Taxonomy of European Damaeidae X. Description of Coronabelba unicornis n. gen., n. sp. (Acari, Oribatida, Damaeidae) from Abkhazia, with comments on genus Metabelba Grandjean, 1936
Kolesnikov, Vasiliy B.1 and Miko, Ladislav2
1✉ Federal public budgetary scientific institution All-Russian Research Institute of Protection of Plants, VNIISS, Voronezh Region, 396030, Russia & Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Volodarskogo Str., 625003 Tyumen, Russia.
2Faculty of Science, Institute for Environmental Studies, Charles University in Prague, Prague, Czech Republic.
2022 - Volume: 62 Issue: 2 pages: 340-351
https://doi.org/10.24349/1khj-v25fZooBank LSID: 2717199D-C77A-4A5A-AA88-723C8F553488
Original research
Keywords
Abstract
Introduction
During the taxonomic identification of oribatid mites collected in Caucasus (Georgia, Abkhazia), a new species, very similar to Metabelba (Pateribelba) platynotus Grandjean, 1954 was found. The main goal of this publication is to describe and illustrate this new species and resolve its generic placement within Damaeidae, together with M. (P.) platynotus. Grandjean (1954) described Metabelba platynotus and assigned it to the genus Metabelba Grandjean, 1936, while highlighting that this should be a provisional placement because of several important differences. In the remarks to the description he pointed out: ''I put platynotus in the genus Metabelba because it has characters which currently serve as a definition for this genus. Later it will be necessary to put platynotus in a new genus or subgenus. It differs from other Metabelba not only by its facies and by the shape of its notogaster but by its non-cottony cerotegument.'' (Grandjean, 1954, in French).
In this original definition of the genus Metabelba, Grandjean (1936) used as differential character the presence of companion setae d on tibia II and III, and its absence on tibia IV, where the solenidion is free, long and tactile. In comments to this genus he also highlighted the similarity of solenidion of tibia IV with solenidion φ1 of tibia I and remarked about separate (faster) evolution of leg IV compared to legs II and III. He highlighted similarities to Porobelba and Damaeobelba such as smaller body size, extensive development of ''cottony'' cerotegument and moniliform legs but also mentioned presence of apophyses and absence of spinae adnatae in Metabelba in comparison with Porobelba and Damaeobelba. He did not mention other leg chaetotaxy characters such as increased number of setae on trochanters and femora.
Bulanova-Zachvatkina (1965) first mentioned an increased number of setae on femora and trochanters III-IV in Metabelba as generic character and highlighted that it is shared with some other genera (Metabelbella and Parabelbella). This character was not yet highlighted as generic in first revision of Metabelba by van der Hammen and Strenzke (1953).
Norton (1979) proposed a list of apomorphies in Damaeidae, and his familial concept put Metabelba into a monophyletic group of genera defined by an increased number of setae on femora, not mentioning differences in trochanteral setation. This concept was generally followed by Miko (2006), who defined Metabelba, apart from other characters, by an increased number of setae on trochanters (1-1-4-3 or 1-1-3-3) and femurs (10-10-9-9 or 10-10-9-8), and associated setal formula of tibia 0-1-1-0, mentioning that femur I and II may rarely bear only nine setae. The nominate subgenus was characterized by presence of apophyse P, while species without it were considered as members of the poorly defined subgenus Parametabelba Mihelčič, 1964. This concept was later revised by Mourek et al. (2011), who argued that according to the rules of International Commission of Zoological nomenclature the name Parametabelba is not available and cannot be used as a valid name of this taxon, and proposed new subgenus Pateribelba instead, with the type species Metabelba sphagni Strenzke, 1950. The new definition of Metabelba and both subgenera was provided and discussed in detail.
Subias (2004, online update 2021) synonymized Pateribelba Mourek, Miko et Bernini, 2011 with Neobelba Bulanova-Zachvatkina, 1967, probably on the basis of the redescription of Neobelba pseudopapillipes Bulanova-Zachvatkina, 1967 by Miko and Kolesnikov (2014), where similarities with Metabelba s.str. and Pateribelba were broadly discussed. However, the synonymy between Pateribelba and Neobelba was rejected on the basis of companion seta d present on tibia IV in Neobelba, as setation of leg IV was seen as an important distinguishing character (separate from characters of leg II and III) already by Grandjean, as mentioned above.
Metabelba platynotus Grandjean, 1954 with trochanteral setation 1-1-4-3, associated setation of tibia 0-1-1-0, and femoral setation 9-9-7-7 was placed by Mourek et al. (2011) into the subgenus Metabelba (Pateribelba), even if femoral setation was seen as exceptional within that subgenus. Similarly, Subias (2004, online update 2021) keeps M. platynotus within his concept of subgenus Neobelba. Results of our analysis lead us to accept of original view of Grandjean (1954) and propose for newly found species, together with M. platynotus, a new taxon at the generic level. The definition of a new genus Coronabelba n. gen. and its relations to other taxons within Metabelba sensu lato complex is another aim of this paper.
Material and methods
Two individuals (females) of Coronabelba unicornis n. sp. were collected in Abkhazia (Georgia), East Abkhazian region, Tkvarcheli District, surroundings of the village of Akarmara, beech-laurelcherry forest, 42°51′46″N, 41°46′15″E, 550 m a.s.l, soil, 20.Mar. 2021 (coll. I. Turbanov). Mites were extracted from samples into 75% ethanol using Berlese's funnels with electric lamps in laboratory conditions for 10 days. Type specimens are deposited in the Zoological Institute, Russian Academy of Sciences, St. Petersburg, Russia (ZISP). Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration.
Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster. Notogastral width refers to the maximum width of the notogaster in the dorsal view. Lengths of body setae were measured in the lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu-tibia-tarsus.
Drawings were made with a camera lucida attached to the transmission light microscope ''Biomed 6 variant 3''. General morphological terminology mostly follows that of F. Grandjean: see Travé and Vachon (1975) for references, Norton (1977) for leg setal nomenclature, and Norton and Behan-Pelletier (2009) for overview. Some specific damaeid characters follow the terminology summarized in Miko (2015). Paired structures are described in the singular, unless otherwise noted.
The following abbreviations are used: ro, le, in, bs, ex—rostral, lamellar, interlamellar, bothridial and setae, respectively; bo—bothridium; P—propodolateral apophysis; Ba, Bp, Da, Dp—dorsosejugal tubercles; La—lateral tubercle; Sa, Sp—parastigmatic tubercles; dis—discidium; apt—anteroprodorsal tectum; ptb—medial prodorsal protuberance in the interbothridial region; c, la, lm, lp, h, p—notogastral setae; ia, im, ip, ih, ips—notogastral lyrifissures; gla—opisthonotal gland opening; cs—circumgastric scissure; csb—circumgastric sigillar band; a, m, h—subcapitular setae; or—adoral seta; v, l, d, cm, acm, ul, sul, vt, lt – palp setae; ω—palp and leg solenidion; cha, chb—cheliceral setae; Tg—Trägårdh's organ; 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c, 4d—epimeral setae; Va, Vp—ventrosejugal tubercles; E2a, E2p—epimeral tubercles; g, ag, an, ad—genital, aggenital, anal and adanal setae, respectively; iad—adanal lyrifissure; ian—anal lyrifissure; Tr, Fe, Ge, Ti, Ta—leg trochanter, femur, genu, tibia and tarsus, respectively; p.a.—leg porose area; σ, φ—leg solenidia; ɛ—leg famulus; v, ev, bv, l, d, ft, tc, it, p, u, a, s, pv—leg setae; k—scalp-attachment cornicle.
Systematics
Coronabelba n. gen.
ZOOBANK: CDF6A279-5C92-4A02-8557-39EC9395DA57 ![]()
Diagnosis — Small damaeid mites with thick layer of columnar and amorphous cerotegument. Prodorsum with raised interbothridial region (prodorsal protuberance), with or without tubercles of enantiophysis D; enantiophyses B, L and apophysis P absent. Prodorsal setae medium sized, smooth, broadened, bent up to claw-like (ex). Bothridial seta short, setiform, heavily covered by cerotegument, distally attenuated to slightly flagellate. Parastigmatic tubercles uneven, Sa narrow, long, spiniform and Sp smaller, shorter, spiniform or almost reduced. Notogaster elongated, oval to ovoid in dorsal view, in lateral view conical-pyramidal, with highest point shifted posteriad (roughly at the level of setae lp). Spina adnata absent; all notogastral setae in form of strong, smooth erect spines inserted more-less radially on distinct tubercles or even apophyses, short, mostly distinctly shorter than the distance to next more posterior seta. Ventral side without ridges and apophyses, tubercles E2 and V absent or very weakly developed. Discidium present. Legs moniliform, with tibial solenidia II-III coupled with seta d, seta d on tibia IV absent, tibial solenidion therefore free, but relatively short (about as long as or shorter than length of tibia), setiform. Trochanters III and IV with increased numbers of setae (trochanteral formula 1-1-4-3); femora I-II with nine setae (femoral formulas 9-9-9-9 or 9-9-7-7); genual setation: 4-4-4-4, tibial setation: 4-5-5-4, tarsal setation: 20-17-17-14.
Type species: Coronabelba unicornis n. sp.
Other included species — Coronabelba platynotus (Grandjean, 1954) n. comb. (= Metabelba (Pateribelba) platynotus).
Etymology — The prefix ''Corona-'' is derived from the Latin feminine noun corona = crown, wreath. Notogastral setation inserted on tubercles and apophyses resemble a crown.
Coronabelba unicornis n. sp.
ZOOBANK: 2B812841-973F-46E2-B815-6E682224E61D ![]()
(Figures 1–5)






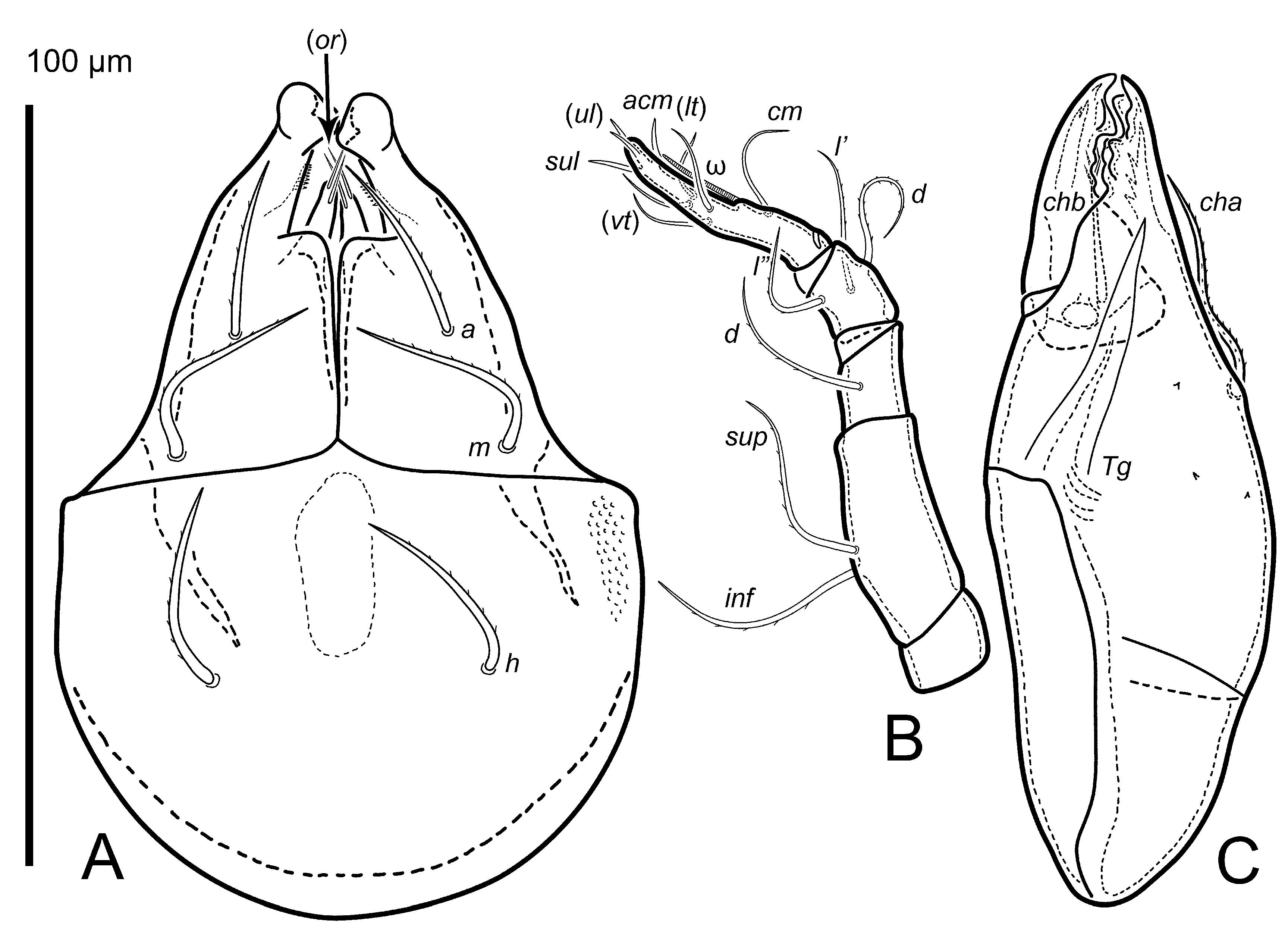








Diagnosis — Body size: 470 × 260. Body surface and legs covered by columnar cerotegument (Figures 1A, 5). Prodorsum without tubercles (Da, Dp, Ba, Bp and La absent), apophysis P absent. With medial peaked prodorsal protuberance in the interbothridial region appearing as flat horn oriented anteriad. Between bases of lamellar setae there is a small sclerotized ridge (anteroprodorsal tectum). Bothridial seta short, smooth, with flagellate tip, covered distally by dense cerotegument creating ''head''. Seta ex particularly strong, curved, claw-like. Notogastral setae spiniform, inserted on distinct apophyses, particularly posterior apophyses (row h) may be as long as the seta or even longer. Ventral tubercles Va absent, Vp faintly distinguishable as indistinct thickening; E2 absent. Parastigmatic tubercle Sa subtle, quite long, straight spiniform and narrow, Sp faintly distinguishable, flat. Discidium quite long, spiniform, slightly curved, pointed, directed laterad. Epimeral setal formula: 3-1-3-4, setae situated on microtubercles. Legs moniliform, short, formulas of leg setation and solenidia: I (1–9–4–4–20) [1–2–2], II (1–9–4–5–17) [1–1–2], III (4–9–4–5–17) [1–1–0], IV (3–9–4–5–14) [0–1–0]. Length of solenidia on genua and tibia II–III slightly longer than respective companion setae; solenidia φ1 three times as long than φ2, φ2 and ω1 on tarsus I curved.
Description of adult — Measurements. Body length: 470, maximum notogastral width 260.
Integument. Body color yellowish brown to medium brown. Cerotegument (Figures 1A, 5) with short, columnar excrescences. All leg femora with uneven mesh cerotegument under the columnar. Underlying cuticle mostly smooth, but faintly granular on lateral part of prodorsum and on tubercles. Genital plate with bumpy cuticle (Figure 1B, C). Gastronotic exuviae (scalps) carried on notogaster (Figures 4A-D, 5E, F), but without significant amounts of organic debris or soil particles.
Prodorsum (Figure 1A, C). Rostrum blunt with weakly defined nose-like protuberance on dorsal side. Propodolateral apophysis absent. Tubercles Da, Dp absent; in Da place there are underdeveloped cuticle eminences; dorsosejugal (Ba, Bp) and lateral (La) tubercles absent. With medial peaked prodorsal protuberance in the interbothridial region. Parastigmatic apophyses Sa, subtle, straight spiniform and narrow, Sp faintly distinguishable, flat. Rostral and lamellar setae similar in length (42–43), smooth, setiform; ro slightly thinner than le. Between the bases le there is a small sclerotized ridge (apt, Figure 1A). Interlamellar seta (41) two times shorter than bothridial setae, setiform, smooth, inserted on tubercular apophysis. Exobothridial seta (20), setiform, curved anteromediad, smooth. Bothridial seta (70–90) smooth, setiform, with flagellate tip. Small, pore-like opening present behind insertion of ex, distinct pore visible also in lateral view behind acetabulum II in sejugal area. Distinct fields of sigillae (muscle insertions) present on prodorsum, two anteriad – anteromediad to bothridium and in, one in interbothridial area (Figure 1A). Posterior edge of two posterior fields strengthened and more sclerotised.
Notogaster (Figures 1A, C, 4D, E). Notogaster conical in lateral view, with highest point shifted posteriad; anterior part of notogaster distinctly flattened. Notogastral setae (c1–h1: 30–35, ps1–ps3: 20–25) shorter than the distance to next more posterior seta, strong, smooth erect spines. Notogastral setae inserted on large protuberances (Figure 4E). Distance c1–c1 shorter than c2–c2. Seta c1 directed forward. Posterior notogastral setae (p1–p3) setiform, with a sharp tip, without large protuberances. Circumgastric row of muscle sigillae distinct, well visible. One pair of minute light spots present on notogaster between to insertions of setae la and lm.
Gnathosoma (Figure 3). Subcapitulum longer than wide (110 × 70–76). Subcapitular setae (a, m, h) similar in length (25–27), setiform, slightly barbed. Adoral seta (8) setiform, thin, smooth. Palp (88–95) with setation 0–2–1–3–9(+ω). Solenidion bacilliform, pressed to surface of palptarsus mediobasally. Postpalpal seta (7) spiniform. Chelicera (110) slightly narrowed, position of teeth is shown in Figure 3C; seta cha (30) curved in the central part, with short barbs on external curvature and attenuated tip, seta chb (23) straight, distally bent and with fringe of diminishing barbs. Trägårdh's organ (36) elongate triangular. Chelicera with three minute teeth on dorsal part.
Epimeral and lateral podosomal regions (Figure 1B, C). Epimeral tubercles (E2) absent. Ventral tubercles Va absent, Vp very weakly expressed, in the form of a slight rounding. Ventrosejugal furrow deep, body distinctly narrowed between acetabula II and III. Epimeral setal formula: 3–1–3–4. Setae 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c and 4d situated on microtubercles. Epimeral setae comparatively long, smooth, thin, with attenuate tips. Epimeres with well visible muscular sigillae, particularly closer to axial part. Discidium well developed, spiniform, pointed, directed laterad.
Anogenital region (Figure 1B, C). Six pairs of genital (20–29), one pair of aggenital (32), two pairs of anal (25) and three pairs of adanal (20–24) setae, all smooth, setiform, subequal in length, similar to epimeral setae. Distance ad3–ad3 more than ad2–ad2. Lyrifissure iad apoanal, oblique and divergent from body axis posteriad. Anal lyrifissure present.
Legs (Figures 2, 5A–D). Legs segments with distinctly swollen distal parts (moniliform), except tarsi, where swollen part is proximal. All legs distinctly shorter than body, leg IV longer than rest of legs (Table 1). Leg setae comparatively long and strong, pointed; smooth or slightly barbed. Formulas of leg setation and solenidia: I (1–9–4–4–20) [1–2–2], II (1–9–4–5–17) [1–1–2], III (4–9–4–5–17) [1–1–0], IV (3–9–4–5–14) [0–1–0]; homologies of setae and solenidia indicated in Table 2. Famulus setiform, emergent, relatively long. Solenidion of genua I, II and III each coupled with companion seta d, as well as solenidion of tibiae II and III. Length of solenidia on genua and tibiae II–III slightly longer than respective companion setae; solenidion φ1 three times as long than φ2, φ2 and ω1 on tarsus I curved; solenidion on tibia IV not longer than tibia itself.






Ontogeny — Unknown. However, larval and nymphal exuvia (Figures 4A-D, 5E, F) carried on the notogaster by adults. Larval notogastral setae strong, moderately long, darkly pigmented, and mostly with short barbs. Larval setae c1 are directed towards each other, h1 long (length as l). Nymphal notogastral setae strong, moderately long, darkly pigmented, and mostly with very short barbs; setae h1 and ps1 short, thin. Cornicle very short, thickened, conical; it uniformly narrowing toward apices, blunt, with the non-split distal part, situated at the level of setae h2–h3 insertions of the adult.
Type material — Holotype (female) and paratype (females) Abkhazia (Georgia), East Abkhazian region, Tkvarcheli District, surroundings of the village of Akarmara, beech-laurelcherry forest, 42°51′46″N, 41°46′15″E, 550 m a.s.l, soil, 20.III.2021 (coll. I. Turbanov).
Type deposition — Holotype (female) and paratype (females) are stored in ZISP. All specimens are preserved in a 70% solution of ethanol with a drop of glycerol.
Etymology — The specific name ''unicornis'' refers to the medial peaked prodorsal protuberance in the interbothridial region.
Remarks — The new species is morphologically similar to Coronabelba platynotus Grandjean, 1954 n. comb. in form of notogaster and notogastral setae, Sa and dis. However, the new species differs from the latter by the absence Da and Dp (versus enantiophysis D present), the presence of median, peaked prodorsal protuberance in the interbothridial region (versus slight gentle elevation only), poorly developed Sp (versus small, but distinct Sp present), long and curved solenidion φ2 on tibia I, three times longer than φ2 (versus φ1 more than 5 times longer than φ2), solenidion ω1 on tarsus I curved (versus straight), dorsal notogastral setae inserted on large apophyses, claw on leg I particularly long, about one third of tarsus I length (versus shorter claw, roughly one fifth of tarsus I length in C. platynotus).
Discussion — Generic concepts of Metabelba highlight usually increased number of setae on moniliform legs, particularly on trochanters III-IV and on femora, and presence of companion seta d on tibia II and III, while on tibia IV the solenidion is usually long, tactile and without companion seta. Together with quite similar body shape and abundant cerotegument in thick layers it puts together quite numerous species, many of them insufficiently known or poorly described. Subias (2004, online amendment 2021) lists 30 species of the genus, equally divided in 2 subgenera. Yet, morphological differences between the species include differences in characters, which were regarded as generic in other damaeid oribatids, for example presence/absence of apophysis P, development of tubercle L, or differences in presence of companion setae d on tibia. As most of the characters seem to be very stable within the group, we argue that observed differences need to be considered at generic level. In Metabelba, as defined by Miko (2006) and Mourek et al. (2011), presence of 10 setae on femora I and II is a typical character, and exemption mentioned in diagnosis (presence of nine setae) was related to Grandjean's M. platynotus only. Already Grandjean (1954) noted different femoral setation of M. platynous, and highlighted also the uniqueness of the general structure of body and cerotegument of his species, indicating that it may belong to different taxon at generic level, as already mentioned. C. unicornis n. sp. is very similar to Grandjean's species and shares all the important characters which differentiate both species from all Metabelba species. They have similar form of notogaster (elongated ovoid, conical in lateral view), spiniform notogastral setae inserted on distinct tubercles or apophyses, same number (9) of setae on femur I and II, and columnar cerotegument, present in sejugal and interbothridial region. There is therefore no doubt about the relationship between the two species, and about clear difference to other Metabelba species. This led us to follow the view of Grandjean (1954) and propose new genus as defined here and discussed further.
From all Metabelba species, only M. (Pateribelba) denscanis Mourek, Miko et Bernini, 2011 is exceptional in having conical notogaster, but clearly different from Coronabelba n. gen., with generally circular shape in dorsal view, without flattening in anterodorsal side and without specific shape and insertions of notogastral setae on apophyses or distinct tubercles. It resembles rather notogaster of Belba species as discussed recently by Miko (2021) In both Coronabelba n. gen. species, notogaster is differently shaped, noticeably more elongated and oval/ovoid in dorsal view, maximum notogastral height is at level setae lp-h3 (in M. (P.) denscanis with maximum notogastral height at level setae lm-lp), and clearly overhangs in posterior part. The form of notogastral setae of C. platynotus n. comb. and C. unicornis n. sp. and their insertions was not observed in all other species of Metabelba. There are also differences in the shape of cerotegument excrescences mentioned by Grandjean (1954), but they are not universal: most species of Metabelba have filamentous (''cottony'') cerotegument, but some have it shaped differently (e.g. M. (P.) denscanis with irregular excrescences, M. (P.) centurion Miko, Mourek et Ermilov, 2014 with granular/tubercular cerotegument or M. (M.) propexa (Kulczynski, 1902) with granular /columnar cerotegument), however still different from columnar form in Coronabelba n. gen., Coronabelba platynotus n. comb. and C. unicornis n. sp. have a well developed and quite deep prodorsal furrow, well visible in lateral view. The prodorsal protuberance, which is absent or only slightly indicated in Metabelba species, is elevated in Coronabelba n. gen., and transformed into a pointed protrusion at C. unicornis n. sp.
Another character supporting the separation of the new genus is the development of cornicle k on nymphal exuvia, which could be observed on our material. According to Ermilov (2012), Metabelba is characterized by long cornicles, with weakly dilated proximal part and split distal part. In C. unicornis n. sp., we observed short cornicles, without split distal part, reminding cornicles of Damaeus Koch, 1835.
Following separation of Coronabelba n. gen., definition of Metabelba as presented in Miko (2006) and Mourek et al. (2011) can be narrowed, with no exceptions from the rule of 10 setae present on femur I and II. At the same time, we believe that presence of well developed seta d on tibia IV on M. (Neobelba) pseudopapillipes (Bulanova-Zachvatkina, 1967) and its absence on all other species placed into subgenus Pateribelba does not allow to consider Neobelba and Pateribelba to be the same taxon, despite of their rather high similarity otherwise. Therefore, we support the concept of Metabelba with nominal subgenus and two other separated subgenera mentioned. Specific differences observed on some Metabelba species (e. g. M. (P.) denscanis and M. (P.) centurion) require further assessment, as regards their subgeneric placement.
Acknowledgements
The authors thank Ilya S. Turbanov (I.D. Papanin Institute of Biology of Inland Waters of Russian Academy of Sciences, Borok, Russia) for collecting the soil substrate. The study of oribatid mites of the family Damaeidae was funded by the Russian Foundation for Basic Research (№ 18-04-00097).
References
- Bulanova-Zachvatkina E.M. 1965. O diagnostike vidov roda Metabelba Grandjean, 1936 (Oribatei, Damaeidae). Zoologicheskij Zhurnal, 36 (9): 1333-1343. [in Russian]
- Bulanova-Zachvatkina E.M. 1967. Pancirnje kleschtschi - Oribatiyi. Moscow: Vysshaya Shkola: 254 pp. [in Russian]
- Enami Y. 1994. A new species of the genus Belba (Acari: Damaeidae) from Japan. Edaphologia, 51: 1-5.
- Ermilov S.G. 2012. On the morphology of cornicles in oribatid mites of the family Damaeidae (Acari, Oribatida). Entomol. Rev., 92 (5): 576-582 https://doi.org/10.1134/S0013873812050107
- Grandjean F. 1936. Les Oribates de Jean Frederic Hermann et de son pere, Ann. Soc. Entomol. Fr., 105: 27-110.
- Grandjean F. 1954. Observations sur les Oribates (29e serie). Bull. Mus. natl. hist. nat., 2e serie, 26 (3): 334-341.
- Hammen L. van der, Strenzke K. 1953. A partial revision of the genus Metabelba Grandjean (Oribatei, Acari). Zool. Meded., 32(14): 141-154.
- Hermann F. 1804. Memoire apterologique. Strassbourg. 1-144. Ex: Grandjean, F. (1936) Les Oribates de Jean Frederic Hermann et de son pere, Ann. Soc. Entomol. Fr., 105: 27-110.
- Koch C.L. 1835. Deutschlands Crustaceen, Myriapoden und Arachniden, Heft 3: F. Pustet, Regensburg, pp 1.
- Kulczynski V. 1902. Species Oribatinarum (Oudms.) (Damaeinarum Michael) in Galicia collectae. Bulletin de l'Académie des Sciences de Cracovie, 2: 89-96.
- Miko L. 2006. Damaeidae. In: Weigmann, G. Hornmilben (Oribatida), Die Tierwelt Deutschlands 76: Goecke & Evers Publ. p. 179-207.
- Miko L. 2015. Taxonomy of European Damaeidae VIII. Contribution to classification of genus Damaeus C. L. Koch, 1835, with a review of Adamaeus Norton, 1978 and Paradamaeus Bulanova-Zachvatkina, 1957 and redescription of three species. Zootaxa, 3980 (2): 151-188. https://doi.org/10.11646/zootaxa.3980.2.1
- Miko L. 2021. Taxonomy of European Damaeidae IX. Contribution to the revision of the genus Belba von Heyden, 1826 (Acari, Oribatida). Syst. Appl. Acarol., 26 (8): 1575-1613. https://doi.org/10.11158/saa.26.8.13
- Miko L., Mourek J., Ermilov S.G. 2014. Taxonomy of African Damaeidae (Acarina: Oribatida) I. Metabelba (Pateribelba) centurion sp. nov. from Ethiopia and redescription of Metabelba (Pateribelba) glabriseta. Int. J. Acarology, 40 (7): 519-534 https://doi.org/10.1080/01647954.2014.951686
- Mourek J., Miko L., Bernini F. 2011. Taxonomy of European Damaeidae (Acari: Oribatida) IV. Partial revision of Metabelba Grandjean, 1936 with proposal of one new subgenus, one new species and redescriptions of two known species. Zootaxa, 3099: 1-42. https://doi.org/10.11646/zootaxa.3099.1.1
- Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal, D.L. (Editor), Biology of oribatid mites. Syracuse, SUNY College of Environmental Science and Forestry, pp. 33-61.
- Norton R.A. 1979. Familial concepts in the Damaeoidea as indicated by preliminary phylogenetic studies. In: Rodriguez, J.G. (Ed.). Recent Advances in Acarology, 2. p. 529-533 https://doi.org/10.1016/B978-0-12-592202-9.50076-8
- Norton R.A., Behan-Pelletier V.M. 2009. Suborder Oribatida. Chapter 15. In: Krantz, G.W. & Walter, D.E. (Editors), A manual of acarology: Lubbock, Texas Tech University Press. p. 430-564.
- Travé J., Vachon M. 1975. François Grandjean. 1882-1975 (Notice biographique et bibliographique). Acarologia, 17(1): 1-19.
- Winkler J. 1955. Nový druh pancířníků, Belba bartoši n. sp., z Pradědu (Acari, Oribatoidea). Ochrana přírody, 10: 306.



2021-11-20
Date accepted:
2022-03-21
Date published:
2022-03-24
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Kolesnikov, Vasiliy B. and Miko, Ladislav
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




