New records of Gigantolaelaps wolffsohni (Mesostigmata: Laelapidae) in Chile, an ectoparasite of Oligoryzomys longicaudatus (Rodentia: Cricetidae): ecological aspects and relation to body size and sex of their host
Fuenzalida-Araya, Karen  1
; González-Aguayo, Felipe
1
; González-Aguayo, Felipe  2
; Moreno, Lucila
2
; Moreno, Lucila  3
; Landaeta-Aqueveque, Carlos
3
; Landaeta-Aqueveque, Carlos  4
; Santodomingo, Adriana
4
; Santodomingo, Adriana  5
; Silva-de la Fuente, Carolina
5
; Silva-de la Fuente, Carolina  6
and González-Acuña, Daniel7
6
and González-Acuña, Daniel7
1Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Concepción, Chile.
2Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Concepción, Chile.
3Departamento de Zoología, Facultad de Ciencias Naturales y Oceanográficas, Universidad de Concepción, Concepción, Chile.
4Departamento de Patología y Medicina Preventiva, Facultad de Ciencias Veterinarias, Universidad de Concepción, Chillán, Chile.
5Departamento de Ciencia Animal, Facultad de Ciencias Veterinarias, Universidad de Concepción, Chillán, Chile.
6Escuela de Medicina Veterinaria, Facultad de Ciencias Agrarias y Forestales, Universidad Católica del Maule, Curicó, Chile.
7Departamento de Patología y Medicina Preventiva, Facultad de Ciencias Veterinarias, Universidad de Concepción, Chillán, Chile.
2022 - Volume: 62 Issue: 4 pages: 965-973
https://doi.org/10.24349/nze0-ju8mOriginal research
Keywords
Abstract
Introduction
The Laelapidae family (Acari: Mesostigmata) is a big and cosmopolitan group of mites with diverse habitats and lifestyles (Dhooria 2016; Lindquist et al. 2009; Wall and Shearer 2001). They are the most common ectoparasites of marsupials and rodents (Lareschi 2017). Usually, mites from this family are associated with New-World rodents (Cricetidae: Sigmodontinae: Oryzomyini) and Old-World rodents (Muridae: Murinae) (Gettinger et al. 2011). The Neotropical region holds a wide diversity of Laelapidae mites (Gettinger et al. 2005), including those from the genus Gigantolaelaps (Abba et al. 2001).
Members of the genus Gigantolaelaps can be easily identified thanks to their large size, known as the largest representatives of the Laelapidae family (Fonseca 1939), and the length of their idiosoma being more than 1400 µm long (Furman 1972). As diagnostic characteristics, these mites have a sternal plate narrow at the front with wide anterior projection, which occupies most or all of the presternal area; a genito-ventral plate slightly expanded posteriorly with only one pair of setae, and their posterior setae of the leg II always longer than the other setae of the legs (Furman 1939). These mites are described as ectoparasite exclusive of Oryzomyini tribe rodents (Gettinger 1987; Lareschi et al. 2004), with male mites inhabiting their host's nest and females living on their host (Radovsky 1994; Gettinger et al. 2011; Lareschi 2017). Because of the latter, only female Gigantolaelaps are dispersed by their hosts (Radovsky 1994).
In Chile, Gigantolaelaps wolffsohni (Oudemans) has been found in the long-tail pygmy rice rat Oligoryzomys longicaudatus Bennet in only four localities: Valparaíso (Valparaíso region), Río Melado (Maule region), Concepción (Biobio region) and Chiloé island (Los Lagos region) (Lareschi and González-Acuña 2010). The first locality belongs to the Mediterranean ecoregion, while the other three localities belong to the Template Forest ecoregion. This rodent is a native species distributed in Chile and Argentina. In Chile, it can be found from Atacama to Tierra del Fuego (Weksler and Bonvicino 2015). Infestation with Laelapidae mites can lead from minor skin irritation in their hosts to transmission of pathogenic microorganisms. Moreover, the role of this mite in epizootics and the maintenance of disease among rodents may be significant but unknown (Carmichael et al. 2007; Lareschi 2017). These effects could vary with different factors such as prevalence and abundance (Carmichael et al. 2007). To date, there is limited information about the distribution of the laelapid G. wolffsohni in Uruguay, Venezuela, and Chile, but the characteristics of its ecology are yet to be studied (Furman 1972; Lareschi et al. 2006; Lareschi and González-Acuña 2010).
The purpose of this research was to provide an update on the distribution of G. wolffsohni mites on O. longicaudatus in Chile and describe the ecological aspects of this parasite and its host. The latter included whether factors such as the ecoregion, season, and weight, and sex of the host influence the abundance and prevalence of the mite G. wolffsohni. These factors were included based on previous studies indicating their influence on (Strandtmann and Wharton 1958; Carmichael et al. 2007; Fernandes et al. 2012, 2015).
Material and methods
Rodent trapping and mite collecting
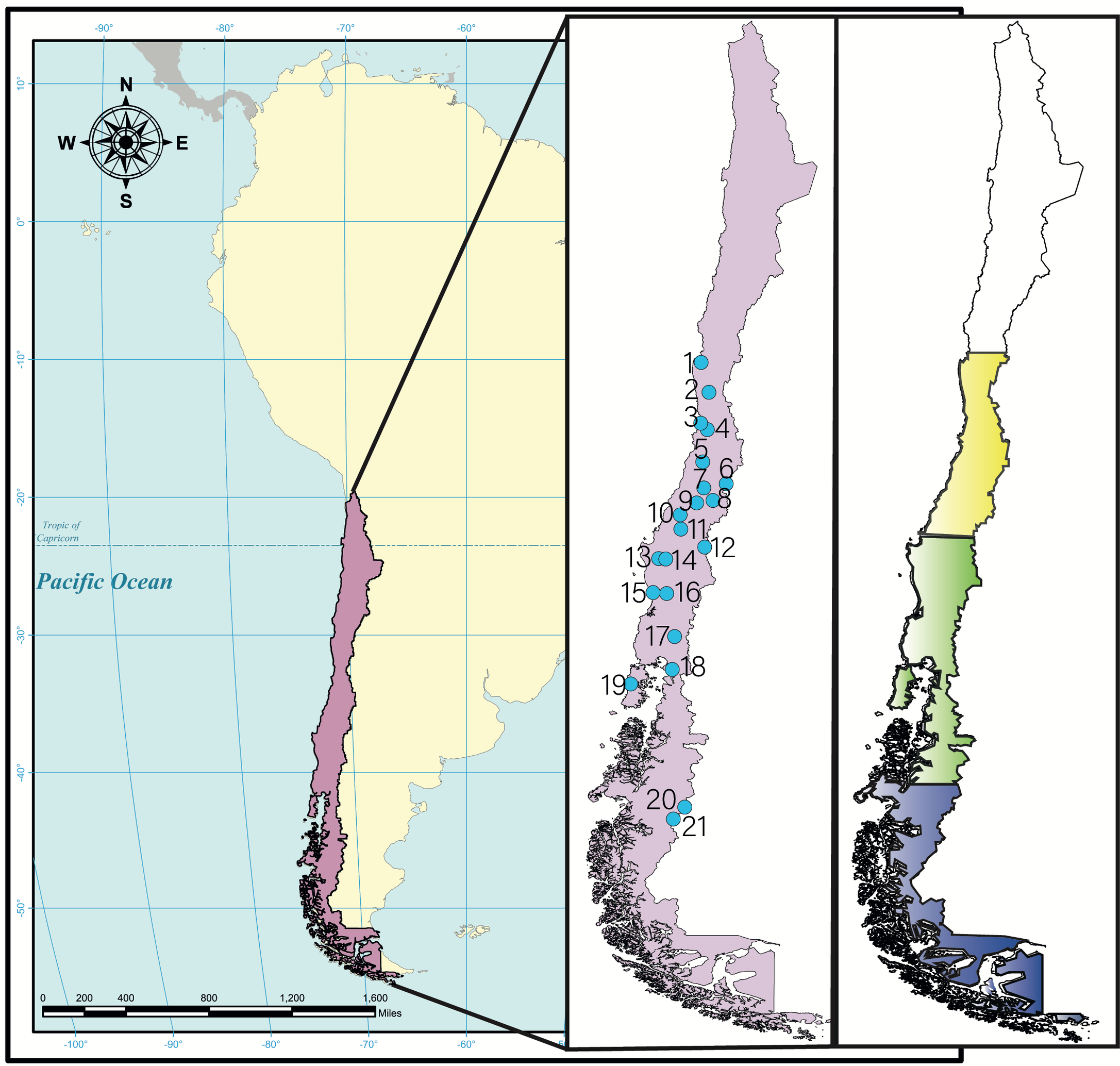
Rodents were captured from Arica to Magallanes regions of Chile (18°28′60″S – 70°19′60″W to 53°10′0″S – 70°55′60″W) for 10 years (2010-2019) in different seasons (summer, autumn, winter, spring). Most of the captures were in National Parks (NP) or National Reserves (NR). The captures were separated in three of the main ecoregions present in Chile: Mediterranean (from latitude 30°S to 38°S) (Villagrán and Hinojosa 2005; Armesto et al. 2007), Template Forest (from latitude 38°S to 47°S) (Villagrán and Hinojosa 2005), and Magellanic Forest (from latitude 47°S to south) (Morrone 2001) (Figure 1). The Mediterranean has high temperatures in summer (from 14 °C to 29 °C) and lower in winter (from 3.7 °C to 13 °C), with relative humidity from 45% to 80%. Likewise, the Temperate Forest has warm temperatures in summer (from 11 °C to 24 °C) and lower in winter (from 3.5 °C to 9 °C), with relative humidity from 66% to 90%. Meanwhile, the Magellanic Forest (in the latitude 47°S) has temperatures from 6 °C to 25 °C in summer and -1.7 °C to 9.6 °C in winter, with relative humidity from 51% to 70% (Dirección Meteorológica de Chile 2021).


Rodents were captured with Sherman traps and oatmeal was used as bait (Mills et al. 1995). Traps remained open throughout the night and they were checked in the early morning. For the handling of rodents, we followed the biosafety standards established by the Pan-American Health Organization (Organización Panamericana de la Salud 1999).
Rodents captured were sedated with xylazine 2% (5mg/kg) and ketamine 100% (75mg/kg) mixed together in the same syringe and injected intramuscularly (Mayer 2013). Then, rodents were identified (Patton et al. 2015), measured (with digital caliper Mitutoyo®), sexed, and weighted (with portable digital scale Pesolac®). Finally, rodents were brushed with a toothbrush over a white tray looking for mites and then were released where they were captured once they woke up from sedation.
Mites' identification
All mites found were stored in microtubes of 1,5 ml with ethanol 96% inside until laboratory examination. Mites were examined under a stereo microscope in the Parasite Ecology Laboratory of Zoology Department in Universidad de Concepción, Concepción, Chile. The mites were separated from other ectoparasites and were cleared in a microtube of 1,5 ml with Nesbitt solution for 15 minutes to 80 °C. After that, they were put in a new microtube with deionized water for 24 hours. Finally, mites were mounted in Berlese medium on a slide where they were identified following the descriptions of Lee (1966) and Furman (1972).
Statistical analysis
A database was established with the following data by rodent specimen: the amount of G. wolffsohni mites per rodent, weight and sex of rodents, season, and the ecoregion.
We used Quantitative Parasitology 3.0 online software (Q.P.3.0) (Reiczigel et al. 2019) to determine ecological indexes (prevalence, abundance, mean intensity, median intensity, crowding mean, and aggregation indexes (variance/mean, Poulin's index and negative binomial exponent k)).
Then, we used negative binomial regressions (NBR) to assess the association of the abundance of G. wolffsohni with body weight, sex, season, and ecoregional distribution of their host, and logistic regressions (LR) to assess the association of the same variables with the presence of the parasite. These analyzes were performed using STATA/BE 17 software. In the case of season and ecoregion of distribution, autumn and Magellanic Forest, respectively, were the base level and the other categories were the dummy comparing variables.
In order to select the best model, we were eliminating the less significant variables and we compared models with likelihood radio tests. The best model was that in which no variable could be removed without significant loss of likelihood. A probability value p < 0.05 was used to indicate statistical significance.
Results
All mites analyzed were identified as G. wolffsohni which were founded in 21 localities in Chile (Figure 1). We captured 4723 rodents of different species which 329 were identified as O. longicaudatus (204 female and 125 male). We found 273 G. wolffsohni mites on O. longicaudatus rodents (150 mites on 62 female rodents and 123 mites on 49 male rodents). All the mites found were female.
We collected G. wolffsohni mites from the hind legs of their host, specifically in the medial side of the thigh. Additionally, a larval stage inside an adult female were observed under microscopical examination (Figure 2). These were visible mainly from June to November.



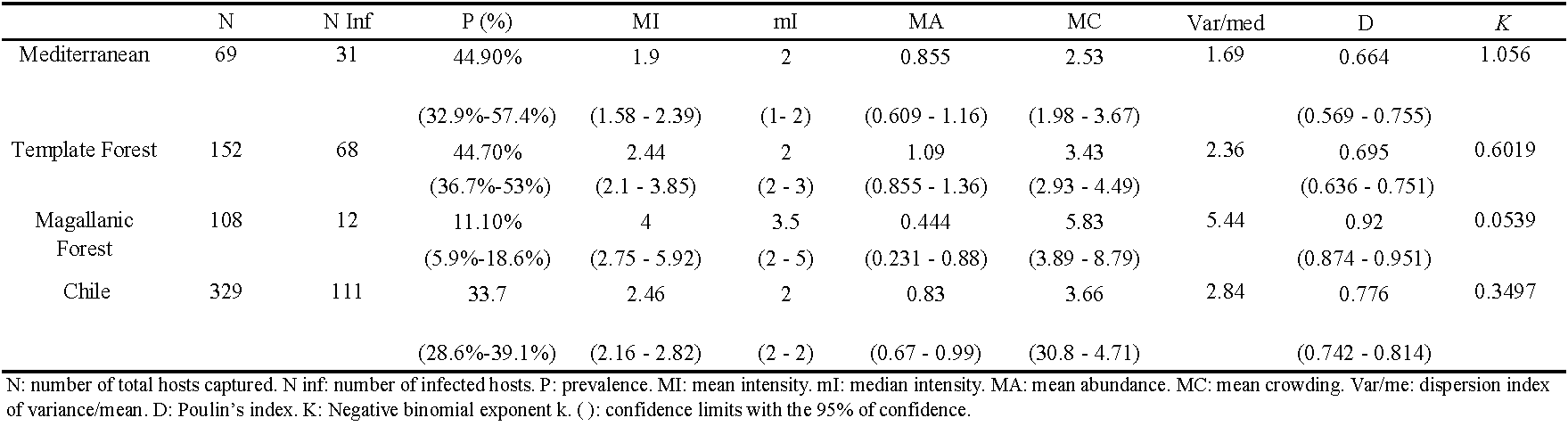
The ecological indexes are presented in Table 1. The total prevalence of G. wolffsohni was 34% (range: 29% - 39%). These mites displayed an aggregated distribution in all ecoregions (Table 1). We did not find association between weight, sex, and biogeographical distribution of host with the presence and the abundance of G. wolffsohni mites. Nonetheless, we determined that the abundance of mites is associated with the season (Table 2). Similar results were found in the analyses of the presence, with season being the sole variable that significantly associated with presence. In both, abundance and presence, the autumn presented parasitism rates significantly lower than the other seasons (Table 2).





Discussion
This research expands the known distribution of G. wolffsohni from latitude 30°S to 47°S in Chile with 19 new localities of distribution. All G. wolffsohni mites found were associated with O. longicaudatus, except for six mites found in one specimen of Rattus rattus in Ocoa, Valparaíso region (32°51′0″S – 71°4′60″O). Since R. rattus has an opportunistic nidification (Coto 2015) and it could share some arboreal habits with O. longicaudatus (González-Ittig et al. 2010; Coto 2015), we believe that this finding is accidental and R. rattus should not be considerate as a new host for G. wolffsohni. Our research states that G. wolffsohni is parasite-specific to O. longicaudatus in Chile. The host specificity for Laelapidae mites had been reported before through an association of a highest and specific prevalence in exclusive hosts (Gettinger 1987; Lareschi et al. 2004; Correia 2015; Gettinger and Owen 2016). Gigantolaelaps wolffsohni has been found in the others no Oryzomyini rodents such as Lundomys molitor (Winge), Akodon azarae Fischer, and Bolomys obscurus Waterhouse in Uruguay (Lareschi et al. 2006), however, these authors found almost all the mites associated with Oligoryzomys rodents. Previously, G. wolffsohni was found on a single Abrothrix sanborni (Osgood) in Chile (Lareschi and González-Acuña 2010). Nevertheless, the authors that reported it, mentioned that it corresponds to an accidental finding. Our results, added to other findings, suggest that G. wolffsohni might use occasionally no Oryzomyini rodents for transportation and dispersion.
We only found female mites which agrees with the hypothesis that only female disperses on rodents (Radovsky 1994; Martins-Hatano et al. 2002). Since the host's nest provides a large and diverse food supply, it is likely that males and nymphs stay in the host nest (Radovsky 1994).
Some laelapines mites have an abbreviation of the general lifecycle by the retention of the egg by the female, or by the retention of both the egg and larva (Radovsky 1994; Cakmak and Da Silva 2018). The suppression of the egg by retention in the female protects the developing mite from mortality factors that otherwise would affect it. However, this suppression could reduce the reproductive capacity of the female since the individual offspring requires more time in uterus (Radovsky 1994). In this research, we observed that G. wolffsohni are, at least, larviparous. The results do not allow establishing that these mites are exclusively larviparous and not nymphiparous. Moreover, the type of reproduction has not been described for this species. Parthenogenesis is a type of asexual reproduction well documented in other mites of the order Mesostigmata (Błoszyk et al. 2004). More research is needed to understand the life cycle of species of the genus Gigantolaelaps.
Even though we did not find association between the abundance and prevalence of G. wolffsohni with the ecoregions, previous studies have determined that these factors can affect its, since different host's densities in different areas influence the frequency of intraspecific contacts (Fernandes et al. 2012, 2015). In this aspect, O. longicaudatus has been reported as a rodent with high vagility (Palma et al. 2007), which means a higher level of dispersion for their parasites (Poulin 2007). Probably, this high dispersion rate of O. longicaudatus maintains G. wolffsohni with low variation in its abundance and prevalence through different ecoregions. Additionally, we do not find an association between the abundance and prevalence of G. wolffsohni and the sex and weight of their host. This agrees with other authors who found that Gigantolaelaps peruviana (Ewing) abundance was not related to the sex and weight of its host; however, they found an association in other Laelapidae mites (Fernandes et al. 2012, 2015).
An important factor of this variation is the distribution of its host and the environmental conditions throughout the ecoregions. It has been reported that the lifecycle of mites is sensitive to humidity and temperature variations (Strandtmann and Wharton 1958). Additionally, it has been observed that these mites prefer warm temperature (23 °C to 35 °C) and high humidity conditions (above 50%) (Strandtmann and Wharton 1958). In this aspect, all ecoregions have high humidity, however the Mediterranean and the Template Forest have upper humidity than Magellanic Forest (Dirección Meteorológica de Chile 2021), which is consistent with the higher prevalence in Mediterranean and Template Forest (Table 1). Gigantolaelaps wolffsohni was not found in the NP. Torres del Paine and NR. Magallanes localities, nonetheless, its host was found there. This may be due to the fact that in these localities the temperatures do not exceed 7 °C in winter, nor 15 °C in summer, and despite presenting high relative humidity (60% - 76%) (Dirección Meteorológica de Chile 2021), the temperature would be a limiting factor for its development (Strandtmann and Wharton 1958).
The distribution of G. wolffsohni is aggregated through ecoregions, which agrees with the most common distribution of parasites where some hosts have several parasites and most of the hosts have none or few parasites (Poulin 2007). The aggregation value is higher in Magellanic Forest than in other ecoregions. This may be due to the fact that in the areas of Patagonia, population densities of 5.4 individuals per hectare have been reported for O. longicaudatus, unlike the template forest, where the population density is from 24 to 47 individuals per hectare (Spotorno et al. 2000). Hence, mites of Magellanic Forest have less possibility to find other hosts, since they are more dispersed in the environment. As a consequence, mites would tend to pass a long time on the same host and it is less likely that they find other individuals to parasitize (Poulin 2007).
We determine that the season influences the abundance and prevalence of G. wolffsohni in Chile, which is consistent with the findings of other authors (Carmichael et al. 2007; Lareschi and Krasnov 2010). They found that Gigantolaelaps mattogrossesnsis has a variation of its abundance related to the humidity and precipitation factors in different seasons (Carmichael et al. 2007). Meanwhile, a variation in the abundance of G. wolffsohni throughout the season was found in Buenos Aires province, Argentina (Lareschi and Krasnov 2010). Humidity and precipitation are important factors for the host O. longicaudatus as well because they are highly dependent on water, moreover it is one of the rodents with lowest survival rate in drought conditions (Palma et al. 2007). As a consequence, if the environmental conditions are unfavorable for the host, it would not be favorable for the parasite (Poulin 2007).
This research represents a contribution to the parasitology and acarology studies of Chile and South America. Additionally, this study provides a better understanding of ecological aspects of G. wolffsohni and their relation with its host O. longicaudatus. This rodent represents an important reservoir Hantavirus in Chile (Belmar-Lucero et al. 2009; Spotorno et al. 2000), consequently, the knowledge about this rodent has importance in public health. An important part of understanding the population and community dynamics around O. longicaudatus is knowing its parasites, to which this research contributes.
References
- Abba A.M., Udrizar Sauthier D.E., Bender J.B., Lareschi M. 2001. Mites (Acari: Laelapidae) associated with sigmodontinae rodents in Entre Ríos province, Argentina. Mem. Inst. Oswaldo Cruz, Rio Janeiro, 96: 1171-1172. https://doi.org/10.1590/S0074-02762001000800025
- Armesto J.J., Arroyo M.T.K., Hinojosa L. 2007. The Mediterranean environment of Central Chile. In: Veblen T. T., Young K. R., Orme A. R. (Eds). The Physical Geography of South America. Oxford University Press. p. 184-199. https://doi.org/10.1093/oso/9780195313413.003.0019
- Belmar-Lucero S., Godoy P., Ferrés M., Vial P., Palma R.E. 2009. Range expansion of Oligoryzomys longicaudatus (Rodentia, Sigmodontinae) in Patagonian Chile, and first record of Hantavirus in the region. Rev. Chil. Hist. Nat., 82(2): 265-275. https://doi.org/10.4067/S0716-078X2009000200008
- Bloszyk J., Adamski Z., Napierala A., Dylewska M. 2004. Parthenogenesis as a life strategy among mites of the suborder Uropodina (Acari: Mesostigmata). Can. J. Zool. 82(9): 1503-1511. https://doi.org/10.1139/z04-133
- Cakmak I., Da Silva F.R. 2018. Maternal care, larviparous and oviparous reproduction of Hypoaspis larvicolus (Acari: Laelapidae) feeding on astigmatid mites. Exp. Appl. Acarol., 75: 457-465. https://doi.org/10.1007/s10493-018-0282-7
- Carmichael J.A., Strauss R.E., Mcintyre N.E. 2007. Seasonal Variation of North American Form of Gigantolaelaps mattogrossensis (Acari: Laelapidae) on Marsh Rice Rat in Southern Coastal Texas. J. Med. Entomol., 44(1): 80-84. https://doi.org/10.1093/jmedent/41.5.80
- Correia A.P. 2015. Levantamento de ectoparasitos de pequenos mamíferos não voadores em duas unidades de conservação no estado de Santa Catarina [Undergraduated Thesis]. Universidade Federal Santa Catarina. pp. 73.
- Coto H. 2015. Protocolos para la vigilancia y control de roedores sinantrópicos [Internet]. Organización Panamericana de Salud; Organización Mundial de la Salud. Available from: https://iris.paho.org/bitstream/handle/10665.2/50507/protocolosvigilancia_spa.pdf?sequence=1&isAllowed=y

- Dhooria M.S. 2016. Fundamentals of Applied Acarology. Springer. pp. 471. https://doi.org/10.1007/978-981-10-1594-6
- Dirección Meteorológica de Chile. Reporte anual de humedad y temperatura [Internet].[1 March 2021]. Available from: https://climatologia.meteochile.gob.cl/application/index/menuTematicoProductos
 .
. - Fernandes F.R., Cruz L.D., Linhares A.X. 2012. Effects of sex and locality on the abundance of lice on the wild rodent Oligoryzomys nigripes. Parasitol. Res., 111: 1701-1706. https://doi.org/10.1007/s00436-012-3009-4
- Fernandes F.R., Cruz L.D., Linhares A.X., Von-Zuben C.J. 2015. Effect of body size on the abundance of ectoparasitic mites on the wild rodent Oligoryzomys nigripes. Acta parasitológica, 60(3): 515-524. https://doi.org/10.1515/ap-2015-0073
- Fonseca F. da. 1939. Notas de Acarologia. XXV. Os laelaptidae gigantes, parasitas de roedores sul-americanos; gênero e espécies novos (Acari) [Internet]. Mem. Inst. Butantan, Available from: https://bibliotecadigital.butantan.gov.br/arquivos/83/PDF/3.pdf

- Furman D.P. 1972. Mites of the family Laelapidae in Venezuela (Acarina: Laelapidae) [Internet]. Brigham Young University Science. Bulletin, Biological Series: 17(3): 1-58. Available from: https://scholarsarchive.byu.edu/byuscib/vol17/iss3/113(3)
 : article 1.
: article 1. - Gettinger D. 1987. Host associations of Gigantolaelaps (Acari: Laelapidae) in the Cerrado Province of Central Brazil. J. Med. Entomol., 24(5): 559-565. https://doi.org/10.1093/jmedent/24.5.559
- Gettinger D., Martins-Hatano F., Gardner S.L. 2011. Some laelapine mites (Acari: Laelapidae) ectoparasitic on small mammals in the Galapagos Islands, including a new species of Gigantolaelaps from Aegialomys galapagoensis. J. Parasitol., 97(4): 574-576. https://doi.org/10.1645/GE-2426.1
- Gettinger D., Martins-Hatano F., Lareschi M., Malcolm J.R. 2005. Laelapine mites (Acari: Laelapidae) associated with small mammals from Amazonas, Brazil, including a new species from marsupials. J. Parasitol., 91(1): 45-48. https://doi.org/10.1645/GE-3401
- Gettinger D.D., Owen R.D. 2016. Laelapine mite (Acari: Laelapidae) morphometric analysis reflects taxonomic and geographic clusters of South American Oryzomyines (Rodentia: Sigmodontinae). Manter: Journal of Parasite Biodiversity, 1(2): 1-22. https://doi.org/10.13014/K23X84KM
- González-Ittig R.E., Salazar-Bravo J., Barquez R.M., Gardenal C.N. 2010. Phylogenetic relationships among species of the genus Oligoryzomys (Rodentia, Cricetidae) from Central and South America. Zool. Scr., 39(6): 511-526. https://doi.org/10.1111/j.1463-6409.2010.00446.x
- Lareschi M. 2017. Artrópodos ectoparásitos. En: Drago F. B. (Ed). Macroparásitos. Diversidad y Biología. Argentina: Editorial de la Universidad Nacional de La Plata (EDULP). p. 167-185.
- Lareschi M., Gettinger D., Venzal J.M., Arzua M., Nieri-Bastos F.A., Barros-Battesti D.M., Gonzalez E.M. 2006. First report of mites (Gamasida: Laelapidae) parasitic on wild rodents in Uruguay, with new host records. Neotrop. Entomol., 35: 596-601. https://doi.org/10.1590/S1519-566X2006000500005
- Lareschi M., González-Acuña D. 2010. Acari, Laelapidae (ectoparasitic mites), central and southern Chile. Check-List., 6(4): 546-548. https://doi.org/10.15560/6.4.546
- Lareschi M., Krasnov B.R. 2010. Determinants of ectoparasite assemblage structure on rodent host from South American marshlands: the effect of host species, locality and season. Med. Vet. Entomol., 24: 284-292. https://doi.org/10.1111/j.1365-2915.2010.00880.x
- Lareschi M., Nieri-Bastos F., Barros-Battesti D.M., Nava S., Beldomenico P., Autino A., Gettinger D. 2004. Gigantolaelaps gilmorei Fonseca, 1939 (Acari: Laelapidae): taxonomic status, lectotype and paralectotype designation and new distributional records. Syst. Parasitol., 59(3): 235-236. https://doi.org/10.1023/B:SYPA.0000048104.83914.9e
- Lee D. 1966. The neotropical mite genus Gigantolaelaps Fonseca, 1939 [Master Thesis]. Texas Technological College. pp: 122.
- Lindquist E.E., Krantz G.W., Walter D.E. 2009. Order Mesostigmata. In: Krantz G. W., Walter D. E. (Eds). A Manual of Acarology. Texas Tech University Press. p. 124-132.
- Martins-Hatano F., Gettinger D., Bergallo H. 2002. Ecology and host specificity of Laelapine mites (Acari: Laelapidae) of small mammals in an Atlantic Forest area of Brazil. J. Parasitol., 88: 36-40. https://doi.org/10.1645/0022-3395(2002)088[0036:EAHSOL]2.0.CO;2
- Mayer J. 2013. Rodent. In: Carpenter J. W. (Ed). Animal Exotic Formulary. Elsevier. p. 476-516.
- Mills, J.N., Childs, J.E., Ksiazek, T.G., Peters, C.J., Velleca, W.M. 1995. Methods for trapping and sampling small mammals for virologic testing [Internet]. United States Department of health and human services. Available from: https://stacks.cdc.gov/view/cdc/11507.
- Morrone J. 2001. Biogeografía de América Latina y el Caribe. Zaragoza: M&T- Manuales & Tesis SEA. pp. 148.
- Organización Panamericana de la Salud. 1999. Hantavirus en las Américas: Guía para el diagnóstico, el tratamiento, la prevención y el control [Internet]. Washington, D.C.: OPS. Available from: https://iris.paho.org/handle/10665.2/31081.
- Palma R.E., Torres-Pérez F., Boric-Barguetto D. 2007. The ecology and evolutionary history of Oligoryzomys longicaudatus in southern South America. In: Kelt D. A., Lessa E. P., Salazar-Bravo J., Patton J. L. (Eds). The Quintessential Naturalist. University of California publications. p. 671-693.
- Patton, J.L., Pardiñas, U.F.J., D'Elía, G. 2015. Mammals of South America: Volumen II Rodents. University of Chicago Press. pp: 1384. https://doi.org/10.7208/chicago/9780226169606.001.0001
- Poulin, R. 2007. Evolutionary ecology of parasites. Princeton University Press. pp. 360. https://doi.org/10.1515/9781400840809
- Radovsky F. 1994. The evolution of parasitism and the distribution of some Dermanyssoid mites (Mesostigmata) on vertebrate hosts. In: Houck M. A. (Ed). Mites: Ecological and evolutionary analyses of life-history patterns. Chapman & Hall. p. 186-217. https://doi.org/10.1007/978-1-4615-2389-5_8
- Reiczigel J., Marozzi M., Fábián I., Rózsa L. 2019. Biostatistics for parasitologists: A primer to quantitative parasitology. Trends Parasitol., 35(4): 277-281. https://doi.org/10.1016/j.pt.2019.01.003
- Spotorno A.E., Palma R.E., Valladares J.P. 2000. Biología de los roedores reservorios de hantavirus en Chile. Rev. Chil. infectología, 17(3): 197-210. https://doi.org/10.4067/S0716-10182000000300003
- Strandtmann R.W., Wharton G.W. 1958. A manual of mesostigmatid mites parasitic on vertebrates. The institute of acarology, department of Zoology, University of Maryland. pp. 69.
- Villagrán C., Hinojosa L.P. 2005. Esquema biogeográfico de Chile. In: Llorente-Bousquets J., Morrone J. J. (Eds). Regionalización biogeográfica en Iberoámeríca y tópicos afines. Ediciones de la Universidad Nacional Autónoma de México, Jiménez Editores. p. 55-577.
- Wall R. L., Shearer D. 2001. Veterinary ectoparasites: Biology, pathology & control. Wiley-Blackwell. pp. 304. https://doi.org/10.1002/9780470690505
- Weksler M., Bonvicino C.R. 2015. Genus Oligoryzomys Bangs, 1900. In: Patton J. L., Pardiñas U. F. J., D'Elía G. (Eds). Mammals of South America: Volumen II. Rodents. The University of Chicago Press. p. 417-436.



2022-04-21
Date accepted:
2022-09-14
Date published:
2022-09-23
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Fuenzalida-Araya, Karen; González-Aguayo, Felipe; Moreno, Lucila; Landaeta-Aqueveque, Carlos; Santodomingo, Adriana; Silva-de la Fuente, Carolina and González-Acuña, Daniel
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



