Ontogenetic instars of the oribatid mite Scheloribates arsizonensis n. sp. (Acari, Oribatida, Scheloribatidae) from Ethiopia
Ermilov, Sergey G.  1
and Rybalov, Leonid B.
1
and Rybalov, Leonid B.  2
2
1✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
2Institute of Ecology and Evolution, Russian Academy of Sciences, Laboratory of Soil Zoology and General Entomology, Moscow, Russia.
2023 - Volume: 63 Issue: 1 pages: 122-135
https://doi.org/10.24349/o0ta-ustmZooBank LSID: 974FC473-BCD3-40FE-A45C-865BABCAF4D3
Original research
Keywords
Abstract
Introduction
The oribatid mite genus Scheloribates (Acari, Oribatida, Scheloribatidae) was proposed by Berlese (1908), with Zetes latipes Koch, 1844 as type species. According to Subías (2022), it comprises 282 species and 20 subspecies belonging to six subgenera, which have a cosmopolitan distribution.
The subgenus Scheloribates (Scheloribates) comprises 210 species and 19 subspecies (Subías 2022). The main subgeneric traits for adult can be found in: e.g., Pletzen (1963), Coetzer (1968), Corpus-Raros (1980), Balogh and Balogh (1990, 1992), Weigmann (2006), Bayartogtokh (2010), Ermilov and Anichkin (2014). The identification keys to selective species of the subgenus were presented in: e.g., Shaldybina (1975), Balogh and Balogh (1992), Weigmann (2006), Bayartogtokh (2010), Ermilov et al. (2011); Ermilov and Starý (2017). The development of Scheloribates was described (completely or partially) in nine known species and one unknown species (Norton and Ermilov 2014) (see General remarks below).
Presently, nine species of Scheloribates (Scheloribates) are registered in the Ethiopian fauna (e.g., Mahunka 1982; Ermilov et al. 2011, 2012; Ermilov and Rybalov 2018, 2019): S. acutirostrum Ermilov, Rybalov and Franke, 2011, S. aethiopicus Mahunka, 1982, S. discifer Balogh, 1959, S. fimbriatus Thor, 1930, S. latipes (Koch, 1844), S. leleupi Balogh, 1959, S. maximus Balogh, 1962, S. perisi Pérez-Íñigo, 1982, S. praeincisus (Berlese, 1910).
In the course of taxonomic identification of materials collected from litter and green moss in Arsi Mountains National Park, Ethiopia (Fig. 1), we found a new species of Scheloribates (from nominate subgenus). The main goal of the paper is to describe and illustrate this new species based on adult and juvenile instars, and compare its juveniles with those of other Scheloribates species.



Methods
Sampling — Substrate samples (litter and mosses) containing oribatid mites were collected using a stainless-steel frame (50 × 50 cm) with a sieve (mesh size 2 × 2 cm). Mites were extracted into 75% ethanol using Berlese's funnels with electric lamps in laboratory conditions (in Institute of Ecology and Evolution, Moscow, Russia). The collection locality is provided in the Material examined section.
Juvenile specimens were associated with adults using criteria outlined by Norton & Ermilov (2014). In particular, they were found in the same samples and had appropriate size and proportions. If more than one similar-sized species was represented in a sample, juveniles were ignored.
Observation and documentation — Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster (in adult)/gastronotic region (in juveniles). Notogastral/gastronotic width refers to the maximum width of the notogaster in adult (behind pteromorphs) and gastronotic region (in juveniles) in dorsal view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu-tibia-tarsus. Drawings were made with a camera lucida using a Leica transmission light microscope ''Leica DM 2500''.
Terminology — Morphological terminology used in this paper follows that of Grandjean: see Travé and Vachon (1975) for references; Norton (1977) for leg setal nomenclature; and Norton and Behan-Pelletier (2009) for overview.
Abbreviations — Prodorsum: lam = lamella; tlam = translamella; slam = sublamella; Al = sublamellar porose area; kf = keel-shaped ridge; lc = lateral carina; pc = postbothridial carina; ro, le, in, bs, ex = rostral, lamellar, interlamellar, bothridial, and exobothridial seta, respectively; D = dorsophragma; P = pleurophragma. Notogaster/Gastronotic region: c, da, dm, dp, la, lm, lp, h, p = setae; Sa, S1, S2, S3 = sacculi; ecl = ecdysial cleavage line (line of dehiscence); gf = gastronotic fold; ia, im, ip, ih, ips = lyrifissures/cupules; gla = opisthonotal gland opening. Gnathosoma: a, m, h = anterior, middle seta of gena and hypostomal seta of mentum, respectively; or = adoral seta; d, l, cm, acm, ul, su, lt, vt, inf, sup = palp setae; ω = palp tarsal solenidion; ep = postpalpal seta; p.a. = porose area; cha, chb = cheliceral setae; Tg = Trägårdh's organ. Epimeral and lateral podosomal regions: Cl = Claparède's organ; 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c = setae; z = aperture of supracoxal gland; PdI, PdII = pedotectum I and II, respectively; dis = discidium; cir = circumpedal carina. Anogenital region: g, ag, an, ad = genital, aggenital, anal, and adanal seta, respectively; iad = adanal lyrifissure/cupule; Amar = marginal porose area; p.o. = preanal organ. Legs: Tr, Fe, Ge, Ti, Ta = trochanter, femur, genu, tibia, tarsus, respectively; trt = trochanteral tooth; ω, φ, σ = solenidia; ɛ = famulus; d, l, v, bv, ev, ft, tc, it, p, u, a, s, pv, pl, = setae; p.a. = porose area. Instars: LA = larva; PN, DN, TN = proto-, deuto- and tritonymph, respectively; AD = adult.
Taxonomy
Family Scheloribatidae Jacot, 1935
Genus Scheloribates Berlese, 1908
Scheloribates arsizonensis n. sp.
ZOOBANK: E8A5C7BD-7725-45C9-BD85-CA94E8C231C0 ![]()
(Figures 2-7)
Diagnosis — (adult) Body length: 675–735 × 450–510. Rostrum protruding, rounded. Prolamella absent; translamella represented by two short ridges. Rostral, lamellar and interlamellar setae long, setiform, barbed; ro shortest, in longest; exobothridial seta minute, simple; bothridial seta comparatively short, fusiform, pointed distally, barbed. Anterior notogastral margin convex medially. One pair of simple notogastral setae (p1) developed; other setae represented by alveoli. All epimeral setae setiform, slightly barbed. Circumpedal carina comparatively long. All anogenital setae setiform, slightly roughened. Marginal porose area complete. Ventroantiaxial part of trochanter III with tooth.





Description of adult — (Figures 2a-d, 3a-g) Measurements – Body length: 705 (holotype: female), 675–735 (43 paratypes: 21 males and 22 females); body width: 465 (holotype), 450–510 (43 paratypes). No differences between males and females in body size.
Integument – Body color brown. Cuticle microsculpturing granulate (visible in dissected specimens under high magnification, ×1000); lateral part of body (between sublamella and pedotecta I, II) with granulate cerotegument.
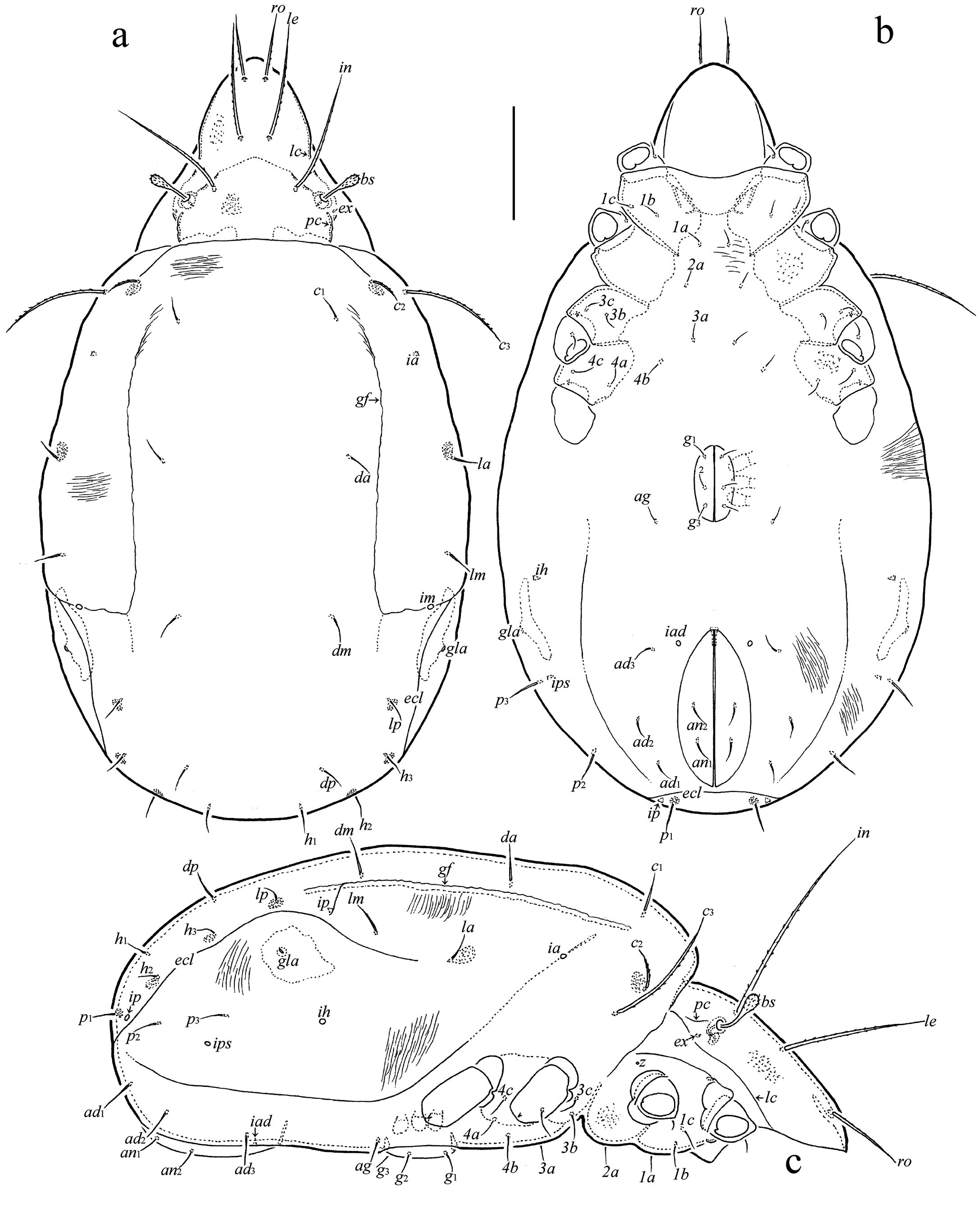
Prodorsum (Figures 2a, c) – Rostrum slightly protruding, narrowly rounded. Lamella about 1/2 length of prodorsum; prolamella absent; sublamella similar to lamella in length; sublamellar porose area rounded (6); translamella represented by two short ridges developed near lamellae. Rostral (76–94), lamellar (124–161) and interlamellar (180–221) setae setiform, barbed; exobothridial seta (9–11) setiform, smooth, often directed backwards; bothridial seta comparatively short (60–71), with fusiform, pointed distally, barbed head. Dorsosejugal porose area diffuse. Dorsophragma slightly elongate.
Notogaster (Figures 2a, c, d) – Anterior notogastral margin slightly convex medially. One pair of notogastral setae (p1: 15–22) developed, setiform, smooth; other setae represented by alveoli. Four pairs of sacculi drop-like. Opisthonotal gland opening and all lyrifissures distinct.
Gnathosoma (Figures 3a-c) – Subcapitulum size: 142–150 × 116–130; subcapitular (a: 30–34; m: 28–30; h: 37–41) and adoral (19) setae setiform, barbed; m thinner than a and h. Palp (length: 82–90) setation: 0-2-1-3-9 (+ω); solenidion bacilliform, not swollen distally; postpalpal seta (9) spiniform, roughened. Chelicera (length: 161–169) with two setiform, barbed setae (cha: 52–56; chb: 35–37).
Epimeral and lateral podosomal regions (Figures 2b, c) – Epimeral formula: 3-1-3-3; setae (1b, 3b: 37–41; 3c: 30–34; others: 19–26) setiform, slightly barbed. Pedotectum II rounded distally in ventral aspect. Discidium triangular, rounded distally. Circumpedal carina comparatively long, anteriorly directed to pedotectum II.
Anogenital region (Figures 2b-d) – Genital (g1: 26–30; g2–g4: 19–26), aggenital (19–26), anal (19–26), and adanal (22–30) setae setiform, slightly roughened. Adanal lyrifissure distinct. Marginal porose area complete, narrowly band-like.
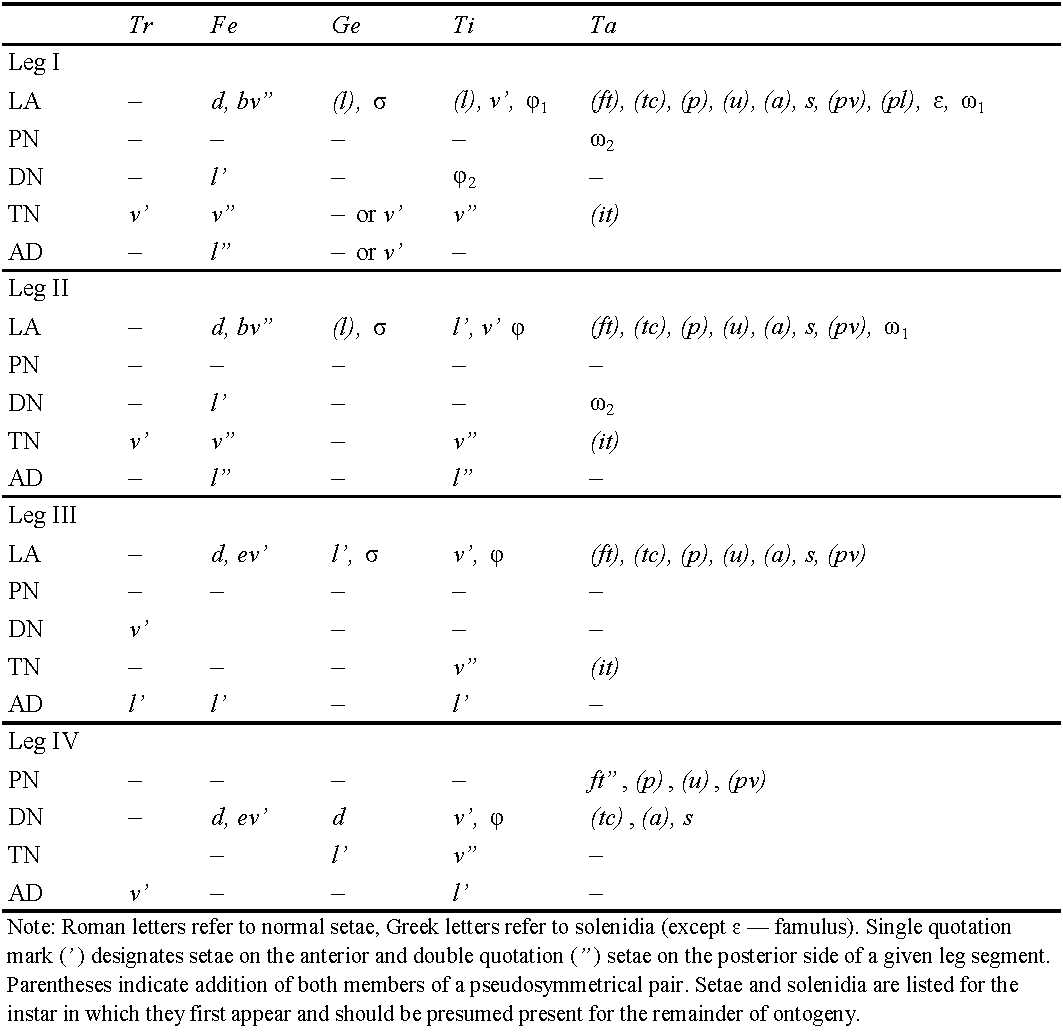
Legs (Figures 3d-g) – Median claw distinctly thicker than lateral claws; all claws slightly barbed on dorsal side; lateral claws with small tooth distoventrally. Ventroantiaxial part of trochanter III with tooth; tibiae I, II with slightly developed tubercle proximoventrally. Dorsoparaxial porose area on femora I-IV and on trochanters III, IV, proximoventral porose area on tarsi I-IV, and distoventral porose area on tibiae I-IV well visible. Formulas of leg setation and solenidia: I (1-5-3-4-18) [1-2-2], II (1-5-2-4-15) [1-1-2], III (2-3-1-3-15) [1-1-0], IV (1-2-2-3-12) [0-1-0]; homology of setae and solenidia indicated in Table 1; famulus short, slightly swollen distally, inserted posterior to solenidion ω2; solenidion ω1 on tarsus I and σ on genu III slightly bacilliform, ω1 and ω2 on tarsus II rod-like; other solenidia setiform.


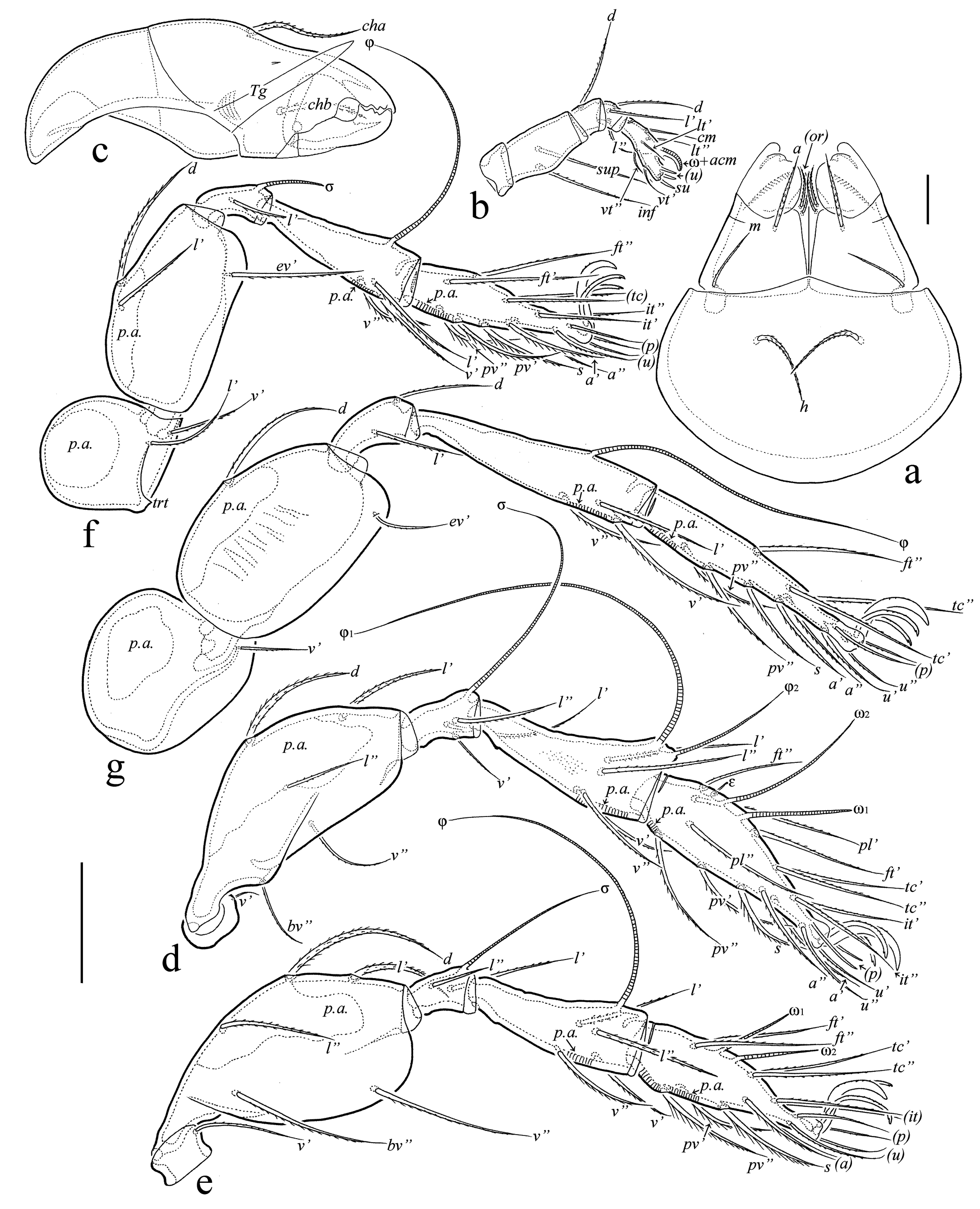
Description of juvenile instars — (Figures 4a-c, 5a, b, 6a-c,7a-h) Measurements – Total length of larva: 315–330 (n=5), protonymph: 390–420 (n=4), deutonymph: 465–510 (n=12), tritonymph: 555–645 (n=20); total width of larva: 135–150 (n=5), protonymph: 195–210 (n=4), deutonymph: 210–240 (n=12), tritonymph: 270–375 (n=20).








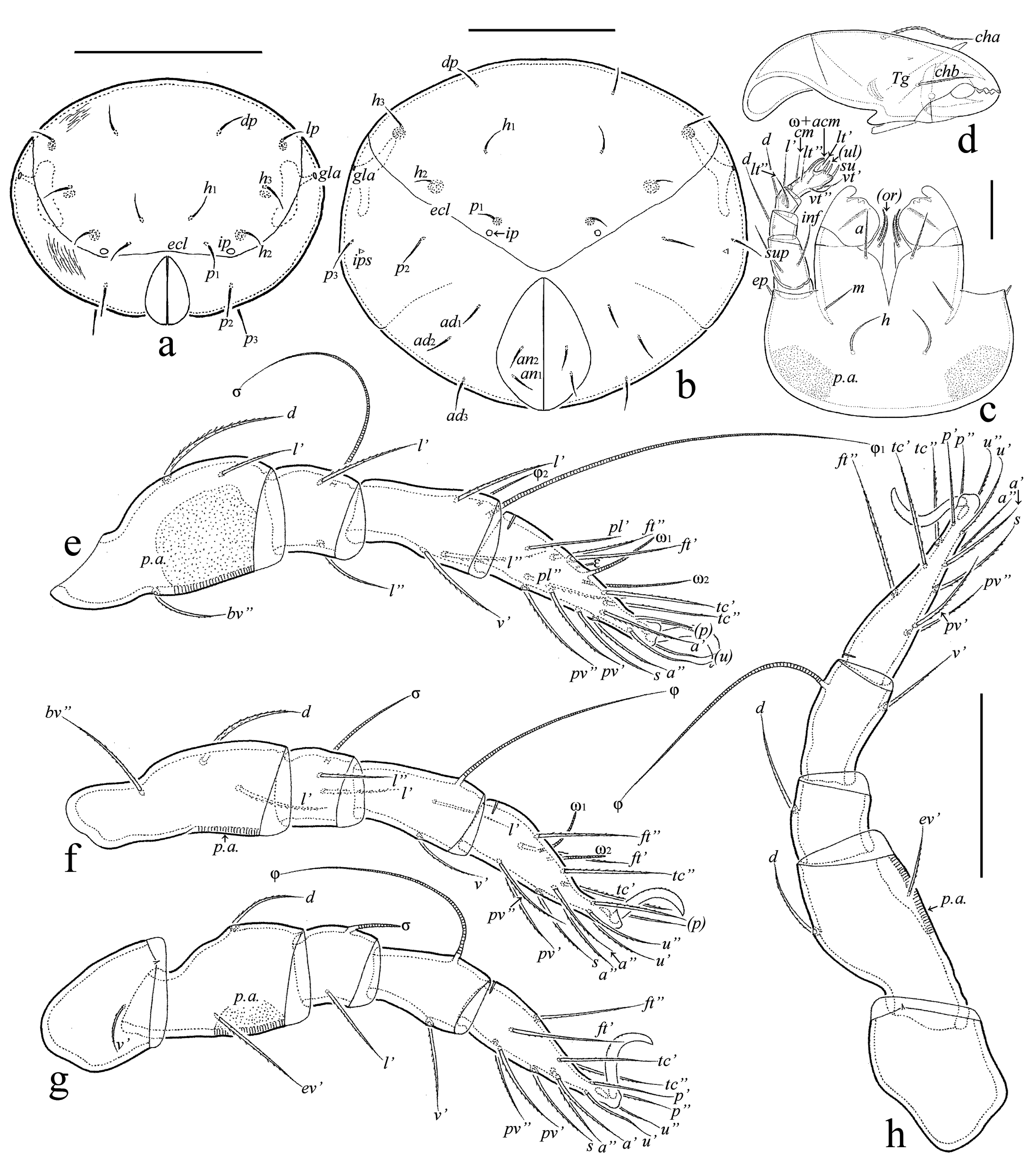


Integument (Figures 4a-c, 5a, b, 6a-c, 7a-c) – Body colorless to light yellowish, legs, gastronotic sclerites, lateral parts of epimeres, and dorsal prodorsal sclerite light brown in deuto- and tritonymphs. Body surface densely porose (visible under high magnification in dissected specimens); additionally, gastronotum and anogenital region slightly folded; gastronotic region with two strong, long, longitudinal, dorsolateral folds.
Prodorsum (Figures 4a, c, 5a, b, 6a, c) – Relatively short, about 1/2 (in larva) and 1/3 (in nymphal instars) length of gastronotic region. Rostrum rounded. Dorsal side of prodorsum with large sclerite bordered by lateral and postbothridial carinae. Rostral (LA: 34–37; PN: 41–45; DN: 53–57; TN: 64–67), lamellar (LA: 41–45; PN: 60–64; DN: 82–86; TN: 116–120) and interlamellar (LA: 60–64: PN: 97–105; DN: 131–139; TN: 172–176) setae setiform, barbed; exobothridial seta (LA: 1; PN: 2; DN, TN: 4) simple, smooth; bothridial seta (LA: 26–30; PN: 30–34; DN: 41–45; TN: 52–56) clavate, rounded distally, barbed.
Gastronotic region (Figures 4a-c, 5a, b, 6a-c, 7a, b) – Posteriorly rather rounded. Nymphal instars with distinct ecdysial cleavage line (line of dehiscence). Larva with 12, nymphal instars with 15 pairs of setiform (except h3 represented by alveolus in LA), slightly barbed setae; setae c1, da, dm, dp thinner than others in larva; relative thickness of gastronotic setae in nymphal instars: c3˃c2˃others; all setae with sclerite at the base, large sclerites at the base of c2, la, lp, h1 in larva, c2, la, lp, h2, h3 in protonymph, c2, la, lp, h2, h3, p1 in deuto- and tritonymphs. Length of gastronotic setae: LA: c3: 32–34, h1, h2: 26–30, others: 17–22; PN: c3: 71–75, c2: 22–26, others: 17–22; DN: c3: 86–90, c2: 26–30, others: 22–26; TN: c3: 105–131, c2: 26–37, lp, h1, h2, h3, p1, p2, p3: 19–22, others: 26–30. Opisthonotal gland opening and all cupules distinct.
Gnathosoma (Figures 7c, d) – Subcapitulum size: LA: 57 × 49; PN: 67–71 × 60–64; DN: 86–90 × 79–82; TN: 105–109 × 97–101; length of subcapitular setae a, h: LA, PN: 15; DN: 17–19; TN: 22–26; length of subcapitular seta m: LA, PN: 11; DN: 13–15; TN: 17–19; length of adoral seta: LA, PN: 7; DN: 9–11; TN: 11–13; all subcapitular and adoral setae setiform, slightly barbed. Posterolateral part of subcapitular mentum with porose area. Palp length: LA: 34; PN: 45; DN: 52–56; TN: 64–67; palp formula: 0-2-1-3-9(+ω); solenidion connected with eupathidium in all instars; length of postpalpal seta: LA, PN: 4; DN, TN: 5–7. Chelicera length: LA: 56; PN: 75–82; DN: 94–97; TN: 112; length of seta cha: LA: 22; PN: 30; DN: 34–37; TN: 41–43; length of seta chb: LA: 15; PN: 19; DN: 20–22; TN: 26; both cheliceral setae setiform, barbed.
Epimeral and lateral podosomal regions (Figures 4b, c, 5a, b, 6b, c) – Setal formulas for epimeres: larva: 3-1-2 (1c as typical scale covering Claparède's organ); protonymph 3-1-2-1; deutonymph 3-1-2-2, tritonymph 3-1-3-3; length of setae: LA: 9–11; PN: 11–13; DN: 13–19; TN: 19–22; all setae setiform, slightly roughened.
Anogenital region (Figures 4b, c, 5a, b, 6b, c,7a, b) – Ontogeny of genital (PN: 11–13; DN: 13–19; TN: 17–19), aggenital (TN: 17–19), anal (TN: 17–19), and adanal (DN: 19–22; TN: 26) setal formulas, proto- to tritonymph: 1-2-3, 0-0-1, 0-0-2, 0-2-2, respectively; all setae setiform, roughened; paraproctal setae absent. There is an unusual development of aggenital setae: they only appear in tritonymph instead in deutonymph. Adanal cupule distinct; anal cupule not observed.
Legs (Figures 7e-h) – Claw of each leg dorsally slightly barbed. Femora I-IV with distoventral porose area. Formulas of leg setation and solenidia: larva I (0-2-2-3-16) [1-1-1], II (0-2-2-2-13) [1-1-1], III (0-2-1-1-13) [1-1-0]; protonymph I (0-2-2-3-16) [1-1-2], II (0-2-2-2-13) [1-1-1], III (1-2-1-1-13) [1-1-0], IV (0-0-0-0-7) [0-0-0]; deutonymph I (0-3-2-3-16) [1-2-2], II (0-3-2-2-13) [1-1-2], III (1-2-1-1-13) [1-1-0], IV (0-2-1-1-12) [0-1-0]; tritonymph I (1-4-2[or 3]-4-18) [1-2-2], II (1-4-2-3-15) [1-1-2], III (1-2-1-3-15) [1-1-0], IV (0-2-2-2-12) [0-1-0]; homologies of setae and solenidia indicated in Table 1.
Material examined — Holotype (female), 43 paratypes (21 males and 22 females) and 41 juvenile instars (five larvae, four protonymphs, 12 deutonymphs, 20 tritonymphs): Ethiopia, Oromia Region, Arsi Zone, Arsi Mountains National Park, 07º17′49′′N, 039º23′40′′E, 3847 m a.s.l., litter and green moss Rhytidiadelphus triquetrus under mixed plants (Erica arborea, Helichrysum spp., Lobelia rhynchopetalum [giant lobelia], Alchemilla pedata, Haplocarpha rueppelii) (Fig. 1), 03.12.2021 (L.B. Rybalov).
Type deposition — The holotype and two paratypes are deposited in the collection of the Senckenberg Museum of Natural History, Görlitz, Germany; 41 paratypes and juvenile instars are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia. All specimens are preserved in 70% solution of ethanol with a drop of glycerol.
Etymology — The species name arsizonensis refers to the Arsi Zone, where the new species was collected.
Remarks — Although, Scheloribates is a large genus (210 species and 19 subspecies) and contains many similar species, adults of S. arsizonensis n. sp. can be distinctly distinguished from other representatives of the genus by the combination of morphological traits, e.g.: 1) body large: 675–735; 2) distinct sculpturing and ornamentation absent; 3) rostrum protruding, rounded; 4) prolamella absent; 5) translamella present, represented by two short ridges; 6) rostral, lamellar and interlamellar setae long, setiform, barbed, in˃le˃ro; 7) bothridial seta comparatively short, fusiform, pointed distally, barbed; 8) anterior notogastral margin convex medially; 9) one pair of notogastral setae (p1) developed, others represented by alveoli. The new species is morphologically most similar to Scheloribates praestantissimus praestantissimus (Berlese, 1916) from Somalia (see Berlese 1916; Mahunka and Mahunka-Papp 1995) in having large body size, protruding, rounded rostrum, long rostral, lamellar and interlamellar setae (with relative length: in˃le˃ro), interrupted medially translamella, and the comparatively short bothridial seta, but differs from the later in having fusiform, pointed distally (versus clavate, rounded distally) bothridial seta, the absence (versus presence) of prolamella, and the presence (versus represented alveoli) of well-developed notogastral setae p1.
General remarks
Main ontogenetic transformations in juvenile instars — Measurements. Size of body increases with each successive instar; length from 315–330 in larva to 555–645 in tritonymph. Integument. Body of colorless to light yellowish but legs, gastronotic sclerites, lateral parts of epimeres, and dorsal prodorsal sclerite light brown in deuto- and tritonymphs. Cuticle porose; additionally, gastronotum and anogenital region folded; gastronotic region with two strong, long, longitudinal, dorsolateral folds. Prodorsum. Rostrum rounded. Lateral and postbothridial carinae present. With five pairs of prodorsal setae: rostral, lamellar and interlamellar long, setae setiform, barbed; exobothridial seta minute, simple; relative length: in˃le˃ro˃ex; bothridial seta clavate, barbed. Gastronotum. Elongate oval in dorsal view. Larva with 12, nymphal instars with 15 pairs of setiform (except h3 represented by alveolus in LA), slightly barbed setae; c3, h1, h2 longest in larva versus c3 longest in nymphal instars; all setae with sclerite at the base but well visible only large sclerites at the base of c2, la, lp, h1 in LA, c2, la, lp, h2, h3 in PN, c2, la, lp, h2, h3, p1 in DN and TN. Gnathosoma. Subcapitulum with three pairs of subcapitular and two pairs of adoral setae; all setiform, barbed. Palp formula: 0-2-1-3-9(+ω); solenidion connected with eupathidium. Both cheliceral setae setiform, barbed. Epimeral and lateral podosomal regions. Larva with six (1a, 1b, 1c, 2a, 3a, 3b), protonymph with seven (plus 4a), deutonymph with eight (plus 4b), tritonymph with 10 (plus 3c and 4c) pairs of epimeral setae. Anogenital region. In protonymph, one pair of genital setae; in deutonymph, one pair of genital and three pairs of adanal setae added; in tritonymph, one pair of genital, one pair of aggenital and two pairs of anal setae added; paraproctal valves without setae. Legs. Juvenile instars monodactylous; leg setation increasing from instar to instar as shown in Table 1.
Comparison of juvenile instars — As noted above, the ontogeny of juvenile instars in Scheloribates was described in nine known species and one unknown species but the morphology of juvenile instars are known (completely or partially) only for seven representatives (see Norton and Ermilov 2014 for explanations and references): 1) S. (Hemileius) initialis (Berlese, 1908); 2) S. (H.) nicki (Denmark and Woodring, 1965); 3) S. (S.) laevigatus (Koch, 1835); 4) S. (S.) latipes (Koch, 1844); 5) S. (S.) nudus Woodring, 1965; 6) S. (S.) pallidulus (Koch, 1841); and 7) S. (Topobates) holsaticus (Weigmann, 1969).
In general, juveniles of Scheloribates are similar to each other, however, larva and nymphal instars of S. arsizonensis n. sp. can be distinguished from those of the other above listed species by the: 1) presence of two strong, long, longitudinal, dorsolateral folds on the gastronotum in all instars; 2) long gastronotic seta c3 which is distinctly longer than other gastronotic setae in nymphal instars; 3) presence of vestige of gastronotic seta h3 in larva; 4) absence of aggenital setae in deutonymph.
Acknowledgements
The work was performed within the framework of the Joint Russian-Ethiopian Biological Expedition, financially supported by the Russian Academy of Sciences. We are grateful to Dr. Gezahegn Degefe for supporting the field studies and organizing laboratory works and two anonymous reviewers for valuable comments. Collecting of materials was conducted under the current Agreement of 17.12.2020 between the Russian Academy of Sciences and the Ministry of Science and Technology (Ministry Innovation and Technology) of the Federal Democratic Republic of Ethiopia. This research was supported by the cooperative agreement No. FEWZ-2021-0004 from the Russian Ministry of Science and Higher Education.
References
- Balogh J. 1959. Oribates (Acari) nouveaux d'Angola et du Congo Belge (1ère série). Companhia de Diamantes de Angola. Lisboa, 48: 91-108.
- Balogh J. 1962. Mission zoologique de l′I.R.S.A.C. en Afrique orientale (P. Basilewsky et N. Leleup, 1957). LXXV. Acari Oribates. Annales du Musêe Royal de l′Afrique Centrale Tervuren Belgium, Zoologie, 110: 90-131.
- Balogh J., Balogh P. 1990. Oribatid mites of the Neotropical region. II. Budapest: Akadémiai Kiadó Press. 333 p.
- Balogh J., Balogh P. 1992. The oribatid mite genera of the World. Vol. 1. Budapest: Hungarian National Museum Press. 263 p.
- Bayartogtokh B. 2010. Oribatid mites of Mongolia (Acari: Oribatida). Moscow: KMK. 372 p.
- Berlese A. 1908. Elenco di generi e specie nuove di Acari. Redia, 5: 1-15.
- Berlese A. 1910. Brevi diagnosi di generi e species nuovi di Acari. Redia, 6: 346-388.
- Berlese A. 1916. Centuria terza di Acari nuovi. Redia, 12: 289-338.
- Coetzer A. 1968. New Oribatulidae Thor, 1929 (Oribatei, Acari) from South Africa, new combinations and a key to the genera of the family. Memórias do Instituto de Investigaçāo Cientifica de Moçambique, 9(A): 15-126.
- Corpuz-Raros L. 1980. Philippine Oribatei (Acarina) V. Scheloribates Berlese and related genera (Oribatulidae). Kalikasan, 9(2-3): 169-245.
- Denmark M.A., Woodring G.P. 1965. Feeding habits of Hemileius new species (Acari: Cryptostigmata, Oribatulidae) on Florida orchids. The Florida Entomologist, 48(1): 9-16. https://doi.org/10.2307/3493516
- Ermilov S.G., Anichkin A.E. 2014. A new species of Scheloribates (Scheloribates) from Vietnam, with notes on taxonomic status of some taxa in Scheloribatidae (Acari, Oribatida). International Journal of Acarology, 40(1): 109-116. https://doi.org/10.1080/01647954.2014.885564
- Ermilov S.G., Rybalov L.B. 2018. New faunistic and taxonomic data on oribatid mites (Acari, Oribatida) of Ethiopia. Systematic and Applied Acarology, 23(9): 1827-1837. https://doi.org/10.11158/saa.23.9.9
- Ermilov S.G., Rybalov L.B. 2019. Oribatid mites from the vicinities of Dhati-Walal National Park, Ethiopia (Acari, Oribatida). Spixiana, 42(2): 235-252.
- Ermilov S.G., Starý J. 2017. A new species of Scheloribates (Acari, Oribatida, Scheloribatidae) from Vietnam, with key to the striolatus-group. Ecologica Montenegrina, 10: 14-21. https://doi.org/10.37828/em.2017.10.3
- Ermilov S.G., Sidorchuk E.A., Rybalov L.B. 2012. Oribatid mites (Acari: Oribatida) of Ethiopia. Zootaxa, 3208(1): 27-40. https://doi.org/10.11646/zootaxa.3208.1.2
- Ermilov S.G., Rybalov L.B., Franke K. 2011. Ethiopian oribatid mites of the family Scheloribatidae (Acari: Oribatida). African Invertebrates. 52(2): 311-322. https://doi.org/10.5733/afin.052.0207
- Koch C.L. 1835. Deutschlands Crustaceen, Myriapoden und Arachniden, Heft 3. F. Pustet, Regensburg.
- Koch C.L. 1841. Deutschlands Crustaceen, Myriapoden und Arachniden, Heft 31. F. Pustet, Regensburg.
- Koch C.L. 1844. Deutschlands Crustaceen, Myriapoden und Arachniden, Heft 38. F. Pustet, Regensburg.
- Mahunka S. 1982. Oribatids from the Eastern Part of the Ethiopian Region (Acari) I. Acta Zoologica Academiae Scientiarum Hungaricae, 28(3-4): 293-336.
- Mahunka S., Mahunka-Papp L. 1995. The oribatid species described by Berlese (Acari). Budapest: Hungarian Natural History Museum. 325 p.
- Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal D.L. (Ed.). Biology of oribatid mites. Syracuse: SUNY College of Environmental Science and Forestry. pp. 33-61.
- Norton R.A., Behan-Pelletier V.M. 2009. Oribatida. In: Krantz G.W., Walter D.E. (Eds). A Manual of Acarology (TX). Lubbock: Texas University Press. Chapter 15. pp. 430-564.
- Norton R.A., Ermilov S.G. 2014. Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida). Zootaxa, 3833(1): 1-132. https://doi.org/10.11646/zootaxa.3833.1.1
- Pérez-Íñigo C. 1982. Resultados de la expedición Peris-Alvarez a la Isla de Annobón. (13). Oribatid mites (3rd part). Eos, Revista Espanola de Entomologia, 58: 223-236.
- Pletzen van R. 1963. Studies on South African Oribatei (Acarina). Acarologia, 5(4): 690-703.
- Shaldybina E.S. 1975. The family Scheloribatidae. In: Ghilyarov M.S. (Ed.). Key to Soil Inhabiting Mites. Sarcoptiformes. Moscow: Nauka Press. pp. 262-268.
- Subías L.S. 2022. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Monografías Electrónicas S.E.A., 12: 1-538.
- Thor S. 1930. Beiträge zur Kenntnis der Invertebratenfauna von Svalbard. Skrifter om Svalbard og Ishavet, Oslo, 27: 1-156.
- Travé J., Vachon M. 1975. François Grandjean. 1882-1975 (Notice biographique et bibliographique). Acarologia, 17(1): 1-19.
- Weigmann G. 1969. Zur Taxonomie der europäischen Scheloribatidae mit der Beschreibung von Topobates holsaticus n. sp. (Arachnida: Acari: Oribatei). Senckenbergiana Biologica, 50: 421-432.
- Weigmann G. 2006. Hornmilben (Oribatida). Die Tierwelt Deutschlands. Teil 76. Keltern: Goecke and Evers. 520 p.
- Woodring J.P. 1965. The biology of five new species of oribatids from Louisiana. Acarologia, 7(3): 564-576.



2022-11-26
Date accepted:
2023-01-23
Date published:
2023-01-26
Edited by:
Pfingstl, Tobias

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Ermilov, Sergey G. and Rybalov, Leonid B.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



