handling time. Yazdanpanah et al. (2022) showed that the handling time of N. cucumeris decreased with mass-rearing up to 20 generations and then increased in G30. Behavioral changes in natural enemies under long-term rearing may occur due to environmental or genetic adaptability (Evenden et al. 2002). When the phytoseiids were transferred to a new diet, they were presumably not identical with respect to satiation levels, because the previous food, even though it had been supplied ad libitum, did not have the same nutritional quality. In fact, N. californicus shows different rates of increase when continuously fed on each of these food types (Castagnoli and Simoni 1991). Furthermore, a phytoseiid subjected to any change needs some time to adapt to the new condition (Castagnoli et al. 2001). In G20 N. californicus reared on the thorn apple pollen showed the highest attack rate (a) (0.182 prey/d) with a handling time of 1.611 h. But Khanamani et al. (2017) showed the attack rate and handling time of N. californicus reared on the almond pollen was 0.114 (prey/d) and 0.333 (h), respectively in G20, differently from our results. The difference between these findings may be due to the differences in nutritional histories of the predator, rearing techniques, the quality of the pollen used, difference in genetic populations, the difference in age and stage of the tested predator, and differences in the quality of T. urticae used as food.
Similar to our findings, Yazdanpanah et al. (2022) showed that the relationship between the ECI of female predator and prey density was not significant in all tested generations. Our findings showed a significant relationship between the number of eggs laid by N. californicus and the density of T. urticae deutonymphs in G0, G10 and G40. The oviposition rate of phytoseiid mites is related to predation because they always devote a major fraction of food ingested to egg production (Sabelis and Janssen 1994). The relationship between the number of eggs laid and the prey density was linear in G10 and the number of eggs increased by increasing the prey number; the rate of oviposition rapidly increased with increasing prey density in G0 and G40 and the relationship between them was nonlinear, with increasing rate slow at higher densities. The plateau of oviposition rate of predatory mites at high prey densities is possibly due to the satiation of the nutrient constraints for egg production and the limitation of females to lay not more than a certain number of eggs at high prey densities (Omkar and Pervez 2004; Fathipour et al. 2020). Considering that this predator is nutritionally type II (capable of controlling two-spotted spider mites on various crops), it can also consume other mite species, small insects, or pollen when the primary prey is unavailable (McMurtry and Croft 1997). The differences observed over generations can be explained by the food habits of the predator because long-term rearing of biological agents on alternative foods may alter their different physiological and behavioral properties including foraging behaviors and the predation rate of the reared biocontrol agents under constant conditions may be influenced when exposed to the main prey (Pratissoli et al. 2004; Ansari Shiri et al. 2022; Castagnoli and Simoni 1999). The results of these findings in G0 and G40 are similar to those reported for N. californicus on T. urticae (Khanamani et al. 2017) and for A. swirskii on T. urticae (Bazgir et al. 2020). Furthermore, there was a significant relationship between predator generation and prey density on oviposition of N. californicus: after long-term rearing (40 generations) of N. californicus on thorn apple pollen, the oviposition rate at all densities is not significantly different between G0 and G40. Therefore, the thorn apple can be a suitable alternative food for the long-term rearing of N. californicus.
Our findings suggested that mean prey consumption of N. californicus increased with increasing prey density in all generations. Our results also indicated that at the highest prey density in all generations, there was a significant difference between the G40 and other generations. On the other hand, the results showed the longest handling time was recorded in G0 (1.670 h), G3 (1.657 h), G20 (1.611 h), and G10 (1.594 h), showing a lower mean consumption rate than G40 (0.890 h) at the higher prey densities. The results of these findings are similar to those observed for N. cucumeris on eggs of T. urticae (Dalir et al. 2021), N. cucumeris on nymphs of T. urticae (Yazdanpanah et al. 2022) N. californicus on T. urticae (Zheng et al. 2017), A. swirskii on T. urticae in presence and absence of pollen (Fathipour et al. 2020).
The long-term rearing of the predator on alternative foods and supplementary diet is one of the influencing factors on the functional response (Fathipour et al. 2020; Khanamani et al. 2017). Also, Sarwar et al. (2009) indicated that the predator has adapted to the prey that has been its usual diet for a long time. Our findings show that long-term rearing on thorn apple pollen did not affect the type of functional response of N. californicus, but could affect the number of eggs laid and the number of prey consumed. However, the efficiency of the predator mite was not significantly different in the first and last generations. Neoseiulus californicus could be an efficient biocontrol agent of T. urticae in long-term rearing on thorn apple pollen under controlled conditions. However, more research such as greenhouse studies are needed to investigate the efficacy of mass-reared predators under natural conditions.
Acknowledgments
This study is a part of the Ph.D. dissertation of the first author that was funded by Lorestan University, which is greatly appreciated.
References
- Ahn J.J., Kim K.W., Lee J.H. 2010. Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J. Appl. Entomol., 134: 98-104. https://doi.org/10.1111/j.1439-0418.2009.01440.x
- Ansari-Shiri H., Fathipour Y., Hajiqanbar H., Riahi E., Riddick E.W. 2022. Quality control of the predatory mite Amblyseius swirskii during long-term rearing on almond Prunus amygdalus pollen. Arthropod Plant Interact, 16(6): 645-655. https://doi.org/10.1007/s11829-022-09929-6
- Badii M.H., McMurtry J.A. 1988. Effect of prey density on functional and reproductive response of the predatory mite Phytoseiulus longipes (Acari: Phytoseiidae). Int. J. Acarol., 14: 61-69. https://doi.org/10.1080/01647958808683789
- Bazgir F., Shakarami J., Jafari S. 2020. Prey-stage preferences, functional and numerical responses, and mutual interference of Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Eotetranychus frosti (Tetranychidae). Int. J. Acarol., 46: 185-191. https://doi.org/10.1080/01647954.2020.1734657
- Castagnoli M., Simoni S. 1999. Effect of long-term feeding history on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 23: 217-234.
- Castagnoli M., Simoni S., Nachman G. 2001. Short-term changes in consumption and oviposition rates of Neoseiulus californicus strains (Acari: Phytoseiidae) after a diet shift. Exp. Appl. Acarol., 25: 969-983. https://doi.org/10.1023/A:1020639301898
- Castagnoli M., Simoni S. 1991. Influenza della temperatura sull′incremento delle popolazioni di Amblyseius californicus (McGregor) (Acari: Phytoseiidae). Redia LXXIV, 621–640.
- Carrillo D., Pena J.E. 2012. Prey-stage preferences and functional and numerical responses of Amblyseius largoensis (Acari: Phytoseiidae) to Raoiella indica (Acari: Tenuipalpidae). Exp. Appl. Acarol., 57: 361-372. https://doi.org/10.1007/s10493-011-9488-7
- Cédola C.V., Sánchez N.E., Liljesthröm G.G. 2001. Effect of tomato leaf hairiness on functional and numerical response of Neoseiulus californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 25: 819-831. https://doi.org/10.1023/A:1020499624661
- Dalir S., Hajiqanbar H., Fathipour Y., Khanamani M. 2021. Age-dependent functional and numerical responses of Neoseiulus cucumeris (Acari: Phytoseiidae) on two-spotted spider mite (Acari: Tetranychidae). J. Econ. Entomol., 114: 50-61. https://doi.org/10.1093/jee/toaa266
- Dicke M., Sabelis M.W. Groeneveld A. 1986. Vitamin-A deficiency modifies response of predatory mite Amblyseius potentillae to volatile kairomone of two-spotted spider mite Tetranychus urticae. J. Chem. Ecol., 12: 1389-1396. https://doi.org/10.1007/BF01012358
- Eini N., Jafari S., Fathipour Y., Zalucki M.P. 2022. How pollen grains of 23 plant species affect performance of the predatory mite Neoseiulus californicus. BioControl., 1-15. https://doi.org/10.1007/s10526-022-10129-7
- Eini N., Jafari S., Fathipour Y. 2023. The quality assessment of Neoseiulus californicus (Phytoseiidae) reared on thorn apple pollen for 40 generations. Biocontrol Sci. Technol. https://doi.org/10.1080/09583157.2023.2173144
- Escudero L.A., Ferragut F. 2005. Life-history of predatory mites Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae) on four spider mite species as prey, with special reference to Tetranychus evansi (Acari: Tetranychidae). Biolog. Control., 32: 378-384. https://doi.org/10.1016/j.biocontrol.2004.12.010
- Eveleigh E.S., Chant D.A. 1981. Experimental studies on acarine predatory prey interactions: effect of predator age and feeding history on the prey consumption and functional response (Acarina: Phytoseiidae). Can. J. Zool., 59: 1387-1406. https://doi.org/10.1139/z81-191
- Evenden M.L., Spohn B.G., Moore A.J., Preziosi R.F., Haynes K.F. 2002. Inheritance and evolution of male response to sex pheromone in Trichoplusia ni (Lepidoptera: Noctuidae). Chemoecol., 12: 53-59. https://doi.org/10.1023/A:1020499624661
- Everson P. 1979. The functional response of Phytoseiulus persimilis (Acarina: Phytoseiidae) to various densities of Tetranychus urticae (Acarina: Tetranychidae). Can. Entomol., 111: 7-10. https://doi.org/10.4039/Ent1117-1
- Fathipour Y., Karimi M., Farazmand A., Ali A.T. 2018. Age-specific functional response and predation capacity of Phytoseiulus persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia, 58: 31-40. https://doi.org/10.24349/acarologia/20184225
- Fathipour Y., Maleknia B. 2016. Mite predators. In Ecofriendly pest management for food security pp. 329-366. https://doi.org/10.1016/B978-0-12-803265-7.00011-7
- Fathipour Y., Maleknia B., Bagheri A., Soufbaf M., Reddy G.V. 2020. Functional and numerical responses, mutual interference, and resource switching of Amblyseius swirskii on two-spotted spider mite. Biolog. Control., 146: 104266. https://doi.org/10.1016/j.biocontrol.2020.104266
- Fathipour Y., Sedaratian A. 2013. Integrated management of Helicoverpa armigera in soybean cropping systems. Soybean-Pest Resistance. InTech, Rijeka, Croatia pp. 231- 280. https://doi.org/10.5772/54522
- Ganjisaffar F., Perring T.M. 2015. Prey stage preference and functional response of the predatory mite Galendromus flumenis to Oligonychus pratensis. Biolog. Control., 82: 40-45. https://doi.org/10.1016/j.biocontrol.2014.12.004
- Hassell M.P., Lawton J.H., Beddington J.R. 1977. Sigmoid functional response by invertebrate predators and parasitoids. J. Animal. Ecol., 46: 249-262. https://doi.org/10.2307/3959
- Holling C.S. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol., 91: 385-398. https://doi.org/10.4039/Ent91385-7
- Jafari S., Fathipour Y., Faraji F. 2012. Temperature-dependent development of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) at seven constant temperatures. Insect. Sci., 19: 220-228. https://doi.org/10.1111/j.1744-7917.2011.01444.x
- Jafari S. 2010. Phytoseiid mites of the Lorestan province and determining the predation efficiency of Neoseiulus barkeri (Phytoseiidae) [Ph.D. thesis]. Faculty of Agriculture, Tarbiat Modares University. 192 pp.
- Jafari S., Fathipour Y., Faraji F., Bagheri M. 2010. Demographic response to constant temperatures in Neoseiulus barkeri (Phytoseiidae) fed on Tetranychus urticae (Tetranychidae). Syst. Appl. Acarol., 15: 83-99. https://doi.org/10.11158/saa.15.2.1
- Jafari S., Fathipour Y., Faraji F. 2011. The influence of temperature on the functional response and prey consumption of Neoseiulus barkeri (Phytoseiidae) on two-spotted spider mite. JESI 31: 39-52.
- Jafarian F., Jafari S., Fathipour Y. 2022. Functional response of the predatory mite, Typhlodromus bagdasarjani (Acari: Phytoseiidae) to protonymphs of Eotetranychus frosti (Acari: Tetranychidae) on four apple cultivars. Acarologia, 62(2): 454-464. https://doi.org/10.24349/7ejy-uk7s
- Jervis M.A., Kidd N.A., Jervis M., Kidd N. 1996. Practical approaches to their study and evaluation. Chapman and Hall, London p.375-394. https://doi.org/10.1007/978-94-011-0013-7_6
- Juliano S.A. 2001. Nonlinear curve fitting: predation and functional response curves, pp. 178–196. In S. M. Scheiner and J. Gurevitch (eds.), Design and analysis of ecological experiments. Oxford University Press, New York.
- Khanamani M., Fathipour Y., Talebi A.A., Mehrabadi M. 2017. Quantitative analysis of long-term mass rearing of Neoseiulus californicus (Acari: Phytoseiidae) on almond pollen. J. Econ. Entomol., 110: 1442-1450. https://doi.org/10.1093/jee/tox116
- Kolokytha P.D., Fantinou A.A., Papadoulis, G.T. 2011. Effect of several different pollens on the bio-ecological parameters of the predatory mite Typhlodromus athenas Swirski and Ragusa (Acari: Phytoseiidae). Environ. Entomol., 40(3): 597-604. https://doi.org/10.1603/EN10276
- Laing J.E., Osborn J.A. 1974. The effect of prey density on the functional and numerical responses of three species of predatory mites. Entomophaga., 19: 267-277. https://doi.org/10.1007/BF02371052
- Madadi H., Enkegaard A., Brodsgaard H. F., Kharrazi-Pakdel A., Mohaghegh J., Ashouri A. 2007. Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. J. Appl. Entomol., 131: 728-733. https://doi.org/10.1111/j.1439-0418.2007.01206.x
- McMurtry J.A. 1977. Some predaceous mites (Phytoseiidae) on citrus in the Mediterranean region. Entomophaga., 22: 19-30. https://doi.org/10.1007/BF02372986
- McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annu Rev Entomol., 42:291–321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., De Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- McMurtry J., Huffaker C., Van de Vrie M. 1970. Ecology of tetranychid mites and their natural enemies: A review. I. Tetranychidae enemies: their biological characters and the impact of spray practices. Hilgardia., 40: 331-390. https://doi.org/10.3733/hilg.v40n11p331
- Minitab. 2017. Minitab 18 statistical software. State College (PN): Minitab Inc.
- Mohaghegh J., De Clercq P., Tirry L. 2001. Functional response of the predators Podisus maculiventris (Say) and Podisus nigrispinus (Dallas) (Het., Pentatomidae) to the beet armyworm, Spodoptera exigua (Hubner) (Lep., Noctuidae): effect of temperature. J. Appl. Entomol., 125: 131-134. https://doi.org/10.1046/j.1439-0418.2001.00519.x
- Moraes G.J., McMurtry J.A., Denmark H.A., Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa., 434: 1-494. https://doi.org/10.11646/zootaxa.434.1.1
- Omkar O., Pervez A. 2004. Functional and numerical responses of Propylea dissecta (Mulsant) (Coleoptera: Coccinellidae). J. Appl. Entomol., 128: 140-146. https://doi.org/10.1111/j.1439-0418.2004.00824.x
- Parajulee M. N., Phillips T. W., Hogg D. B. 1994. Functional response of lyctocoris campestris (F.) adults: Effects of predator sex, prey species and experimental habitat. Biological Control, 4 (1): 80-87. https://doi.org/10.1006/bcon.1994.1014
- Pavela R. 2017. Extract from the roots of Saponaria officinalis as a potential acaricide against Tetranychus urticae. J. Pest. Sci., 90: 683−692. https://doi.org/10.1007/s10340-016-0828-6
- Pervez A., Omkar O. 2006. Ecology and biological control application of multicoloured Asian ladybird, Harmonia axyridis: a review. Biocontrol. Sci. Technol., 16: 111-128. https://doi.org/10.1080/09583150500335350
- Pereira de Sousa Neto E., Filgueiras R.M., Mendes J.A., Melo J.W. 2019. Functional and numerical responses of Neoseiulus idaeus and Neoseiulus californicus to eggs of Tetranychus urticae. Int. J. Acarol., 45: 6-7. https://doi.org/10.1080/01647954.2019.1638965
- Pratissoli D., Oliveira H.N., Gonc alves J.R., Zanuncio J.C. Holtz A.M. 2004. Changes in biological characteristics of Trichogramma pretiosum (Hym.: Trichogrammatidae) reared on eggs of Anagasta kuehniella (Lep.: Pyralidae) for 23 generations. Biocontrol. Sci. Technol, 14(3): 313-319. https://doi.org/10.1080/09583150310001639196
- Rogers D. 1972. Random search and insect population models. J. Animal. Ecol., 41: 369-383. https://doi.org/10.2307/3474
- Sabaghi R., Hosseini R., Sahragard A. 2011. Functional and numerical responses of Scymnus syriacus Marseul (Coleoptera: Coccinellidae) to the black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae) under laboratory conditions. J. Plant. Prot. Res., 51: 423-428. https://doi.org/10.2478/v10045-011-0070-4
- Sabelis M.W., Janssen A. 1994. Evolution of life-history patterns in the Phytoseiidae. In: Houck MA, editor. Mites. New York, NY: Chapman and Hall; p. 70-98. https://doi.org/10.1007/978-1-4615-2389-5-4
- Sabelis M.W., Janssen A. 1993. Evolution of life-history patterns in the Phytoseiidae. In: Houck M.A. (ed.), Mites: ecological and evolutionary analyses of life-history patterns. Chapman & Hall, New York, pp. 70-98. https://doi.org/10.1007/978-1-4615-2389-5_4
- Sadat F., Nazari A., Jafari S., Karahroudi Z.R. 2021. How long‑term mass rearing affects the quality of the Trichogramma embryophagum (Hartig) (Hymenoptera: Trichogrammatidae) reared on Sitotroga cerealella (Olivier) eggs. Egypt. J. Biol. Pest. Control., 31: 1-12. https://doi.org/10.1186/s41938-021-00456-9
- Sarwar M. 2016. Comparative life history characteristics of the mite predator Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) on mite and pollen diets. Int. J. Pest Manag, 62(2): 140-148. https://doi.org/10.1080/09670874.2016.1146806
- SAS Institute. 2003. GLM: a guide to statistical and data analysis, version 9.1. Cary (NC): SAS Institute.
- Schabenberger O., Gregoire T.G., Kong F. 2000. Collections of simple effects and their relationship to main effects and interactions in factorials. American. Statistician., 54: 210-214. https://doi.org/10.1080/00031305.2000.10474547
- Shipp J.L., Whitfield G.H. 1991. Functional response of the predatory mite, Amblyseius cucumeris (Acari: Phytoseiidae), on western flower thrips, Frankliniella occidentalis (Thysanoptera: Thripidae). Environ. Entomol., 20: 694-699. https://doi.org/10.1093/ee/20.2.694
- Skirvin D.J., Fenlon J.S. 2001. Plant species modifies the functional response of Phytoseiulu spersimilis (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae): implications for biological control. Bull. Entomol. Res., 91: 61–67. https://doi.org/10.1079/BER200063
- Skirvin D.J., Fenlon J.S. 2003. The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol, 31: 37-49. https://doi.org/10.1023/B:APPA.0000005107.97373.87
- Solomon M.E. 1949. The natural control of animal populations. J. Animal. Ecol., 18: 1-35. https://doi.org/10.2307/1578
- Sørensen J.G., Addison M.F., Terblanche J.S. 2012. Mass-rearing of insects for pest management: challenges, synergies and advances from evolutionary physiology. Crop. Prot., 38: 87-94. https://doi.org/10.1016/j.cropro.2012.03.023
- Van Leeuwen T., Tirry L., Yamamoto A., Nauen R., Dermauw W. 2015. The economic importance of acaricides in the control of phytophagous mites and an update on recent acaricide mode of action research. Pestic. Biochem. physiol., 121: 12-21. https://doi.org/10.1016/j.pestbp.2014.12.009
- Walzer A., Schausberger P. 1999. Predation preferences and discrimination between con and heterospecific prey by the phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus. BioControl 43: 469-78. https://doi.org/10.1023/A:1009974918500
- Xiao Y., Fadamiro H.Y. 2010. Functional response and prey-stage preferences of species of predacious mites (Acari: Phytoseiidae) on citrus red mite, Panonychus citri (Acari: Tetranychidae). Biolog Control 53: 345-352. https://doi.org/10.1016/j.biocontrol.2010.03.001
- Yazdanpanah S., Fathipour Y., Riahi E., Zalucki M.P. 2022. Generation-dependent functional and numerical responses of Neoseiulus cucumeris (Acari: Phytoseiidae) long-term reared on almond pollen. Biocontrol Sci Technol 1-13. https://doi.org/10.1080/09583157.2021.2023831
- Zheng Y., De Clercq P., Song Z.W., Li D.S., Zhang B.X. 2017. Functional response of two Neoseiulus species preying on Tetranychus urticae Koch. Syst Appl Acarol 22: 1059-1068. https://doi.org/10.11158/saa.22.7.13



2022-11-02
Date accepted:
2023-05-03
Date published:
2023-05-04
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Eini, Narges; Jafari, Shahriar; Fathipour, Yaghoub and Prager, Sean M.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



