Effects of temperature on life table parameters of a newly described phytoseiid predator, Neoseiulus neoagrestis (Acari: Phytoseiidae) fed on Tyrophagus putrescentiae (Acari: Acaridae)
Moradi, Maryam  1
; Joharchi, Omid
1
; Joharchi, Omid  2
; Döker, Ismail
2
; Döker, Ismail  3
; Khaustov, Vladimir A.
3
; Khaustov, Vladimir A.  4
; Salavatulin, Vladimir M.
4
; Salavatulin, Vladimir M.  5
; Popov, Denis A.6
and Belyakova, Natalia A. 7
5
; Popov, Denis A.6
and Belyakova, Natalia A. 7
1✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
2Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
3✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia & Agricultural Faculty, Department of Plant Protection, Acarology Laboratory, Cukurova University, Adana, Turkey.
4Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
5Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
6Institute of Applied Entomology, St. Petersburg, Russia.
7All-Russian Institute of Plant Protection, St. Petersburg, Russia.
2023 - Volume: 63 Issue: 1 pages: 31-40
https://doi.org/10.24349/n3ej-nn6sOriginal research
Keywords
Abstract
INTRODUCTION
Predatory mites belonging to the family Phytoseiidae (Acari: Mesostigmata) are important biological control agents of some phytophagous mites and insects such as tetranychoid and eriophyoid mites, whiteflies, thrips and free-living nematodes (McMurtry et al. 2013). Although about 2800 species of phytoseiid mites are currently known, only a few have been studied with regard to their potential as biological control agents (McMurtry et al. 2013; Tsolakis et al. 2013; Knapp et al. 2018). Among them A. swirskii Athias-Henriot, Neoseiulus californicus (McGregor), N. cucumeris (Oudemans) and Phytoseiulus persimilis Athias-Henriot are mass produced predators and commercially available for the biological control of spider mites, thrips, and whiteflies (Knapp et al. 2018). Despite many successful cases obtained by using the aforementioned predators in the biological control, the determination of natural or naturalized populations of phytoseiid mites are of considerable importance due to their adaptations to local environmental conditions (Gerson 2014). The cosmopolitan mould mite, also known as copra or cheese mite, Tyrophagus putrescentiae (Schrank) (Acari: Acaridae) is found in a variety of habitats including stored products, decaying organic matter, plant seeds, medicinal plants and mushroom beds (Eraky 1995; Chmielewski 1999). In addition, T. putrescentiae is a common pest of stored foods with relatively high fat and protein content, such as cheese, copra, dried egg, groundnuts, ham, herring meal and linseed as well as dried bananas, wheat spillage, oats, barley and flour (Hughes 1976; Mullen and O'Connor 2009). Besides, it can also colonize in human dwellings especially in areas with relatively high humidity and temperature such as in carpets, sofas, and blankets, and considered as one of the main sources of allergens in house dust worldwide (Fernandez-Caldas et al. 1990). Furthermore, T. putrescentiae is one of the most important food sources which is commonly used for mass rearing of generalist phytoseiid mites, due to its low cost of production on inexpensive food sources such as bran, yeast, and flour (Vangansbeke et al. 2014; Barbosa and de Moraes 2015). Knowledge about the biological characteristics and life table parameters of phytoseiid predators, on the other hand, is considered being one of the most important steps for evaluation of their potential on preys (Tsolakis et al. 2016; Ben Chaaban et al. 2018; Tsolakis et al. 2019; Mortazavi et al. 2022). Previous studies revealed that the life table parameters of phytoseiid mites are differentially affected by several important factors such as temperature, relative humidity, host plant, prey species, age and pesticide application (Sugawara et al. 2017; Tsolakis et al. 2019; Ersin et al. 2021).
In this study, as a first step to determine the optimum temperature among the three temperatures tested (20, 25, and 30 °C) under laboratory conditions, we studied biological characteristics and life table parameters of Neoseiulus neoagrestis (as a recently described phytoseiid species), fed on T. putrescentiae with the aim of mass rearing.
Material and methods
Mite colonies
Initial colony of T. putrescentiae were obtained from soil samples collected from Tyumen, Russia. The mites were extracted by using Berlese-Tullgren funnels into tap water and transferred into the plastic containers (12 X 8 X 4 cm in size) sealed with a mesh (< 100 micrometers) on the lid for ventilation. Sufficient amount of wheat bran was provided as a food source for the mites. The predatory mite, Neoseiulus neoagrestis was collected from moss in Sochi, Russia (Khaustov et al. 2022). The stock culture of the predator was maintained in the plastic containers described above using T. putrescentiae as a food source. The prey and the predator rearing units were kept separately in incubators (SANYO Electric Biomedical co., Japan) at 25 °C and 90 ± 5% RH and 12: 12 L: D. The predatory mites were reared for several generations, before being used in experiments.
Immature development and survival
The immature development and survival of the predators were individually observed by using transparent plastic containers (3 cm in diameter and 1.8 cm deep). The lid of the experimental test units was drilled (2 cm in diameter) and covered with a fine mesh (\textless100 micrometers in size) to allow for ventilation. Two to three gravid females of the predator obtained from the stock culture were transferred to experimental arenas using a brush (number 000) under a stereo microscope (Zeiss, Stemi-305, Germany). Subsequently, egg laying was observed in the arenas at two-hour intervals. The eggs (when there was more than one egg) and adult females of N. neoagrestis were removed from the experimental arenas using a brush, and only one egg was kept in each arena. Sufficient amounts of T. putrescentiae mixed with bran was provided as a food source for the predators at daily intervals. The duration of the immature stages including egg, larva, protonymph, and deutonymph were separately determined at three temperatures (20, 25 and 30 °C, and a constant humidity 90 ± 5% RH and photoperiod 12: 12 L: D), based on the observations conducted at 12-hour intervals.
Adult survival and fecundity
The female and male individuals successfully reached the adult stages were individually monitored at daily intervals to obtain the preoviposition, oviposition, and postoviposition periods as well as the number of eggs produced, in the same experimental units and conditions described earlier. In order to increase the mating likelihood, each female was paired with two males that were obtained from the stock culture. The observations were finalized when the last individual of the original cohort had died. The sex ratio of the offspring was determined by rearing all eggs deposited by the predator`s females to adulthood.
Life table parameters
The life table parameters of N. neoagrestis were estimated based on age-stage, two-sex life table analysis (Chi and Liu 1985). The age-stage-specific survival rate (sxj ) (where x and j are age and stage, respectively); the age-stage specific fecundity (fxj ) of adult females; the age-specific survival rate (lx ); the age-specific fecundity (mx ), adult preoviposition period (APOP), total preoviposition period (TPOP), and the life table parameters including the net reproductive rate (R0), intrinsic rate of increase (r), finite rate of increase (λ), gross reproductive rate (GRR), DT doubling time and mean generation time (T) were calculated in TWOSEX-MSChart computer program (Chi, 2020). The standard errors were estimated by using bootstrap method, and the differences were determined by using paired bootstrap test (Efron and Tibshirani 1993).
Results
Immature development and survival
Although immature stages of N. neoagrestis successfully reach adulthood at all temperatures tested, the temperature had significant effect on immature development and survival of this predator (Table 1). In general, the duration of immature stages obtained at each temperature was significantly different from each other for both sexes except for larva duration for females. The larva duration of females obtained at 25 °C and 30 °C were similar but significantly shorter than those obtained at 20 °C. In addition, no significant difference was detected between immature survival rates among the temperatures tested.



Adult survival and fecundity
Similar to the immature development, total pre-oviposition, adult pre-oviposition, and oviposition periods (TPOP, APOP, OP), and longevity of N. neoagrestis fed on T. putrescentiae were significantly affected by the temperatures tested (Table 2). The shortest TPOP (6.81 days), APOP (1.38 days) and OP (20.27 days), and female (39.88 days) and male (47.20 days) longevities were detected at 30 °C. In addition, these values were significantly different than those obtained at 20 °C and 25 °C (except APOP, and OP). Furthermore, the longevity of females was shorter than males in all temperatures tested. The highest total number of eggs (62.29) laid by per female was determined at 25 °C and this value is significantly different from that obtained at 20 °C (41.46), but similar to that obtained at 30 °C (58.65).



Life table parameters
The highest intrinsic rate of increase (r= 0.241 day–1), finite rate of increase (λ= 1.272 day–1), and the shortest mean generation time (T= 13.416 days), and doubling time (DT= 2.874 days) obtained at 30 °C, and these values are significantly different from those obtained at two other temperatures (Table 3). In contrast, no significant difference was detected between the net reproductive rates (R0), and gross reproductive rates (GRR) among the temperatures. However, numerically higher values were determined at 25 °C (R0= 29.066 offspring/individual, GRR= 41.543 offspring/individual).



The age-stage survival rate curves (sxj ) show the probabilities of survival rate of newly emerged N. neoagrestis individuals to age x and stage j (Figure 1A). The variations in development rates resulted in overlaps between different developmental stages at three different temperatures. The highest survival rates of females and males are 43.33 and 0.30 at 20 °C, 0.46 and 0.26 at 25 °C, 0.43 and 0.33 at 30 °C, respectively.
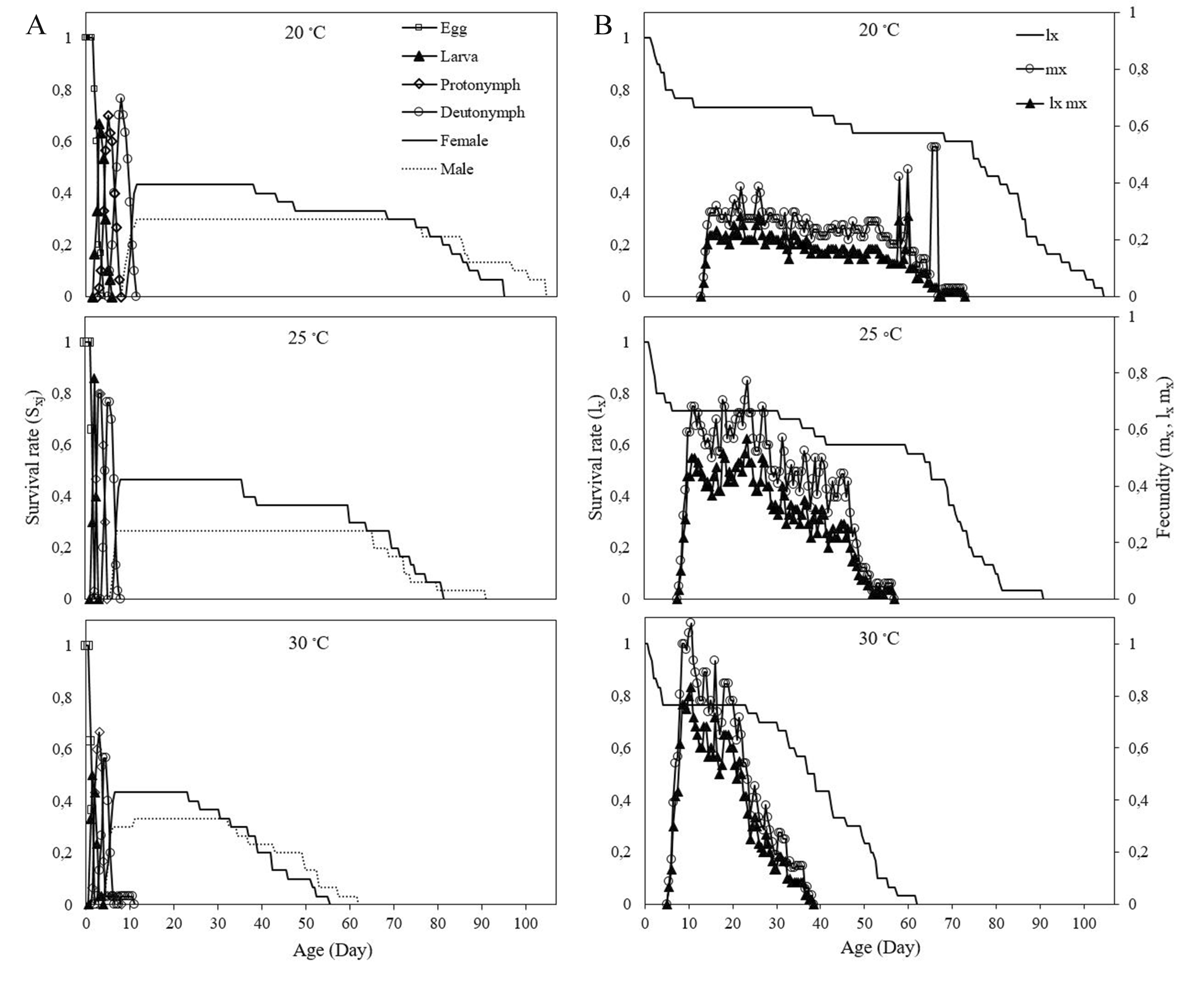


The age-specific survival rate (lx ) showed more or less a similar pattern of gradual decline during the development at three different temperatures tested, but (lx ) declined faster at 30 °C compared to the other two temperatures (Figure 1B). The first oviposition occurred on the days 13th, 7th, and 6th at 20, 25 and 30 °C, respectively. The highest values for mx and lxmx were observed at 30 °C.
The age-stage-specific life expectancy (exj ) shows the future expected life span of an individual of age x and stage j (Figure 2A). The life expectancies of a newly emerged individual were 60.59, 49.49 and 33.59 days at 20, 25 and 30 °C, respectively. In general, the age-stage-specific life expectancy (exj ) values of adult females of N. neoagrestis are lower than those of males at all temperatures tested. In addition, the (exj ) values obtained at 30 °C was lower compared to the other two temperatures, which demonstrated that life expectancies might be shorter at high temperatures. The highest age-stage-specific reproductive values (vxj ) of N. neoagrestis fed on T. putrescentiae were 9.75 on 20th day, 11.50 on 11th day, and 13.96 on 8th day, at 20 °C, 25 °C, and 30 °C, respectively (Figure 2B).



Discussion
Temperature is known to be one of the most important stressors for all organisms including insect and mite species (Wojda 2017). Many previous studies reported that the biological characteristics and life table parameters of phytoseiid mites, one of the most important groups of predators for their potential to be used in biological control, are also influenced by the temperature (Tsoukanas et al. 2006; Vangansbeke et al. 2013; Yazdanpanah et al. 2022). Therefore, the determination of a suitable temperature for the development and reproduction of phytoseiid mites is the first step for their mass rearing and usage in biological control.
Neoseiulus neoagrestis is a recently described phytoseiid species and nothing is known concerning its biological parameters on its prey (Khaustov et al. 2022). However, our preliminary observations showed that it might be a promising candidate for the biological control of spider mites. Therefore, this study includes the first and preliminarily data regarding biological characteristics and life table parameters of N. neoagrestis on T. putrescentiae at different temperatures. According to results, both males and females of the predator could develop to adults on the prey T. putrescentiae at all temperatures tested. Nevertheless, several biological characteristics, especially adult longevity and fecundity as well as life table parameters were differentially affected by the temperature.
The total developmental periods of females and males of the predator obtained at 25 °C were 7.07 days for females and 6.56 days for males. The total developmental period of N. neoagrestis females was higher than those obtained for A. eharai Amitai & Swirski (4.9 days), A. swirskii (5.1 days) and N. cucumeris (5.9 days) fed on Carpoglyphus lactis (L.) (Acari: Carpoglyphidae) at the same temperature (Ji et al. 2015). According to Ibrahim and Palacio (1994) the total immature developmental period for both sexes of N. longispinosus fed on Tetranychus urticae Koch (Acari: Tetranychidae) was 4.3 days at 25 - 28 °C. It was 9.2 days for males and 9.6 days for females of N. barkeri fed on Oligonychus afrasiaticus (McGregor) (Acari: Tetranychidae) at 25 °C (Negm et al. 2014). However, these values were decreased to 8.5 and 9 days for males and females, respectively, when the temperature increased to 35 °C. In addition, Gotoh et al. (2004) reported the total immature developmental period for both sexes of Neoseiulus californicus (McGregor) fed on T. urticae as 7.2 and 4.3 days at 20 °C and 25 °C, respectively and 3.3 days for males and 3 days for females at 30 °C. In addition, the immature development of Neoseiulus neoagrestis males were shorter than those of the females. Similar results were reported in a number of phytoseiid species such as N. barkeri Hughes (Jafari et al. 2011), Euseius finlandicus (Oudemans) (Broufas and Koveos 2001), Phytoseius plumifer (Canestrini & Fanzago) (Louni et al. 2014) and N. longispinosus (Evans) (Rahman et al. 2013).
Percent survival rate from egg to adult at all temperatures tested, was lower in present study than those reported for N. longispinosus by Rahman et al. (2013) and for N. californicus by Gotoh et al. (2004). Ji et al. (2015) reported that the percent immature survival for N. cucumeris at 25 °C was 58.54, which is also lower when compared to the results of the present study.
Similar to immature development, adult longevity was also found to be significantly different between constant temperatures for both males and females. A similar trend has been reported for two other Neoseiulus species, N. longispinosus (Rahman et al. 2013) and N. barkeri (Xia et al. 2012). In another study, Negm et al. (2014) reported that the longevity of N. barkeri fed on O. afrasiaticus at 25 °C was 35.6 and 30.3 days for females and males, respectively. In addition, average female longevity for N. cucumeris and Amblyseius swirskii fedding on C. lactis were 67.3 and 45.7 days, respectively (Ji et al. 2015).
The preoviposition, the oviposition and adult longevity determined in this study are generally higher than those reported for N. longispinosus (Rahman et al. 2013) and N. californicus (Gotoh et al. 2004).
The highest total number of the eggs laid by the female N. neoagrestis was 62.29 at 25 °C followed by 58.65 at 30 °C and 41.46 at 20 °C. Neoseiulus californicus fed on Panonychus ulmi (Koch) (Acari: Tetranychidae) produced 51.88, 63.94 and 48.74 at 20, 25 and 30 °C, respectively (El Taj and Jung 2012). However, much lower values, 41.6, 38.4 and 28.4 eggs at 20, 25 and 30 °C, were reported for the same species fed on Tetranychus urticae (red form), respectively (Gotoh et al. 2004). Bond (1989) reported that the total number of the eggs laid by N. barkeri females fed on Thrips tabaci Lind. (Thysanoptera: Thripidae) was 47.1 at 25 °C. Jafari et al. (2011) found that N. barkeri fed on T. urticae produced 38.62 eggs during its lifespan. In addition, Ibrahim and Palacio (1994) determined the total number of eggs laid by N. longispinosus 50.7 at 25-28 °C. These results clearly showed that the effects of temperature on egg production of phytoseiid mites are variable depending on species.
In addition to other biological characteristics, the life table parameters are also variable and influenced by the temperature. The intrinsic rate of increase (r = 0.241 day–1) and finite rate of increase (λ = 1.272 day–1) determined at 30 °C in our study were more or less similar to those reported for N. longispinosus (r = 0.268 day–1, λ = 1.3 day–1) at the same temperature (Rahman et al. 2013). In contrast, the r value (0.172 day–1) determined at 25 °C in the current study, was lower than those determined for A. eharai (0.253 day–1), A. swirskii (0.232 day–1) and N. cucumeris (0.212 day–1) fed on C. lactis (Ji et al. (2015). The net reproductive rate (R0 = 29.06 offspring/individual) determined at 25 °C in the current study, was more or less similar to that reported for N. longispinosus preying on T. urticae (R0 29.4 offspring/individual) (Kolodochka 1983). However, the mean generation times (T=13.41 - 29.09 days) of N. neoagrestis were longer than those determined for N. longispinosus (9.0 - 9.8 days) (Ibrahim and Palacio 1994; Kolodochka 1983). The doubling time determined (DT= 2.87 days at 30 °C) in the present study, is also shorter than that reported for N. longispinosus (3.2 days) (Rahman et al. (2013), but lower than that found for N. californicus (2.37 days) (El Taj and Jung 2012).
In conclusion, this study reports, the biological characteristics and life table parameters of a newly described and a potential predatory mite to be used in biological control, for the first time. The results showed that the performance of N. neoagrestis seems to be better at 25 °C and 30 °C compared to 20 °C. By its fecundity, N. neoagrestis is close to such successful biocontrol agents as N. californicus and N. cucumeris. In addition, a prolonged lifespan potentially lets the predator persist in greenhouses for two months. Further studies should be conducted to determine biology of this predator on agriculturally important pests including spider mites, thrips and whiteflies. In addition, the efficacy of the predator against aforementioned pests should also be determined in field and greenhouse conditions.
Acknowledgements
The present research was supported by the grant from the Russian Science Foundation, project number 20–64–47015.
References
- Barbosa M.F.C., de Moraes G.J. 2015. Evaluation of astigmatid mites as factitious food for rearing four predaceous phytoseiid mites (Acari: Astigmatina; Phytoseiidae). Biol. Control., 91: 22-26. https://doi.org/10.1016/j.biocontrol.2015.06.010
- Ben Chaaban S., Chermiti B., Kreiter S. 2018. Biology and life-table of Typhlodromus (Anthoseius) athenas (Acari: Phytoseiidae) fed with the Old-World Date Mite, Oligonychus afrasiaticus (Acari: Tetranychidae). Acarologia, 58: 52-61. https://doi.org/10.24349/acarologia/20184229
- Bond J. 1989. Biological studies including population growth parameters of the predatory mite Amblyseius barkeri (Acarina: Phytoseiidae) at 25 °C in the laboratory. Entomophaga, 34: 275-287. https://doi.org/10.1007/BF02372676
- Broufas G.D., Koveos D.S. 2001. Development, survival and reproduction of Euseius finlandicus (Acari: Phytoseiidae) at different constant temperatures. Exp. Appl. Acarol., 25: 441-460. https://doi.org/10.1023/A:1011801703707
- Chmielewski W. 1999. Acceptance of buckwheat grain as food by Tyrophagus putrescentiae (Schrank) (Acari: Acaridae). Fagopyrum, 16: 95-97.
- Chi H. 20220 TWOSEX-MSChart: a computer program for the age- stage, two-sex life table analysis (Ver. 2020.06.16). http://140.120.197.173/Ecology/Download/Twosex-MSChart.zip
 . National Chung Hsing University, Taichung, Taiwan.
. National Chung Hsing University, Taichung, Taiwan. - Chi H., Liu H. 1985. Two new methods for the study of insect population. Bull. Inst. Zool. Acad. Sin. , 24: 225-240.
- Efron B., Tibshirani, R.J. 1993. An introduction to the bootstrap. Chapman & Hall, New York, NY, 430 pp. https://doi.org/10.1007/978-1-4899-4541-9
- El Taj H.F., Jung C. 2012. Effect of temperature on the life-history traits of Neoseiulus californicus (Acari: Phytoseiidae) fed on Panonychus ulmi. Exp. Appl. Acarol., 56: 247-260. https://doi.org/10.1007/s10493-012-9516-2
- Eraky S.A. 1995. Some biological aspects of Tyrophagus putrescentiae. In: Kropcyzynska D., Boczek J., Tomczyk A. (Eds.), The Acari. Oficyna Dabor, Warszawa: Publisher. p. 127-133.
- Ersin F., Turanli F., Cakmak I. 2021. Development and life history parameters of Typhlodromus recki (Acari: Phytoseiidae) feeding on Tetranychus urticae (Acari: Tetranychidae) at different temperatures. Syst. Appl. Acarol., 26: 496-508. https://doi.org/10.11158/saa.26.2.12
- Fernandez-Caldas E., Fox R.W., Bucholtz G.A., Trudeau W.L., Ledford D.K., Lockey R.F. 1990. House dust mite allergy in Florida. Mite survey in households of mite-sensitive individuals in Tampa, Florida. Allergy Proceedings, 11: 263-267. https://doi.org/10.2500/108854190778879710
- Gerson U. 2014. Pest control by mites (Acari): Present and future. Acarologia, 54: 371-394. https://doi.org/10.1051/acarologia/20142144
- Gotoh T., Yamaguchi K., Mori K. 2004. Effect of temperature on life history of the predatory mite Amblyseius (Neoseiulus) californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 32: 15-30. https://doi.org/10.1023/B:APPA.0000018192.91930.49
- Hughes B.O. 1976. Preference decisions of domestic hens for wire or litter floors. Appl. Anim. Ethol., 2: 155-165. https://doi.org/10.1016/0304-3762(76)90043-2
- Ibrahim Y.B., Palacio V.B. 1994. Life history and demography of the predatory mite, Amblyseius longispinosus Evans. Exp. Appl. Acarol., 18: 361-369. https://doi.org/10.1007/BF00116317
- Jafari S., Fathipour Y., Faraji F. 2011. Temperature-dependent development of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) at seven constant temperatures. Insect. Sci., 19: 220-228. https://doi.org/10.1111/j.1744-7917.2011.01444.x
- Ji J., Zhang Y.X., Lin J.Z., Chen X., Sun L., Saito Y. 2015. Life histories of three predatory mites feeding upon Carpoglyphus lactis (Acari, Phytoseiidae; Carpoglyphidae). Syst. Appl. Acarol., 20 (5): 491-496. https://doi.org/10.11158/saa.20.5.5
- Khaustov V.A. Doker I., Joharchi O., Kazakov D.V., Khaustov A.A., Moradi M., Fang X-D., Klimov P. 2022. A new, broadly distributed species of predacious mites, Neoseiulus neoagrestis sp. nov., (Acari: Phytoseiidae) discovered through GenBank data mining and extensive morphological analyses. Syst. Appl. Acarol., 27: 2038-2061. https://doi.org/10.11158/saa.27.10.14
- Knapp M., van Houten Y., van Baala E., Groot T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia, 58: 72-82. https://doi.org/10.24349/acarologia/20184275
- Kolodochka L.A. 1983. Ecological features of the predaceous mite Amblyseius longispinosus. Vestn. Zool. 5: 36-42. (In Russian)
- Louni M., Jafari Sh., Shakarami J. 2014. Life table parameters of Phytoseius plumifer (Phytoseiidae) fed on Rhyncaphytoptus ficifoliae (Diptilomiopidae) under laboratory conditions. Syst. Appl. Acarol., 19: 275-282. https://doi.org/10.11158/saa.19.3.3
- McMurtry J.A., de Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Mortazavi N., Fathipour Y., Talebi A.A., Riahi E. 2022. Suitability of monotypic and mixed diets for development, population growth and predation capacity of Typhlodromus bagdasarjani (Acari: Phytoseiidae). Bull. Entomol. Res., 28: 1-11. https://doi.org/10.1017/S0007485322000372
- Mullen G.R., O'Connor B.M. 2009. Mites. In: Mullen G.R., Durden L. (Eds). Medical and Veterinary Entomology (2nd ed.). Academic Press. p. 423-482.
- Negm M.W., Alatawi F.J., Aldryhim Y.N. 2014. Biology, predation and life table of Cydnoseius negevi and Neoseiulus barkeri (Acari: Phytoseiidae) on the old world date mite Oligonychus afrasiaticus (Acari: Tetranychidae). J. Insect Sci., 14 (177): 1-6. https://doi.org/10.1093/jisesa/ieu039
- Rahman V. J., Babu A., Roobakkumar A., Perumalsamy K. 2013. Life table and predation of Neoseiulus longispinosus (Acari: Phytoseiidae) on Oligonychus coffeae (Acari: Tetranychidae) infesting tea. Exp. Appl. Acarol., 60: 229-240. https://doi.org/10.1007/s10493-012-9649-3
- Sugawara R., Ullah M.S., Ho C.C., Gökçe A., Chi H., Gotoh T. 2017. Temperature-dependent demography of two closely related predatory mites Neoseiulus womersleyi and N. longispinosus (Acari: Phytoseiidae). J. Econ. Entomol., 110: 1533-1546. https://doi.org/10.1093/jee/tox177
- Tsolakis H., Jordà Palomero R., Ragusa E. 2013. Predatory performance of two Mediterranean phytoseiid species, Typhlodromus laurentii and Typhlodromus rhenanoides fed on eggs of Panonychus citri and Tetranychus urticae. Bull. Insectology., 66: 291-296.
- Tsolakis H., Principato D., Jordà Palomero R., Lombardo A. 2016. Biological and life table parameters of Typhlodromus laurentii and Iphiseius degenerans (Acari, Phytoseiidae) fed on Panonychus citri and pollen of Oxalis pes-caprae under laboratory conditions. Exp. Appl. Acarol., 70: 205-218. https://doi.org/10.1007/s10493-016-0076-8
- Tsolakis H., Sinacori M., Ragusa E., Lombardo A. 2019. Biological parameters of Neoseiulus longilaterus (Athias-Henriot) (Parasitiformes, Phytoseiidae) fed on prey and pollen in laboratory conditions. Syst. Appl. Acarol., 24: 1757-1768. https://doi.org/10.11158/saa.24.9.12
- Tsoukanas V.I., Papadopoulos G.D., Fantinou A.A., Papadoulis G.Th. 2006. Temperature-dependent development and life table of Iphiseius degenerans (Acari: Phytoseiidae). Environ. Entomol., 35: 212-218. https://doi.org/10.1603/0046-225X-35.2.212
- Vangansbeke D., De Schrijver L., Spranghers T., Audenaert J., Verhoeven R., Nguyen D.T., Gobin B., Tirry L., Clercq P. 2013. Alternating temperatures affect life table parameters of Phytoseiulus persimilis, Neoseiulus californicus (Acari: Phytoseiidae) and their prey Tetranychus urticae (Acari: Tetranychidae). Exp. Appl. Acarol. 61: 285-298. https://doi.org/10.1007/s10493-013-9704-8
- Vangansbeke D., Nguyen D.T., Audenaert J., Verhoeven R., Gobin, B., Tirry L., De Clercq P. 2014. Performance of the predatory mite Amblydromalus limonicus on factitious foods. Biol. Control., 59: 67-77. https://doi.org/10.1007/s10526-013-9548-5
- Wojda I. (2017) Temperature stress and insect immunity. J. Therm. Biol., 68: 96-103. https://doi.org/10.1016/j.jtherbio.2016.12.002
- Xia B., Zou Z., Li P., Lin P. 2012. Effect of temperature on development and reproduction of Neoseiulus barkeri (Acari: Phytoseiidae) fed on Aleuroglyphus ovatus. Exp. Appl. Acarol., 56: 33-41. https://doi.org/10.1007/s10493-011-9481-1
- Yazdanpanah S., Fathipour Y., Riahi E., Zalucki M.P. 2022. Modeling Temperature-Dependent Development Rate of Neoseiulus cucumeris (Acari: Phytoseiidae) Fed on Two Alternative Diets. Environ. Entomol., 51: 145-152. https://doi.org/10.1093/ee/nvab130



222-10-05
Date accepted:
2022-12-22
Date published:
2023-01-09
Edited by:
Marčić, Dejan

This work is licensed under a Creative Commons Attribution 4.0 International License
2023 Moradi, Maryam; Joharchi, Omid; Döker, Ismail; Khaustov, Vladimir A.; Salavatulin, Vladimir M.; Popov, Denis A. and Belyakova, Natalia A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



