Could phytoseiid mites impair biological control of the invasive plant, Ailanthus altissima?
Toldi, Maicon  1
; Juarez Ferla, Noeli
1
; Juarez Ferla, Noeli  2
; Evangelho Silva, Darliane
2
; Evangelho Silva, Darliane  3
; de Andrade Rode, Priscila
3
; de Andrade Rode, Priscila  4
; de Azevedo Meira, Anderson
4
; de Azevedo Meira, Anderson  5
and de Lillo, Enrico
5
and de Lillo, Enrico  6
6
1Laboratory of Acarology, University of Vale do Taquari – Univates, Avelino Talini, 171, Lajeado, 95900-000, Brazil.
2Laboratory of Acarology, University of Vale do Taquari – Univates, Avelino Talini, 171, Lajeado, 95900-000, Brazil.
3Laboratory of Acarology, University of Vale do Taquari – Univates, Avelino Talini, 171, Lajeado, 95900-000, Brazil.
4Laboratory of Acarology, University of Vale do Taquari – Univates, Avelino Talini, 171, Lajeado, 95900-000, Brazil.
5✉ Laboratory of Acarology, University of Vale do Taquari – Univates, Avelino Talini, 171, Lajeado, 95900-000, Brazil.
6Department of Soil, Plant and Food Sciences (Di.S.S.P.A.), University of Bari Aldo Moro, via Amendola, 165/a, 70126, Bari, Italy.
2022 - Volume: 62 Issue: 4 pages: 892-897
https://doi.org/10.24349/2o0d-2ri4Original research
Keywords
Abstract
Introduction
Ailanthus altissima (Miller) Swingle (Simaroubaceae), commonly known as tree of Heaven, is a native tree to China and North Vietnam, thanks to its rapid global spread has become invasive in several places (Kowarik and Säumel 2007). With rapid growth, it invades natural areas establishing populations with high density, thus shading native species. It exhibits root allelopathy that inhibits growth and development of other plant species occupying the same environment (Heisey 1996). It is considered invasive in Europe and in all continents, except for Antarctica (Kowarik and Säumel 2007).
The introduction of herbivorous insects, mites and pathogens for the management of pests, weeds (or plants) and diseases that affect ecosystems, has been intensified over the years in order to decrease environmental impacts by controlling the density of invasive plants like tree of Heaven. Therefore, the biological control of weeds is aimed at reducing the population of some species with either ecological or economic importance (Seastedt 2015).
Aculus taihangensis Hong & Xue (Eriophyidae) described from Hebei Province, China (Hong and Xue 2005) as vagrant on Ailanthus altissima, has recently been reported also in Italy, Croatia, Greece, Serbia, Hungary, Romania, Bulgaria, Austria, Slovenia, and France on the same host (de Lillo et al. 2022). It seems to be an interesting candidate for the biological control of this exotic plant (de Lillo et al. 2017). Eriophyoidea generally causes damage to countless agricultural crops, foresting, and ornamental plants (Lindquist et al. 1996). The severity of the symptoms they induce consists of reduction of biomass production and reproductive performance of the host plant, and they depend on mite population density and on the morphology of the plant attacked (Oldfield 1996; Smith et al. 2010; de Lillo and Skoracka 2010). Approximately 80% of Eriophyoidea are exclusively associated to unique host plant species (Skoracka et al. 2010), which means they are a possible candidate for the control of invasive plants (Smith et al. 2010), ensuring the protection of the native plants in places of introduction.
Among the predatory mites that might be associated with eriophyid mites, phytoseiid mites are the most common and abundant on plants (Tixier 2018). Several species are important control agents in greenhouses (Zhang 2003) and orchards (Parra et al. 2002). They feed on mites, insects, nematodes, fungi, pollen, and plant exudates (McMurtry et al. 2013). Amblyseius swirskii Athias-Henriot, for example, fed efficiently on Aculops lycopersici (Tryon) on tomato leaves in laboratory trials (Park et al. 2010). Typhlodromus (T.) exhilaratus was reported to be a type III generalist predator (McMurtry et al. 2013), feeding on tetranychids, eriophyids, and pollen (Ragusa 1981). Typhlodromus (T.) exhilaratus has already been reported associated with Aceria caulobia (Nalepa) inside the stem galls induced by this mite in Apulia (de Lillo 1987; de Lillo and Monfreda 2004), and it is a quite common species in Southern Italy. Mites of Euseius are commonly found associated with plants with smooth leaves or little pubescent surface (Seelmann et al. 2007, McMurtry et al. 2013). They have been reported on eucalyptus, coffee, and forest fragments (Queiroz and Flechtmann 2011), and have also been associated with grapevines (Tixier et al. 2013). Euseius stipulatus (Athias-Henriot) is a generalist species, capable of developing when feeding on pollen as alternative food (McMurtry et al. 2013). This species has already been reported in Italy (Ragusa and Swirski 1976), Spain (Ferragut et al. 1988), and Portugal (Silva et al. 2019) in environments with temperate climate environments, where it provides a significant level of control of Panonychus ulmi (Koch) (Rodrigues 2005).
To date, nothing has been found about predatory mites controlling A. taihangensis. The present study hypothesizes that predatory mites found in European natural environments could reduce the level of biological control of this weed by eriophyoid. Therefore, the aim is to evaluate the predation capacity of E. stipulatus and T. (T.) exhilaratus feeding on A. taihangensis.
MATERIAL AND METHODS
The experiments were carried out in the Acarology Laboratory of Universitá degli Studi di Bari Aldo Moro, Bari, Italy. Euseius stipulatus and Aculus taihangensis were collected from Ailanthus altissima in the city of Bari, Italy (41°06′33.3″N 16°53′04.3″E), while Typhlodromus (T.) exhilaratus was collected from Suaeda vermiculata Forsskål ex J. F. Gmelin (Amaranthaceae) in the city of Margherita di Savóia, Italy (41°22′23.5″N 16°07′38.6″E), where it was found inside the stem galls induced by A. caulobia. Rearing stocks of the predatory mites were maintained on A. altissima contaminated with A. taihangensis and A. caulobia populations. Rearing stocks were maintained in the laboratory throughout the period in a climate chamber at 25 ± 1 °C, 12 hours of photophase, and 70 ± 5% relative humidity.
The study of predator development was initiated with 40 eggs of each species, obtained from isolated females for a period of 6 hours, which were isolated in arenas on Petri dishes with 6 cm containing 4 cm diameter leaf disks of A. altissima, contaminated with more than 20 individuals of A. taihangensis per day. Each leaf disk was attached to a pin, surrounded by distilled water, and replaced daily. Three daily observations were conducted at 8 AM, 1 PM, and 6 PM, using a stereo microscope Zeiss Stemi 305, during immature period to determine the duration of each of the immature stages. During adulthood, females were paired with males obtained from the stock colonies and evaluations were conducted once a day at 1 PM, checking the number of eggs laid and mite survival. Eggs were collected and transferred to other arenas to determine sex ratio. The males were kept isolated in the arenas until death.
The data were compared using an ANOVA test and post-hoc Tukey's test at a significance level of 5% using R Studio (R Development Core Team 2010). Life-table calculations were performed according to Silveira-Neto et al. (1976).
Net reproductive rate (Ro = Σmx.lx, where mx: the number of offsprings/ female x sex ratio; lx: specimens alive/total specimens), mean generation time (T = Σ mx.lx.x/ Σ mx.lx), innate ability to increase (rm = log Ro/ T.0.4343), and finite growth rate (λ = erm) were calculated.
Results
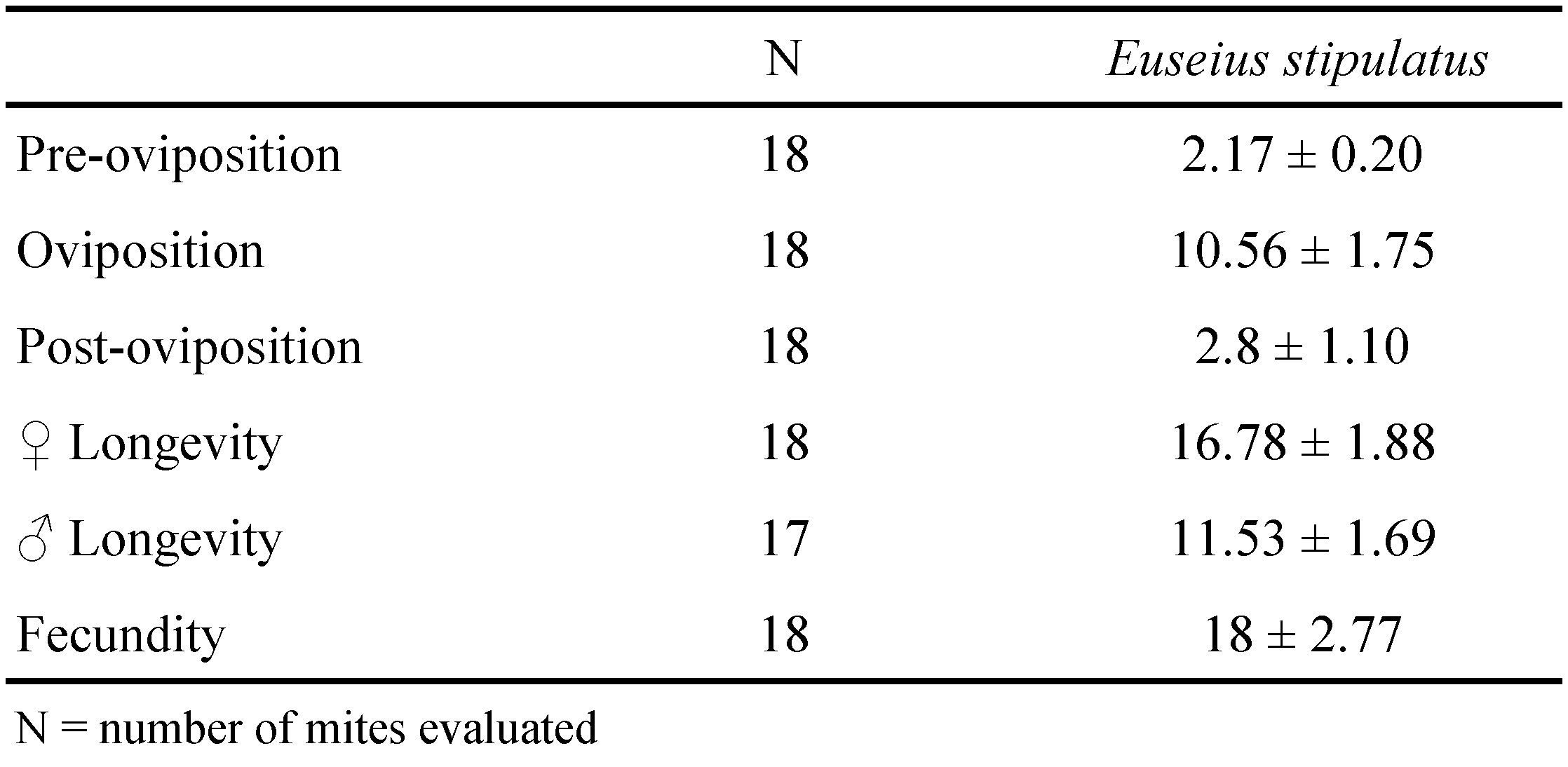
Only 37.5% of Typhlodromus (T.) exhilaratus reached adulthood, while 87.5% of E. stipulatus did (Table 1). In most stages, E. stipulatus had a shorter duration of stages than T. (T.) exhilaratus, except for the larval stage. The duration of the immature period of Euseius stipulatus was about 5.6 days. The deutonymph stage being the shortest of the stages. The duration of the immature period of T. (T.) exhilaratus was about 7.6 days with the larval stage being the shortest of the stages.






Life table parameters were calculated only for E. stipulatus since, as only 4 females of T. exhilaratus laid 14 eggs. The mean fecundity of E. stipulatus was 18 eggs/female (Table 2). The sex ratio was 0.66 and the number of the males was 17. The results showed that E. stipulatus feed on A. taihangensis, reaches adulthood and lays eggs.


Discussion
The study of the interactions of native predators with native introduced organisms is required when planning weed control programs using the introduced organisms as biological control agent. In our case we supposed the potential biological control of A. altissima using A. taihangensis. The present study showed that both E. stipulatus and T. (T.) exhilaratus fed on A. taihangensis, this one being a more suitable prey for E. stipulatus. Aculus taihangensis populations were not suitable to keep T. (T.) exhilaratus populations.
The development time of E. stipulatus feeding on A. taihangensis was similar to that of the same predator feeding on Carpobrotus edulis (L.) N. E. Brown (Aizoaceae) pollen (Ferragut et al. 1987). However, it was longer when feeding on apple, almond, pear, apricot, plum, walnuts and cherry pollen (Bouras and Papadoulis 2005). The same was the case when feeding on Aleurothrixus floccosus Maskell (Aleyrodidae), Panonychus citri (McGregor), Tetranychus urticae Koch (Tetranychidae), Lorryia formosa Cooreman (Tydeidae), and Planococcus citri Risso (Pseudococcidae) (Ferragut et al. 1987). However, this time was longer than when the same mite feeds on alternatives presented above. These pollen diets could be considered, in the future, as complementary diets that have been shown in other cases to increase predator fitness and, at the end, the control. To date, there has only been one study on the biology of T. (T.) exhilaratus (Ragusa 1981) feeding on different diet types: Borago officinalis (L.) (Boraginaceae), Salvia rosmarinus Spenner (Lamiaceae), Bougainvillea spp. (Nyctaginaceae), Jasminum spp. (Oleaceae), Oxalis spp. (Oxalidaceae), and Duranta ellisia Jacquemin (Verbenaceae) pollen, and on P. citri and T. urticae in which the duration of the egg-adult period was between 5.5-8 days. The development time of this mite in this study is within this range reported above, suggesting that this predator is a generalist, surviving on a wide range of food sources. These results suggest that this predator is a generalist, surviving on a broad range of food.
The intrinsic rate, oviposition period and fecundity of E. stipulatus were higher when feeding on C. edulis pollen (0.19; Ferragut 1987), indicating that it is a suitable diet, while A. taihangensis could be considered as a complementary food.
The predator T. (T.) exhilaratus was expected to be an efficient biological control agent ofA. taihangensis, since, according to Ragusa (1981), the diet of this predator includes tetranychids, eriophyids, and pollen. However, the results obtained did not corroborate the initial hypothesis.
The potential of E. stipulatus for biological control increases in more humid environments and milder temperatures (Thurman et al. 2017). This mite is considered tolerant to pesticides compared to other species usually found in the same environment, such as Neoseiulus californicus (McGregor) and Phytoseiulus persimilis Athias-Henriot (Argolo et al. 2014). Nonetheless, E. stipulatus has only been found in preserved vineyards environments (Silva et al. 2019). Several studies provided information on the occurrence of predatory mites on plants. However, little is known about the parameters that explain this occurrence (Tixer 2018). The biological control of A. taihangensis on A. altissima will be more efficient only under unfavorable conditions for E. stipulatus.
In conclusion, A. taihangensis was not a suitable prey for T. (T.) exhilaratus, suggesting that there is no risk of impairing the action of this eriophyid in the control of A. altissima in the field. On the other hand, A. taihangensis is a suitable prey for E. stipulatus, so it should be considered that this predatory mite may be a factor impeding the success of a biological control program using the eriophyid for biological control of A. altissima. However, further studies are needed to know the real risk in the field as this also depends on environmental conditions, and the ability of the predator to search for prey.
References
- Argolo, P. S., Jacas, J. A., Urbaneja, A. 2014. Comparative toxicity of pesticides in three phytoseiid mites with different life-style occurring in citrus: Euseius stipulatus, Neoseiulus californicus and Phytoseiulus persimilis. Experimental and applied acarology, 62(1): 33-46. https://doi.org/10.1007/s10493-013-9726-2
- Bouras S.L., Papadoulis G.T. 2005. Influence of selected fruit tree pollen on life history of Euseius stipulatus (Acari: Phytoseiidae). Exp. Appl. Acarol., 36: 1-14. https://doi.org/10.1007/s10493-005-2381-5
- de Lillo E. 1987. L'acarocecidio indotto da Aceria caulobius (Nalepa) n. comb. (Acari: Eriophyoidea) su Suaeda fruticosa Forsk., serbatoio naturale del predatore Typhlodromus exhilaratus Ragusa (Acari: Phytoseiidae). Entomologica, 22: 5-14.
- de Lillo E., Monfreda R. 2004. `Salivary secretions' of eriophyoids (Acari: Eriophyoidea): first results of an experimental model. Exp. Appl. Acarol., 34: 291-306. https://doi.org/10.1023/B:APPA.0000049219.93796.11
- de Lillo E., Panzarino O., Loverre P., Valenzano D., Mattia C., Marini F., Augé, M., Cristofaro M. 2017. New eriophyoid mites from Italy. IV. Mites associated with weed plants. Syst. Appl. Acarol., 22: 2256-2272. https://doi.org/10.11158/saa.22.12.15
- de Lillo, E., Skoracka, A. 2010. ''What's ''cool" on eriophyoid mites?." Exp. Appl. Acarol. 51.1: 3-30. https://doi.org/10.1007/s10493-009-9297-4
- Ferragut F., Costa-Comelles J., Garcia-Mari F., Laborda R., Roca D., Marzal C. 1988. Population dynamics of the phytoseiid Euseius stipulatus (Athias-Henriot) and its prey Panonychus citri (McGregor) (Acari: Phytoseiidae, Tetranychidae), in Spanish citrus (in Spanish). Bol. San. Veg. Plagas, 14: 45-54.
- Ferragut F., Garcia-Mari F., Costa-Comelles J., Laborda R. 1987. In Xuence of food and temperature on development and oviposition of Euseius stipulatus and Typhlodromus phialatus (Acari: Phytoseiidae). Exp. Appl. Acarol., 3: 317-329. https://doi.org/10.1007/BF01193168
- Heisey R.M. 1996. Identification of an allelopathic compound from Ailanthus altissima (Simaroubaceae) and characterization of its herbicidal activity. Am. J. Bot., 83: 192-200. https://doi.org/10.1002/j.1537-2197.1996.tb12697.x
- Hong, X.Y., Xue, X.F. 2005. Four new species of Aculops keifer (Acari: eriophyoidea: eriophyidae) from China. Oriental Insects, v. 39, n. 1, p. 203-211. https://doi.org/10.1080/00305316.2005.10417433
- Kowarik I., Säumel I. 2007. Biological flora of Central Europe: Ailanthus altissima (Mill.) Swingle. Perspectives in Plant Ecology, Evol. Syst., 8: 207-237. https://doi.org/10.1016/j.ppees.2007.03.002
- Lindquist E.E., Sabelis M.W., Bruin J. (Eds.). 1996. Eriophyoid mites: their biology, natural enemies and control. Elsevier, Amsterdam, World Crop Pests, 6. pp. 790.
- McMurtry J.A., Moraes G.J.de, Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Oldfield G.N. 1996. Diversity and host plant specificity. In: Eriophyoid mites. Their biology, natural enemies and control. Elsevier, Amsterdam, World Crop Pests, 6. p. 199-216. https://doi.org/10.1016/S1572-4379(96)80011-X
- Park H.H., Shipp L., Buitenhuis R. 2010. Predation, development, and oviposition by the predatory mite Amblyseius swirkii (Acari: Phytoseiidae) on tomato russet mite (Acari: Eriophyidae). J. Econ. Entomol., 103: 563-569. https://doi.org/10.1603/EC09161
- Parra J.P.R., Botelho P.S.M., Corrêa-Ferreira B.S., Bento J.M.S. (Eds.). 2002. Controle biológico no Brasil. São Paulo: Manole. p. 225-238.
- Queiroz D.L.de, Flechtmann, C.H.W. 2011. Ácaros associados ao eucalipto. Embrapa Florestas-Documentos (INFOTECA-E), 26.
- Ragusa S. 1981. Influence of different kinds of food substances on the developmental time in young stages of the predacious mite Typhlodromus exhilaratus Ragusa (Acarina: Phytoseiidae). Redia, 64: 237-243.
- Ragusa S., Swirski E. 1976. Notes on predacious mites of Italy, with a description of two new species and of an unknown male (Acarina: Phytoseiidae). Redia, 59: 179-196.
- R Development Core Team. 2010. R: A language and environment for statistical computing. Computer programme. http://www.R-project.org/
 (Accessed 26 March, 2019).
(Accessed 26 March, 2019). - Ripka G., Érsek L. 2014. A new Aculops species (Acari: Prostigmata: Eriophyoidea) on Ailanthus altissima from Hungary. Acta Phytopathol. Entomol. Hungarica, 49: 49-56. https://doi.org/10.1556/APhyt.49.2014.1.5
- Rodrigues J.R.O. 2005. Os ácaros fitoseídeos na limitação natural do aranhiço-vermelho em fruteiras e vinha. Ponte de Lima: Instituto Politécnico de Viana do Castelo. Escola Superior Agrária de Ponte de Lima, D.L. pp. 179.
- Seastedt T.R. 2015. Biological control of invasive plant species: a reassessment for the Anthropocene. New Phytol., 205: 490-502. https://doi.org/10.1111/nph.13065
- Seelmann L, Auer A, Hoffmann D, Schausberger P. 2007. Leaf pubescence mediates intraguild predation between predatory mites. Oikos, 116: 807-817. https://doi.org/10.1111/j.0030-1299.2007.15895.x
- Silva D.E., Nascimento J.M.do, Meira A.A., Johann L., Corrêa L.L.C., Rodrigues R., Ferla N.J. 2019. Phytoseiid mites under different vineyard managements in the subregions of Lima and Cávado of the Vinho Verde region in Portugal. Syst. App. Acarol., 24(5): 918-928. https://doi.org/10.11158/saa.24.5.13
- Silveira-Neto S., Nakano O., Barbin D., Nova N.A.V. 1976. Manual de ecologia dos insetos. São Paulo, Agronômicas Ceres. pp. 419.
- Skoracka A., Smith L., Oldfield G., Cristofaro M., Amrine J.W.Jr. 2010. Host-plant specificity and specialization in eriophyoid mites and their importance for the use of eriophyoid mites as biocontrol agents of weeds. Exp. Appl. Acarol., 51:93-113 https://doi.org/10.1007/s10493-009-9323-6
- Smith L., de Lillo E., Amrine J.W.Jr. 2010. Effectiveness of eriophyid mites for biological control of weedy plants and challenges for future research. Exp. Appl. Acarol., 51: 115-149. https://doi.org/10.1007/978-90-481-9562-6_7
- Thurman J.H., Crowder D.W., Northfield T.D. 2017. Biological control agents in the Anthropocene: current risks and future options. Curr. Opin.Ins. Sci., 23: 59-64. https://doi.org/10.1016/j.cois.2017.07.006
- Tixier M.-S. 2018. Predatory mites (Acari: Phytoseiidae) in agro-ecosystems and conservation biological control: a review and explorative approach for forecasting plant-predatory mite interactions and mite dispersal. Frontiers Ecol. Evol., 6: 1-21. https://doi.org/10.3389/fevo.2018.00192
- Tixier M.-S., Baldassar A., Duso C., Kreiter S. 2013. Phytoseiidae in European grape (Vitis vinifera L.): bioecological aspects and keys to species (Acari: Mesostigmata). Zootaxa, 3721: 101-142. https://doi.org/10.11646/zootaxa.3721.2.1
- Xue, X.F., Hong, X.Y. 2006. Eriophyoid mite fauna from Henan Province, central China (Acari: Eriophyoidea) with descriptions of five new species. Zootaxa, v. 1204, n. 1, p. 1-30-1-30. https://doi.org/10.11646/zootaxa.1204.1.1
- Zhang, Z.Q. 2003. Mites of greenhouses: identification, biology and control. CABI Publishing, Biddles Ltd, Guildford and King's Lynn, UK. pp. 244.



2021-08-22
Date accepted:
2022-08-23
Date published:
2022-09-06
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Toldi, Maicon; Juarez Ferla, Noeli; Evangelho Silva, Darliane; de Andrade Rode, Priscila; de Azevedo Meira, Anderson and de Lillo, Enrico
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



