Caeculus fedrae sp. nov., a new fossil species of rake-legged mite (Acari: Caeculidae) from Baltic amber.
Porta, Andrés O.  1
; Michalik, Peter
1
; Michalik, Peter  2
and Ramírez, Martín J.
2
and Ramírez, Martín J.  3
3
1✉ División Aracnología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Av. Ángel Gallardo 470 C1405DJR, Buenos Aires, Argentina & Universidad de Buenos Aires, Facultad de Ciencias Exactas y Naturales, Departamento de Ecología, Genética y Evolución, Instituto de Ecología, Genética y Evolución de Buenos Aires (IEGEBA, UBA-CONICET), Pabellón II, Ciudad Universitaria, Buenos Aires C1428EGA, Argentina & Departamento de Ciencias Exactas, Universidad Nacional del Oeste, Belgrano 369 C1718, San Antonio de Padua, Buenos Aires, Argentina.
2Zoological Museum, University of Greifswald, Loitzer Str. 26, 17489, Greifswald, Mecklenburg-Vorpommern, Germany.
3División Aracnología, Museo Argentino de Ciencias Naturales “Bernardino Rivadavia”, Av. Ángel Gallardo 470 C1405DJR, Buenos Aires, Argentina.
2022 - Volume: 62 Issue: 4 pages: 1154-1170
https://doi.org/10.24349/n718-9sxsZooBank LSID: 5B10E209-4973-41A2-9815-4746C9AA5E1F
Original research
Keywords
Abstract
Introduction
Mites of the family Caeculidae Berlese 1883 are medium to large sized and common in arid habitats. One of the main characteristics are the spines on the legs, which are used to capture small arthropods (Walter et al. 2009). Currently, the family comprises about 100 extant species in seven accepted genera (Taylor et al. 2013). With regard to the fossil record, only four species are known, all belonging to the genus Procaeculus, from different amber deposits: P. eridasonae Coineau & Magowski, 1994, from Baltic amber (Middle Eocene); P. dominicensis Coineau & Poinar, 2001, from Dominican amber (Upper Eocene), Procaeculus sp. (Rivas et al. 2016) from Chiapas amber (Early Miocene) from mines in Simojovel, Mexico, and P. coineaui Porta, Proud, Franchi, Porto, Epele & Michalik, 2019, from Burmese amber (Upper Cretaceous).
The nominal genus of the family, Caeculus Dufour, 1932, comprises 21 extant species (Taylor et al. 2013; Bernard et al. 2020; Porta and Vazquez, 2020); only C. echinipes Dufour, 1832 is found in Europe while the rest of the species are distributed in North America. Yves Coineau (1974) proposed the genus Pseudocaeculus for some American species including C. americanus Banks, 1899. However, Taylor et al. (2013) pointed out that Pseudocaeculus is not an available name, since Coineau did not designate a type species. Bernard et al. (2000) published the first phylogeny of the genus based on morphological data and suggested that the common ancestor of Caeculus inhabited southwestern North America and that the lineage spread from North America to Europe. Here, we describe the first fossil species of the genus Caeculus from Baltic amber (Middle Eocene) which is also the first fossil caeculid mite not belonging to the genus Procaeculus, and the second species of the genus known from Europe.
Material and methods
The specimens are preserved in cut pieces of amber. Cutting of the amber pieces was made using a mini grinder Pro'sKit PT-5205U equipped with dental discs. For optical observation, specimens were prepared by sanding with a series of waterproof sandpapers and polishing with a fine cerium oxide paste and Chamois leather. To refer to each specimen of the type series we introduce the following abbreviations: Hol for the holotype, SMF Be 14264a, Par1 for the paratype SMF Be 14265a and Par2 for the paratype SMF Be 14264b. Hol and Par2 (SMF Be 14264b) belong originally to the same piece of Baltic amber. In Hol (Figs. 1A–B, 2) there are bubbles clinging on to the specimen's idiosoma, and the aspidosoma is broken in its anterior portion precluding the observation of the gnathosoma (Fig. 2). In Par1 (Fig. 3) the dorsal plates are partially degraded precluding the observation of chaetotaxy (Fig. 4B). Dorsal to Par2 (Fig. 1C) there is a fault in the amber piece precluding the observation of dorsal plates and setae with optical instruments, and the bubbles in the ventral side preclude the observation; some segments of legs I and II are missing. Specimens were studied and measured on Olympus BH-2, CH-2 and Reichert Zetopan compound microscopes. Drawings were made with a camera lucida mounted on an Olympus microscope using both transmitted and incident light. Photographs were taken with a camera on a Leica D2500, the Olympus BH-2 or the Reichert Zetopan. Terminology follows Coineau (1974) with the addition of notation of measures and ratios of leg segments as in Porta et al. (2019) and Porta and Vazquez (2020). Following Porta et al. (2021), a rational number, p, associated to an event is the estimation of the probability of this event obtained as the count of favorable cases divided by the count of total cases. Measurements are given in micrometers. Abbreviations Hol, Par1 and Par2 after or before some observation indicates that it corresponds to a specific exemplar; for example: tibia III with solenidium (Par1) means that the solenidium has been observed in the tibia III of the paratype 1. All specimens are deposited in the Senckenberg Naturmuseum Frankfurt (SMF).



Results
Family Caeculidae Oudemans, 1902
Genus Caeculus Dufour, 1832
Type species: Caeculus echinipes Dufour, 1832 by monotypy.
Diagnosis — (based on Coineau 1974) – Caeculus corresponds to caeculids with dorsal plates without intensive neotrichy, ω solenidium dorsally located, famulus ε dorsal and hidden, famulus κ″ recessive, hidden; eupathidia with the typical shape of ′baguette de tambour′ (stick drum) of most of the genera of the family, legs with lateral claws equally sized in each tarsus, with trichobothria on all tarsi and tridactyly evident.
Remark — Except for the species C. echinipes, which has been studied in detail by Y. Coineau in his monograph on the family (1974), and two species recently described (Bernard et al. 2020; Porta and Vazquez 2020), all the other species of the genus need to be redescribed.
Caeculus fedrae sp. nov.
ZOOBANK: 006725F4-64A2-4343-B548-A896C4E8DC47 ![]()
(Figs. 1–11)
Etymology
The specific name is a patronym in honor to Dr. Fedra Bollatti (IDEA-Conicet).
Type material
Holotype, SMF Be 14264a, adult, sex indeterminate in Baltic amber (Eocene), and 2 paratypes, SMF Be 14264b and SMF Be 14265a, both adults of sex indeterminate also from Baltic amber.
Type locality
Baltic Sea coast, Yantarny settlement (formerly Palmnicken), Sambia (Samland) Peninsula, the Kaliningrad Region, Russia.
Diagnosis
Aspidosoma relatively short, slightly downturned (Fig. 3A), with anterior border with a median concavity (Fig. 5C–D), with two setae in the region Pp (Fig. 2); seta Po impair and short and trichobothria bo spatulate (Fig. 5F–G). The regressive chaetotaxy of the dorsal plates is unique for the genus Caeculus, plate D with only a pair of setae in each the series a, b and c; b2 absent in plate L; M plate with only 4 setae and ds absent. Other diagnostic characters are dorsal plates cuticle with alveoli (Fig. 4A–B), plate M (Figs. 2, 3) fused; leg I trochanter (Fig. 6A, D) with two pedunculated seta on l′ series, femur undivided in all legs (Figs. 6A, 9D, 10C–D); two setae in the series v′ of femur I, with v′ spiny, straight, with blunt end (Fig. 6A, C) and v1′ much shorter, curved and peciolated, two subequal spiny setae (the two distal) on genu I series v′ (Fig. 6A–B) and two on tibia (Fig. 7A, C).
Remarks
The three fossil specimens are considered adults based on the relative sizes of the anal and genital plates (Figs. 1B, 3C) and the presence of at least six setae on the genital plate of the holotype (Coineau 1974). The new fossil species is assigned to the genus Caeculus based in the chaetotaxy of the dorsal plates (Fig. 2), the shape of the leg eupathidia (e.g. Fig. 6); the presence of trichobothria and tridactyly in all leg tarsi (Figs. 8, 9A–C, 10E, 11F–G) and the position of the ω solenidium and the famulus ε in the dorsal facies of the tarsi I (Fig. 8). It must be noted here that C. fedrae sp. nov. differs from other fossil caeculids, all belonging to the genus Procaeculus, by the shape of eupathidia, the evident tridactyly and the bases of the trichobothria bo (Porta et al. 2019).
Description
Body color: grayish brown.












Idiosoma — subtrapezoidal in dorsal view (Figs. 1A, 2, 3A), 1120 (940–1100) long, 640 (640) wide at the level of coxa IV, cuticle of dorsal plates, coxae and hypostome with alveoli (Fig. 4), membranous integument striate.
Aspidosoma — slightly downturned (Figs. 2, 3A), anterior margin 235 long in Par1, with a median concavity (Fig. 5C–D), posterior margin 451 wide in Hol. Seta Po (Fig. 5F–G) impair, peciolated and small, 41 long, observable in Par1; trichobothria bo in Par1 spatulate, broadening in the distal end, 46–60 long. Apparently, one small seta Pa, in the left anterior margin of Par1 (Fig. 5D), only one insertion Pm observable in the left lateral margin of Hol (Fig. 2B), sector of the Pp with 2 setae in Hol, 23–32 long (Fig. 2B). Lateral eye plates not separated from other dorsal plates; anterior pair of eyes diameter 18–20, posterior pair 23.
Hysterosoma — Dorsal view – with 5 dorsal plates D, (L), M and P (Figs. 2, 3). Unpaired dorsal plate D trapezoidal, 438 long, anterior margin 307 long, posterior margin 446 long in Hol (Fig. 2B), series a1, b1, c1, only observable in Hol, each with a pair of setae situated near the median axis of the plate, length of setae a1: 41, b1: 42–46, c1: 46; distance between setae insertions, a1-a1: 64, b1-b1: 69, c1-c1: 80, a1-b1: 67, b1-c1: 90. Paired plates L 460 (400 Par1) long, 124 (115 Par1) wide; a2:30 (37 Par2), b2 absent, c2: 37 long. Median posterior plate M with a median posterior concavity (Figs. 2, 3), 64 (77 Par1) long at median axis and 115 in the lateral margin of the body, 653 (531 Par1) wide, not divided in two plates, with 2 pairs of long setae, d1 and d2, 60 and 46–57 long, respectively, ds absent, distance between setae insertions, d1-d1: 184, d1-d2: 156-161, d2-d2: 510. Posterior plate P, 64 (69 Par2) long, 483 (484 Par2) wide; with 4 setae (only insertion observable in some cases), e1 and e2, 64 and 46 long, respectively; distance between setae insertions, e1-e1: 161, e1-e2: 96. Seta hs on posterior border of idiosoma, 35 long.
Ventral view – (Figs. 1B–C, 3C, 4C), coxae and hypostome cuticle with alveoli, membranous integument striate. Coxal setae clavate, coxa I with at least 4 setae, II and IV with at least 2, III and IV with at least 1. Genital opening 284 (189 Par1) long, 185 wide with 6 pairs of genital setae in Hol, 14–16 long. Anal opening 192 (169) long, with at least a pair of clavate setae, 32 long. Only ps3 and ps2 observable in pseudoanal plates of Hol and Par1.
Gnathosoma — Chelicerae not observable. Palp only partially observed in Par1 in ventral (Fig. 5A–B) and lateral view, trochanter, femur and genu not observed, tibia with at least 5 setae, only seta d calcar, the rest of them clavate, only the contour of the tarsus visible in lateral view as well as two terminal eupathidia in ventral view. Subcapitulum (Fig. 4C) in Par1 posteriorly rounded, anteriorly subconical, 231 wide at level of palp insertion, 250 long, setae m, 28 long in Hol, n not visible, two pairs of adoral setae, or 1-2, 14–16 long.
Legs — (Figs. 6–11)









Leg I – (Figs. 6–8). Trochanter (Figs. 6A, C, 7A, C) 253 (220–253) long, 166 (142–156) wide with two setae on series l′ (only one seta in addition an elevation of the cuticle in one setal insertion observable in Hol), 64 (46–60), 83+ (64–83) long, Hol with one elongated and peciolated seta on d, 60–64 long. Femur (Figs. 6A, C, 7A, C) 253–262 (230–240) long, 115–133 (115) wide with two setae on series v′, v′ seta spiny, straight, with distal end blunt, 124 (101), 156+ (138+) long, ratios Rf: 1.08 (0.88), RPref+: 1.36 (1.2), seta v1′ peciolated, curved, 46–51 long (27); with a spiny and blunt ended seta on series v", 54 long in Par1; 4 peciolated setae on series d, 3 in series l′ with l′ eupathidial and the rest peciolated, 28–38 long, 3–4 setae on series l″ with setae l″ and l1″ eupathidial in Hol and l2″ also eupathidial en Par1. Genu (Figs. 6A–B, 7A–B), 253–267 long, 87–92 wide in Par1 with the 2 distal setae on series v′, straight and spine shaped, subequal, 133–146 (124–138), 174-184+ (166–184) long, seta v2′, peciolated and curved, 32 long; 1 spiny seta observable in series v″, 138 (108), 175+ long; 4 peciolated setae on the series d; 5 and 5–6 setae on each of the lateral series l′ and l″, respectively, setae l′, l2′ (p=1/2), l3′, l″ and l2″ (p=2/3) and l3″ (p=1/2) eupathidial. Tibia (Figs. 6A, 7) 338 long in Par1, with 3 setae in series v′, the 2 distal pointed and subequal, in Par1 133–138, 175–193+ long, the proximal, v2′, peciolated, 41–46, 64–69+ long in Par1, 2 subequal distal spiny setae observable on series v″, 138-140, 161+ long, in addition to 2 shorter, v2» and v3″, 62 and 31 long, respectively; Par1 with 5 setae in lateral series, setae l″ and l3″ eupathidial, series l′ not observable solenidium φ hidden inserted in the habitual dorsoantiaxial position in the distal part of the tibia observable in Hol (Fig. 8A), κ″ not observed. Tarsus (Fig. 8), 253 (246 in Par1) long, 41 wide at base, with 5 setae in series l′ and 4 in l″ series, with l1″, l2″ and l′ eupathidial; hidden solenidium ω inserted dorsally (Fig. 8A) at the level of l2′, ε famulus hidden, located in dorsal facies, near the solenidium; series v′ with at least 4 setae; (er) non eupathidial, (st) not observed, trichobothria bt short, 26 long; tridactyly present, with two the two lateral claws, ol′ and ol", subequal and the median, oc, shorter.
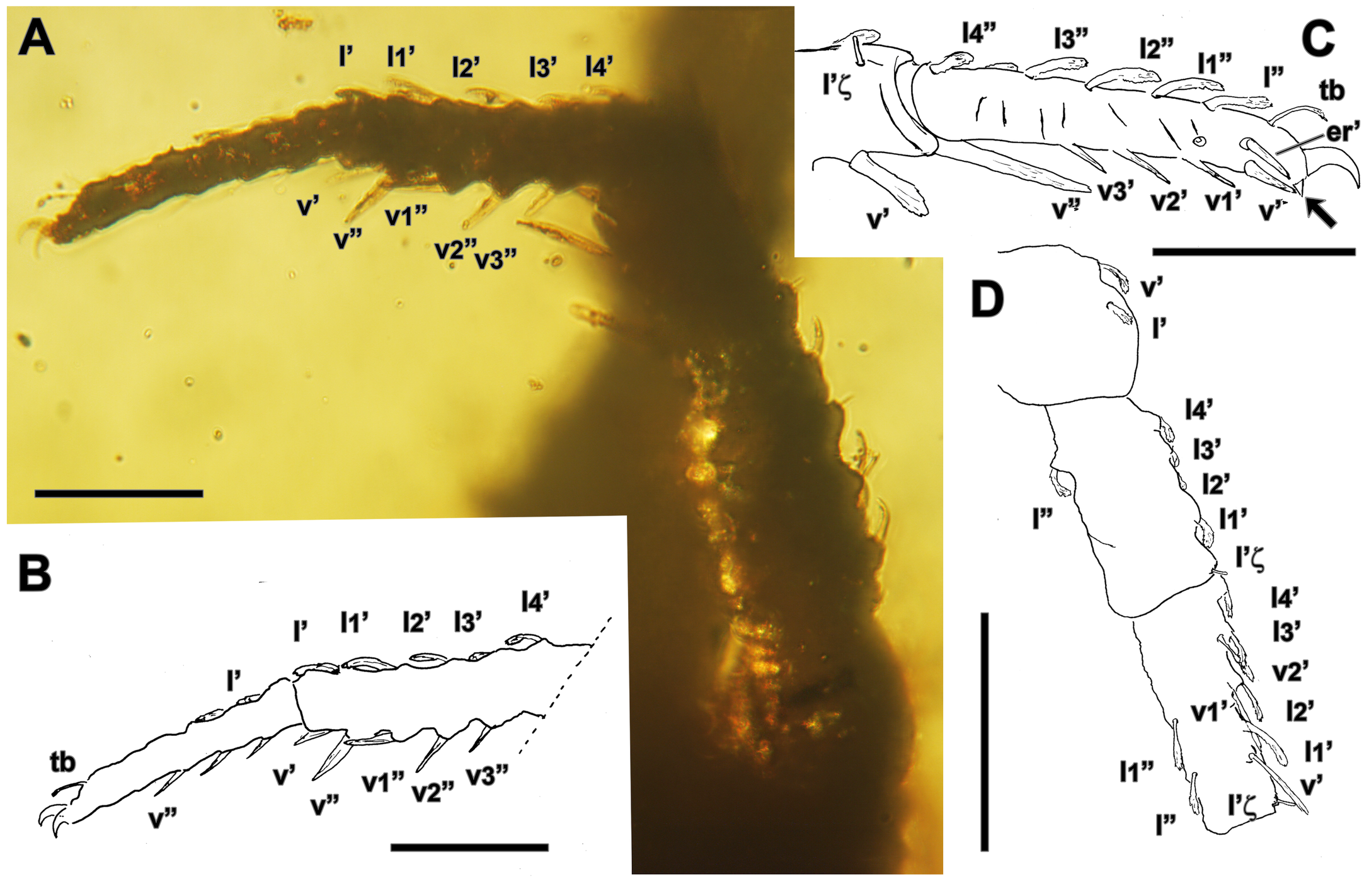


Leg II – (Fig. 9) Trochanter (Fig. 9D) in Par1 189 long, 92 wide with only one seta observable in each series l′ and d'. Femur (Fig. 9D) 170 long, 87 wide, with 5 setae in series l′ and at least 1 in l″, respectively; l′ eupathidial. Genu (Fig. 9A, D) in Par1 184 long, 69 long, with 4 setae in series l′ all peciolated, Hol with 2 setae in ventral series v″, v″ seta elongated, 78, 92+ long. Tibia (Figs. 9A–B) in Par1 207 long, with 4 setae on ventral series v″ (6 in Par2), 5 setae on lateral series l″, l′ eupathidial in Par2, solenidium φ not observable. Tarsus (Fig. 9B–C) with 5 setae on lateral series l″ and l′ (4 in Par2); solenidium ω and famulus ε as in leg I (Hol), each ventral series v′, with at least 4 setae; er′ non eupathidial, trichobothria bt 25 (32 in Par2) long; tridactyly present (Fig. 9C, arrow) with the two claws ol′ and ol" subequal.



Leg III – (Figs. 10, 11) Trochanter (Fig. 10A, C, F–G) in Par1 130–156 long, 115–124 wide, with 2 setae in series l′. Femur in Par1 133–147 long, 81–87 wide, with 2 setae in series l′ with l′ eupathidial. Genu 147 long, 64 wide in Par1, with 4 setae on series d (Hol); 4 setae in lateral series l′ with only seta l′ eupathidial. Tibia (Fig. 11A, D), 285–317 (322 Par2) long, 46 (46 Par2) wide, RTi 6.2–6.3 (7 in Par2) with 6 setae on ventral series v″ (5 in Par2), 3 setae in series d, 6 setae in lateral series l′ in Hol, 5 in Par1; 5 setae in series l″ with setae l1″, l2″ (p=1/3), l3″ (p=2/3) and l4″ (p=1/2); l′ eupathidial in Hol, Par1 and Par2 and l4′ eupathidial only in Hol, solenidium φ inserted in dorsoantiaxial position in the distal part of the tibiae. Tarsus 202 (221 Par2) long, (32 Par) wide at base, with 3 setae in ventral series v′, pair (er) non eupathidial, trichobothria bt 110 (92 Par2) long; tridactyly present, with the two lateral claws ol′ and ol" subequal.



Leg IV – (Figs. 10, 11) Trochanter (Fig. 10A, D) in Par1 253 long, 120 wide, with 1 seta on each d and v series. Femur 161 long, 78 wide, with 2 setae on series l′, distal seta l′ eupathidial. Genu of Par1 166 long, 55 wide, with 4 setae in lateral series l′; setae l″ and l′ eupathidial in Hol. Tibia (Figs. 10B, D, 11B, E), 345 (253–381) long, 60 wide, with 4–5 setae in ventral series v′, 5–6 setae in lateral series l′ and 5 on l″, setae l″, l3″, l′ and l3′ eupathidial, solenidium φ apparently absent. Tarsus (Figs. 10B, E, 11B–C, F–G) 207–216 (230 in Par2) long, 37 wide at base, with 5 setae in ventral series v″ (4 in Par2), 3 on lateral series l″ with l1″ eupathidial in the left leg of Hol (p=1/2); (er) non eupathidial; trichobothria bt 115 (106 in Par1, 124 in Par2) long; tridactyly observable (Fig. 11F, arrow), with the two claws ol′ and ol" subequal, 28 long.
Discussion
Morphology and Systematics
The new species present some morphological features that are remarkable for the genus Caeculus and deserves to be further discussed, namely: the aspidosomal shape; the leg chaetotaxy with entire femora, the chaetotaxy of the dorsal idiosomal plates and the presence of an impair seta Po.
In contrast to most of the extant species of Caeculus the aspidosoma of C. fedrae sp. nov. is short, slightly downturned, and not horizontally extended over the gnathosoma, compared with most of the extant species of Caeculus. However, we refrain from proposing a new genus for this fossil species based on this peculiar morphology as C. kerrulius Mulaik, 1945 seems to have a similar morphology of the aspidosoma (see Mulaik 1945: Figs. 1, 17), and species of Neocaeculus Coineau 1967 have great interspecific variation in this character (Taylor 2014).
The entire femora of all legs and the morphology of the series v′ of leg I in the new species is also very unusual for the genus; only two species of Caeculus have undivided femora, according to the original descriptions, C. americanus, Banks 1899 and C. clavatus Banks 1905 (it will be interesting to reexamine Banks' specimens to confirm that their femora are truly entire). The series v′ on the femur I is, however, very different in Banks' species: while in these species v′ and v1′ have similar shape and size, in C. fedrae sp. nov. v1′ is peciolated and much smaller than v′, which is spine-shaped (Fig. 6A, C). On the other hand, the chaetotaxy of the series v′ in leg I of C. fedrae sp. nov. exhibits a high level of concordance with those of leg I of other species in the genus Procaeculus, namely P. dominicensis Coineau and Poinar, 20019; P. eridanosae Coineau and Magowski, 1994; P. mexicanus (Mulaik & Allred, 1954); P. oregonus (Mulaik & Allred, 1954) and P. willmanni (Vitzthum, 1933) (see Coineau 1969). All species of Procaeculus also present undivided femora. It must be mentioned, however, that most species of the genus Procaeculus have been collected from trees, as was noted for Coineau and Magowski (1994) in order to explain why this is the only genus present in fossil amber. Then, considering that the fossil arthropods found in Baltic amber seem to be mainly bark-dwelling species (Dunlop et al., 2018), the similarity between the legs I of C. fedrae sp. nov. and those of some Procaeculus may be a convergent morphological adaptation to bark-dwelling lifestyle (although undivided femora I are found in many Neocaeculus species that are terrestrial, e.g. Taylor et al. (2014), they present a different pattern of the v′ series). The oligotrichy of the dorsal plates L and D of C. fedrae sp. nov. is unique for the genus. Following the studies of the ontogeny of the setae of the dorsal plates in the Coineau (1974), the setae present in C. fedrae sp. nov. constitutes the minimal set possible in adults caeculids. The same condition has been reported for some psammophile caeculids belonging to Microcaeculus (Coineau 1974) and Andocaeculus Coineau, 1974 (Porta et al. 2020) and is considered a derived condition, resulting from a process of ''évolution régressive par retardement″ of the dorsal plates chaetotaxy. Furthermore, the other caeculid species described from Baltic amber, Procaeculus eridanosae, shares the same condition, unique also for its genus. It could be interesting to study if there is some correlation between the presence of oligotrichy in the dorsal plates and some ecological constraints in modern caeculids in order to make paleoecological inferences.
According to the proposed character evolution by Coineau (1974), the presence of a single seta Po in C. fedrae sp. nov., must be considered derived especially for the genus Caeculus, where the plesiomorphic condition would be a pair of setae. This author even reported the transition from a pair to a impair seta Po in the course of the ontogeny of C. echinipes. The same condition as in C. fedrae sp. nov. occurs at least in three North American species of the genus. The presence of this derived condition in the new taxon, a putative internal node in the phylogeny of the family, is of great value for the dating and study of the morphological changes during the evolution of this family.
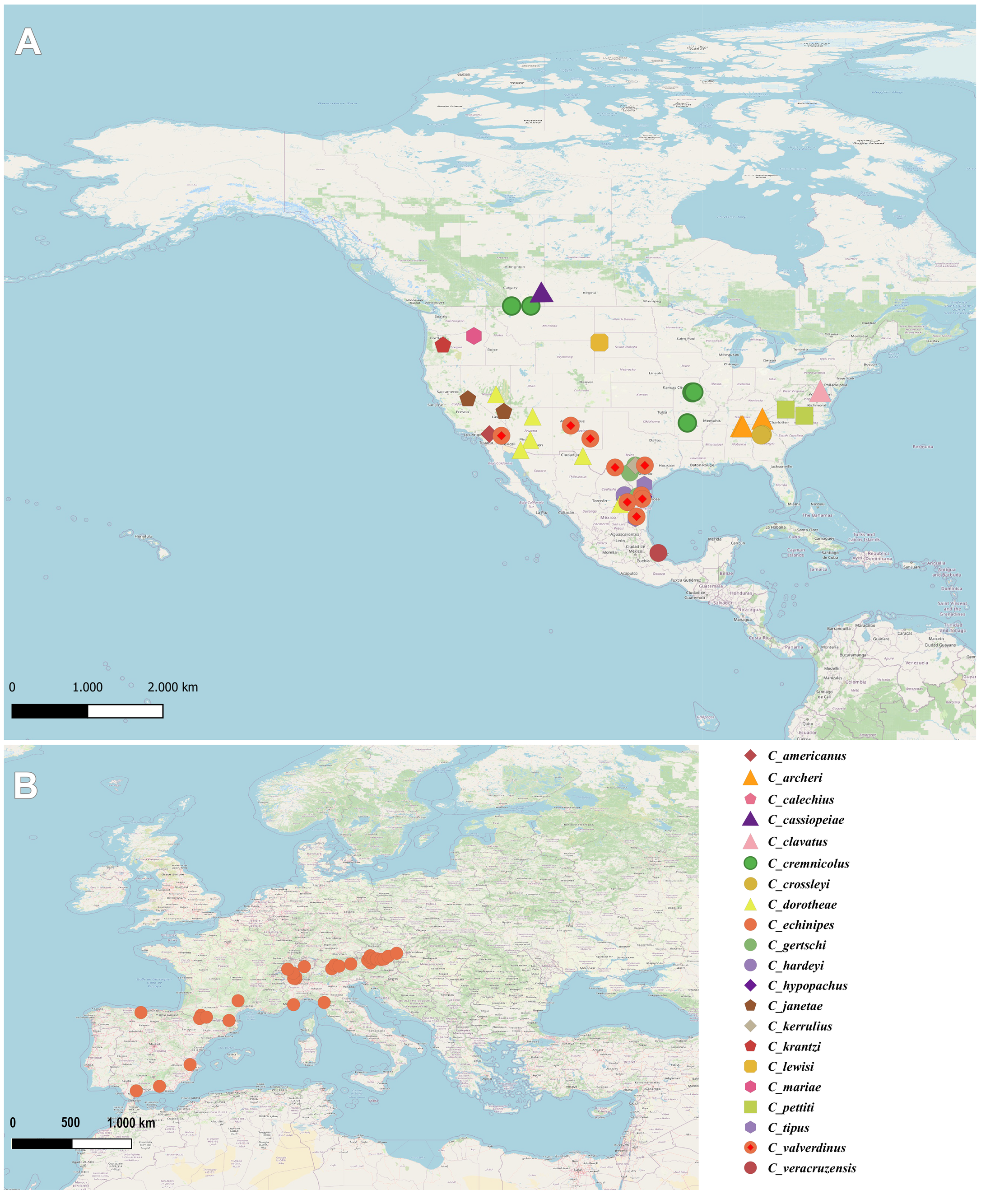
Distribution of the genus Caeculus
So far, only one species of the genus, C. echinipes, is reported from Europe. It has been suggested that its wide distribution (Fig. 12B) may be a consequence of a postglacial distribution process (Franz 1952). In contrast, the genus Caeculus is rather diverse in the Nearctic Region (Fig. 12A) with 20 species covering, as a whole, a wide range (data from Banks 189; 1905; Bernard et al. 2020; Enns 1958; Franz 1952; Hagan 1985; Higgins and Mulaik, 1957a, b; McDaniel and Boe 1990; Mulaik 1945; Mulaik and Alfred 1954; Nevin 1943; Porta and Vazque Rojas 2020). Based on their first phylogeny of the genus, Bernard et al. (2020) suggest the possibility of the North American southwest origin of the genus, followed by a dispersal event to Europe of an ancestor of C. echinipes. The discovery of a new fossil species in Europe presenting so many morphological particularities suggest that modifications of the hypothesis about the origins of the genus may be necessary. From our point of view, based on the empirical data of the genus Andocaeculus, ecological constrains take a prevalent role in the evolution of some morphological characters as leg and idiosoma chaetotaxy, the introduction of molecular data may be necessary for robust phylogenetic inference concerning this family.


Paleoecology
The paleoenvironment from where the Baltic amber originated, was traditionally considered as a tropical forest (Ander 1942; Czeczott 1961). Consequently, at first glance, the presence of mites of the genus Caeculus, usually associated with dry habitats, could be considered unusual. However, the ecology of the extant taxa corresponding to fossil taxa described from this amber in recent years agrees with the new conception of this paleoenvironment as a mosaic of diverse environments with warm temperature (Sadowski et al. 2017). In addition to taxa associated with moist habitats as labidostommatids mites (Sidorchuk and Bertrand 2013), pseudotyrannochthonid pseudoscorpions (Schwarze et al. 2022) and harvestmen (Dunlop 2007; Dunlop and Mitov 2015; Dunlop et al. 2018), other taxa currently restricted to arid habitats have been described from this amber, including teneriffid and opilioacarid mites and solifuges (Sayre 199; Dunlop et al. 2004).
Acknowledgments
We would like to thank to Jason Dunlop (Museum für Naturkunde, Berlin), Christopher Taylor (University of Western Australia) and Philippe Auger (CBGP-INRAE) for their very valuable suggestions in the previous versions of the manuscript, to Marius Veta for providing information regarding de origin of the samples and Mónica M. Solórzano Kraemer (Senckenberg Gesellschaft für Naturforschung, Frakfurt) for the loan of the samples.
References
- Ander K. 1942. Die Insektenfauna des Baltischen Bernsteins nebst damit verknüpften zoogeographischen Problemen. Lunds Universitets Årsskrift, 38: 1-82.
- Banks N. 1899. An American species of the genus Caeculus. Proceedings of the Entomological Society of Washington, 4: 221-222.
- Banks N. 1905. Descriptions of some new mites. Proceedings of the Entomological Society of Washington, 7: 135-136.
- Bernard J., Lumley L., Buck M., Cobb, T. 2020. A new species of rake-legged mite, Caeculus cassiopeiae (Prostigmata, Caeculidae), from Canada and a systematic analysis of its genus. ZooKeys, 926: 1-23. https://doi.org/10.3897/zookeys.926.48741
- Coineau Y. 1969. Contribution à l′étude des Caeculidae. Cinquième série. Procaeculus aitkeni, une nouvelle espèce de Trinidad, B. W. l. Revue d'Ecologie et de Biologie du Sol, 6(1): 53-67.
- Coineau Y. 1974. Éléments pour une monographie morphologique, écologique et biologique des Caeculidae (Acariens). Mémoires du Muséum National d'Histoire Naturelle, Série A, Zoologie, 81: 1-299, pls 1-24.
- Coineau Y., Magowski W.Ł. 1994. Caeculidae in amber. Acarologia, 35(3): 243-246.
- Coineau Y., Poinar G. 2001. Un Caeculidae de l′ambre de la République Dominicaine. Acarologia, 41(1-2): 141-144.
- Czeczott H. 1961. The flora of the Baltic amber and its age. Prace Muzeum Ziemi, 4: 119-145.
- Dunlop J.A. 2007. Paleontology. In: Pinto-da-Rocha R., Machado G. & Giribet G. (eds): Harvestmen. The biology of Opiliones. Harvard University Press, https://doi.org/10.4159/9780674276833-007
- Cambridge, MA. p. 247-265.
- Dunlop J.A., Wunderlich J., Poinar Jr. G.O. 2004. The first fossil opilioacariform mite (Acari: Opilioacariformes) and the first Baltic amber camel spider (Solifugae). Transactions of the Royal Society of Edinburgh, Earth Sciences, 94: 261-273. https://doi.org/10.1017/S0263593300000663
- Dunlop J.A., Mitov P.G. 2015. The first fossil cyphophthalmid harvestman from Baltic amber. Arachnologische Mitteilungen, 40: 47-54. https://doi.org/10.5431/aramit4006
- Dunlop J.A., Kothoff U., Hammel J.U., Ahrens J., Harms D. 2018. Arachnids in Bitterfeld amber: a unique fauna of fossils from the heart of Europe or simply old friends? Evolutionary Systematics. 2: 31-44. https://doi.org/10.3897/evolsyst.2.22581
- Enns W.R. 1958 A new species of rake-legged mite from Missouri (Acarina, Caeculidae). Journal of the Kansas Entomological Society, 31(2): 107-113.
- Franz H. 1952 Revision der Caeculidae Berlese 1883 (Acari). Bonner Zoologische Beiträge, 3(1-2): 91-124.
- Hagan D.V. 1985. Caeculus crossleyi n. sp. (Acari: Caeculidae) from granite outcrops in Georgia, U.S.A. International Journal of Acarology, 11(4): 241-245. https://doi.org/10.1080/01647958508683424
- Higgins H.G., Mulaik, S.B. 1957a. A new Caeculus from Oregon (Acarina: Caeculidae). Great Basin Naturalist, 17(1-2): 27-29. https://doi.org/10.5962/bhl.part.6226
- Higgins H.G., Mulaik S.B. 1957b. Another Caeculus from southwestern United States (Acarina:Caeculidae). Texas Journal of Science, 9: 267-269.
- McDaniel B., Boe A. 1990. A new species and distribution record for the genus Caeculus Dufour (Acari: Caeculidae) from South Dakota. Proceedings of the Entomological Society of Washington, 92(4): 716-724.
- Mulaik S. 1945. New mites in the family Caeculidae. Bulletin of the University of Utah, 35(17): 1-23.
- Mulaik S., Allred D.M. 1954. New species and distribution records of the genus Caeculus in North America. Proceedings of the Entomological Society of Washington,
- 56: 27- 40.
- Nevin R.R. 1943. Caeculus pettiti, a new species of mite from Virginia. Annals of the Entomological Society of America, 36(3): 389-393. https://doi.org/10.1093/aesa/36.3.389
- Porta A.O., Proud D.N., Franchi E., Porto, W., Epele, M.B., Michalik P. 2019. The first record of caeculid mites from the Cretaceous amber of Myanmar with notes on the phylogeny of the family. Zootaxa, 4647(1): 023-043. https://doi.org/10.11646/zootaxa.4647.1.5
- Porta A.O., Vázquez Rojas I.M. 2020. A new Caeculus (Prostigmata: Caeculidae) from Mexico, with an updated key for the genus. Systematic and Applied Acarology, 25(4): 743-758. https://doi.org/10.11158/saa.25.4.13
- Porta A.O., Pizarro-Araya J. & Ramírez M.J. 2021. Revision and phylogeny of the genus Andocaeculus (Acari:Caeculidae) I: the group A. weyrauchi. Zootaxa, 4945(1). https://doi.org/10.11646/zootaxa.4945.1.1
- Rivas G., Serrano L., Vega F. 2016. First record of Procaeculus (Acari: Caeculidae) in Miocene amber from Chiapas, Mexico. Boletín de la Sociedad Geológica Mexicana, 68: 87-92. https://doi.org/10.18268/BSGM2016v68n1a10
- Sadowski E.-M., Schmidt A.R., Seyfullah L.J., L. Kunzmann. 2017. Conifers of the ′Baltic amber forest′ and their palaeoecological significance. Stapfia, 106: 1-73.
- Sayre R.M., Smiley R. L., Walter D. E. 1992. Report of a teneriffiid mite (Acari) in Baltic amber and notes on recent discoveries. International Journal of Acarology 18: 303-305. https://doi.org/10.1080/01647959208683964
- Schwarze D., Harms D., Hammel J.U., Kotthoff U. 2022. The first fossils of the most basal pseudoscorpion family (Arachnida: Pseudoscorpiones: Pseudotyrannochthoniidae): evidence for major biogeographical shifts in the European paleofauna. Paläontologische Zeitschrift, 96: 11-27. https://doi.org/10.1007/s12542-021-00565-8
- Sidorchuk E.A, Bertrand, M. 2013. New fossil labidostomatids (Acari: Labidostomatidae) from Eocene amber and presence of an apustulate species in Europe. Acarologia, 53: 25-39. https://doi.org/10.1051/acarologia/20132079
- Taylor C.K., Gunawardene N.R., Kinnear A. 2013. A new species of Neocaeculus (Acari: Prostigmata: Caeculidae) from Barrow Island, Western Australia, with a checklist of world Caeculidae. Acarologia, 53(4): 439-452. https://doi.org/10.1051/acarologia/20122105
- Taylor C.K. 2014. Two further Neocaeculus species (Acari: Prostigmata: Caeculidae) from Barrow Island, Western Australia. Acarologia. 54(3): 347-358. https://doi.org/10.1051/acarologia/20142136
- Walter D.E., Lindquist E.E., Smith I.M., Cook D.R., Krantz G.W. (2009) Order Trombidiformes. In: Krantz, G.W. & Walter, D.E. (Eds.), A manual of acarology (3rd ed.). Texas Tech University Press, Texas, p. 233-429.



2022-06-21
Date accepted:
2022-10-25
Date published:
2022-10-28
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Porta, Andrés O.; Michalik, Peter and Ramírez, Martín J.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



