Mites of the genus Oulenziella Fan & Zhang (Acari: Winterschmidtiidae), with description of a new species from Kenya
Fan, Qing-Hai  1
and Faraji, Farid
1
and Faraji, Farid  2
2
1Plant Health and Environment Laboratory, Ministry for Primary Industries, Auckland, New Zealand.
2Eurofins MITOX BV, Science Park 408, 1098 XH Amsterdam, The Netherlands & Institute for Biodiversity and Ecosystem Dynamics, Section Population Biology, University of Amsterdam, The Netherlands.
2022 - Volume: 62 Issue: 1 pages: 148-160
https://doi.org/10.24349/i2bo-zeicZooBank LSID: 1FF85B7C-B11A-4E45-A6D7-AC2D921B3196
Original research
Keywords
Abstract
Introduction
The genus Oulenziella was established by Fan and Zhang in 2015 based on Calvolia bakeri Hughes collected from India (Fan et al. 2015). It remained monotypic until the recent publication of Oulenziella longiseta Barbosa and Moraes, collected from the states of Mato Grosso and Bahia, Brazil (Barbosa & Moraes 2021). Both species are arboreal in tropical and subtropical areas (Fan et al. 2020; Barbosa & Moraes 2021). Because it is arboreal O. bakeri was considered a potential alternative food for biological control agents such as species in the family Phytoseiidae. Oulenziella bakeri is fungivorous (Fan et al. 2015) and has been successfully cultured in the laboratory on yeast (Liu & Zhang 2016) and mass reared on substrate containing yeast or a combination of yeast, flour, wheat bran, vermiculite and sawdust for the commercial production of Neoseiulus californicus (McGregor) (Phytoseiidae) in China (Jiang 2014; Zhu et al. 2019) for control of the two spotted spider mite, Tetranychus urticae (Koch) (Tetranychidae).
The life cycle of Oulenziella bakeri consists of five stages: egg, larva, protonymph, tritonymph and adult (no heteromorphic deutonymph observed). Fan et al. (2020) studied all postembryonic stages of O. bakeri except the deutonymph and provided ontogenetic data useful in understanding structural homologies, making taxonomic decisions, and reconstructing phylogenies. The present study proposes a third species of Oulenziella collected from mango trees in Kenya, provides a record of O. bakeri from Kenya, redefines the genus, and provides a diagnostic key to species of Oulenziella as well as an updated key to genera of the subfamily Oulenziinae.
Material and methods
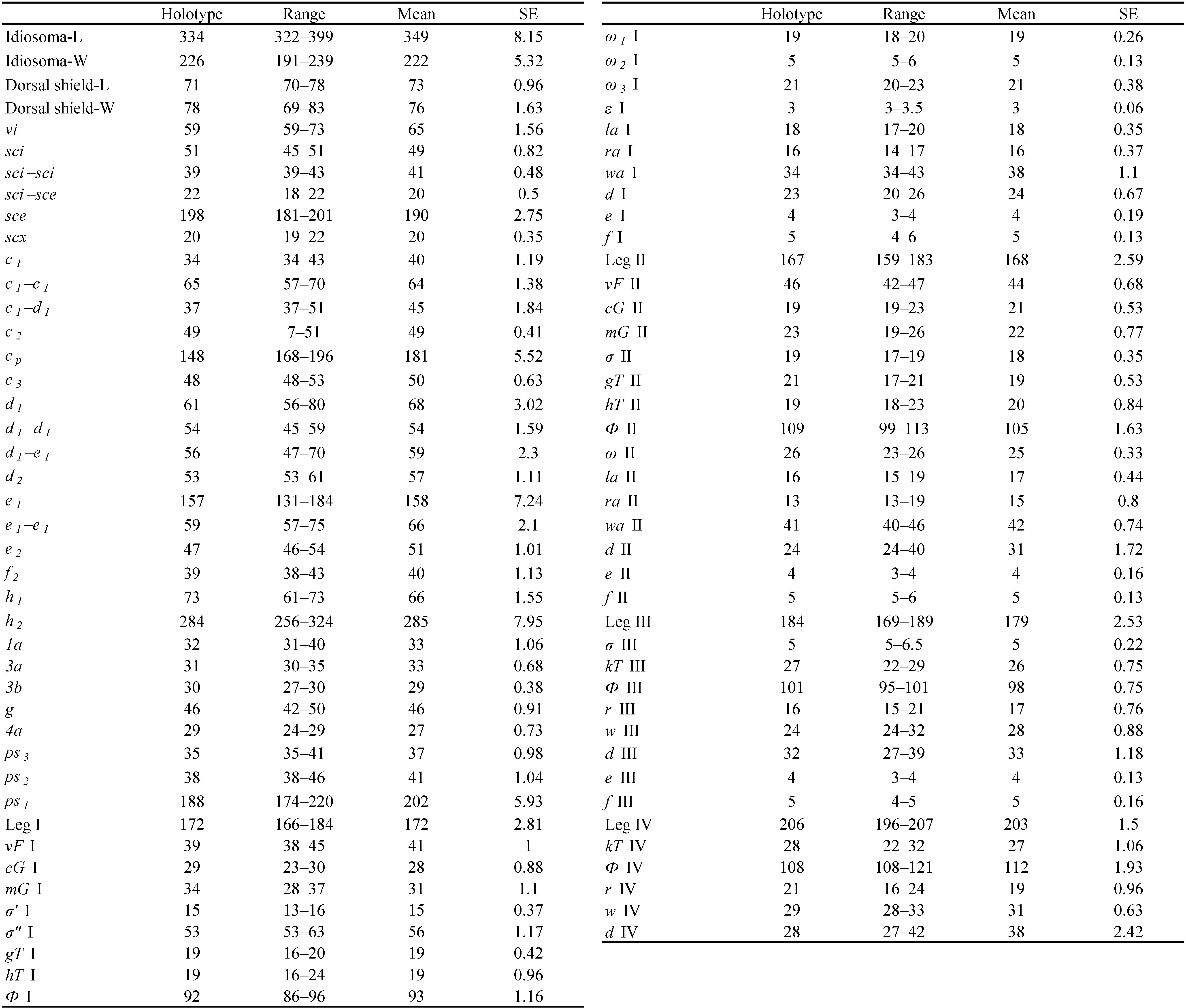
Specimens were slide-mounted using modified Hoyer's medium as described by Faraji and Bakker (2008) and examined using differential interference contrast microscopy. Illustrations were made using a drawing tube attached to an interference-phase contrast microscope (Nikon eclipse 80i). The tarsal ventro-terminal setae and other minute structures, e.g., spermatheca, famulus and solenidia, were re-examined and confirmed by means of a Zeiss Axio Imager 2 microscope. Images were taken using a Zeiss AxioCamHRc camera attached to the microscope, montaged with the Helicon Focus, and edited with Photoshop 2020. Idiosomal length was measured from the anterior rim to the posterior margin of the idiosoma and the width measured at the maximum width of the idiosoma between legs II and III. Setae were measured from the alveolus to the tip, and legs measured from the base of the trochanter to the tip of the claw. All measurements are given in micrometers (μm). Terminology of idiosomal chaetotaxy follows the hypothesis VI of Griffiths et al. (1990), that for palp and leg chaetotaxy follows Grandjean (1939) and Griffiths (1970). The holotype (circled) and three paratype females are deposited in the New Zealand Arthropod Collection, Landcare Research, Auckland, New Zealand (NZAC), and four paratype females are deposited in the Plant Health and Environment Laboratory, Auckland, New Zealand (PHEL). Acronyms of repositories follow Zhang (2018).
Systematics
Oulenziella africana Fan and Faraji, sp. n.
ZOOBANK: 0BF45932-8131-4395-8A69-CD50F70EB6A9 ![]()
(Figures 1–7, Table 1)
Material examined
Holotype female and 3 paratype females on the same slide, mango tree, 5 km west of Thika, Kenya, 23 Nov. 2016, by H. Wainwright; 4 paratype females one a slide, same collection data as holotype.
Diagnosis
ADULT FEMALE. Idiosomal setae sce about 2.8–3.4× as long as vi and 3.6–4.2× as long as sci; sci–sci 1.8–2.3× as wide as sci–sce; h2 1.3–1.9× as long as cp and 4.7–6.9× as long as h1 ; cp 4.2–5.4× as long as c1 ; e1 2.6–3.4× as long as e2 . Opisthonotal gland opening gla located much closer to e2 than to d2 . Leg I with genual solenidia σ″ 3.5–4.2× as long as σ′.
Male, larva and nymphs: Unknown.
Description
Adult female
(Figures 1–6)









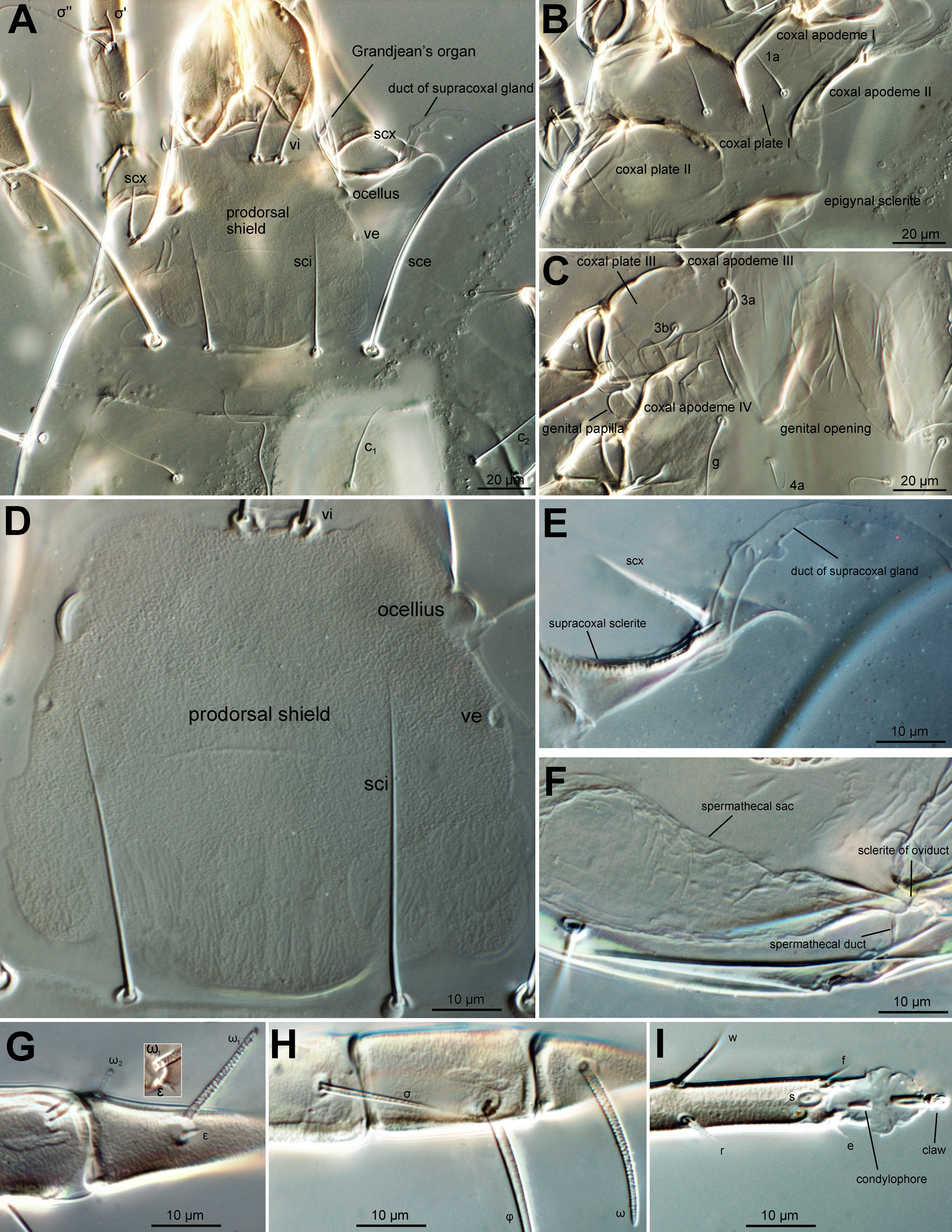
Chelicerae robustly chelate (Figure 3C): fixed digit bearing 1 subterminal tooth, 3 large medial teeth and 1 small proximal tooth; movable digit bearing one small subterminal tooth, 3 large medial teeth, and 1 small proximal tooth; cheliceral setae cha small, conical, chb absent. Subcapitulum: setae m filiform (Figure 3D); palpal supracoxal setae elcp absent; dorsal and lateral palptibial setae filiform; dorsal palptarsal setae filiform, terminal palptarsal solenidion ω indistinct, present as rudimentary lines (Figure 3D).
Idiosoma (Figures 1 and 6A) oval, cuticle smooth. Prodorsal shield (Figures 1, 3A and 6D) nearly trapezoidal, finely stippled, posterior one forth areas with faint longitudinal to oblique wrinkles (Figure 6D), posterior margin medially convex and sublaterally concave. Supracoxal sclerite (Figures 3B and 6E) elongate; Grandjean's organ (Figures 1 and 3B) horn-shaped, smooth and short; supracoxal setae scx tapering from base to tip, medially barbed. Ocelli (Figures 1, 3A and 6D) situated close to anterior corners of prodorsal shield; seta vi slightly longer than 4/5 of length of prodorsal shield; setae ve represented by alveoli, situated at lateral margins of prodorsal shield, posterior to ocelli; sce about 2.8–3.4× as long as vi and 3.6–4.2× as long as sci; sci–sci 1.8–2.3× as wide as sci–sce. Opisthonotal gland openings gla located closer to e2 than to d2 , not distinctively coloured. Tiny opisthosomal tubercles visible on some specimens. Hysterosomal setae h3 absent; h2 longest, sce, cp and e1 obviously longer than others; c1 , c2 , c3 , d1 , d2 , e2 and h1 similar in length, f2 positioned on ventral side of idiosoma and shorter than others; e1 2.6–3.4× as long as e2 , h2 1.3–1.9× as long as cp and 4.7–6.9× as long as h1 ; cp 4.2–5.4× as long as c1 .
Coxal apodemes I (Figures 2 and 6B) joined at midline, forming a prosternal apodeme directed posteromedially; coxal plates I posteriorly extending beyond apex of prosternal apodeme, slightly concave posteromedially; coxal apodemes II (Figures 2 and 6B) directed posteromedially, coxal plates II large, extending far beyond apex of apodeme II, posterior margin concave posteromedially; sejugal apodeme faint, a simple ridge. Genital opening (Figures 2 and 6C) inverted V-shaped, situated medially between coxae III and IV, epigynal sclerite (Figure 2) situated immediately anterior to genital opening, umbrella-shaped in middle, extending laterally beyond inner tips of apodemes III; apodemes III directed medially; apodemes IV directed anteromedially and jointed with post-apodemes of coxae III. Ventral setae 1a inserted lateral to prosternal apodeme, 3a lateral to genital opening, g posterior to second pair of genital papillae, 4a posterior to lateral arms of genital opening. Anal opening far posterior to genital opening, slightly shorter than genital opening, surrounded by 3 pairs of pseudanal setae, ps1 longest, about 4.6–5.5× as long as ps2 and 4.8–6.1 ps3 . Copulatory opening close to posterior margin of idiosoma (Figures 3E and 6F); spermathecal duct (inseminatory canal) a thin cylindrical tube, slightly widening as it reaches spermathecal sac (seminal receptacle); sclerotised base of spermathecal sac bell-shaped, a pair of bell-shaped sclerites of oviducts situated at end of spermathecal sac, sclerites nearly as large as spermathecal base.
Legs. All setae on trochanters, femora, genua and tibiae smooth and attenuate.



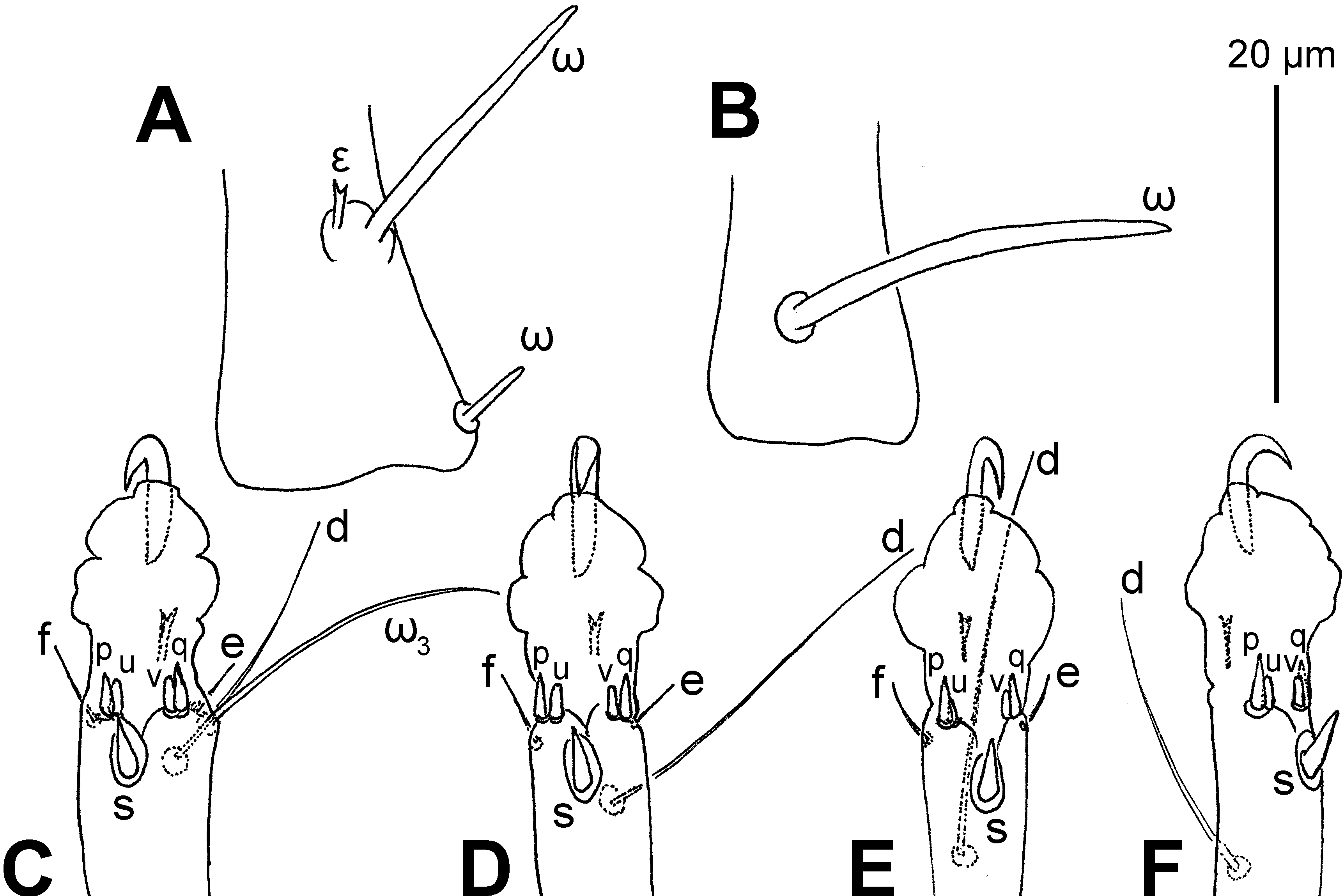




Leg I (Figures 4A, 5A, C and 6G). Trochanter bearing 7–9 minute teeth on anteromedial edge; femoral seta vF nearly extending to tip of genu; genual solenidia σ″: σ′= 3.5–4.2, setae cG as long as mG; tibial solenidion φ obviously extending beyond tarsal claw tip, gT and hT subequal; tarsus about 3.7–4.1× as long as its basal width, ω1 parallel sided and tapered at its apex, ε apically bifurcate and slightly shorter than ω2 , ω3 slightly shorter than d, seta wa 1.9–2.4× as long as la and 2.1–2.6× as long as ra, d nearly extending to tarsal claw tip; e and f very small and e slightly longer than f; ventro-terminal spines s (4.5–5.0) conical; p and q subequal (3.0–3.5) and conical; u and v subequal (2.0) and apically truncate.
Leg II (Figures 4B, 5B, D and 6H). Trochanter bearing 7–9 minute teeth on anteromedial edge; femoral vF nearly extending to base of genual solenidion (σ), σ nearly extending to half-length of tibia, seta cG as long as or slightly shorter than mG; tibial solenidion φ obviously extending beyond tarsal claw tip, gT about as long as hT; tarsus about 4.1–4.6× as long as its basal width, ω tapering towards its apex, seta wa more than 2.3–3.2× as long as la and ra, d nearly extending to tarsal claw tip; e and f very small; ventro-terminal spines s (4.0–5.0) conical; p and q subequal (3.0–3.5) and conical; u and v subequal (2.0) and apically truncate.
Leg III (Figures 4C, 5E and 6I). Femur nude; genual solenidion σ very small, not extending to apical rim of genu; tibial solenidion φ slightly extending beyond tarsal claw tip, kT about as long as w on tarsus; tarsus about 6.1–6.9× as long as its basal width; r and w situated at same level, d nearly extending to tarsal claw tip; e and f very small but e slightly longer; ventro-terminal spines s (4.0–5.0) conical; p (3.5–4.5) longer than q (3.0–3.5), both conical; u (2.5–3.5) longer than v (2.0–3.0), both apically truncate.
Leg IV (Figures 4D and 5F). Trochanter, femur and genu nude; tibial solenidion φ about extending to tarsal claw tip, kT about as long as w on tarsus; tarsus more than 5.9–6.8× as long as its basal width; r situated proximal to w, d extending to base of tarsal claw; e and f absent; ventro-terminal spines s (4.0–5.0) conical; p (3.5–4.0) longer than q (2.5–3.0), both conical; u and v subequal (2.0–2.5) and both apically truncate.


Male, larva and nymphs
Unknown.
Egg
(Figure 7)
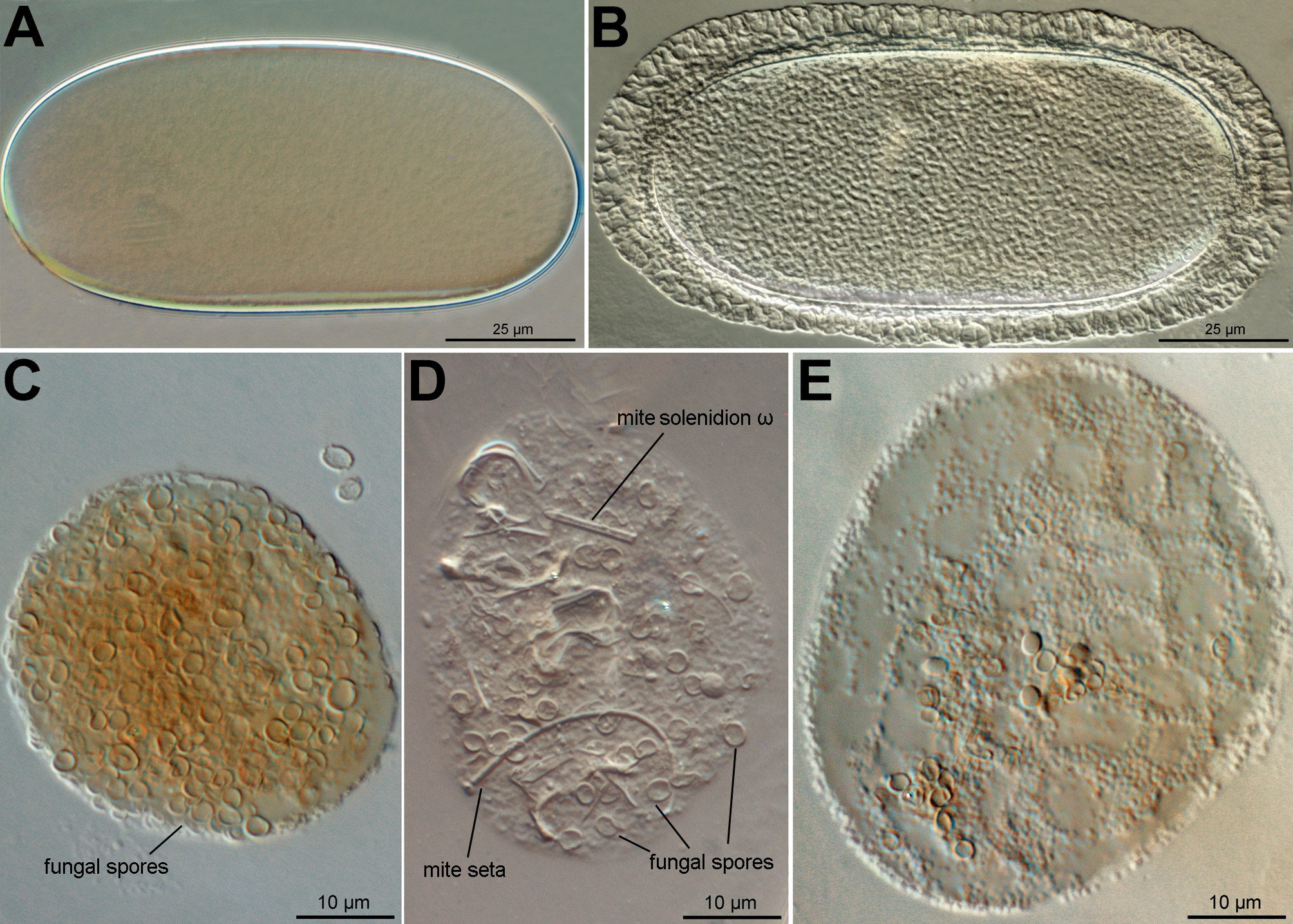


(n=5). Oblong in shape, nearly twice as long (105–116) as wide (53–61); shell of newly formed egg (Figure 7A) smooth; shell of fully developed egg (Figures 7B) ornamented with scattered punctations and surmounted longitudinally by a reticulated band forming an envelope which is wider at the ends (12–15) and narrower in middle parts (8–10). Five mites each was found inside an egg.
Etymology
The species name africana refers to the origin of this species in Africa.
Biology
Digested spores of Penicillium sp. (Eurotiales: Trichocomaceae) and fragmented mite body parts were found in the food boli (Figures 7C–E), suggesting that O. africana sp. n. is likely to be detritivores. The mite solenidion and setae in the food boli (Figure 7D), are possibly from the shed exoskeletons of members of the mite population.
Remarks
This species can be readily distinguished from the known species by the following key.
Key to adult females of Oulenziella
1. Genus I with solenidion σ″ 3–4× as long as σ′; idiosomal seta h2 4–7× as long as h1
...... 2
— Genus I with σ″ 6–10× as long as σ′; h2 12–15× as long as h1
...... O. longiseta Barbosa and Moraes
2. Idiosomal seta e1 about as long as or slightly longer than e2
...... O. bakeri (Hughes)
— Idiosomal seta e1 2.6–3.4× as long as e2
...... O. africana Fan and Faraji, sp. n.
Oulenziella bakeri (Hughes, 1962)
(Table 2)
New record for Kenya.



Material examined
One adult female, ex mango tree, five km west of Thika, Kenya, 23 Nov. 2016, by H. Wainwright.
Oulenziella bakeri is characterized by having idiosomal setae sce 3.5–4.4× as long as sci, cp 3.1–4.5× as long as c1 , e1 as long as or slightly longer than d1 , e1 0.9–1.5× as long as e2 , h2 4.7–6.9× as long as h1 , and genu I with solenidion σ″ 3.2–4.0× as long as σ′ in adult females. The Kenyan specimen is consistent with all these key characters of females of O. bakeri described by Fan et al. (2015; 2020). Measurements on the adult female collected from Kenya are presented in Table 2.
Redefinition of Oulenziella Fan and Zhang, 2015
Due to the recent research (Barbosa & Moraes 2021; Fan et al. 2021; this work), a redefinition of the genus Oulenziella is presented below.
FEMALE. Ocelli strongly bulging, positioned on lateral margins of prodorsal shield in a position slightly posterior to vi; setae sce from nearly 3× to more than 8× as long as sci; supracoxal setae scx slender and scarcely barbed; hysterosomal setae e1 1.0–10.0× as long as e2 ; h2 from nearly 4.0× to more than 12× as long as h1 ; each tarsus (excluding pretarsus) more than 4× as long as its basal width. Genu I with solenidion σ″ 3.0–9.5× as long as σ′; σ on genu III small, usually not extending beyond anterior rim of segment; seta d on tarsi III and IV positioned at level of apical 1/8 to 1/6 of segment. Chaetotaxy and solenidiotaxy of legs I–IV: trochanter 1, 1, 1, 0; femora 1, 1, 0, 0; genua 2 + 2σ, 2 + 1σ, 1σ, 0; tibiae 2 + 1φ, 2 + 1φ, 1 + 1φ, 1 + 1φ; tarsi I with 4 long setae (wa, ra, la and d), 2 small apical setae (f and e) + 1 subterminal ventral spine (s) + 4 terminal ventral spines (u, p, v and q) + ω1 + ω2 + ω3 + 1ε; tarsus II similar to tarsus I but without ω2, ω3 and ε; tarsus III with 3 long seta (w, r and d), 2 small apical setae (f and e) + 1 subterminal ventral spine (s) + 4 terminal ventral spines (u, p, v and q); tarsus IV with 3 long seta (w, r and d) + 1 subterminal ventral spine (s) + 2 terminal ventral spines (u, p, v and q).
MALE. Similar to female but genital opening with an aedeagus situated between coxae IV; sclerite anterior to genital opening contiguous with medial part of apodemes IV; ventral setae 3a absent; genital setae tiny, about as long as basal alveolus; tarsi I and II (excluding pretarsi) less than 3× as long as their basal width, their apicoventral portion modified into suckers; subterminal ventral spine (s) of tarsi I–II indiscernible; terminal ventral spines p and q of tarsi I and II blunt.
Oulenziinae currently comprises five genera. A key to genera of the subfamily (adult females) is presented as follows (Modified from Fan et al. 2015).
Key to genera of the subfamily Oulenziinae (adult females)
1. Prodorsal shield with a pair of ocelli on its anterolateral margins
...... 2
— Prodorsal shield without ocelli
...... 4
2. Tibiae I and II each with one seta (gT); tibia IV without ventral seta; tarsus II without setae la or ra, tarsus III without seta w
...... Oulenzia Radford, 1950
— Tibiae I and II each with 2 setae (gT and hT); tibia IV with a ventral seta (kT); tarsus II with setae la and ra, tarsus III with seta w
...... 3
3. Genu I with σ″ more than 3× as long as σ′; ocellus with lateral margin strongly bulging
...... Oulenziella Fan and Zhang, 2015
— Genu I with σ″: σ′=1 to 2; ocellus with lateral margin slightly recurved
...... Procalvolia Fain, 1971
4. Spermathecal duct long, forming 5–6 loops
...... Psylloglyphus Fain, 1966
— Spermathecal duct short, not forming loops
...... Acalvolia Fain, 1971
Discussion
There are relatively few morphological characters for identifying species of Oulenziella based on current knowledge. Useful characters include the comparative lengths of dorsal idiosomal setae (i.e., e1 : e2 , h2 : h1 ) and the comparative length of solenidia σ″ and σ′ of genu I. The male genitalia of O. bakeri and O. longiseta don′t exhibit significant variability and is not considered a key character here. The structure of the female genitalia such as the base of spermatheca is potentially a useful character to separate O. longiseta from the other two species.
It is worth noting that the famulus (ε) on tarsus I of O. africana sp. n. is apically cleft (Figures 5A and 6G) though it is not an obvious feature. We re-examined ten females and ten males of O. bakeri described by Fan et al. (2020) and found that ε is cleft but can be inapparent when it is not in the correct visual position. We also examined six females and two males of Acalvolia americana Fan, George and Kumarasinghe (Fan et al. 2010), an undescribed female of Oulenzia from Malaysia (Fan et al. 2012), and four adult females of Czenspinskia transversostriata (Oudemans) collected in Turkey. Solenidion ε is generally conical with an obscure subapical barb on these specimens. Further observation on species of other genera needs to be done to determine whether this is a generic character for Oulenziella.
Barbosa and Moraes (2020) incorrectly listed ′Oulenzia arboricola: Baker & Warton, 1952, 342; Meyer & Rodriguez 1966: 24; Fan et al. 2012: 334.′ under the species Oulenzia arboricola (Oudemans, 1928)′ which should be removed from page 1042 of Barbosa and Moraes (2020). However, the authors did correctly treat Oulenzia arboricola sensu Baker & Warton, 1952 as Oulenziella bakeri (Hughes, 1962) on page 1043 (Barbosa & Moraes 2020). We confirm that, as in O. bakeri, the apicoventral portion of tarsi I and II of male O. longiseta is modified into a tarsal sucker and seta s is indiscernible based on images kindly provided by Barbosa and Moraes on 7 July 2021.
It is possible that mites of Oulenziella don′t have a heteromorphic deutonymphal stage as discussed in Fan et al. (2020) who studied more than one thousand specimens of all life stages of O. bakeri in the collection of the Plant Health and Environment Laboratory, New Zealand.
Acknowledgements
We would like to thank Dr Henry Wainwright (Real IPM Company Ltd. Madaraka, Thika, Kenya) for providing us the mite material described in this study, Drs Gilberto J. de Moraes and Marina F. C. Barbosa (Universidade de São Paulo, Brazil) for re-examining type specimens of Oulenziella longiseta, and Dr W. Ho (Plant Health & Environment Laboratory, Ministry for Primary Industries) for identifying the fungus in the mite guts. The senior author is indebted to Drs Sherly George and Lalith Kumarasinghe and colleagues in Ministry for Primary Industries, New Zealand for encouraging the senior author's research.
References
- Barbosa M.F.C., Moraes G.J. de. 2021. Mites of the family Winterschmidtiidae (Acari: Sarcoptiformes: Astigmatina) from agricultural habitats in Brazil, with description of a new species and a key to species reported. Syst. Appl. Acarol., 26(6): 1040−1054. https://doi.org/10.11158/saa.26.6.3
- Fan Q.-H., George S., Kumarasinghe L. 2010. Genus Acalvolia (Acari: Winterschmidtiidae), with the description of a new species from the USA. Zootaxa, 2719: 41−61. https://doi.org/10.11646/zootaxa.2719.1.4
- Fan Q.-H., George S., Kumarasinghe L. 2012. Redescription of Oulenzia arboricola (Oudemans, 1928), type species of Oulenzia Radford, 1950 (Acari: Astigmata: Winterschmidtiidae). Syst. Appl. Acarol., 17(3): 333–338. https://doi.org/10.11158/saa.17.3.11
- Fan Q.-H., George S., Li D., Zhang Z.-Q. 2015. Establishment of Oulenziella gen. nov. for Oulenzia bakeri (Hughes, 1962) (Acari: Winterschmidtiidae). Zootaxa, 3949(2): 191-202. https://doi.org/10.11646/zootaxa.3949.2.2
- Fan Q.-H., Li D., George S. 2020. Ontogenetic stages of Oulenziella bakeri (Hughes) (Acari: Winterschmidtiidae). Zootaxa, 4900(1): 62-101. https://doi.org/10.11646/zootaxa.4900.1.7
- Faraji F., Bakker F. 2008. A modified method for clearing, staining and mounting plant-inhabiting mites. Eur. J. Entomol., 105: 793-795. https://doi.org/10.14411/eje.2008.105
- Grandjean F. 1939. La chaetotaxie des pattes chez les Acaridae. Bull. Soc. Zool. Fr., 64: 50-60.
- Griffiths D.A. 1970. A further systematic study of the genus Acarus L., 1758 (Acaridae, Acarina), with a key to species. Bull. Nat. Hist. Mus., Zool., 19 (2): 85-118.
- Griffiths D.A., Atyeo W.T., Norton R.A., Lynch C.A. 1990. The idiosomal chaetotaxy of astigmatid mites. J. Zool., 220: 1-32. https://doi.org/10.1111/j.1469-7998.1990.tb04291.x
- Hughes A.M. 1962. The genus Calvolia Oudemans, 1911 (Acari: Sarcoptiformes). Acarologia, 4 (1): 48-63.
- Jiang H.-B. 2014. Cultivation method and application of Oulenzia bakeri (Hughes). China patent, CN201410143238.X. filed (11 April 2014), and issued (25 June 2014).
- Liu J.-F., Zhang Z.-Q. 2016. Effects of short-term exposure to low temperature on survival, development and reproduction of banana-associated Oulenziella bakeri (Acari: Winterschmidtiidae). Syst. Appl. Acarol., 21(8): 1078-1086. https://doi.org/10.11158/saa.21.8.8
- Zhang Z.-Q. 2018. Repositories for mite and tick specimens: acronyms and their nomenclature. Syst. Appl. Acarol., 23 (12): 2432–2446. https://doi.org/10.11158/saa.23.12.12
- Zhu R., Guo J.-J., Yi T.-C., Xiao R., Jin D.-C. 2019. Functional and numerical responses of Neoseiulus californicus (McGregor) to eggs and nymphs of Oulenziella bakeri and Tetranychus urticae. Syst. Appl. Acarol., 24(7): 1225-1235. https://doi.org/10.11158/saa.24.7.7



2021-07-12
Date accepted:
2022-01-28
Date published:
2022-02-02
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Fan, Qing-Hai and Faraji, Farid
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



