Mite fauna on Dittrichia species (Asteraceae) in Syrian costal region: new records and primary observations on the behavior of Typhloseiella isotricha (Athias-Henriot) (Meostigmata: Phytoseiidae)
Ebrahim, Walaa  1
and Barbar, Ziad
1
and Barbar, Ziad  2
2
1Department of Plant Protection, Faculty of Agriculture, Al-Baath University, P.O. Box 77, Al-Sham St., Homs, Syria.
2✉ Department of Plant Protection, Faculty of Agriculture, Al-Baath University, P.O. Box 77, Al-Sham St., Homs, Syria.
2023 - Volume: 63 Issue: 2 pages: 529-538
https://doi.org/10.24349/ma1r-1i1nOriginal research
Keywords
Abstract
Introduction
Predatory mites of the family Phytoseiidae (Acari: Mesostigmata) are of great importance for integrated pest management in various agroecosystems. Some Phytoseiidae species are widely used as biological agents for controlling phytophagous mites and other pests (Chant and McMurtry 2007; McMurtry et al. 2013). Researches about this family were progressively advanced in Syria during the last years. In total, thirty-one species of these predators were recorded until now, particularly in Latakia governorate (Barbar and Negm, 2022). These predators were almost collected from citrus orchards, vegetables crops, and from about forty plant species of natural vegetation surrounding agro-ecosystems (e.g. Barbar, 2013, 2014, 2016; Barbar et al. 2022). Of these natural vegetation, Dittrichia viscosa (L.) Greuter (Asteraceae) seems to be one of the most abundant species in the coastal region of Syria, and D. graveolens (L.) Greuter is another species accompanied the former in few localities. The two Dittrichia species are common in the Mediterranean region (Gökbulut et al. 2013; Parolin et al. 2014) and the former has been investigated against insects and phytophagous mites, and as the host plant of efficient generalist predators (Topakci et al. 2005; Alexenizer and Dorn, 2007; Ingegno et al. 2011). However, the number of predatory mite species (in particular those of the family Phytoseiidae) collected from these plant species were very low worldwide. In fact, only Amblyseiella setosa Muma and Typhloseiella isotricha (Athias-Henriot) were observed on D. graveolens and D. viscosa, respectively (Demite et al. 2022). In Syria, T. isotricha is the unique species collected from D. viscosa (Barbar, 2016). Although this predator was observed in 13 countries of the Mediterranean region (Demite et al. 2022), nothing is known about its ecology or associated prey.
The objectives of the present work were to determine phytophagous and predatory mites on D. viscosa and D. graveolens plants in the coastal region of Syria (Tartous and Latakia governorates), and to identify, the eventual source of feeding of most abundant predator for future evaluations of predation capacity and feeding habits.
Material and methods
Sampling and identification of mites
Surveys of mite fauna present on D. viscosa and on D. graveolens were conducted in Tatrus and Latakia governorates in June, August and September 2021, and in June, July and August 2022. About 150 leaves of Dittrichia species were collected in each locality. Sampling dates, geographic coordinates and altitude above sea level (a.a.s.l. in meter) for each sampling location are presented in table 1.



The mites were collected from leaves of Dittrichia species using the ''dipping-checking-washing-filtering'' method (Boller, 1984), and then mounted on slides in Hoyer's medium and dried at 45-50 °C for a week.
Mite taxa were identified to family levels using the key of Krantz and Walter (2009). Identification to infra-family level was carried out mainly using the following works: (1) for Mesostigmata: Phytoseiidae (Chant and McMurtry, 2007); (2) for Sarcoptiformes: Acaridae (Smiley 1991; Zhang 2003); (3) for Trombidiformes: Camerobiidae (Du Toit et al. 1998; Khanjani and Ueckermann, 2002) Erythraeidae (Southcott, 1961; Xu et al. 2019); Raphignathidae (Khanjani and Ueckermann, 2003; Fan and Zhang, 2005; Nasrollahi et al. 2018), Tenuipalpidae (Meyer 1979; Mesa et al. 2009), and Tetranychidae (Bolland et al. 1998). The specimens were deposited in the Arthropod Collection of the Department of Plant Protection, Faculty of Agriculture, Al-Baath University, Homs, Syria.
Phytoseiids on leaves infected by the rust fungus Coleosporium inulae
In the above surveys, relatively high number of phytoseiid specimens was observed in some collected samples of D. viscosa leaves infected by the rust fungus Coleosporium inulae Rabenhorst. To verify the effect of the presence of this fungus on phytoseiid mite abundances, a sample of 160 D. viscosa leaves were collected, divided them into four groups (40 leaves in each group): 1. small leaves (dimensions ranged from 1.8-6.0 long X 0.4-1.3 cm wide) without rust spots, 2. small leaves with rust spots, 3. big leaves (dimensions ranged from 6.4-10.3 long X 1.8-3.4 cm wide) without rust spots, and 4. big leaves with rust spots. The number of phytoseiid mites and rust spots were then counted on each leaf in each group using a binocular microscope. As no mite specimens were observed on small leaves without rust spots, this group of leaves was excluded from statistical analysis. As data were not normally distributed, a Kruskal-Wallis non-parametric analysis of variance followed by multiple comparisons between ranks (IBM® SPSS® version 20, 2011) were carried out to compare phytoseiids abundance between the three remaining groups of leaves. Generalized linear models initially showed that the mites on the three groups of D. viscosa leaves mentioned above followed a negative binomial distribution. Therefore, two aggregation indexes were studied. The first index was the variance/mean ratio (I), (I = s2/m), in which I = 1 indicates a random spatial distribution; I < 1, uniform distribution and I < 1, aggregate distribution. The second index was the k exponent of the negative binomial distribution (k = m2/S2-m), in which negative value of k indicates a uniform distribution, low and positive value (0 < k < 2) suggests a highly aggregate distribution, value between two and eight refers to a moderate aggregation, and value higher than eight is related with random distribution (Fernandes et al. 2019). To describe quantitatively the eventual effects of the presence of C. inulae rust spots on patterns of phytoseiids distribution, the data (numbers of mites and rust spots) were used to estimate the coefficient of negative bionomial distribution (B) and its exponent [Exp (B)].
Results and discussion
New mite records
Mites were found in 31 of 38 sampled localities. A total of 13 mite species belonging to seven families (Acaridae, Camerobiidae, Erythraeidae, Phytoseiidae, Raphignathidae, Tenuipalpidae, and Tetranychidae) are reported on Dittrichia species in the present study (Table 2). Of these, three identified species are recorded for the first time from Syria and belong to Raphignathidae (one species), Erythraeidae (one species) and Tenuipalpidae (one species). These new records are presented thereafter.



Erythraeus (Zaracarus) passidonicus Haitlinger, family Erythraeidae: two larvae were collected from D. viscosa, Al-Matin, Tartous governorate, 17.7.2021. This species was described from a unique larva in Greece and was re-described from three larvae in Turkey (Haitlinger, 2006, 2010).
Raphignathus collegiatus Atyeo, Baker and Crossley, family Raphignathidae: one female and two males were collected from D. viscosa, Almderjat, Tartous governorate, 25.6.2022. This species is known from China, Egypt, former USSR, USA and Turkey (Doğan and Ayyildiz, 2003).
Brevipalpus rotai (Castagnoli & Pegazzano), family Tenuipalpidae: one female was collected from D. viscosa, Hosn Souliman, Tartous governorate, 13.6.2022. This species is known from Greece, Italy and Turkey on Olea europaea L. (Castro et al. 2022). This is the first report of this species on D. viscosa although the absence of olive orchards near plants from which the samples were taken.
Domination of Phytoseiidae on Dittrichia species



The most abundant family observed on Dittrichia species was Phytoseiidae with four species representing about 86% of total collected mite specimens. Typhloseiella isotricha (Athias-Henriot) was the dominant species with about 73% and 85% of total collected mite specimens and total collected phytoseiids, respectively (Figure 1). This species was collected from D. viscosa in 16 locations and from D. graveolens in one location (Table 2). It was found in association with the phytophagous mite, B. obovatus, B. rotai and Tetranychus sp. It has been already reported on D. viscosa in Latakia governorate, Syria (Barbar, 2016) and was exclusively found on D. viscosa worldwide (Demite et al. 2022).
Typhlodromus (Anthoseius) rhenanus (Oudemans) was relatively abundant. All specimens of this species were collected from D. viscosa in nine locations in Tartous governorate (Table 2). These specimens represent 11% and 15% of total collected mite specimens and total collected phytoseiids, respectively (Figure 1). This is the first record of this predator on D. viscosa in the world (Demite et al. 2022). It was found in association with the phytophagous mite B. obovatus in the present study and it has been already reported on various herbaceous, shrub and arboreal plants in Syria (Barbar and Negm, 2022). Other phytoseiid species were sporadic and its presence on Dittrichia seems to be accidental.
Other mites on Dittrichia species



Excluding the mite species mentioned above, three predaceous and three phytophagous mite species were also recorded on Dittrichia in the present study. For the first group, only one female of the predatory mite Neophyllobius sp. (Camerobiidae) was collected from D. graveolens in Tartous governorate. As some leg segments were missed in the collected specimen and the failed tentative to re-collect additional ones, it was impossible to identify this specimen to species level. A unique species of this predatory mite family is known from Syria: Neophyllobius persiaensis Khanjani & Ueckermann, collected from Citrus limon (L.) Osbeck and from soil litter in Latakia governorate (Barbar, 2016). The second species was Erythraeus (Erythraeus) adanaensis (Saboori & Çobanoğlu) (Erythraeidae). Two larvae were observed on D. graveolens and D. viscosa (Table 2). It has been already collected from leaves of eggplant Solanum melongena L. in Latakia governorate (Barbar, 2018). The third species, Raphignathus gracilis (Rack) (Raphignathidae) was collected only on D. viscosa (Table 2). This predator was reported also from C. limon and from ground litter in Latakia governorate (Barbar, 2016).


Concerning phytophagous mite species, seven specimens of the mould mite Tyrophagus putrescentiae (Schrank) (Acaridae) were collected from D. viscosa in five locations (Table 2). It has already been observed on some plant species, and was collected from ground litter in Latakia governorate (Barbar, 2016). The second species was B. obovatus (Tenuipalpidae) found only on D. viscosa (Table 2). It has already been collected from Eriobotrya japonica (Thunb.) Lindl. and Punica granatum L. in Latakia governorate (Barbar, 2016, 2018). On the other hand, nine Tetranychus sp. (Tetranychidae) specimens were collected from D. graveolens and D. viscosa in six locations (Table 2). It was impossible to identify the specimens to species level as they were collected in female stage.
Coleosporium inulae seems as eventual source of alimentation of T. isotricha
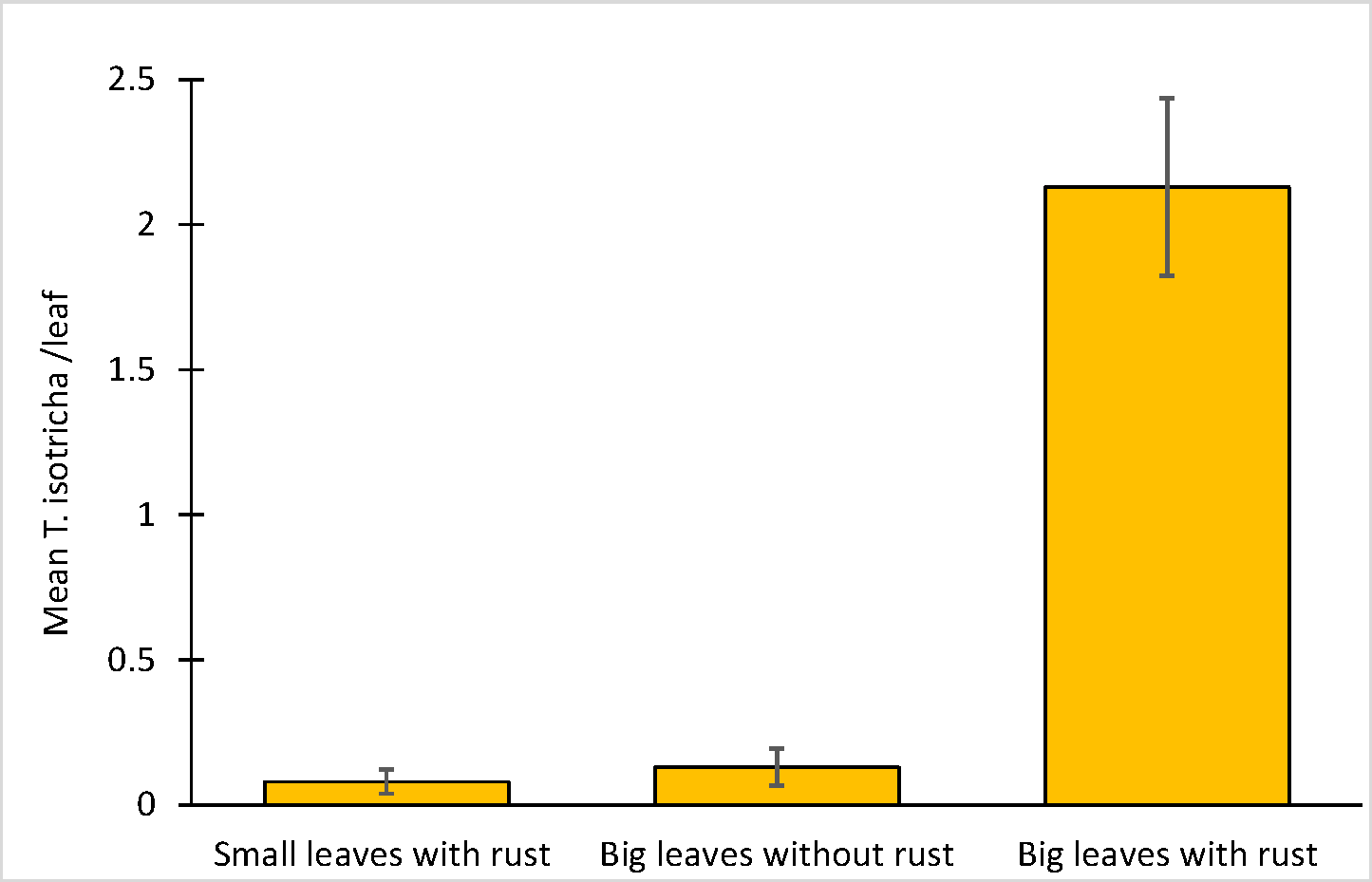
Typhloseiella isotricha was observed on D. viscosa near or close to the opened spots of rust (Figure 2). The highest density of this predator was observed on big leaves (mean ± SE mites per leaf: 2.13 ± 0.31) (Figure 3) having high density of rust spots (3.30 ± 0.24 spot per leaf). It was significantly different from big leaves without rust spots (0.13 ± 0.06 mites/leaf) (H = 61.1; df = 1; P < 0.001), but also from small leaves with low density of rust spots (0.13 ± 0.07) (0.08 ± 0.04 mites/leaf) (H = 57.8; df = 1; P < 0.001). No significant differences were recorded between small leaves with rust and big leaves without rust (H = 3.5; df = 1; P < 1.00).
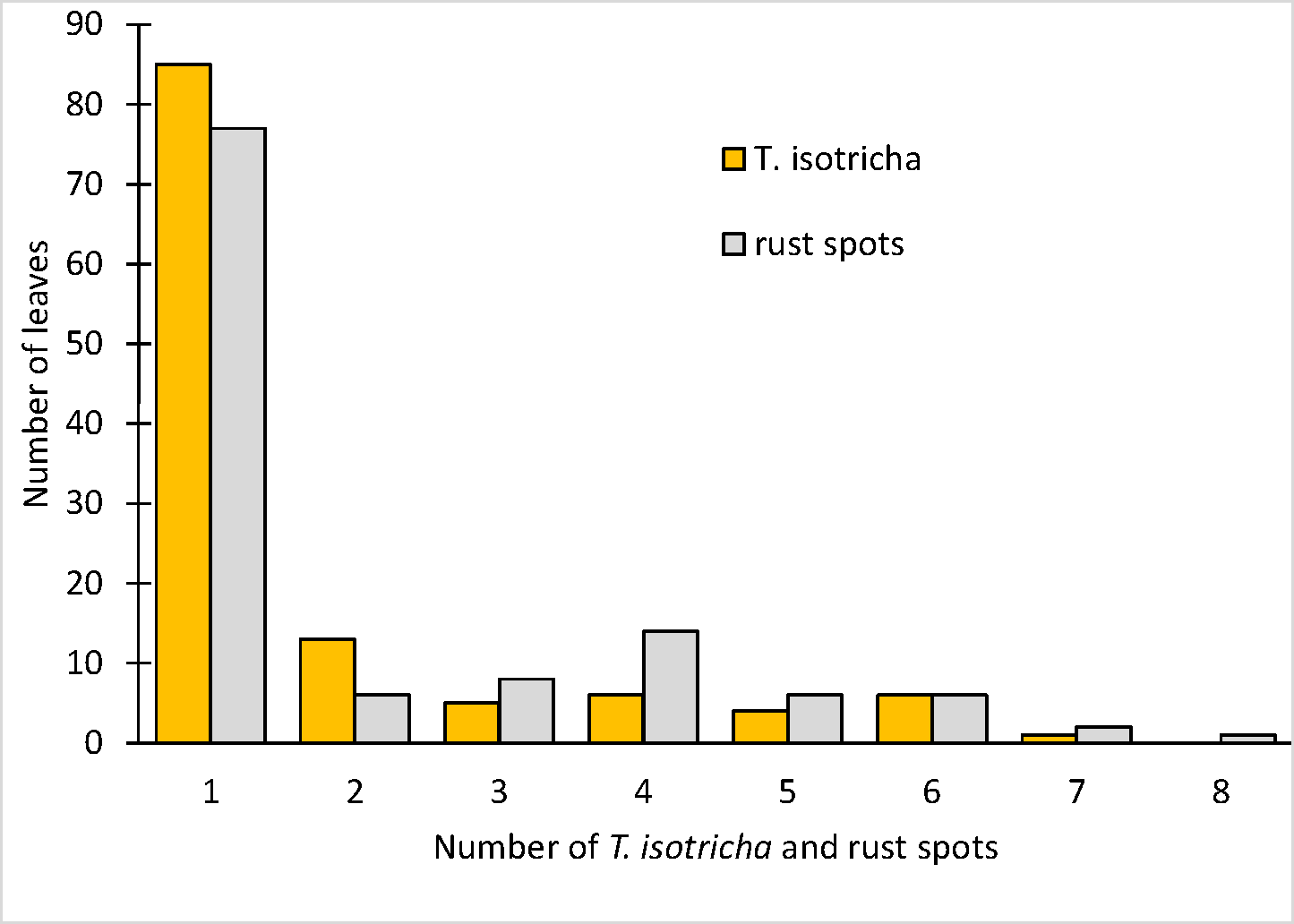


The distribution of T. isotricha on different groups of D. viscosa leaves follows a negative binomial distribution and very similar to this of rust spots (Figure 4). The value of the index of aggregation (I = 2.9; S2 = 2.226 and m = 0.77) is < 1, and the k exponent of the negative binomial distribution (k = 0.41) suggests a highly aggregate distribution. Moreover, the presence of rust spots on leaves of D. viscosa seems a significant predictor of the number of T. isotricha on this plant. Actually, the coefficient estimate ''B'' = 0.752, SE = 0.01, P < 0.001 and the incidence rate ratio, Exp (B) = 2.12, indicates that for every one unit increase on the predictor (rust spots), the number of T. isotricha increases by a percent of 21.2%.
These results seem to show that T. isotricha prefers big leaves of D. viscosa that are highly infested by the rust spots of C. inulae. These results suggest that rust spores could be a potential food source for T. isotricha. The presence of all developmental stages of this predator on leaves infected by the rust and, the absence of other sources of feeding such as phytophagous mites on examined leaves would indicate that the predator could feed, survive, develop and probably reproduce on rust spores. As already reported, several phytoseiid species have the ability to feed on fungi: Typhlodromus pyri Scheuten and Amblyseius andersoni (Chant), for example, could survive, develop and oviposit on grape downy mildew Plasmopara viticola (Berk. & Curtis) Berlese & De Toni (Pozzebon and Duso, 2008) and Ricoseius loxocheles (De Leon) can feed and develop successfully when feeding exclusively on coffee rust Hemileia vastatrix Berk. & Broome (Oliveira, 2012). Therefore, experiments under laboratory conditions should be carried out in order to clarify the biology and to test the predation ability and feeding habits of T. isotricha on different preys and sources of alimentation.
Acknowledgements
The authors thank M. Fawaz Azmeh for his valuable help in the identification of the fungus; also thank M. Almokdad for his valuable comments on statistical analysis.
References
- Alexenizer M., Dorn A. 2007. Screening of medicinal and ornamental plants for insecticidal and growth regulating activity. Journal of Pest Science, 80(4): 205-215. https://doi.org/10.1007/s10340-007-0173-x
- Barbar Z. 2013. Survey of phytoseiid mite species (Acari: Phytoseiidae) in citrus orchards in Lattakia governorate, Syria. Acarologia, 53(3): 247-261. https://doi.org/10.1051/acarologia/20132098
- Barbar Z. 2014. Occurrence, population dynamics and winter phenology of spider mites and their phytoseiid predators in a citrus orchard in Syria. Acarologia, 54(4): 409-423. https://doi.org/10.1051/acarologia/20142143
- Barbar Z. 2016. The mite fauna (Acari) of two Syrian citrus orchards, with notes on their morphology and economic importance. Systematic & Applied Acarology, 21(8): 991-1008. https://doi.org/10.11158/saa.21.8.1
- Barbar Z. 2018. New mite records (Acari: Mesostigmata, Trombidiformes) from soil and vegetation of some Syrian citrus agrosystems. Acarologia, 58(4): 919-927. https://doi.org/10.24349/acarologia/20184298
- Barbar Z., Negm M.W. 2022. Mesostigmata (Acari: Parasitiformes) mites of Syria: new records and species list. International Journal of Acarology, 48(4-5): 429-431. https://doi.org/10.1080/01647954.2022.2066720
- Barbar Z., Parker B., Skinner M. 2022. Phytoseiidae (Acari: Mesostigmata) of Syria: new records and first description of the male of Eharius stathakisi Döker. Acarologia, 62(1): 12-21. https://doi.org/10.24349/2y2g-zk3m
- Bolland H.R., Gutierrez J., Flechtmann C.H.W. 1998. World catalogue of the spider mite family (Acari: Tetranychidae). Leiden: Brill Academic Publishers. pp. 392.
- Boller E.F. 1984. Eine anfache Ausschwemm-Methode zur schellen Erfassung von Raumilben, Trips und anderen Kleinathropoden im Weinbau - Schweiz Zeitschrift für Obst-und Weinbau, 120: 249-255.
- Castro E.B., Mesa N.C., Feres R.J.F., Moraes G.J.de, Ochoa R., Beard J.J., Demite P.R. 2021. Tenuipalpidae Database. Available from: http://www.tenuipalpidae.ibilce.unesp.br (accessed 05/12/2022).
- Chant D.A., McMurtry J.A. 2007. Illustrated Keys and Diagnoses for the Genera and Subgenera of the Phytoseiidae of the World (Acari: Mesostigmata). West Bloomfield: Indira Publishing House. pp. 220.
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R. de. C. 2021. Phytoseiidae Database. Available from http://www.lea.esalq.usp.br/phytoseiidae (accessed 20 December 2022).
- Doğan S., Ayyildiz N. 2003. Mites of the genus Raphignathus (Acari: Raphignathidae) from Turkey. New Zealand Journal of Zoology, 30(2): 141-148. https://doi.org/10.1080/03014223.2003.9518332
- Du Toit B.J., Theron P.D., Ueckermann E.A. 1998. A new genus and four new species of the family Camerobiidae (Acari: Raphignathoidea) from South Africa. International Journal of Acarology, 24(1): 3-19. https://doi.org/10.1080/01647959808684122
- Fan Q-H., Zhang Z-Q. 2005. Raphignathoidea (Acari: Prostigmata). Fauna of New Zealand, 52: 1-400.
- Fernandes M.G., Costa E.N., Cavada L.H., Mota T.A., da Fonseca P.R.B. 2019. Spatial distribution and sampling plan of the phytophagous stink bug complex in different soybean production systems. Journal of Applied Entomology, 143(3): 236-249. https://doi.org/10.1111/jen.12584
- Gökbulut A., Özhan O., Satılmış B., Batçıoğlu K., Günal S., Şarer E. 2013. Antioxidant and antimicrobial activities, and phenolic compounds of selected Inula species from Turkey. Natural Product Communications, 8(4): 475-478. https://doi.org/10.1177/1934578X1300800417
- Haitlinger R., 2006. New records of mites (Acari: Prostigmata: Erythraeidae, Trombidiidae) from Samos, Greece, with descriptions of six new species. Systematic & Applied Acarology, 11(1): 107-123. https://doi.org/10.11158/saa.11.1.12
- Haitlinger R., 2010. New records of mites (Acari: Prostigmata: Erythraeidae, Trombidiidae) from turkey, with descriptions of four new species. Zeszyty Naukowe Uniwersytetu Przyrodniczego we Wrocławiu, Biologia i Hodowla Zwierząt, LX 577: 49-61.
- IBM Corp. Released 2011. IBM SPSS Statistics for Windows, Version 20.0. Armonk, NY: IBM Corp.
- Ingegno B.L., Pansa M.G., Tavella L. 2011. Plant preference in the zoophytophagous generalist predator Macrolophus pygmaeus (Heteroptera: Miridae). Biological Control, 58(3): 174-181. https://doi.org/10.1016/j.biocontrol.2011.06.003
- Khanjani M., Ueckermann, E.A. 2002. Camerobiidae of Iran with descriptions of three new species (Acari: Camerobiidae). Systematic & Applied Acarology, 7: 159-166. https://doi.org/10.11158/saa.7.1.17
- Khanjani M., Ueckermann E.A. 2003. Two new species of the genus Raphignathus Dugés (Acari: Raphignathidae) from Iran. Acarologia, 43(3): 299-306.
- Krantz G.W., Walter D.E. 2009. A Manual of Acarology. Third Edition. Lubbock: Texas Tech University Press. pp. 807.
- McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the life styles of phytoseiid mites and implications for biological control strategies. Systematic & Applied Acarology, 18(4): 297-320. https://doi.org/10.11158/saa.18.4.1
- Mesa N.C., Ochoa R., Welbourn W.C., Evans G.A., Moraes G.J. de. 2009. A catalog of the Tenuipalpidae (Acari) of the world with a key to genera. Zootaxa, 2098(1): 1-185 https://doi.org/10.11646/zootaxa.2098.1.1
- Meyer M.K.P. 1979. The Tenuipalpidae (Acari) of Africa with keys to the world fauna. Entomology Memoir, Department of Agriculture Republic South Africa, Pretoria, 50: 1-133.
- Nasrollahi S., Khanjani M., Mirfakhraei S.M. 2018. A new species of the genus Raphignathus (Acari: Raphignathidae) from Kurdistan, Iran, with a key to world species. Systematic & Applied Acarology, 23(10): 2070-2081. https://doi.org/10.11158/saa.23.10.14
- Oliveira, C.M. de. 2012. Interactions of Ricoseius loxocheles (Acari: Phytoseiidae) and coffee leaf rust. MSc. Dissertation, Universidade Federal de Viçosa, Minas Gerais, Brazil. pp. 49.
- Parolin P., Scotta M.I., Bresch C. 2014. Biology of Dittrichia viscosa, a Mediterranean ruderal plant: a review. ΦYTON, 83: 251-262. https://doi.org/10.32604/phyton.2014.83.251
- Pozzebon A., Duso C. 2008. Grape downy mildew Plasmopora viticola, an alternative food for generalist predatory mites occurring in vineyards. Biological Control, 45(3): 441-449. https://doi.org/10.1016/j.biocontrol.2008.02.001
- Smiley R.L. 1991. Mites (Acari). In: Gorham, J.R. (Eds.), Insect and mite pests in food. An illustrated key. USDA Agricultural Handbook. pp 767.
- Southcott R.V. 1961. Studies on the systematics and biology of the Erythraeoidea (Acarina), with a critical revision of the genera and subfamilies. Australian Journal of Zoology, 9(3): 367-610. https://doi.org/10.1071/ZO9610367
- Topakci N., İkten C., Göçmen H. 2005. A research on some effects of Inula viscosa (L.) Ait (Asteraceae) leaf extract on carmine spider mite, Tetranychus cinnabarinus (Boısd.) (Acarı:Tetranychidae). Akdeniz Üniversitwsi Ziraat Fakültesi Dergisi, 18(3): 411-415. [In Turkish]
- Xu S.Y., Yi T.C., Guo J.J., Jin D.C. 2019. The genus Erythraeus (Acari: Erythraeidae) from China with descriptions of two new species and a key to larval species of the genus worldwide. Zootaxa, 4647(1): 54-82. https://doi.org/10.11646/zootaxa.4647.1.7
- Zhang Z.Q. 2003. Mites of greenhouses: Identification, biology and control. Wallingford, UK: CABI Publishing. pp. 244.



2023-01-05
Date accepted:
2023-04-24
Date published:
2023-05-04
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Ebrahim, Walaa and Barbar, Ziad
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



