Variability of dorsal shield in different populations of Amblyseius ishizuchiensis Ehara (Acari: Phytoseiidae)
Döker, Ismail  1
; Khaustov, Vladimir A.
1
; Khaustov, Vladimir A.  2
; Joharchi, Omid
2
; Joharchi, Omid  3
; Jung, Chuleui
3
; Jung, Chuleui  4
and Marchenko, Irina I.
4
and Marchenko, Irina I.  5
5
1✉ Tyumen State University, Institute of Environmental and Agricultural Biology (X-BIO), Tyumen, Russia & Cukurova University, Agricultural Faculty, Department of Plant Protection, Acarology Lab, Adana, Turkey.
2Tyumen State University, Institute of Environmental and Agricultural Biology (X-BIO), Tyumen, Russia.
3Tyumen State University, Institute of Environmental and Agricultural Biology (X-BIO), Tyumen, Russia.
4✉ Research Institute of Agricultural Science and Technology, Andong National University, Andong, Republic of Korea & Department of Plant Medicals, Andong National University, Andong, Republic of Korea.
5Institute of Systematics and Ecology of Animals, Novosibirsk, Russia.
2022 - Volume: 62 Issue: 4 pages: 916-926
https://doi.org/10.24349/9cvj-j2iaOriginal research
Keywords
Abstract
Introduction
The tribe Amblyseiini Wainstein (Acari: Phytoseiidae) is the largest not only in the subfamily but also in the entire family with more than 600 valid species (Chant and McMurtry 2007; Demite et al. 2022). According to the generic concepts of Chant and McMurtry (2004) regarding subtribal diagnosis, the tribe consists of three subtribes, Amblyseiina Chant & McMurtry, Arrenoseiina Chant & McMurtry, and Proprioseiopsina Chant & McMurtry. All of them are characterized by the combinations of the following characters, i) the ratio of setae s4 and Z1 at least equal, greater, and usually much greater than 3.0:1.0, ii) the lengths of certain dorsal setae, especially j3, s4, Z4, and Z5 which become progressively longer, and the other dorsal setae which become progressively shorter, iii) with an increasing number of teeth on the fixed digit of the chelicera, and iv) with an increased presence of macrosetae on legs in addition to leg IV. The authors also suggested that the species in the subtribe Amblyseiina can be basically separated from the members of the other two subtribes by having sternal shield narrower, length/width ratio usually ca. 1.0:1.0, ventrianal shield usually longer than wide, length/width ratio usually greater than 1.0:1.1; all shields lightly sclerotized.
During surveys carried out to determine the mite fauna in various localities of Russia and South Korea as well as during a detailed examination of the materials deposited in the Institute of Systematics and Ecology of Animals, Novosibirsk, Russia we encountered a large number of specimens of Amblyseius ishizuchiensis Ehara, 1972. This species was originally described from Japan, and then reported from China and Russia (Kolodochka 2006; Fang and Wu 2017). Because of variations in dorsal shield punctuation, we redescribed this species based on female and male specimens, with strongly punctate dorsal shield of both sexes, collected from several localities of Far East Russia, and South Korea. According to the aforementioned taxonomical system used to separate phytoseiid species, this species is included in the genus Amblyseius. However, as reported by Chant and McMurtry (2004), our observation of the current material showed that this species is an anomaly based on several morphological characteristics and it does not really fit with the diagnosis of the subtribe Amblyseiina. We thus also discussed the position of this species to the known tribes/subtribes and genera, as well as its taxonomical status.
Material and methods
Soil and litter samples were collected from various localities in Far East Russia as well as mount Ilwolsan, Gyoungbuk province, Republic of Korea. The mites were extracted using Berlese-Tullgren funnels. Specimens were cleared in lactic acid solution and mounted in Hoyer's medium. The taxonomic system proposed by Chant and McMurtry (2007) is followed. Setal nomenclature for the dorsal idiosoma follows that of Lindquist and Evans (1965) as adapted by Rowell et al. (1978), and Chant and Yoshida-Shaul (1991). For designation of dorsal solenostomes (gland pores), the system developed by Athias-Henriot (1975) is used, as proposed by Papadoulis et al. (2009). Measurements are given in micrometers (µm). Morphological observations, illustrations and measurements were made using compound microscope Axio Imager A2 (Carl Zeiss, Germany), equipped with differential interference contrast (DIC) optical system. Pictures were taken with Axiocam 506 color (Carl Zeiss, Germany). Dorsal shield length was measured along the midline.
Results
Amblyseius ishizuchiensis Ehara
Amblyseius ishizuchiensis Ehara, 1972: 162.
Iphiseius wangi Yin, Bei & Lu, 1992: 281.
Amblyseius wangi (Yin, Bei & Lu), suspected as junior synonym by Chant & McMurtry (2004) and Fang & Wu (2017).
Amblyseius grandisimilis Ma, 2004: 72. Suspected as junior synonym (in this study).
(Figures 1–5)
Diagnosis
Dorsal setal pattern 10A:9B; dorsal shield either smooth or punctate, with lateral reticulations, with six pairs of solenostomes (gd1, gd2, gd4, gd6, gd8 and gd9). Setae Z4, Z5 and s4 greatly elongated; setae j1 and j3 at least three to four times longer than other dorsal setae; others short and minute. Peritreme extending beyond setae j1. Sternal, genital and ventrianal shields strongly sclerotized and reticulated, wider than long; ventrianal shield with three pairs of setae and a pair of rounded pores (gv3) in addition to circumanal setae. Setae JV5 inserted on a platelets. Calyx of spermatheca cup-shaped, slightly constricted near vesicle; atrium nodular, attached to calyx without neck. Fixed and movable digits of chelicera with 14 and four teeth, respectively. Genu II with seven setae. All legs with macrosetae. Erect setae present on basitarsus I. Male ventrianal shield with three pairs of setae. Male spermatophoral process L-shaped with well-developed toe.
Redescription
Female
(n = 10)
Dorsum (Figures 1A, 2A–D) – Dorsal setal pattern 10A:9B (r3 and R1 off shield). Dorsal shield rotund, heavily sclerotized, brownish in color, heavily punctate in some specimens or smooth in some other specimens; all with lateral reticulation; with six pairs of solenostomes (gd1, gd2, gd4, gd6, gd8 and gd9) and fifteen pairs of poroids (sensillae) visible. Muscle-marks (sigillae) visible mostly on podosoma. Length of dorsal shield 414 (387–434), width at level of s4 292 (267–306). Dorsal shield setae smooth, except Z4 and Z5, serrated. Measurements of setae as follows: j1 28 (25–31), j3 39 (36–41), j4 6 (5–7), j5 6 (5–8), j6 8 (5–9), J2 12 (10–14), J5 9 (8–10), z2 10 (8–11), z4 8 (6–9), z5 6 (5–7), Z1 12 (10–14), Z4 190 (184–199), Z5 228 (215–241), s4 162 (152–172), S2 11 (10–12), S4 11 (9–13), S5 10 (9–12), r3 11 (10–13) and R1 10 (9–11).






Peritreme (Figures 1A, 5B) – Extending beyond setae j1. Posterior end of peritrematal plate enlarged with ectal strip, with posterior end broadly rounded.
Venter (Figures 1B, 5A) – Ventral setal pattern JV-3:ZV. Sternal shield strongly sclerotized and reticulated, wider than long; with three pairs of setae (st1–st3) and two pairs of poroids (iv1 and iv2); distance between st1– st3 64 (61–67), and st2– st2 82 (78–85); metasternal setae st4 and a pair of poroids (iv3) on metasternal plates. Genital shield strongly reticulated; width at level of genital setae (st5) 96 (90–102), broadly truncate posteriorly, slightly narrower than ventrianal shield, ratio width ventrianal shield to genital shield about 1.15; para-genital poroids iv5 on integument. Ventrianal shield wider than long, strongly reticulated with three pairs of pre-anal setae (JV1, JV2 and ZV2), one pair of para-anal (Pa) and a post-anal seta (Pst); and a pair of rounded pores (gv3) posteromesad setae JV2. Length of ventrianal shield 112 (103–122), width at level of setae ZV2 138 (131–147). Setae ZV1, ZV3, JV4 and JV5 and five pairs of poroids on integument surrounding ventrianal shield. Setae JV5 inserted on platelets, smooth, much longer than other ventral setae 69 (59–83).
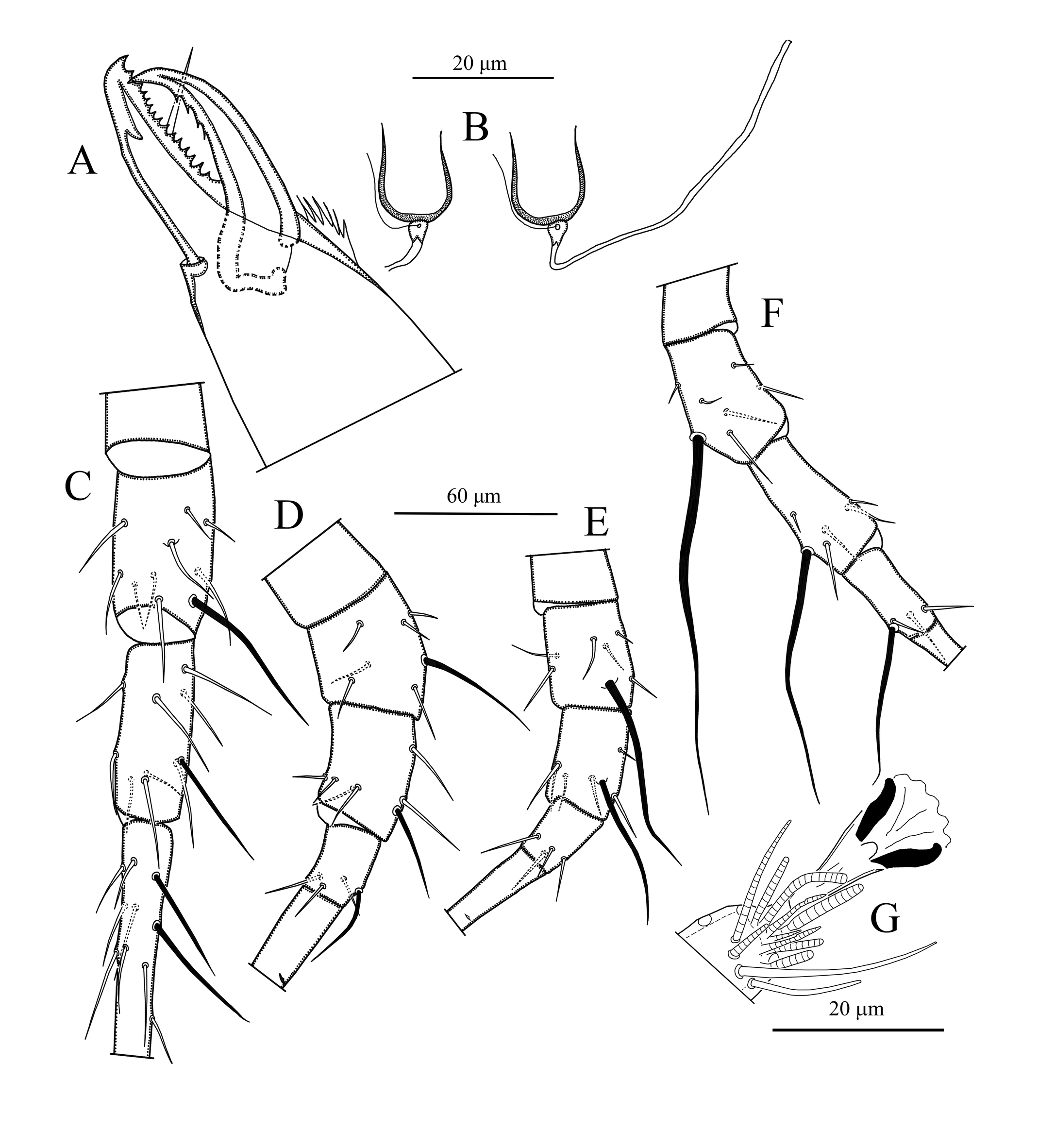


Chelicera (Figure 3A) – Fixed digit of chelicera 36 (35–37) long, with 14 teeth and pilus dentilis; movable digit 36 (36–38) long, with four teeth.
Spermatheca (Figure 3B, 5C) – Calyx of spermatheca cup-shaped, slightly constricted near vesicle, 12 (10–15) in length; atrium nodular, attached to the calyx without neck.
Legs (Figures 3C–G) – Length of legs (excluding pretarsus): leg I 348 (330–365); leg II 298 (267–332); leg III 306 (287–341); leg IV 382 (359–399). All legs with macrosetae. Tarsus I with two erect setae. Measurements of macrosetae as follows: SgeI 50 (47–55), StiI 39 (34–43), SgeII 40 (38–43), StiII 28 (26–30), StII 33 (32–34), SgeIII 75 (67–82), StiIII 50 (46–55), SgeIV 142 (128–157), StiIV 89 (81–100), StIV 62 (55–92). Apical sensorial setal cluster of tarsus I includes 10 modified setae. Chaetotaxy as follows, Leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/0 1, tibia 1 2/1 1/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 1/1 0/2 0, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 1/1 0/2 0, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/0 2/1 1.
Male
(n = 5)



Dorsum (Figure 4A) – Dorsal setal pattern 10A:9B (r3 and R1 on shield). Dorsal shield rotund, heavily sclerotized, brownish in color, punctate in some specimens and smooth in others; all with lateral reticulation; with six pairs of solenostomes (gd1, gd2, gd4, gd6, gd8 and gd9) and fifteen pairs of poroids (sensillae) visible. Muscle-marks (sigillae) visible mostly on podosoma. Length of dorsal shield 338 (318–352), width at level of s4 249 (234–262). Dorsal setae smooth, except Z4 and Z5, serrated. Measurements of dorsal setae: j1 22 (21–23), j3 35 (33–37), j4 5 (4–6), j5 5, j6 6 (5–6), J2 8 (7–8), J5 8 (7–8), z2 6 (5–7), z4 6, z5 5 (5–6), Z1 9 (8–10), Z4 132 (125–139), Z5 177 (168–182), s4 103 (101–105), S2 7 (7–8), S4 8 (7–10), S5 7 (7–8), r3 9 (8–10) and R1 8 (8–9).
Peritreme – As in female.
Venter (Figure 4B) – Sternogenital shield strongly sclerotized and reticulated; with five pairs of setae (st1, st2, st3, st4 and st5) and three pairs of poroids (iv1, iv2 and iv3); 117 (113–119) long and 70 (68 72) wide at level of setae ST2. Ventrianal shield wider than long, strongly reticulated with three pairs of pre-anal setae (JV1, JV2 and ZV2), one pair of para-anal (Pa) and a post-anal setae (Pst); and a pair of rounded pores (gv3) posteromesad setae JV2. Length of ventrianal shield 130 (121–137), width at level of anterior corners 169 (164–173). Setae JV5 smooth, much longer than other ventral setae 29 (27–30) in length.
Chelicera (Figure 4C, 5D) – Fixed digit with 11 teeth and pilus dentilis; movable digit with one tooth, spermatophoral process L-shaped with well-developed toe.



Legs – Length of legs (excluding pretarsus): leg I 303 (283–330); leg II 257 (258–280); leg III 262 (243–287); leg IV 327 (319–337). All legs with macrosetae. Tarsus I with two erect setae. Measurements of macrosetae as follows: SgeI 35 (34–37), StiI 26 (26–27), SgeII 29 (28–31), StiII 21 (19–22), StII 21 (20–22), SgeIII 45 (42–49), StiIII 34 (34–35), SgeIV 90 (86–95), StiIV 57 (54–60), StIV 47 (41–49). Chaetotaxy as in female.
Material examined
Specimens with punctate dorsal shield: four females from litter in mixed forest, vicinity of LZP-3 village, Anuchinsky district, Primorsky Krai, Russia, 12 September 1978, coll. Bakurov. Nine females and five males from litter under Fraxinus sp. (Oleaceae), Ussuri Nature Reserve, Primorsky Krai, Russia, 15 August 1988, coll. Grishina. Four females and one male, in litter, Silinsky park, Komsomolsk-on-Amur, Khabarovsky Krai, Russia, 15 August 2011, coll. Dubatolov. One female, litter in mixed forest, Botanical garden, Vladivostok, Primorsky Krai, Russia, 09 September 2012, coll. Potapov. Two females from soil-litter in Mount Ilwolsan (36°80′N, 129°09′E, 1140 m), Gyoungbuk Province, Republic of Korea, 19 July 2020, coll. Chuleui Jung.
Specimens with smooth dorsal shield: six females and two males from litter in deciduous forest, Boytsovo village, Khabarovsky Krai, Russia, 05 September 1991, coll. Volonihina. Ten females from litter in oak forest, Zeya Nature Reserve, Amur region, Russia, 25 August 2010, coll. Dubatolov. Voucher slides are deposited in the mite collections of Tyumen State University, Zoology Museum, Tyumen, Russia; Department of Plant Medicals, Andong National University (PMANU), Republic of Korea; Institute of Systematics and Ecology of Animals, Novosibirsk, Russia; Cukurova University, Agricultural Faculty, Department of Plant Protection, Acarology Lab, Adana, Turkey.
World distribution
China (Fang and Wu 2007), Japan (Ehara 1972), Russia (Kolodochka 2006 and this study), and South Korea (this study).
Remarks
Amblyseius ishizuchiensis was described by Ehara (1972) based on the material collected from litter in Mount Kamegamori, Shikoku, Japan. This study reports A.ishizuchiensis for the first time in South Korea. Morphological characters and measurements of the Korean and the Russian specimens are almost identical to those reported in the original description and the subsequent redescriptions (Denmark and Muma 1989; Fang and Wu 2017). However, we observed a strongly punctate dorsal shield in both sexes in the specimens originated from South Korea as well as from Anuchinsky district, Ussuri Nature Reserve, Vladivostok (Primorsky Krai) and Komsomolsk-on-Amur (Khabarovsky Krai), Russia. In addition, the populations collected from Boytsovo village (Khabarovsky Krai) and from Amur region have smooth dorsal shield as illustrated in the previous descriptions (Ehara 1972; Denmark and Muma 1989; Fang and Wu 2017). Although specimens with smooth versus punctate dorsal shield are never found in a single population, we considered on a provisional basis this difference to represent an intraspecific and interpopulation variation, until further, perhaps molecular, studies are conducted. There is no other morphological difference including the dorsal setae lengths between the populations having smooth or punctate dorsal shields. Based on our best knowledge, a variation on dorsal shield punctuation has never been reported for phytoseiid mites within the same species. In addition, nothing is known on which environmental / biological features can be attributed this phenotypic variation. Chant and McMurtry (2004) reported that A. wangi (Yin, Bei & Lu, 1992) which was described from China may be a junior synonym of A. ishizuchiensis. In addition to A. wangi, we were not able to find any distinct morphological difference between A. ishizuchiensis and A. grandisimilis which was also described from China (Ma 2004). Therefore, we suspected that these three species are conspecific. However, punctuation on the dorsal shield of all these species should be confirmed in further studies.
Discussion
As reported in previous descriptions, all ventral shields of the examined specimens of A. ishizuchiensis are wider than long. In their key to the subtribes in the tribe Amblyseiini, however, Chant and McMurtry (2004, 2007) basically separated the subtribe Amblyseiina from the other two subtribes, Arrenoseiina and Proproseiopsina, by having sternal shield narrower, length/width ratio usually ca. 1.0:1.0, ventrianal shield usually longer than wide, length/width ratio usually greater than 1.0:1.1; all shields lightly sclerotized. Amblyseius ishizuchiensis shared several important morphological characters with the members of the genus Amblyseius (Amblyseiina) by having dorsal setal pattern 10A:9B (seta J2 present) and macrosetae on other legs in addition to leg IV. However, it has distinct differences and does not really fit with all diagnostic characteristics of this subtribe, instead apparently fitting better the characteristics of the subtribe Arrenoseiina, still with some exceptions. The keys presented by Chant and McMurtry (2007) for the separation of genera and subgenera of Phytoseiidae seem to point in the same direction. The characteristics that make these specimens most similar to the Arrenoseiina include the rotund, broad dorsal shield, broad ventral shields, the strong sclerotization of all shields, and the brown color. Within the subtribe Arrenoseiina, this species fit well to the genus Arrenoseius based on the presence of dorsal seta j5, and normally inserted ventral setae JV2 and ZV2 in position. However, when we consider a diagnostic character for this genus, the absence of macroseta on legs I-III, we fail to accommodate this species to the genus Arrenoseius. The biological and ecological features of the species included in the same genus can also provide some useful information, however, members of all genera mentioned above are known to inhabit soil litter habitats (Chant and McMurtry 2004).
If we ignore the placement of ventral seta, on the other hand, A. ishizuchiensis can be considered as a member of Iphiseiodes, another genus in this subtribe. However, species of this genus have so far been described and reported only from the Neotropical region, particularly from Brazil, but not in the Palearctic region (Denmark and Muma 1973; El-Banhawy 1984; Gondim Jr and Moraes 2001; Ferla and Silva 2011; Nuvoloni et al. 2015; Mineiro et al. 2011; Demite et al. 2022). Moreover, it is almost impossible and may not be correct to ignore the nature of the placement of ventrianal setae, listed among the apomorphic characters of this genus.
Interestingly, if we ignore several morphological characteristics in addition to the presence of dorsal setae j5, such as the presence/absence of macroseta on leg II and large-robust posterior projection on peritrematal plate, this species also shows a close affinity to the species listed in the genus Phytoscutus Muma, a third genus in the same subtribe. In addition, some members of this genus such as P. japonicus Ehara & Kishimoto 2007, and P. salebrosus (Chant, 1960) were reported from the East Palearctic region (Yoshida-Shaul and Chant 1997; Ehara and Kishimoto 2007; Demite et al. 2022). Among the other possible genera discussed above, Phytoscutus is the only genus that showed great variability in terms of the presence/absence of certain idiosomal setae such as J2, S2, S4 and ZV3. Based on this variability, Chant and McMurtry (2007), established four species groups in this genus. Therefore, one option would be the definition of a fifth species group with the presence of dorsal seta j5 in this genus. However, in this case, certain modifications in the identification keys are needed at tribal and subtribal level and much more observations would be required.
Finally, based on the discussion provided above, we can formulate the hypothesis that A. ishizuchiensis might be a ''transitional'' species between the subtribes Amblyseiina and Arrenoseiina. However, we decided to keep it in Amblyseius on a provisional basis due to the absence of more evidences such as molecular data. Furthermore, Chant and McMurtry (2004) mentioned this anomaly but also decided to include this species within the genus Amblyseius subtribe Amblyseiina. Therefore, a comprehensive molecular study based on a series of species belonging to the aforementioned genera is highly recommended in order to accommodate this species into the most correct genus to eliminate adaptative radiations and to clearly understand the phylogenetic relationships between them.
Acknowledgements
We are grateful to JS Oh (ANU and SNU) due to help for sampling and extraction processes. The present research was supported by the cooperative agreement No. FEWZ-2021–0004 from the Russian Ministry of Science and Higher Education. Also, this study was partly supported by National Institute of Biological Resources (NIBR), MoE (NIBR201902204) and National Research Foundation of Korea (NRF), MoEd (NRF-2018R1A6A1A03024862) of Republic of Korea. Ismail Döker was supported by the Cukurova University Scientific Projects Foundation Units, grant number: FAY-2022-14495.
References
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le releve organotaxique de La face dorsale adulte (Gamasides protoadeniques, Phytoseiidae). Acarologia, 17(1): 20-29.
- Chant D.A. 1960. Descriptions of five new species of mites from India (Acarina: Phytoseiidae, Aceosejidae). Can. Entomol., 92: 58-65. https://doi.org/10.4039/Ent9258-1
- Chant D.A., McMurtry J.A. 2004. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part IV. Tribe Amblyseiini Wainstein, subtribe Arrenoseiina Chant & McMurtry. Int. J. Acarol., 30: 291-312. https://doi.org/10.1080/01647950408684399
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). West Bloomfield: Indira Publishing House. pp. 219.
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Int. J. Acarol., 17: 187-199. https://doi.org/10.1080/01647959108683906
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2022. Phytoseiidae Database. Available from: https://www.lea.esalq.usp.br/phytoseiidae
 (accessed 19 May 2022).
(accessed 19 May 2022). - Denmark H.A., Muma M.H. 1973. Phytoseiid mites of Brazil (Acarina: Phytoseiidae). Revista Brasil. Biol., 33: 235-276.
- Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occasional Papers of the Florida State Collection of Arthropods, 4, 149 pp.
- Ehara S. 1972. Some phytoseiid mites from Japan, with descriptions of thirteen new species (Acarina: Mesotigmata). Mushi, 46: 137-173.
- Fang X.-D., Wu W.-N. 2017. A new species of the genus Neoseiulus Hughes (Acari: Phytoseiidae) and the male of Amblyseius ishizuchiensis Ehara, 1972 from China. Syst. Appl. Acarol., 22: 1574-1584. https://doi.org/10.11158/saa.22.10.3
- Ehara S., Kishimoto H. 2007. Description of a new species of Phytoscutus (Acari: Phytoseiidae) from Kyushu, Japan. Int. J. Acarol., 33: 111-113. https://doi.org/10.1080/01647950708684509
- El-Banhawy E.M. 1984. Description of some phytoseiid mites from Brazil (Acarina: Phytoseiidae). Acarologia, 25: 125-144.
- Ferla N.J., Silva G.L. da 2011. Description of a new species of Iphiseiodes De Leon (Acari: Phytoseiidae) on Ilex paraguariensis (Aquifoliaceae) from Rio Grande do Sul, Brazil. Int. J. Acarol., 37: 106-109. https://doi.org/10.1080/01647954.2010.496373
- Gondim Jr. M.G.C., Moraes G.J. de 2001. Phytoseiid mites (Acari: Phytoseiidae) associated with palm trees (Arecaceae) in Brazil. Syst. Appl. Acarol., 6: 65-94. https://doi.org/10.11158/saa.6.1.11
- Kolodochka L.A. 2006. Phytoseiid mites of the Palaearctic Region (Parasitiformes, Phytoseiidae): faunistic, taxonomy, ecomorphology, evolution. Vestn. Zool., suppl. 21, 250pp. [in Russian].
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina Acarina: Mesostigmata. Mem Ent Soc Can., 47: 1-64. https://doi.org/10.4039/entm9747fv
- Ma L.-M. 2004. Descriptions on two new species of the genus Amblyseius and male and nymphs of Typhlodromus orientalis (Acari: Gamasina: Phytoseiidae). Acta Arachnol., 13: 71-76 [in Chinese with English abstract].
- Mineiro J.L. de C., Castro T.M.M.G. de, Moraes G.J. de 2011. Description of a new species and complementary description of a known species of Iphiseiodes De Leon (Acari: Phytoseiidae). Zootaxa, 2876: 30-34. https://doi.org/10.11646/zootaxa.2876.1.3
- Nuvoloni F.M., Lofego A.C., Rezende J.M., Feres R.J.F. 2015. Phytoseiidae mites associated with Hevea spp. From the Amazon region: a hidden diversity under the canopy of native trees. System. Biodivers., 13: 182-206. https://doi.org/10.1080/14772000.2014.985344
- Papadoulis G.Th., Emmanouel N.G., Kapaxidi E.V. 2009. Phytoseiidae of Greece and Cyprus (Acari: Mesostigmata). West Bloomfield, Indira Publishing House, 200 pp.
- Rowell H.L., Chant, D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. https://doi.org/10.4039/Ent110859-8
- Yoshida-Shaul E., Chant D.A. 1997. A world review of the genus Phytoscutus Muma (Phytoseiidae: Acari). Acarologia, 38: 219-238.
- Yin S., Bei N., Lu, C. 1992. Two new species of the genus Iphiseius from China (Acari: Phytoseiidae). J. Shenyang Agric. Univ., 23: 281-285 [in Chinese].



2022-06-01
Date accepted:
2022-09-06
Date published:
2022-09-13
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Döker, Ismail; Khaustov, Vladimir A.; Joharchi, Omid; Jung, Chuleui and Marchenko, Irina I.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



