A new species of Rhynchohydracarus Lundblad 1936 (Acariformes: Hydrachnidiae: Hydryphantoidea: Rhynchohydracaridae) from central Brazil, with DNA barcodes, a key for known species and a proposal for the homologies of dorsalia, ventralia, lateralia and glandularia for the family
Castro, Luiz A. S. de  1
; Proctor, Heather C.
1
; Proctor, Heather C.  2
and Lofego, Antonio C.
2
and Lofego, Antonio C.  3
3
1✉ Department of Biological Sciences, Institute of Biosciences, Humanities and Exact Sciences (IBILCE), São Paulo State University (UNESP), São José do Rio Preto, SP, 15054-000, Brazil.
2Department of Biological Sciences, University of Alberta, Edmonton, AB, T6G 2E9, Canada.
3Department of Biological Sciences, Institute of Biosciences, Humanities and Exact Sciences (IBILCE), São Paulo State University (UNESP), São José do Rio Preto, SP, 15054-000, Brazil.
2022 - Volume: 62 Issue: 1 pages: 161-173
https://doi.org/10.24349/0lg5-xg0lZooBank LSID: 2B893E42-84B8-412E-B23F-A4EE014788EC
Original research
Keywords
Abstract
Introduction
The Rhynchohydracaridae is a water mite family that inhabits riffles, springs, and streams in the New World (Cook 1974; Goldschmidt 2004, 2006, 2009; Goldschmidt et al. 2016; Proctor et al. 2015; Goldschmidt and Ramírez-Sánchez 2020) and is composed of three subfamilies: Rhynchohydracarinae Lundblad, 1936, Clathrosperchontinae Lundblad, 1936, and Santiagocarinae Valdecasas, 2001 (Walter et al. 2009; Goldschmidt and Ramírez-Sánchez 2020; Smit 2020). For a brief checklist of all genera, species, type localities, and known distribution see Castro et al. (2020). The monogeneric subfamily Rhynchohydracarinae is considered endemic to the Neotropical region (Di Sabatino et al. 2008) and, until now, has included only three species in the genus Rhynchohydracarus, with sparse and distant localities of occurrence (Fig. 1). In South America, Rhynchohydracarus testudo Lundblad, 1936 was described from Brazil (Lundblad 1936, 1941) and Rhynchohydracarus dividuus Lundblad, 1941 from Paraguay (Lundblad 1941), whereas in Central America, Rhynchohydracarus carmenae Valdecasas, 2001 is known only from Panama (Valdecasas 2001). Some other records of unidentified or undescribed Rhynchohydracarus species are mentioned for Costa Rica (Goldschmidt 2004, 2006, 2009), Panama (Goldschmidt et al. 2016) and Ecuador (Goldschmidt and Ramírez-Sánchez 2020). Rhynchohydracarus species are often found associated with substrate such as sand and small pebbles collected from hyporheic habitats (L.A.S. de C., personal observation). The reduction of ocular pigments, the dorso-ventrally flattened body and strong sclerotization suggest that they are adapted for living in the interstitial zone. Concerning habitat preferences, Fernández and Fossati-Gaschignard (2011) classified the preferences of two Rhynchohydracarus species as ''unknown'' (based on the lack of ecological information in Lundblad's descriptions, these two species are probably R. testudo and R. dividuus) and R. carmenae as ''benthic'' given that this species was collected by kick-sampling the substrate (Valdecasas 2001). Here we increase the knowledge of Rhynchohydracaridae by describing a fourth species of Rhynchohydracarus from central Brazil using integrative taxonomy, including complete morphological descriptions of adult female and male together with their barcodes, which represent the first genetic information for the family Rhynchohydracaridae. A proposal for the homologies of dorsalia, ventralia, and glandularia in Rhynchohydracaridae is also provided, followed by an identification key for the known species of Rhynchohydracarus.
Material and methods
Sampling
Water mites were collected by digging, stirring, and removing substrate with a small shovel, followed by washing detritus and dislodged organisms into a plankton dip net (250 μm mesh) from a shallow and sandy stream, ''Córrego da Santa'', 15.788083° S, 48.872194° E, located in the Cerrado biome, Pirenópolis, Goiás State, central Brazil. After collecting, the substrate was transferred with water to plastic vials and taken to a laboratory at São Paulo State University (Piracicaba, Brazil), where living mites were separated from the fine gravel under a stereomicroscope using forceps and a pipette and preserved in 96% ethanol. Vouchers, including holotype and paratypes of the new species, are deposited in the Acari collection of the Department of Biological Sciences (DCBSJRP), State University of São Paulo (UNESP), São José do Rio Preto, Brazil.



Molecular analysis
DNA extraction
Non-destructive DNA extraction was carried out individually on four collected Rhynchohydracarus specimens, applying an adapted and modified protocol from Gilbert et al. (2007). The digestion buffer consisted of 1M CaCl2 (3 µl), 2% sodium dodecyl sulfate (SDS) in powder (20 mg), dithiothreitol (DTT) (40 µl), Tris buffer pH 8 (100 µl), NaCl (20 µl). Water was added to bring the volume to 1 ml. Then each water mite was transferred from 96% ethanol to individual 1.5 ml Eppendorf® tubes containing 250 µl of digestion buffer and 5 µl of proteinase K (20 mg/ml), and incubated overnight (12–14 hours) at 65°C. After incubating, mite specimens were removed from the buffer and placed in new 1.5 ml tubes with absolute ethanol to stop further digestion and for subsequent morphological studies of the exoskeletons.
DNA purification
From the total volume of each tube (255 µl) of the products of extraction, 200 μl were transferred to new 1.5 ml tubes and the same volume of Phenol:Chloroform:Isoamyl Alcohol (25:24:1) was added. Then the tubes were closed and vortexed for 2 s and finally centrifuged at 13000 RPM for 10 minutes at room temperature. After centrifugation, two phases were obtained with the supernatant being transferred to new 1.5 ml tubes with the addition of 1 µl of glycogen (5 mg/mL), 20 µl of 3M pH 5.2 sodium acetate, and 154.7 µl (0.7 volume of the supernatant) of 100% ice-cold isopropanol. The mixture was gently inverted and immediately stored at -20°C for two hours, followed by centrifugation at 4°C and 13000 RPM for 30 minutes to pellet the nucleic acids. The liquid was then discarded and the pellet obtained was washed twice in 500 µl of ice-cold 70% and 95% ethanol, allowed to air-dry in a fume hood, and eluted in 30 µl of mQ water.
PCR parameters and sequencing
The standard COI barcoding fragment (Hebert et al. 2003) was amplified using primers LCO1490 and HCO2198 (Folmer et al. 1994). PCR reactions were conducted in a final volume of 25 μl, containing 12.4 μl of mQ water, 2.5 μl 10x PCR Buffer, 1 μl of MgCl2 (50 mM) (Sinapse®), 1.0 μl of each primer, 2.0 μl of dNTPs (Sinapse®), 0.1 μl of Taq polymerase Platinum (Sinapse®) and 5.0 μl of template DNA. PCR was performed using an initial denaturation step at 94°C for 120 s, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 45 s, elongation at 72°C for 60 s, and finishing with a final extension at 72°C for 600 s, followed by a pause at 4°C. The purification process was performed using 2.5 μl of Exonuclease I (EXO1) (Thermo Fisher Scientific®) and 5.0 μl of FastAP® Thermosensitive Alkaline Phosphatase (Thermo Fisher Scientific®). The forward and reverse sequences obtained were manually edited and aligned using the software Sequencher 4.1.4 (Gene Codes Corporation, Ann Arbor, MI, USA). COI sequences have been deposited in the Barcode of Life Data System (BOLD: http://www.barcodinglife.org/ ![]() ) and GenBank (https://www.ncbi.nlm.nih.gov/
) and GenBank (https://www.ncbi.nlm.nih.gov/ ![]() ). All steps of molecular analysis were conducted in the Laboratory of Arthropod Molecular Ecology, and bidirectional sequencing was performed by Sanger method in the Agricultural Biotechnology Center (CEBTEC), both located at ''Escola Superior de Agricultura Luiz de Queiroz'' (ESALQ), University of São Paulo (USP), Piracicaba, Brazil.
). All steps of molecular analysis were conducted in the Laboratory of Arthropod Molecular Ecology, and bidirectional sequencing was performed by Sanger method in the Agricultural Biotechnology Center (CEBTEC), both located at ''Escola Superior de Agricultura Luiz de Queiroz'' (ESALQ), University of São Paulo (USP), Piracicaba, Brazil.
Morphological analysis
After DNA extraction, all four mite exoskeletons preserved in 100% ethanol were transferred to 85% lactic acid overnight to clear them as suggested in Proctor et al. (2015), and then slide-mounted in PVA medium (BioQuip, CA, USA) as vouchers. All specimens have been deposited in the mite collection of DCBSJRP-UNESP. Morphological analysis and photography were conducted at the Laboratory of Acarology of ESALQ-USP, using a Nikon® Eclipse 80i microscope, with differential interference contrast (DIC) lighting and equipped with a Nikon® digital camera. Microphotographs were processed with the imaging software Nikon® NIS-Elements. Line drawings were made using the software Adobe Illustrator® CC 2018. Idiosomal structures are named according to the terminology used in Lundblad (1927; 1941), except for coxoglandularia (called epimeroglandularia by Lundblad 1941), which are named cxgl-1 and cxgl-2 according to Proctor et al. (2015), Smith and Cook (2016) and Goldschmidt and Ramírez-Sánchez (2020). These same structures are named in European literature as cxgl-2 and cxgl-4, respectively (Bartsch 2007). Dorsal region: dc—dorsocentralia, dl—dorsolateralia, dgl—dorsoglandularia, lgl—lateroglandularia, prefr—prefrontalia, postfr—postfrontalia, pr—preocularia, po—postocularia. Ventral region: cxgl—coxoglandularia, pregen—pregenital plate, postgen—postgenital plate; vgl—ventroglandularia, v—ventralia. We also propose a new term for the side of the idiosoma, the ''lateral region'', and designate its sclerites as lat—lateralia. Gnathosoma: P-1 to P-5—palp segments from proximal to distal. The following additional abbreviations are used: alt–altitude; m–meters; asl–above sea level; H–height; L–length; W–width; I, II, III or IV–Leg-1 to 6–leg segments from proximal to distal; n–number of specimens measured. All measurements are given in μm.
Results
Systematics
Family Rhynchohydracaridae Lundblad, 1936
Genus Rhynchohydracarus Lundblad, 1936
Rhynchohydracarus armiger n. sp.
ZOOBANK: E8F2D15C-5CC9-4EAF-981F-63EA83531D46 ![]()
(Figures 2–4)
Type series
Holotype female (DCBSJRP-1741), dissected and slide-mounted in PVA, Brazil, Goiás, Pirenópolis, 18 Jul. 2019; 15.788083 S, 48.872194 E, 1123 m asl. Paratypes: one female (DCBSJRP-1742 and two males (DCBSJRP-1743–1744); collector: Luiz A. S. de Castro.
Description. Female



Body slightly flattened dorsoventrally; colour in life yellowish-brown.
Dorsal — Integument lineated, almost completely covered by three unpaired dorsocentralia (dc-1, dc-2+3, dc-4), one paired dorsocentralia (dc-5) and four pairs of dorsolateralia (dl-1–4); these sclerites ranging from densely to scarcely punctate (Fig. 2A). Two pairs of anterior platelets on the dorsum: prefr bearing dgl-1 and pr; postfr bearing two eye lenses and lgl-1; lgl-2, dgl-3 and dgl-4 each lying on individual platelets lateral to the large dc-2+3 and dc-4. Median eye absent. Camerostome margin wrinkled, medially separated, and bearing protuberances on the tips (Fig. 2A).
Lateral — Six pairs of lateralia (lat-1–6): lat-1 bearing lgl-3; lat-3 bearing lgl-4, lat-5 bearing vgl-3, and lat-6 bearing dgl-5; lat-2 and lat-4 without glandularia (Fig. 2A).
Ventral — Three coxal groups; coxae I and II fused medially, forming an anterior plate, bearing cxgl-1; coxae III and IV on each side forming two lateral plates, longer than wide; all coxae densely reticulate (Fig. 2B, 4C). Pregen long, slightly tapering on the anterior margin, with long and thin postero-lateral margins embracing the genital field (Fig. 2B, 4C). Genital flaps with several rounded acetabula and four pairs of setae along the inner margin of flaps (three pairs were lost during slide mounting); cxgl-2 located lateral to genital flaps, lying on trapezoidal plates, with concave anterior margins; postgen well-developed (Fig. 2B, 4C). Four pairs of ventralia (v-1–4), with v-4 bearing vgl-3; vgl-1 without gland openings, lying on small platelets, located between postgen and v-4; vgl-2 absent; excretory plate hexagonal, followed by an additional smaller hexagonal post-excretory platelet (Fig. 2B); legs without swimming setae; I–Leg-1-5 with slender setae; II-IV–Leg-4-5 with stocky setae.
Gnathosoma — Capitulum long and attached to a protrusible trunk-like tube of flexible integument (Fig. 2C); palp stout, number of setae on P-1–5: 0, 1, 1, 5, 2; P-5 with three claws (Figs. 2D-E); chelicera slender, claw stylet-like (Fig. 2C).
Measurements — Holotype (single female paratype in parentheses). Idiosoma, L 551 (556), from tips of dorsal face of camerostome to posterior margin of dc-4, W 250 (246); genital flaps (both sides) L 118 (120), W 130 (127); capitulum L 160 (156), H 40 (43); chelicera: basal segment L 140 (137), H 13 (12), claw L 29 (33); palp segments (P-1–5) L: 9 (8), 36 (40), 40 (42), 29 (34), 19 (17), H: 22 (20), 26 (29), 20 (22), 14 (16), 8 (7), L/H: 0.40 (0.40), 1.38 (1.37), 2.0 (1.9), 2.07 (2.12), 2.37 (2.42); leg segments L: I–Leg-1–6: 35 (37), 72 (73), 61 (56), 79 (70), 97 (84), 90 (80); II–Leg-1–6: 38 (36), 75 (67), 69 (65), 77 (71), 89 (87), 90 (85); III–Leg-1–6: 25 (26), 43 (45), 69 (57), 71 (65), 82 (74), 80 (73); IV–Leg-1–6: 58 (55), 62 (60), 60 (52), 122 (124), 94 (96), 81 (76).
Description. Male



n = 2 paratypes
Dorsal — Similar to female (Fig. 3A, 4A).
Ventral — Similar to female, except that the plates bearing cxgl-2 are fused with pregen and almost surround the genital field (Fig. 3B, 4B).
Gnathosoma — Capitulum (Fig. 3C), palps (Figs. 3D-E) and chelicera (Fig. 3C) similar to female.
Measurements — n=2. Idiosoma L 520, 550, from tips of dorsal face of camerostome to posterior margin of dc-4, W 240, 252; genital flaps (both sides) L 98, 102; W 112, 118; capitulum L 145, 162; rostrum L 39, 47; chelicera: basal segment L 126, 142; H 11, 12; claw L 29, 36; palp segments (P-1–5) L: 8, 10; 38, 39; 34, 39; 25, 30; 16, 17; leg segments L: I-Leg-1–6: 31, 36; 69, 72; 50, 52; 74, 76; 75, 85; 80, 90; II-Leg-1–6: 32, 35; 64, 68; 50, 52; 74, 76; 80, 87; 83, 93; III-Leg-1–6: 24, 28; 41, 47; 40, 42; 64, 71; 78, 86; 72, 75; IV-Leg-1–6: 49, 54; 56, 65; 48, 52; 122, 124; 88, 94; 77, 86.



Etymology
From the Latin armiger, meaning ''arms-bearer'' in reference to the idiosoma, fully armoured by plates
Differential diagnosis
Rhynchohydracarus armiger n. sp. differs from the three already described species in the following three diagnostic characters: 1) anterior margin of the prodorsum (i.e., roof of camerostome) wrinkled, medially separated, and bearing protuberances on the tips; 2) excretory plate hexagonal, accompanied by a similar and smaller post-excretory platelet; 3) in males, the plates that bear cxgl-2 are fused with pregen, almost surrounding the genital field. In other named species of Rhynchohydracarus, prodorsum is smooth, having a median split in R. dividuus shorter than that of R. armiger. Excretory plate is pentagonal in R. testudo and oval in both R. dividuus and R. carmenae. Pregen and postgen fused in males of R. dividuus and R. testudo, forming a unique plate surrounding genital flaps as well as cxgl-2. In R. carmenae these structures are separated, both in male and female.
DNA Barcoding
DNA was successfully amplified and mitochondrial cytochrome C oxidase subunit I gene (COI) sequenced from two of the four R. armiger specimens; sequence data have been deposited in BOLD Systems (https://www.boldsystems.org/ ![]() ) and GenBank (https://www.ncbi.nlm.nih.gov/
) and GenBank (https://www.ncbi.nlm.nih.gov/ ![]() ), with the following accession codes: holotype female, 679 base pairs (BOLD: ENBRA012-21, GenBank: MZ444663) and paratype male, 679 base pairs (BOLD: ENBRA011-21, GenBank: MZ444679).
), with the following accession codes: holotype female, 679 base pairs (BOLD: ENBRA012-21, GenBank: MZ444663) and paratype male, 679 base pairs (BOLD: ENBRA011-21, GenBank: MZ444679).
Remarks
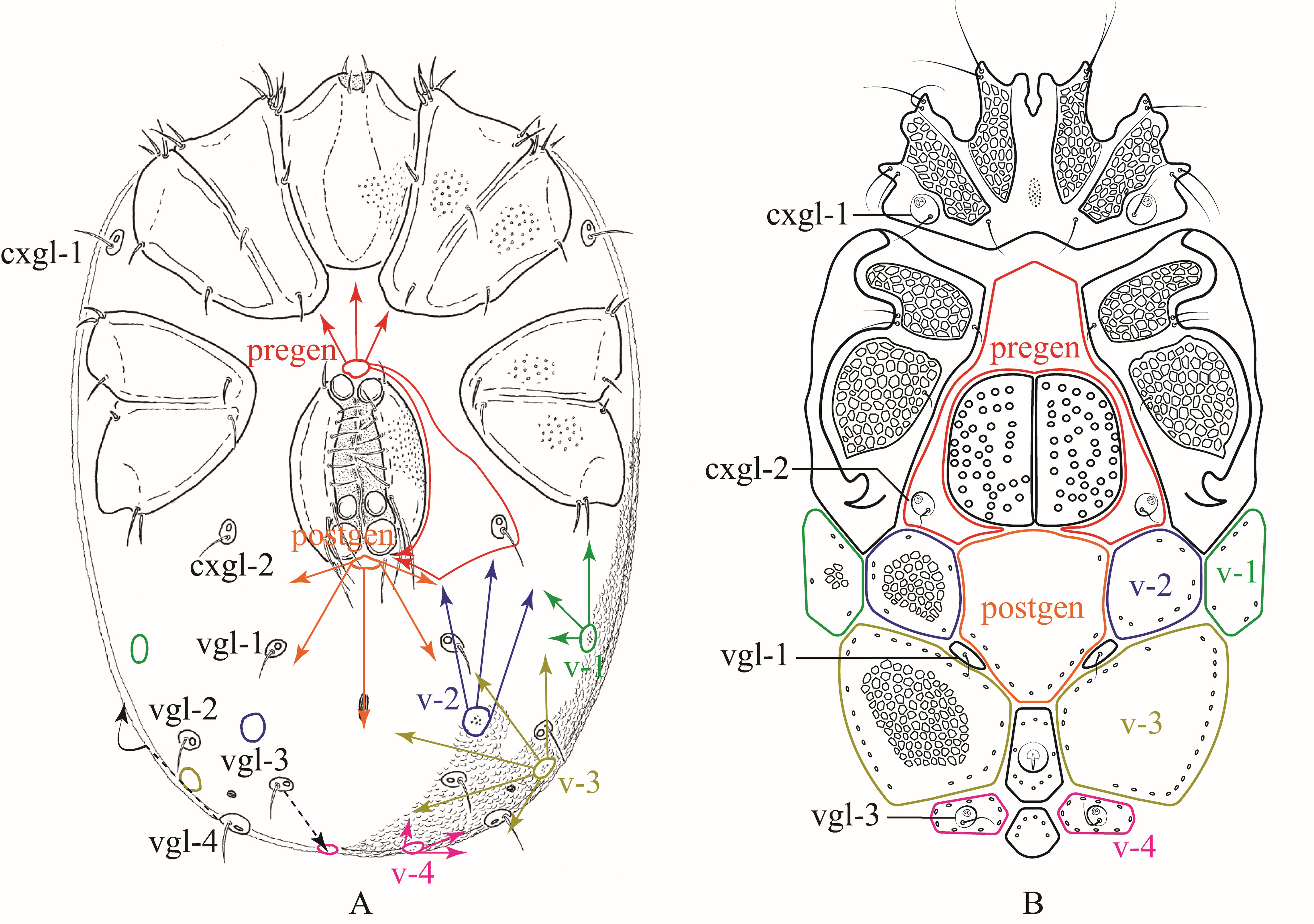
Based on the plesiotypical idiosoma organization of Hydrachnidiae discussed in Davids et al. (2006) (Fig. 5A, 6A), we propose the following homologies for dorsalia (Fig. 5B) and ventralia (Fig. 6B) in Rhynchohydracaridae, including their respective glandularia and the new term ''lateralia''. This proposal should help to facilitate and standardize future morphological studies and descriptions of new species in this family.





Dorsalia
In Rhynchohydracarus the dorsocentralia 1-4 may have fused and expanded in the following way: paired dc-1 fused and involved dgl-2, becoming a single anterior plate; paired dc-2 fused, expanded and involved postocularia (as in Clathrosperchon Lundblad, 1936 and Clathrosperchonella Lundblad, 1937, though in those genera they remained as a separated pair), and also fused with both paired dc-3 (in R. dividuus, R. carmenae, and R. armiger n. sp.) and paired dc-3+4 (in R. testudo) forming, in consequence, 1 or 2 central plate(s), respectively (if not mentioned otherwise, data taken from original descriptions). In Clathrosperchon and Clathrosperchonella, paired dc-3 also fused, becoming a single plate, right below paired dc-2. Therefore, the 13 smaller lateral plates mentioned by Lundblad (1941) for Rhynchohydracarus are: a single dc-1 + paired dl-1 + paired dl-2 + paired dl-3 + paired dl-4 + a pair of plates that bear lgl-2 + paired dc-5. Moreover, dgl-3 and dgl-4 lay on small plates that were not considered in Lundblad's account. Thus, these plates may have been originated by sclerotization of their respective glandularia (Fig. 5B). In Gledhillia Valdecasas, 2001 and Santiagocarus Valdecasas, 2001 (Valdecasas 2001) the fusion of dc-1–4 was complete, giving origin to a unique central plate, whilst paired dc-5 and the dl-1–4 remained separated. The plesiotypic prefr and postfr also may have fused with paired dc-1 and frontale (Fig. 5A), giving rise in all genera of Rhynchoydracaridae to a single dc-1 (Fig. 5B), also known as frontal plate, as discussed in Davids et al. (2006). Dgl-6 (Fig. 5A) are not present in rhynchohydracarids and, therefore, may have been lost in all genera of this family.
Lateralia
Laterally, there are 6 pairs of lateralia in Rhynchohydracarus: lat-1 bearing lgl-3, lat-2, lat-3 bearing lgl-4, lat-4, lat-5 bearing vgl-4 (lgl-5 sensu Lundblad 1941), and lat-6 bearing dgl-5 (Fig. 5B). In the description of R. testudo, Lundblad (1941) mentions that although lgl-2 are dorsal, they shifted slightly to the side and all other lateroglandularia are in fact on the side of the body, enclosed in plates. However, Lundblad neither counted those sclerites nor illustrated them. When mounting water mites on slides without dissection, i.e., with no separation of dorsal and ventral body surfaces of the mite, lateral plates remain better preserved in their natural orientation, tending to fold up and thus can be clearly seen laterally to dorsolateralia (Figs, 2A, 3A, 4A, 5B). None of these lateralia is present in Clathrosperchon and Clathrosperchonella (Lundblad 1941); however, two small plates can be noticed in Gledhillia and Santiagocarus (Valdecasas 2001), located right below posterior margins of paired dc-5 and bearing vgl-4 and dgl-5, similarly to Rhynchohydracarus. Thus, we can assume that these sclerites can also be homologized with lat-5 and -6.
Ventralia
Ventrally, in Rhynchohydracarus testudo and R. dividuuus, the pre- and postgenital plates also got greatly expanded and fused, surrounding completely cxgl-2 and the genital field. In contrast, these genital plates remained separated in R. carmenae and R. armiger n. sp. with cxgl-2 lying on individual plates in females of both species, and fused with the pregenital plate in males of R. armiger (Fig. 6B). In all other genera of Rhynchohydracaridae, these plates are not well-developed, except in Santiagocarinae, where they are fused with the ventral shield. Regarding the ventroglandularia, we noted that in all described species of Rhynchohydracaridae vgl-1 are always present, located medially right below postgen and deprived of glands (Fig. 6B), as discussed in Wiles (1997). The vgl-2 are also present in the family, located laterally and slightly above vgl-1, but without setae in Gledhillia and Santiagocarus, as it was depicted in Valdecasas (2001, Figs. 2 and 5), yet not discussed. However, in all species of Rhynchohydracarus vgl-2 are absent and may have been lost, being considered an autapomorphy of this genus. As illustrated in Davids et al. (2006) and according to Lundblad's nomenclature (1927), vgl-1 and vgl-3 are located medially, while vgl-2, and vgl-4 are located laterally. Therefore, we conclude that lgl-5 (sensu Lundblad 1941) are, in fact,'migratory' vgl-4 (Fig. 6A) in all genera of the Rhynchohydracaridae. Similar to vgl-1, vgl-3 are ventral and positioned posterior to the excretory pore and on the paired v-4 (Fig. 6B); however, Lundblad (1941) described the latter as just ''vgl'' for Clathrosperchon crassipalpis Lundblad, 1936 and only ''v'' for Rhynchohydracarus testudo, and Castro et al. (2020) described vgl-2 as being vgl-1 for Clathrosperchonella olovi Castro, Proctor & Lofego, 2020. Here we correct these mistakes. In Clathrosperchontinae and Rhynchohydracarinae, v-1–3 are found in pairs, with the position of v-1 varying from immediately posterior to coxa IV to almost lateral to coxa IV, as in Clathrosperchon crassipalpis, C. punctatus, and Rhynchohydracarus testudo. Paired v-4 got fused in Clathrosperchontinae whereas in Rhynchohydracarinae remained separate as small plates, bearing vgl-3. Conversely, in Santiagocarinae there was a complete fusion of ventralia, pregen and postgen, giving rise to a single heavily sclerotized ventral shield as described by Valdecasas (2001).
Key to Rhynchohydracarus species based on known adults
1 Excretory plate penta- or hexagonal
...... 2
— Excretory plate oval
...... 3
2 Excretory plate pentagonal; dc-2–4 fused, forming a single dorsal plate; prodorsum integrate and smooth
...... R. testudo Lundblad (only male known)
— Excretory plate hexagonal; dc-2–3 fused, separated from dc-4, forming two dorsal plates; prodorsum medially divided and wrinkled
...... R. armiger n. sp. male and female
3 Dorsal and ventral plates covering almost all the body surfaces but leaving narrow gaps of striated integument; pre and postgen plates fused to form a complete ventral plate, surrounding the genital field; dl-4 present
...... R. dividuus Lundblad (only male known)
— Dorsal and ventral plates covering almost all body surfaces but leaving wide gaps of striated integument; pre and postgen plates neither fused nor forming a ventral plate surrounding the genital field; dl-4 absent
...... R. carmenae Valdecasas (male and female)
Conclusions
The description of Rhynchohydracarus armiger n. sp. from central Brazil brings new data for the known distribution of the endemic Rhynchohydracarinae in the Neotropical region, establishing a connection between the single record of Central American R. carmenae and South American R. dividuus and R. testudo (Fig. 1). It also fills a knowledge gap in a Brazilian area hitherto considered without any information regarding water mite fauna (Goldschmidt 2002). This area covers, in part, an important Brazilian biome - the Cerrado - a central plateau where abundant pristine running-water habitats still await exploration in terms of invertebrate fauna. Prospective work focusing on sampling wider varieties of freshwater biotopes will certainly increase our knowledge of this family, including new information about larvae, nymphs, and host associations. Phylogenetic analysis based on both morphological and molecular characters will clarify relationships within the Rhynchohydracaridae and of this family within Hydryphantoidea.
Acknowledgements
LASDC thanks CAPES (Process 88887.342428/2019-00) for the financial support. Alberto S. Corrêa and Gilberto J. de Moraes, of Department of Entomology and Acarology, ESALQ-USP, kindly provided access to their laboratories and facilities. We thank Tom Goldschmidt and Vladimir Pešić for their careful revision and valuable comments for improving the quality of the MS.
References
- Bartsch I., Davids C., Deichsel R., Di Sabatino A., Gabrys G., Gerecke R., Gledhill T., Jäger P., Mąkol J., Smit H., van der Hammen H., Weigmann G., Wohltmann A., Wurst E. 2007. Chelicerata: Acari I. In: Gerecke, R. (Ed). Süßwasserfauna von Mitteleuropa 7/2-1. Heidelberg: Spektrum Akademischer Verlag. p. 1-388.
- Castro L.A.S.de, Proctor H.C., Lofego A.C. 2020. A new species of Clathrosperchonella Lundblad 1937 (Acariformes: Hydrachnidiae: Hydryphantoidea: Rhynchohydracaridae) from Brazil, with descriptions of the female, male and larva. Syst. Appl. Acarol., 25: 1745-1753. https://doi.org/10.11158/saa.25.10.3
- Cook D.R. 1974. Water mite genera and subgenera. Mem. Am. Entomol. Inst., 21: 1- 860.
- Davids C., Di Sabatino A., Gerecke R., Gledhill T., Smit H., van der Hammen H. 2006. Acari: Hydrachnidia. In: Gerecke R. (Ed). Süßwasserfauna Mitteleuropas, 7/2-1, Chelicerata, Acari I. München: Spektrum Elsevier. p. 241-376. https://doi.org/10.1007/978-3-662-55958-1_7
- Di Sabatino, A., Smit H., Gerecke R., Goldschmidt T., Matsumoto N., Cicolani B. 2008. Global diversity of water mites (Acari, Hydrachnidia; Arachnida) in freshwater. Hydrobiologia, 595: 303-315. https://doi.org/10.1007/s10750-007-9025-1
- Fernández H.R., Fossati-Gaschignard O. 2011. An initial classification of Neotropical water mites (Acari: Hydrachnidia) based on habitat preferences. Int. J. Ecol., 2011: Article ID 910540: 1-11.. https://doi.org/10.1155/2011/910540
- Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol., 3: 294-299.
- Gilbert M.T.P., Moore W., Melchior L., Worobey M. 2007. DNA extraction from dry museum beetles without conferring external morphological damage. PLoS One, 2: e272. https://doi.org/10.1371/journal.pone.0000272
- Goldschmidt T. 2002. The biodiversity of Neotropical water mites. In: Bernini F., Nannelli R., Nuzzaci G., de Lillo E. (Eds). Acarid phylogeny and evolution: adaptation in mites and ticks; Siena: EURAAC. p. 91-99. https://doi.org/10.1007/978-94-017-0611-7_10
- Goldschmidt T. 2004. Environmental parameters determining the water mite communities in Costa Rican freshwater habitats. Exp. Appl. Acarol., 34: 171-197. https://doi.org/10.1023/B:APPA.0000045250.06565.72
- Goldschmidt T. 2006. Diversity of Costa Rican freshwater mites (Arachnida: Acari: Hydrachnidia). Species Divers., 11: 157-175. https://doi.org/10.12782/specdiv.11.157
- Goldschmidt T. 2009. Water mites (Acari, Hydrachnidia) in tropical springs - diversity, specificity, monitoring possibilities. Int. Ver. Theor. Angew. Limnol. Verh., 30: 669-672. https://doi.org/10.1080/03680770.2009.11902212
- Goldschmidt T., Helson J.E., Williams D.D. 2016. Ecology of water mite assemblages in Panama - First data on water mites (Acari, Hydrachnidia) as bioindicators in the assessment of biological integrity of neotropical streams. Limnologica, 59: 63-77. https://doi.org/10.1016/j.limno.2016.03.007
- Goldschmidt T., Ramírez-Sánchez M.M. 2020. Introduction and keys to Neotropical water mites (Acari, Hydrachnidia). Spixiana, 43: 203-303.
- Hebert P.D.N., Cywinska A., Ball S.L., deWaard J.R. 2003. Biological identifications through DNA barcodes. Proc. R. Soc. London. Ser. B Biol. Sci., 270: 313-321. https://doi.org/10.1098/rspb.2002.2218
- Lundblad O. 1927. Die Hydracarinen Schwedens. I - Beitrag zur Systematik, Embryologie, Ökologie und Verbreitungsgeschichte der schwedischen Arten. Zool. Bidr. från Uppsala, 11: 181-540,
- Lundblad O. 1936. Neue Wassermilben aus Santa Catharina in Südbrasilien. Zool. Anz., 115: 29-51.
- Lundblad O. 1941. Die Hydracarinenfauna Südbrasiliens und Paraguays. Erster Teil. K. Sven. Vetenskapsakad. Handl., 19: 1-183.
- Proctor H.C., Smith I.M., Cook D.R., Smith B.P. 2015. Subphylum Chelicerata, Class Arachnida. In: Thorp J.H., Rogers D.C. (Eds). Thorp and Covich's Freshwater Invertebrates. Vol. 1. Ecology and general biology. Fourth Edition. London: Academic Press, Elsevier Incorporated. p. 599-660. https://doi.org/10.1016/B978-0-12-385026-3.00025-5
- Smit H. 2020. Water mites of the World, with keys to the families, subfamilies, genera and subgenera (Acari: Hydrachnidia). Monogr. Ned. Entomol. Ver., 12: 1-774.
- Smith I.M., Cook D.R. 2016. Parasitengonina: Hydrachnidiae and Stygothrombiae. In: Thorp J.H., Rogers D.C. (Eds). Thorp and Covich's Freshwater invertebrates. Vol. 2. Keys to Nearctic fauna. Fourth Edition. London: Academic Press, Elsevier Incorporated. p. 312-412.
- Valdecasas A.G. 2001. A new subfamily of Rhynchohydracaridae (Acari, Hydrachnellae) from the island of Coiba (Panama) with descriptions of new taxa. J. Nat. Hist., 35: 1565-1574. https://doi.org/10.1080/002229301317067674
- Walter D.E., Lindquist E.E., Smith I.M., Cook D.R., Krantz, G.W. 2009. Order Trombidiformes. In: Krantz G.W., Walter D.E. (Eds). A Manual of Acarology. Third Edition. Lubbock: Texas Tech University Press. p. 233-420.
- Wiles P.R. 1997. The homology of glands and glandularia in the water mites (Acari: Hydrachnidia). J. Nat. Hist., 31: 1237-1251. https://doi.org/10.1080/00222939700770671



2021-12-05
Date accepted:
2022-02-01
Date published:
2022-02-03
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Castro, Luiz A. S. de; Proctor, Heather C. and Lofego, Antonio C.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



