Sexually dimorphic claws predict courtship and mating sequence in the intertidal oribatid mite Fortuynia atlantica (Acari, Oribatida)
Pfingstl, Tobias  1
and Kerschbaumer, Michaela
1
and Kerschbaumer, Michaela  2
2
1✉ Institute of Biology, University of Graz, Universitaetsplatz 2, 8010 Graz, Austria.
2Institute of Biology, University of Graz, Universitaetsplatz 2, 8010 Graz, Austria.
2022 - Volume: 62 Issue: 3 pages: 666-671
https://doi.org/10.24349/55m8-v2ubShort note
Keywords
Abstract
Introduction
In most species of oribatid mites, dissociated sperm transfer via stalked spermatophores is the common mode of reproduction, and the gender of adults cannot be determined without examining the genitalia which are usually hidden in the genital vestibule (Behan-Pelletier 2015). Only one percent of brachypyline Oribatida are known to show a distinct sexual dimorphism and although proximal or associative mating is assumed for most species of this minority, such a behaviour has been observed only in a handful of them (Behan-Pelletier 2015). Grandjean (1956, 1966b) observed a courtship dance, which he called'promenade à deux', in Erogalumna zeucta Grandjean, 1966 and Centroribates mucronatus (G. & R. Canestrini, 1882), where the male holds on to the gastronotic region of the female with its first pair of legs and then they walk in a chain. Similar observations were made in the non-brachypyline genus Collohmannia, where males and females also walk in a chain, but after this courtship dance, the male secretes a nuptial gift onto specialized structures of the genu-tibia of its fourth pair of legs and then offers it to the female (Schuster 1962; Norton and Sidorchuk 2014). Physical contact in combination with a transfer of a nuptial gift was also reported in an undescribed Mochloribatula species from Brazil (Oliveira et al. 2007). However, in all these cases direct sperm transfer following courtship was only assumed to occur. The only observation of such a transfer in an oribatid mites was in a non-dimorphic species of Pilogalumna that exhibits no courtship behaviour; instead, the male forcefully attaches a stalkless spermatophore close to the genital plate of the female (Estrada-Venegas et al. 1996).
Distinct sexual dimorphism is thought to have evolved in oribatid mites as a response to intermittent dryness, aquatic habitats and spatially discrete habitats (Norton and Alberti 1997, Behan-Pelletier 2015). The littoral environment represents an intermittently dry habitat and consequently the number of sexually dimorphic species is relatively high there (see Behan-Pelletier 2015 and Pfingstl 2015; for details). Fortuynia atlantica Krisper & Schuster, 2008, an intertidal oribatid mite from Bermuda and the Caribbean, represents one of the most striking cases of sexual dimorphism. The males show enlarged porose areas at the bases of most notogastral setae, two of these setae (la, lm) are very long with leaf shaped tips, and there is a pair of strongly protruding and overhanging lateral projections on a level with setae h3 , in the posterior half of the gastronotic area (Krisper and Schuster 2008). This strong dimorphism was suggested to be involved in some kind of mating behaviour, allowing rapid sperm transfer during low tide (Pfingstl 2013). No such behaviour has been observed, but F. atlantica successfully reproduced in the laboratory despite the documented absence of stalked spermatophores that would have indicated typical indirect sperm transfer (Pfingstl 2013).
Herein, we report yet another dimorphic trait in this species—differences in claw morphology—and test with morphometric means if the form of the female claw is appropriate for holding the notogastral protrusions of the male. We then predict the eventual discovery of courtship sequence that encompasses all dimorphic features of males and females.
Material and methods
All investigated material is stored in the personal collection of one of the authors (TP; it comprises 141 adult specimens of 8 different littoral mite species). For the target species Fortuynia atlantica, we compared the shape of the first leg claw of 11 males and 15 females. Geometric morphometric data analyzed in this study is from Pfingstl et al. (2020) and Kerschbaumer and Pfingstl (2021) and is stored at Dryad; methods are given in the respective publication. Data analysis was done in Rstudio vers. 1.3.1093 (R Core Team 2020).
Results and discussion
Differences in claw shape and their possible role
Among eight investigated littoral species (given in supplementary Figure S1) only F. atlantica is known to show distinct sexually dimorphic characters (apart from claws), and it was also the only species showing a considerable difference between male and female claws of the first leg. Despite having larger bodies, claws of the first leg of F. atlantica females are significantly smaller and more curved than those of males (Fig. 1). Fortuynia atlantica females still show the typical'rock-shape' claws (Pfingstl et al. 2020), but nonetheless differ from females of other species, living in the same habitat (see supplementary Figure S1). The claws of the other legs are also smaller in females but their dimorphic character is not as distinct as in leg I.



This dimorphism indicates that female claws might be somehow involved in some kind of physical contact during mating. In Collohmannia gigantea Sellnick, 1922 and E. zeucta, where the male holds on to the flanks of the female for a'chain walk', paraxial tarsal setae of the attaching leg are shaped like long ribbon noodles or long feathers, which are thought to facilitate maintaining physical contact with the female's smooth notogastral cuticle (Grandjean 1966a, b). Interestingly, Collohmannia johnstoni Norton & Sidorchuk, 2014 shows the same'chain walk' without having modified setae on male tarsus I. However, if no such additional adhesive structures exist, strong attachment could be achieved by hooking the claws to a morphological structure of the partner. There are no known examples of claws used for maintaining contact during a courtship or mating in oribatid mites yet, but there are at least two reports of sexual claw dimorphism (Grandjean 1955, Fernandez 1984). Males of the marine associated Podacarus auberti Grandjean, 1955 possess distinctly larger claws than females and males of the aquatic Hydrozetes ringueleti Fernandez, 1984 show a large ventral tooth on the claw of leg III. The latter species additionally shows a modified femur II shape and a large spine on femur IV (Fernandez 1984), which suggests that the legs might be used to clasp the female. However, if claws can hook on to a morphological structure like a cuticular projection, the contact between partners will be steadier. Cuticular notogastral protuberances are shown by males of several dimorphic species but in nearly all cases they are unpaired and equipped with large porose areas (Behan-Pelletier 2015), consequently they might primarily serve to attract females via some kind of secretion rather than being an anchor for claws. Krisper and Schuster (2008) also reported porose areas on the lateral projections of male F. atlantica, but we were not able to confirm their presence and thus an excretory role for these'handles' is questionable. The lyrifissure ih, on the other hand, is located directly on the projections and thus gives sensory feedback if anything touches these lateral structures. Therefore, we think that the lateral projections of the males are used as anchors for the claws of the females.
Morphological conditions for a claw–handle contact
A physical contact between female claws I and notogastral handles is only possible if the following conditions are met: (1) the legs I of the females should be long enough to reach both'handles' simultaneously (they need to bridge the distances from the'handles' to the posterior end of the male notogaster and the distance from the female rostrum to the insertions of leg I) (Fig. 2a) → z < x + y; (2) the claw diameter of female leg I should be larger than the diameter or size of the'handle' (Fig. 2b). Of course, small but sharp claws could still somehow cling to the projections and shorter legs may still be able to reach both handles when the female mounts the notogaster of the male, but we tested optimal conditions, i.e. claws can clasp most effectively and the female can still walk unhindered with the rest of their legs when the first pair is still attached.
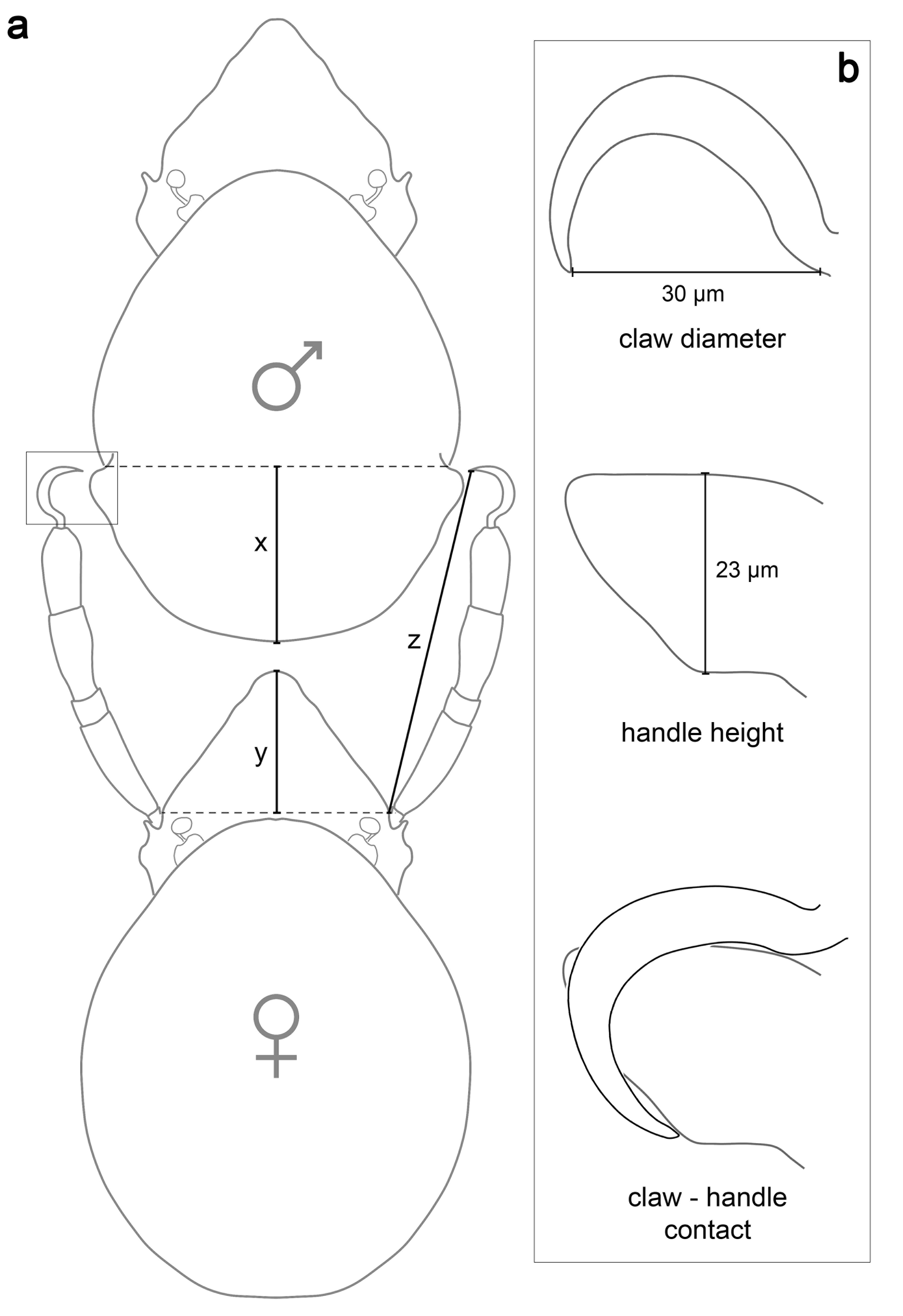


Measurements of the distances to bridge were made in nine specimens of each gender and resulted in the values given in Table 1. The mean distance to bridge is 203 µm and the mean leg length of the females is 252 µm and thus females can easily reach the'handles' simultaneously. Measurements of the claws of 15 females resulted in a mean claw diameter of 28.5 µm and in a mean'handle' height of 23 µm in nine investigated males and thus the second condition is also met.



Hypothetical mode of sperm transfer
The unique dimorphism in claw and the given physical conditions do not necessarily confirm that F. atlantica females indeed clasp the'handles' of the males to perform some kind of'chain walk'. But based on these morphological features and the absence of stalked spermatophores, we hypothesize that a courtship and/or mating behaviour, enabling quick sperm transfer when mites are active during low tide, is shown in this species. The sperm transfer could happen as follows: In a first step (`attraction'), the male needs to attract a willing female, and as sexually dimorphic porose organs are suggested to be secretory organs possibly producing pheromones (Norton and Alberti 1997), the male may secrete pheromones via the enlarged notogastral porose areas at the base of setae la and lm. These long and leaf shaped setae may facilitate pheromone dispersion allowing a transmission over larger distances in air. The female detects the attractant and approaches the male from behind (Fig. 3). The next step is'physical contact'; guided by pheromones, the female raises the first pair of legs and clasps the'love handles' (lateral notogastral projections) of the male with the claws. When the female is properly hooked to the male, the next phase begins with the male depositing a stalkless spermatophore (Fig. 3,'sperm deposition'). In a final step (`lead and uptake'), the male pulls the female over the spermatophore and then the female inserts it into the genital opening.



Conclusions
Direct sperm transfer by a courtship or mating behaviour has never been observed in F. atlantica but sexual dimorphism suggests it exists, and the newly-discovered difference in claw morphology points to a possible scenario. As with other sexually dimorphic oribatid mites, proof will require direct observations of living mites. These are time consuming and difficult to perform, though they are much more needed in acarological research. This short note could be a motivation for acarologists to do so.
Acknowledgments
We would like to thank Roy Norton and an anonymous reviewer for their valuable suggestions and helpful comments.
Funding
This study was supported by the Austrian Science Fund (FWF) under Grant [P 33869-B].
References
- Behan-Pelletier V.M. 2015. Review of sexual dimorphism in brachypyline oribatid mites. Acarologia, 55(2): 127-146. https://doi.org/10.1051/acarologia/20152163
- Estrada-Venegas E., Norton R.A., Moldenke A.R. 1996. Unusual sperm-transfer in Pilogalumna sp. (Galumnidae). In: Mitchell R., Horn D.J., Needham G.R., Welbourn C.W. (Eds.) Acarology IX - Proceedings, Ohio Biological Survey, Columbus, Ohio, vol. 1, p. 565-567.
- Fernandez N.A. 1984. Contribution à la connaissance de la famille Hydrozetidae I. - Hydrozetes (Argentinobates) ringueleti nov. sub-gen., nov. sp. Acarologia, 25: 307-317
- Grandjean F. 1955. Sur un Acarien des iles Kerguélen. Podacarus auberti (Oribate). Memoires du Museum nationale d'histoire naturelle (n.s.), Sèrie A, Zoologie, 8: 109-150.
- Grandjean F. 1956. Sur deux espéces nouvelles d'Oribates (Acariens) apparentées à Oripoda elongata Banks 1904. Archives de zoologie expérimentale et générale, 93: 185-218.
- Grandjean F. 1966a. Collohmannia gigantea Selln. (Oribate). Première partie. Acarologia, 8(2): 328-357.
- Grandjean F. 1966b. Erogalumna zeucta n.g., n.sp. (Oribate). Acarologia, 8: 475-498.
- Kerschbaumer M., Pfingstl T. 2021. Testing for phylogenetic signal in claws suggests great influence of ecology on Caribbean intertidal arthropods (Acari, Oribatida). Scientific Reports, 11:4398. https://doi.org/10.1038/s41598-021-83747-3
- Krisper G., Schuster R. 2008. Fortuynia atlantica sp. nov., a thalassobiontic oribatid mite from the rocky coast of the Bermuda Islands (Acari: Oribatida: Fortuyniidae). Annales Zoologici, 58(2): 419-432. https://doi.org/10.3161/000345408X326753
- Norton R.A., Alberti G. 1997. Porose integumental organs of oribatid mites (Acari, Oribatida). 3. Evolutionary and ecological aspects. Zoologica, 146: 115-143.
- Norton R.A., Sidorchuk E.A. 2014. Collohmannia johnstoni n.sp. (Acari, Oribatida) from West Virginia (U.S.A.) including description of ontogeny, setal variations, notes on biology and systematics of Collohmanniidae. Acarologia, 54: 271-334. https://doi.org/10.1051/acarologia/20142134
- Oliveira A.R., Norton R.A., de Moraes G.J., Faccini J.L.H. 2007. Preliminary observations on courtship behaviour in Mochloribatula (Oribatida: Mochlozetidae). In: Morales-Malacara J.B., Behan-Pelletier V., Ueckermann E., Pérez T.M., Estrada-Venegas E.G., Badii M. (Eds.). Acarology XI: Proceedings of the International Congress, Universidad Nacional Autónoma de México; Sociedad Latinoamericana de Acarología, Mexico, p. 715-718.
- Pfingstl T. 2013. Habitat use, feeding and reproductive traits of rocky-shore intertidal mites from Bermuda (Oribatida: Fortuyniidae and Selenoribatidae). Acarologia, 53(4): 369-382. https://doi.org/10.1051/acarologia/20132101
- Pfingstl T. 2015. An interesting case of sexual dimorphism in intertidal mites: Fortuynia dimorpha sp. nov. (Acari, Oribatida, Fortuyniidae). Systematic and Applied Acarology, 20(5): 567-578. https://doi.org/10.11158/saa.20.5.10
- Pfingstl T., Kerschbaumer M., Shimano S. 2020. Get a grip - evolution of claw shape in relation to microhabitat use in intertidal arthropods (Acari, Oribatida). PeerJ, 8: e8488. https://doi.org/10.7717/peerj.8488
- R Core Team 2020. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.URL https://www.R-project.org/
 .
. - Schuster R. 1962. Nachweis eines Paarungszeremoniells bei den Hornmilben (Oribatei, Acari). Naturwissenschaften, 49(21): 502-503. https://doi.org/10.1007/BF00637051



2022-03-31
Date accepted:
2022-07-01
Date published:
2022-07-05
Edited by:
Roy, Lise

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Pfingstl, Tobias and Kerschbaumer, Michaela
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




