Supplementary descriptions of seven eriophyoid mite species (Acari: Eriophyoidea) recovered from the Viennese Nalepa collection and comparison with Japanese species
Kadono, Fujio  1
; Takei, Madoka
1
; Takei, Madoka  2
; Gotoh, Tetsuo
2
; Gotoh, Tetsuo  3
; Kubota, Kenji
3
; Kubota, Kenji  4
; Hörweg, Christoph
4
; Hörweg, Christoph  5
and Kagiwada, Satoshi
5
and Kagiwada, Satoshi  6
6
1✉ Faculty of Bioscience and Applied Chemistry, Hosei University, Koganei, Tokyo 184-8584, Japan.
2Faculty of Bioscience and Applied Chemistry, Hosei University, Koganei, Tokyo 184-8584, Japan.
3Faculty of Economics, Ryutsu Keizai University, Ryugasaki, Ibaraki 301-8555, Japan.
4Central Region Agricultural Research Center, National Agriculture and Food Research Organization, Kannondai, Tsukuba, Ibaraki 305-8666, Japan.
5Natural History Museum Vienna, 3, zoology, Burgring 7, 1010 Vienna, Austria.
6Faculty of Bioscience and Applied Chemistry, Hosei University, Koganei, Tokyo 184-8584, Japan.
2022 - Volume: 62 Issue: 2 pages: 273-301
https://doi.org/10.24349/z3ie-bf78ZooBank LSID: FFB27F8A-79D9-4870-A4E3-340EA5A7C00F
Original research
Keywords
Abstract
Introduction
In the taxonomic survey of Japanese eriophyoid mites, we found that a species of the genus Aculops transmits the perilla mosaic virus (PerMV) and causes rusting damage to leaves of'Shiso', Perilla frutescens (L.) Britton var. crispa (Benth.) W. Deane (Lamiaceae) (Takei et al., 2019; Kubota et al., 2020). A second species, identified as belonging to the genus Eriophyes, causes edge-rolling damage to leaves of Spiraea thunbergia Siebold ex Blume (Rosaceae). A third species, belonging to the subfamily Rhyncaphytoptinae, was found on leaves of walnut, Juglans mandshurica Maximowicz (Juglandaceae).
The three species are morphologically close to three species collected by Alfred Nalepa over 100 years ago: the Aculops species is morphologically close to Aculops thymi (Nalepa, 1889) collected from Thymus serpyllum L. (Lamiaceae); the Eriophyes species is morphologically close to E. spiraeae (Nalepa, 1893) collected from Spiraea crenifolia (L.) Boiss. (= Spiraea crenata L.) (Rosaceae); and the Rhyncaphytoptinae species is morphologically close to Rhyncaphytoptus gallicolus (Nalepa, 1922) (syn. Phyllocoptyches gallicolus Nalepa, 1922), deutogyne of R. longirostris (Nalepa, 1922) collected from Ulmus campestris L. (Ulmaceae). However. it was difficult to determine if the Japanese specimens are conspecific to the closely related Nalepa species. This is because Nalepa's taxonomic descriptions are not enough detailed to allow species identification.
Recently, Chetverikov et al. (2016) developed a method for recovering mummified materials of eriophyoid mites collected by Nalepa over 100 years ago, which are kept in the Natural History Museum of Vienna (in German: Naturhistorisches Museum Wien, NHMW), Austria, and they successfully made preparation slides capable of species identification. Using this protocol, Marinković et al. (2018) recovered mummified eriophyid mite specimens collected by Nalepa and reported the detailed taxonomic traits for three species of the subfamily Cecidophyinae. Therefore, two of us (F. K. and T. G.) visited the museum and got permission to take a part of the Nalepa mite collection to Japan. According to the protocol of Chetverikov et al. (2016), we recovered eriophyoid mites from the sediment in the vials and made slide preparations.
Here, we provide supplementary descriptions of the topotype specimens of seven eriophyoid species recovered from mummified materials in the Nalepa mite collection. A new combination is proposed for one of them. The taxonomic relationship of Nalepa's species with the Japanese specimens was discussed.
Material and methods
Recovering mites from Nalepa's collection vials and slide preparation



All specimens used in this study were taken from five vials (nos. 340, 341, 117, 355 and 477) of the Nalepa mite collection (Fig. 1) loaned from the NHMW, Austria. The loan was allowed by Dr. Helmut Sattman, director of the Department of Invertebrate Zoology, NHMW, and Dr. Christoph Hörweg (co-author), head of the Department of Invertebrate Zoology and curator of the Collection Arachnoidea. The specimens were examined at the Laboratory of Applied Entomology and Zoology, Hosei University, Tokyo, Japan.
Over the years, the liquid preservative in the vials had evaporated. The preservative was not recorded, but other records indicate that Dr. Nalepa used either a 1:100:2 mixture of picric acid, distilled water and concentrated hydrochloric acid, or a 100:2 mixture of 94% ethanol and concentrated hydrochloric acid (Nalepa, 1906). Following the protocol of Chetverikov et al. (2016), the sediment in each vial was suspended in 5 ml of 70% ethanol, three drops of 5% acetic acid and 10 drops of pure diethyl ether. The vials were then heated for ca. 4 hours at ≥ 75 °C. As a result, within 24 hours, the sediment in the vials was sufficiently dissolved that the mites could be removed. Aliquots of the solutions (300-500 µl) were transferred to a small watch glass. At this stage, the mites were still pigmented, which made it difficult to observe their features under a phase contrast microscope (BX51®, Olympus Co., Tokyo, Japan). The mites were thus treated with lactic acid (a clearing agent) as follows. Mites in the watch glass were picked up with a pig eyelash attached to an insect pin and transferred to a hole slide glass containing about 10 µl of concentrated (\textgreater85%) lactic acid solution (manufacturer) under a dissecting microscope. The hole slide glass was then heated for 4-6 h at 95 °C. This clearly revealed the features of the mites under the phase contrast microscope. The mites were then individually transferred with a pig eyelash to a slide glass containing a drop of modified Berlese medium (Amrine and Manson, 1996) under a dissecting microscope. We were able to make 2-18 high quality, permanent slides for each vial (one intact mite per slide). The remaining solution in the vials was allowed to air dry. The vials and slides will be returned to the museum after publication of the manuscript.
Morphological studies
Mites were examined for their different taxonomic characters using a phase contrast microscope (BX51®) and drawn by a camera lucida (U-DA®, Olympus) attached to the microscope. Measurements and illustrations basically follow Amrine and Manson (1996) and de Lillo et al. (2010), and measurements are given in micrometers (µm) and given as average and ranges in parentheses. Count of dorsal and ventral annuli (including incomplete annuli) was carried out from the prodorsal shield to the anal lobe. Some of the setae have been broken during the preparation process, which were omitted from the setal length values. The morphological nomenclature follows Lindquist and Amrine (1996) and systematic classification follows Amrine et al. (2003). Photos of each character were taken with the phase contrast microscope equipped with a digital microscope camera (DP21®, Olympus). The photos were uploaded to Microsoft PowerPoint 2019 (Microsoft Co., Redmond, WA, USA) and schematic mite drawings were obtained by tracing each character with a drawing tool in PowerPoint.
Results
Two eriophyoid mites collected from Thymus serphyllum (vial nos. 340 and 341)
Aceria thomasi (Nalepa, 1889)
Phytoptus thomasi Nalepa,1889 pp. 135-137 t. 6 f. 1-3.
Phytoptus thomasi: Canestrini,1892 pp. 618-619 t. 49 f. 1; t. 44 f. 8; Cotte,1924 pp. 14-17 pl. 4, f. 19.
Eriophyes thomasi: Liro & Roivainen, 1951 pp. 138-139.
Aceria thomasi: Farkas, 1965 p. 47 fig. 34D; Boczek & Chyczewski, 1978 p. 112; Hellrigl, 2003 p. 100; Amrine & Stasny, 1994 p. 91; Szydło et al., 2010 pp. 144-148 figs. 4-6, 12B, Table 1.
(Figs. 2, 3, Table 1)
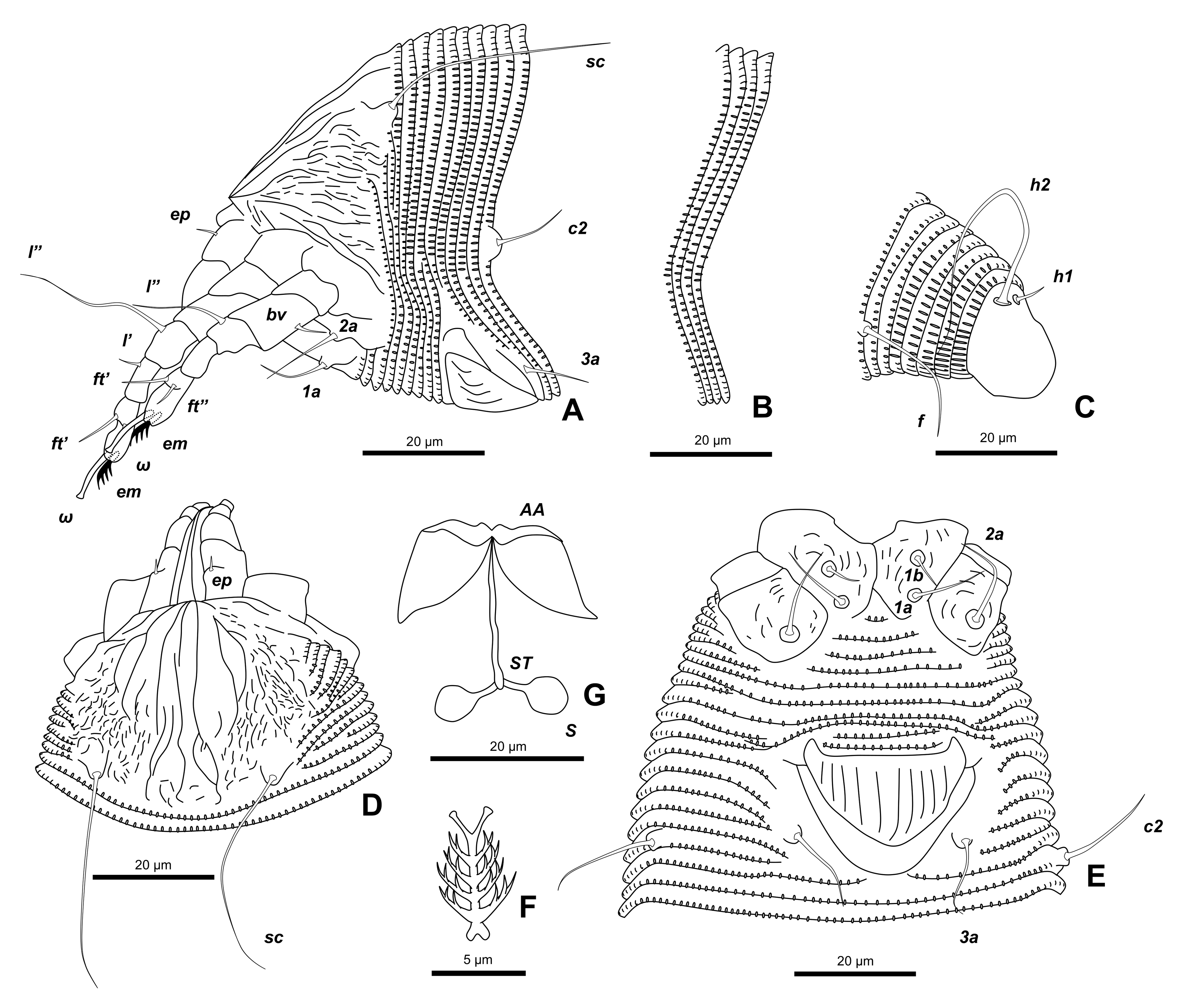





Female (n=10) — Body fusiform, 249 (181-292), 68 (62-74) wide, 65 (61-74) thick.
Gnathosoma 23 (19-26), projecting obliquely downwards; chelicerae 20 (16-27), bent down.
Prodorsal shield semicircular in dorsal view, 36 (31-38), 46 (34-55) wide, with a median line complete, broken at anterior 1/3 of prodorsal shield, admedian lines complete, divergent to rear, first submedian lines on anterior 2/3 of prodorsal shield, curving outwards, second submedian lines on anterior 1/2 of prodorsal shield, curving outwards, joining first submedian lines posteriorly, short lines and dots present in lateral area of prodorsal shield; scapular tubercles, 30 (27-32) apart on rear shield margin; scapular setae sc 32 (23-43, n=8), diverging backward.
Leg I 44 (38-48), femur 13 (7-15), femoral setae bv 5 (4–7, n=8) on 1/2 anterior from the base of femur; genu 7 (6-8), genual setae l″ 15 (10–24, n=7); tibia 9 (8-10), paraxial tibial setae l′ 4 (2–6, n=9) on 1/2 anterior from the base of tibia; tarsus 10 (9-11), tarsal solenidion ω 9 (6-10), slightly curved with knob apically, tarsal empodium em 7 (5-9), simple 4–5–rayed.
Leg II 37 (33-42), femur 10 (8-12), femoral setae bv 5 (4–5, n=8) on 1/2 anterior from the base of femur; genu 6 (5-8), genual setae l″ 6 (4–8, n=9); tibia 7 (5-8); tarsus 9 (7-11), tarsal solenidion ω 8 (6-11), slightly curved with knob apically, tarsal empodium em 6 (4-8), simple 4–5–rayed.
Coxisternal plates with some short lines; prosternal apodeme entire, distinct. Anterior setae on coxisternum I 1b 5 (4-6, n=5), 17 (11-21) apart, ahead of transverse line through proximal setae 1a on coxisternum I, 1a 13 (10-16, n=5), 12 (9-13) apart, ahead of transverse line through proximal setae 2a on coxisternum II, proximal setae 2a 22 (18-27, n=5), 32 (28-33) apart.
External genitalia 19 (17-22), 31 (29-33) wide; coverflap subtriangular, with 10–12 longitudinal striae; setae 3a 13 (8-16, n=8), 25 (23-29) apart. Coxigenital region with 7-10 semiannuli, microtuberculate.
Opisthosoma with subequal annuli dorsoventrally: 83–94 dorsal annuli with semi-ellipsoidal microtubercles on the margin, 78–90 ventral annuli with hemispherical microtubercles on the margin; opisthosomal setae c2 13 (10-20, n=8), 68 (55-77) apart on 12–15th annulus; d 26 (17-35, n=9), 56 (43-64) apart on 29–34th annulus; e 16 (10-22, n=10), 31 (27-34) apart on 47–55th annulus; f 18 (12-24, n=10), 28 (23-32) apart on 7–9th annulus from rear; h1 6 (5-7, n=9), 9 (8-10) apart and h2 49 (35-56, n=4), 13 (12-16) apart.
Male — Not found.
Specimens examined — Ten females on 10 microscopic slides of inventory nos. NHMW29901/1-14 from vial no. 340 and NHMW29902/1 from vial no. 341 in Box TU from the Nalepa mite collection deposited in the NHMW.
Host plant — Thymus serpyllum L.
Other host plants — Thymus pannonicus All. (=T. pulegioides subsp. pannonicus (All.) Kerguélen) (Boža, 1983; Petanović & Stanković, 1999), Thymus praecox Opiz (Szydło et al., 2010), Origanum vulgare L. (Amrine & Stasny,1994) (Lamiaceae)
Type locality — Austria? (Nalepa Collection Locality. Unknown).
Distribution — Austria (Hellrigl, 2003), Finland (Liro & Roivainen, 1951), Germany (Hellrigl, 2003), Iceland (Szydło et al., 2010), Italy (Canestrini, 1892; Hellrigl, 2003), Montenegro (Jočić & Petanović, 2012), Poland (Boczek & Chyczewski, 1978; Skoracka et al., 2005), Serbia (Boža, 1983; Petanović & Stanković, 1999).
Relation to host —According to Nalepa (1889), the mite causes white erineum on blister with a diameter of 5-8 mm on leaves and flowers of Thymus serpyllum.
Remarks — The range of body length and width of Aceria thomasi (Nalepa, 1889) (original data) almost overlapped with both that of the topotype specimens (present data) and that of specimens collected from Iceland (Szydło et al., 2010). The measurements of all taxonomic characters except setae were not almost different between that of topotype specimens (present data) and that of specimens from Iceland (Szydło et al., 2010), but the lengths of most setae measurements of topotype specimens were relatively a little shorter than that of specimens from Iceland (Table 1).



Aculops thymi (Nalepa, 1889)
Phyllocoptes thymi Nalepa, 1889 pp. 137–138, 152–153 pl. 6 f. 4–6.
Phyllocoptes thymi: Canestrini, 1892 pp. 689–690.
Vasates thymi: Roivainen, 1950 p. 27; Farkas, 1965 p. 82.
Aculops thymi: Amrine & Stasny, 1994; Szydło et al., 2010 p. 148–150 f. 7–8.
(Figs. 4, 5, Table 1)






Female (n=11) — Body fusiform, 167 (153-178), 57 (51-62) wide, 56 (53-57) thick.
Gnathosoma 19 (12-30), projecting obliquely downwards; chelicerae 17 (13-24) slightly curved down.
Prodorsal shield 40 (37-44) including frontal lobe 6 (5-8), obtuse triangular with a blunt point, 49 (42-60) wide, subtriangular; frontal lobe 5–8, obtuse triangular with a blunt point. Prodorsal shield smooth, a low bulging centrally and shallow furrow along with the side of shield; scapular tubercles 36 (33-39) apart on the posterior shield margin; scapular setae sc 9 (7-13, n=11), divergent to rear.
Leg I 31 (27-36), femur 10 (7-13), femoral setae bv 8 (5–12, n=7) on anterior 2/5 from the base of femur; genu 5 (4-5), genual setae l″ 12 (9-18, n=7); tibia 7 (6-8), paraxial tibial setae l′ 3 (3-4, n=8) on anterior 1/4 from the base of tibia; tarsus 6 (4-7), tarsal solenidion ω 7 (5-8), slightly curved, with knob apically, tarsal empodium em 6 (3-8), simple, 4–rayed.
Leg II 29 (26-32): femur 8 (7-10), femoral setae bv 6 (5–6, n=6) on anterior 2/5 from the base of femur; genu 5 (4-6), genual setae l″ 6 (4-8, n=7); tibia 6 (5-7); tarsus 7 (5-8), tarsal solenidion ω 7 (7-10), strongly curved, with knob apically, tarsal empodium em 4 (3-5), simple, 4–rayed.
Coxisternal plates smooth; prosternal apodeme entire, distinct. Anterior setae on coxisternum I 1b 5 (3-7, n=7), 10 (9-11) apart, far from proximal setae 1a on coxisternum I, 1a 6 (4-9, n=8), 9 (6-10) apart, a little ahead of line through proximal setae 2a on coxisternum II, proximal setae 2a 17 (13-21, n=7), 25 (20-25) apart.
External genitalia 13 (9-15), 21 (19-22) wide; coverflap subtriangular, with 6-10 longitudinal striae; setae 3a 7 (6-8, n=8), 15 (15-16) apart. Coxigenital region with 3-9 semiannuli, microtuberculate.
Opisthosoma with 21–26 dorsal and 50–58 ventral annuli, dorsal annuli smooth, ventral annuli with ellipsoidal microtubercles on the margin. Opisthosomal setae c2 6 (5-10, n=9), 50 (44-59) apart on 9–14th annulus; d 16 (7-26, n=6), 34 (25-39) apart on 20–26th annulus; e 7 (4-12, n=10), 20 (18-22) apart on 26–38th annulus; f 7 (5-10, n=10), 20 (18-22) apart on 5–6th annulus from rear; h1 4 (3-4, n=8), 7 (4-10) apart and h2 30 (27-32, n=2), 11 (10-12) apart.
Male — Not found.
Specimens examined — 11 females on 11 microscopic slides of inventory nos. NHMW29903/1-13 from vial no. 340 and NHMW29904/1-5 from vial no. 341 in Box TU from the Nalepa mite collection deposited in the NHMW.
Host plant — Thymus serpyllum L.
Other host plants — Thymus vulgaris L. (Kozłowski, 1983; Skoracka et al., 2005), Thymus praecox Opiz (Szydło et al., 2010), Thymus callieri Berb. ex Velen. (Chetverikov et al., 2021), Teucrium chamaedrys L. (Amrine & Stasny, 1994) (Lamiaceae).
Type locality — Austria? (Nalepa Collection Locality. Unknown).
Distribution — Iceland (Szydło et al., 2010), Italy (Nalepa, 1898; Canestrini, 1892), Poland (Kozłowski, 1983; Skoracka et al., 2005), Sweden (Roivainen, 1950), Russia (Chetverikov et al., 2021).
Remarks — According to Nalepa (1889), the mites were always found on erinea caused by Aceria thomasi on leaves of T. serpyllum. The range of body length and width of Aculops thymi (Nalepa, 1889) (original data) were shorter than both that of the topotype specimens (present data) and that of specimens collected from Iceland (Szydło et al., 2010). The measurements of all taxonomic characters except setae were not different between that of topotype specimens and that of specimens from Iceland, but the lengths of most setae of topotype specimens were relatively a little shorter than that of specimens from Iceland (Table 1).
One eriophyoid mite collected from Spiraea crenifolia (vial no. 117)
Eriophyes spiraeae (Nalepa, 1893)
Phytoptus spiraeae Nalepa, 1893 p. 105; Nalepa, 1895 pp. 635-636 t. 3 f. 7-8 (Additional description).
Eriophyes spiraeae: Nalepa, 1898 p. 29; Amrine & Stasny, 1994 p. 209; Xue et al., 2013 p. 18.
(Figs. 6, 7, Table 2)






Female (n=18) — Body vermiform, 169 (125-212), 49 (46-53) wide, 40 (27-48) thick.
Gnathosoma 20 (16-25), projecting obliquely downwards; chelicerae 18 (13-25), slightly curved down.
Prodorsal shield semicircular in dorsal view, 25 (17-36), 34 (29-39) wide, with a median line absent, admedian lines complete, divergent to rear, curving inwards posteriorly, first submedian lines complete, curving outwards at the middle of prodorsal shield, second and third submedian lines on anterior half of prodorsal shield; scapular tubercles 18 (16-20) apart ahead of rear shield margin; scapular setae sc 14 (12-20, n=16), projecting forwards and convergently.
Leg I 32 (27-35), femur 10 (6-12), femoral setae bv 6 (4-9, n=15) on anterior 1/3 from the base of femur; genu 5 (4-6), genual setae l″ 9 (7-15, n=9); tibia 6 (5-7), paraxial tibial setae l′ 4 (3-6, n=17) on anterior 1/2 from the base of tibia; tarsus 7 (4-8), tarsal solenidion ω 8 (6-11), curved with tiny knob apically, tarsal empodium em 6 (3-8), simple, 5–rayed.
Leg II 29 (26-31), femur 9 (7-13), femoral setae bv 5 (4-8, n=12) on anterior 1/3 from the base of femur; genu 5 (3-7), genual setae l″ 7 (5-10, n=6); tibia 5 (3-6); tarsus 7 (6-8), tarsal solenidion ω 9 (8-12), curved with tiny knob apically, tarsal empodium em 5 (4-6), simple, 5–rayed.
Coxisternal plates smooth; prosternal apodeme entire, distinct. Anterior setae on coxisternum I 1b 5 (4-6, n=6), 8 (7-9) apart, near anterior margin of coxisternum I, far from proximal setae 1a on coxisternum I, 1a 6 (3-8, n=5), 8 (7-9) apart, a little ahead of line through proximal setae 2a on coxisternum II, proximal setae 2a 12 (11-13, n=5), 25 (23-26) apart.
External genitalia 13 (11-14), 21 (18-24) wide; coverflap subtriangular, with 7–11 longitudinal striae; setae 3a 12 (7-17, n=17), 17 (13-18) apart. Coxigenital region with 8 semiannuli, microtubercules.
Opisthosoma with subequal annuli: 65–78 dorsal, 64–72 ventral annuli with ellipsoid microtubercles on the margin. Opisthosomal setae c2 11 (7-19, n=8), 48 (45-51) apart on 10-13th annulus; d 11 (10-13, n=16), 33 (31-37) apart on 21–26th annulus; e 9 (7-13, n=17), 16 (15-18) apart on 40–45th annulus; f 9 (7-11, n=15), 25 (24-28) apart on 6–7th annulus from rear; h1 6 (5-7, n=17), 9 (7-12) apart and h2 25 (20-35, n=13), 13 (12-14) apart.
Male — Not found.
Specimens examined — 18 females on 18 microscopic slides of inventory nos. NHMW29905/1-18 from vial no. 117 in Box S3 from the Nalepa mite collection deposited in the NHMW.
Host plant — Spiraea crenifolia (L.) Boiss. (S. crenata L.).
Other host plants — Spiraea densiflora Nutt. ex Rydb. (Keifer, 1952; Amrine & Stasny, 1994; Skoracka et al., 2005), Spiraea salicifolia L. (Xue et al., 2013) (Rosaceae), Taxus baccata L. (Xue et al., 2013) (Taxaceae).
Type locality — Berlin, Germany. (Nalepa Collection Locality. Unknown).
Additional distribution — China (Xue et al., 2013), USA (Keifer, 1952), Poland (Skoracka et al., 2005).
Remarks — According to Nalepa (1895), Eriophyes spiraeae had 6 longitudinal lines with a median line absent on the prodorsal shield and the admedian lines were jointed anteriorly. On the other hand, the topotype specimens had complete admedian lines, a pair of complete submedian lines and two pairs of short submedian lines anteriorly on prodorsal shield and admedian lines were not jointed anteriorly. Direction of scapular setae sc, position of scapular tubercles on the shield, number of abdominal annuli, number of rays on empodium em were not different between E. spiraeae and topotype specimens.



Two eriophyoid mites collected from Ulmus campestris (vial no. 477)
Peralox longirostris (Nalepa, 1922) n. comb
Phyllocoptyches gallicolus Nalepa, 1922 pp. 190-193; Newkirk (1984) as deutogyne.
Phyllocoptes longirostris Nalepa, 1922 pp. 193-194; Newkirk (1984) as protogyne.
Rhyncaphytoptus gallicolus: Amrine et al., 2003 p. 208.
Peralox dentatis Xue et al., 2013 pp. 112-113.
(Figs. 8, 9, Table 2)
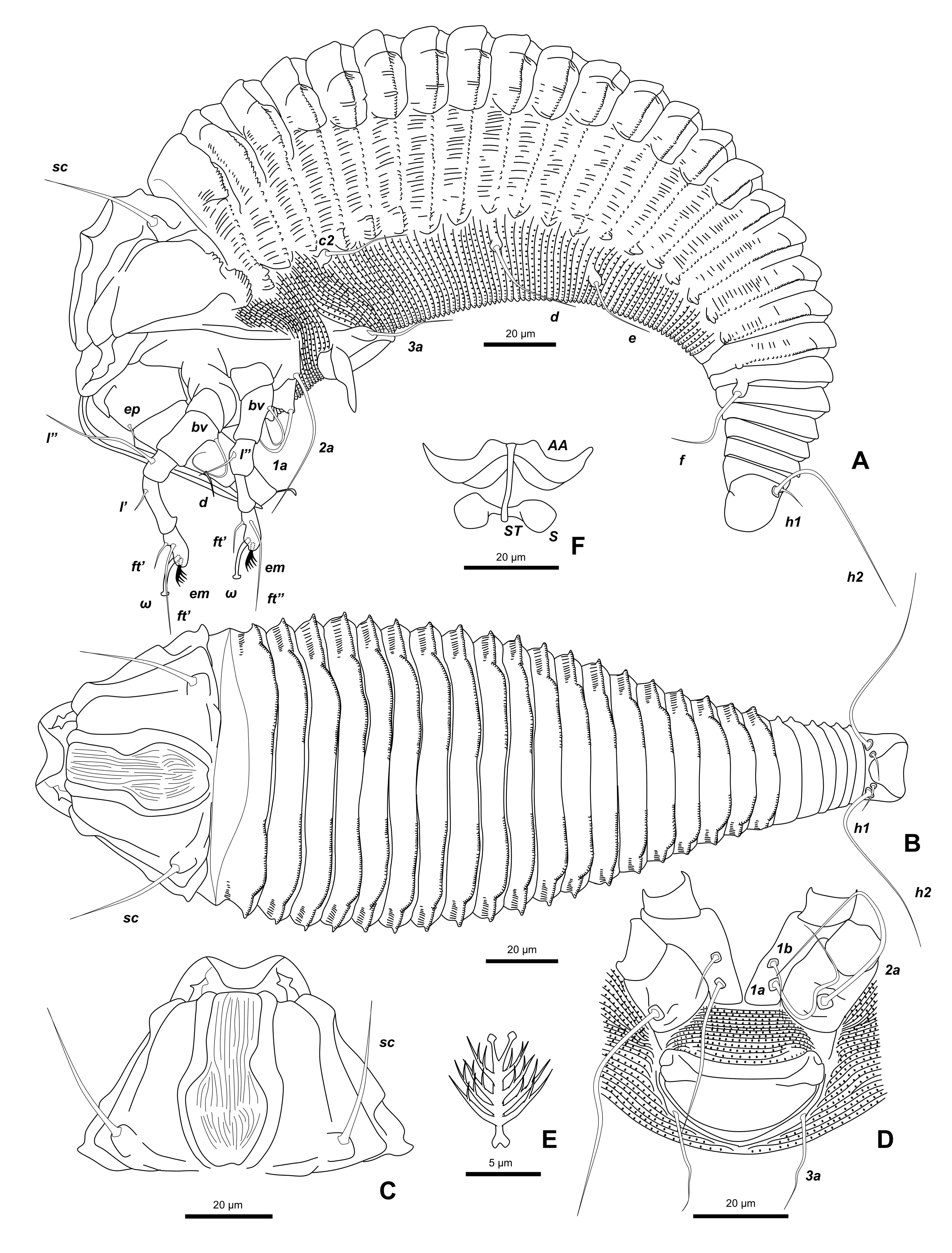





Female (n=8) — Body fusiform 245 (207-302), 87 (86-88) wide, 79 (71-85) thick, a deep groove between posterior part of prodorsal shield and first dorsal annulus; subdorsal and lateral longitudinal ridges from 1st dorsal annulus to 7th annulus from rear.
Gnathosoma 43 (31-61), projecting obliquely downwards; chelicerae 73 (33-86), projecting abruptly downwards a little ahead of gnathosoma base.
Prodorsal shield 49 (37-58), 56 (43-68) wide, trapezoidal; frontal lobe 8 (5-12), arc-shaped apically with a wide base, over gnathosomal base, with median line absent, admedian lines complete, parallel anteriorly, curved outward posteriorly, jointed at rear shield margin, many short lines between admedian lines, submedian lines complete, curving outward posteriorly; scapular tubercles 49 (45-52) apart a little ahead of rear shield margin; scapular setae sc 33 (28-36, n=8), divergent forward.
Leg I 61 (52-69), femur 17 (13-25), femoral setae bv 20 (16-23, n=7) on anterior 1/2 from the base of femur; genu 10 (9-10), genual setae l″ 37 (34-40, n=3); tibia 15 (12-17), paraxial tibial setae l′ 9 (7–10, n=7) on anterior 1/3 from the base of tibia; tarsus 12 (11-13), tarsal solenidion ω 11 (9-12), curved with large knob apically, tarsal empodium em 8 (6-9), simple, 5–6–rayed.
Leg II 53 (45-58), femur 14 (10-17), femoral setae bv 15 (11–21, n=8) on anterior 1/3 from the base of femur; genu 8 (7-10), genual setae l″ 29 (21–38, n=2); tibia 11 (10-13); tarsus 12 (11-12), tarsal solenidion ω 11 (10-13), curved with large knob apically, tarsal empodium em 8 (6-9), simple, 5–rayed.
Coxisternal plates smooth. Coxisternum I separated from each other. Anterior setae on coxisternum I 1b 9 (7-12, n=6), 15 (15-15) apart, ahead of line through proximal setae on coxisternum I, 1a 28 (25-34, n=5), 15 (13-16) apart, a little ahead of line through proximal setae 2a on coxisternum II, proximal setae 2a 44 (38-52, n=5), 36 (35-37) apart.
External genitalia 19 (16-23), 36 (34-37) wide; coverflap bowl-shaped, smooth; setae 3a 24 (17-38, n=8), 28 (26-30) apart. Coxigenital region with 16–18 semiannuli, microtuberculate.
Opisthosoma with 24–27 dorsal, 75–113 ventral annuli. Dorsal annuli with subdorsal ridge on first dorsal annulus running to above opisthosomal setae e and lateral ridges running from above the middle of setae c2 and setae d to above setae f, dorsal annuli smooth between subdorsal ridges, with short lines between subdorsal ridge and lateral ridge and semicircular microtubercles on the margin of annuli, ventral annuli with microtubercles marginally; 7 annuli from rear equal dorsoventrally. Opisthosomal setae c2 22 (19-24, n=7), 77 (75-78) apart on 14–31st annulus; d 62 (53-66, n=6), 67 (60-77) apart on 29–56th annulus; e 16 (13-23, n=8), 38 (34-41) apart on 38–76th annulus; f 30 (25-37, n=7), 27 (26-28) apart on 4–7th annulus from rear; h1 3 (3-4, n=2), 12 (11-13) apart and h2 53 (42-66, n=7), 15 (14-15) apart.
Male — Not found.
Specimens examined — 8 females on 8 microscopic slides of inventory nos. NHMW29906/1-11 from vial no. 477 in Box TU from the Nalepa mite collection deposited in the NHMW.
Host plant — Ulmus campestris L.
Other host plants — Ulmus sp. (Ulmaceae).
Type locality — Austria? (Nalepa Collection Locality. Unknown).
Distribution — China (Xue et al., 2013).
Remarks — Keifer (1962) established the new genus Peralox, with Rhyncaphytoptus ficifoliae as its type species based on having a deep transverse groove behind the prodorsal shield. This species also had a deep groove between the posterior part of the prodorsal shield and the first dorsal annulus, and the rear 7 annuli were completely joined dorsoventrally. So, we concluded this species belongs to the genus Peralox and a new combination was proposed.
Shevtchenkella ulmi (Farkas, 1960)
Oxypleurites ulmi Farkas, 1960 pp. 330–331 figs. 41–43.
Oxypleurites ulmi: Farkas, 1965 p. 105; Huang, 1965 pp. 613–614 figs. 22–28.
Tegonotus ulmi: Newkirk & Keifer, 1971 pp. 7–8.
Shevtchenkella ulmi: Bagdasarian, 1978 p. 938; Amrine & Stasny, 1994 p. 287; Ripka & de Lillo, 1997 p. 151; Xue et al., 2013 pp. 30–31 figs. 18-19; Lotfollahi et al., 2014 pp. 53–54 fig. 2; Denizhan et al., 2015 pp. 39–40.
(Figs. 10, 11, Table 2)






Female (n=2) — Body fusiform, 161 (147-174), 69 (64-74) wide.
Gnathosoma 16 (16-16), projecting downwards; chelicerae 15 (10-19).
Prodorsal shield semicircular in dorsal view, 52 (51-53) (including frontal lobe), 70 (66-74) wide; frontal lobe 8 (7-10), obtuse triangular with a blunt tip. Prodorsal shield almost smooth, with a low bulging between faint admedian lines curving outwards; scapular tubercles 40 (40-40) apart on rear shield margin; scaplar setae sc 6 (5-7), divergent to rear.
Leg I 28 (27-29), femur 8 (8-9), femoral setae bv 7 (5–8) on anterior 2/5 from the base of femur; genu 5 (5-5), genual setae l″ 16 (10–21); tibia 4 (4-4), paraxial tibial setae l′ 4 (3–4) on anterior 1/3 from the base of tibia; tarsus 6 (6-7), tarsal solenidion ω 5 (4-6), slightly curved with knob apically, tarsal empodium em 6 (6-6), simple, 4–rayed.
Leg II 24 (24-24), femur 7 (7-7), femoral setae bv 6 (5–7) on anterior 1/3 from the base of femur; genu 5 (4-5), genual setae l″ 5 (4–5); tibia 4 (4-4); tarsus 5 (5-5), tarsal solenidion ω 6 (6-6), curved with knob apically, tarsal empodium em 6 (6-6), simple, 4–rayed.
Coxisternal plates smooth; prosternal apodeme entire, distinct. Anterior setae on coxisternum I, 1b 7 (6-7), 13 (13-14) apart, ahead of line through proximal setae 1a on coxisternum I; 1a 9 (6-12), 13 (10-15) apart, a little ahead of line through proximal setae 2a on coxisternum II, proximal setae 2a 23 (16-31), 30 (25-35) apart.
External genitalia 12 (12-12), 23 (22-24) wide; coverflap with 8 longitudinal striae; setae 3a 12 (11-12), 17 apart. Coxigenital region with 13 semiannuli, smooth.
Opisthosoma with 17 dorsal annuli projecting laterally and a central longitudinal ridge, 55–64 ventral annuli. Dorsal annuli smooth, projecting laterally; ventral annuli microtuberclate. Opisthosomal setae c2 13 (13-13), 55 (51-59) apart on 10–17th annulus; d 22 (18-26), 32 (32-33) apart on 20–27th annulus; e 8 (6-9), 16 (15-16) apart on 34–41st annulus; f 10 (8-12), 15 (15-16) apart on 4–7th annulus from rear; h1 3 (3-4), 6 (6-7) apart and h2 38 (32-44), 9 (9-9) apart.
Male — Not found.
Specimens examined — 2 females on 2 microscopic slides of inventory nos. NHMW29907/1-3 from vial no. 477 in Box TU from the Nalepa mite collection deposited in the NHMW.
Host plant — Ulmus campestris L.
Other host plants — Ulmus procera Salisb. (= U. minor Mill.) (Xue et al., 2009; Lotfollahi et al., 2014), Ulmus minor Mill. (Xue et al., 2009; Xue et al., 2013; Lotfollahi et al., 2014), Ulmus scabra Mill. (= U. rubra Muhl.) (Ripka & de Lillo, 1997), Ulmus davidiana var. japonica (Rehd.) Nakai (Huang, 1965), Ulmus densa Litv. (Xue et al., 2013), Ulmus glabra Huds. (Xue et al., 2013; Denizhan & Çobanoglu, 2010), Ulmus laevis Pall. (Xue et al., 2013) (Ulmaceae).
Type locality — Nógrádveroee, Hungary. (Nalepa Collection Locality. Unknown).
Additional distribution — Hungary (Farkas, 1960; Ripka & de Lillo, 1997), Great Britain (Farkas, 1965), North America (Farkas, 1965), Japan (Huang, 1965), Iran (Xue et al., 2009; Lotfollahi et al., 2014), China (Xue et al., 2013), Turkey (Denizhan & Çobanoglu, 2010), Serbia (Petanović & Stanković, 1999).
Remarks — The distance between the setae (sc) on the prodorsal shield of the mites was shorter in the original description (Farkas, 1960; 26 µm) than in the topotype specimens (40 µm, present data) .
Two eriophyoid mites collected from Ulmus campestris (vial no. 355)
Aceria filiformis (Nalepa, 1891)
Phytoptus filiformis Nalepa, 1891 p. 370 no. 65b, pp. 374–375 t. 1 f. 5–6.
Eriophyes filiformis: Nalepa, 1898 p. 14; Liro,1941 p. 8; Berezantsev, 1981 p. 453.
Aceria filiformis: Farkas, 1965 p. 22 figs. 15a-c; Amrine & Stasny, 1994 p. 47; Ripka & de Lillo, 1997 p. 149; Hellrigl, 2003 p. 91; Denizhan & Çobanoglu, 2010 p. 545; Denizhan et al., 2015 p. 23.
(Figs. 12, 13, Table 3)






Female (n=13) — Body vermiform, 219 (179-237), 31 (28-40) wide, 31 (25-36) thick.
Gnathosoma 18 (14-27), projecting obliquely forwards; chelicerae 16 (12-20), slightly curved downwards.
Prodorsal shield 23 (18-25), 22 (18-24) wide, semioval in dorsal view, with a median line absent, admedian lines complete, broken in the middle of shield, first and second submedian lines complete, more and less sinuate, convergent posteriorly, some lines in the lateral area of shield; scapular tubercles 15-17 apart on rear shield margin; scapular setae sc 22 (19-26), divergent to rear.
Leg I 26 (21-29), femur 8 (7-11), femoral setae bv 4 (2–5, n=11) on anterior 1/2 from the base of femur; genu 4 (3-5), genual setae l″ 9 (5-14, n=11); tibia 4 (3-5), paraxial tibial setae l′ 4 (3–5, n=11) on anterior 1/3 from the base of tibia; tarsus 6 (5-8), tarsal solenidion ω 7 (6-8), slightly curved with tiny knob apically, tarsal empodium em 6 (5-6), simple, 4–rayed.
Leg II 22 (18-27), femur 8 (5-11), femoral setae bv 4 (3-5, n=11) on anterior 1/2 from the base of femur; genu 3 (2-4), genual setae l″ 4 (3-6, n=11); tibia 4 (3-5), tarsus 5 (3-6), tarsal solenidion ω 7 (6-8), slightly curved with tiny knob apically, tarsal empodium em 6 (5-8), simple, 4–rayed.
Coxisternal plates with short lines; prosternal apodeme entire, distinct. Anterior setae on coxisternum I 1b 3 (2-3, n=3), 11 (11-11) apart, ahead of line through proximal setae 1a on coxisternum I, 1a 7 (6-8, n=5), 6 (5-7) apart, ahead of line through proximal setae 2a on coxisternum II, proximal setae 2a 23 (18-31, n=4), 17 (15-21) apart.
External genitalia 11 (10-12), 13 (12-14) wide, far away from coxisternum II; coverflap bowl- shaped with 6–8 longitudinal striae; setae 3a 5 (5-6, n=9), 11 (10-12) apart. Coxigenital region with 7-12 semiannuli, microtubercles.
Opisthosoma with subequal annuli bearing semi–ellipsoidal microtubercles, 81–94 dorsal, 75–89 ventral annuli. Opisthosomal setae c2 9 (7-11, n=4), 29 (26-31) apart on 10–13th annulus; d 17 (13-23, n=11), 25 (24-26) apart on 19–26th annulus; e 5 (3-6, n=11), 14 (14-15) apart on 33–46th annulus; f 14 (10-22, n=12), 19 (16-20) apart on 5–7th annulus from rear; h1 6 (5-7, n=12), 8 (8-8) apart and h2 33 (22-40, n=12), 12 (11-14) apart.
Male — Not found.
Specimens examined — 13 females on 13 microscopic slides of inventory nos. NHMW29908/1-16 from vial no. 355 in Box TU from the Nalepa mite collection deposited in the NHMW.
Host plant — Ulmus campestris L.
Other host plants — Ulmus campestris L. (= Ulmus glabra Huds.) (Hellrigl, 2003, Denizhan & Çobanoglu, 2010; Denizhan et al., 2015), Ulmus carpinifolia Suckow (= U. minor Mill.) (Janežič, 1982; Petanović & Stanković, 1999), Ulmus montana With. (= U. glabra Huds.) (Nalepa, 1903; Liro, 1941), Ulmus scabra Mill. (= Ulmus montana With.) (Liro, 1941), Ulmus laciniata Mayr (Berezantsev, 1981), Ulmus propinqua Koidz. (= U. davidiana var. japonica (Rehder) Nakai) (Berezantsev, 1981), Ulmus pumila L. (Ripka & de Lillo, 1997) (Ulmaceae).
Type locality — Austria? (Nalepa Collection Locality. Unknown).
Distribution. Italy (Hellrigl, 2003), Hungary (Ripka & de Lillo, 1997), Czech (Vaněčková-Skuhravá, 1996), Finland (Liro, 1941), Serbia (Janežič, 1982; Petanović & Stanković, 1999), Turkey (Denizhan & Çobanoglu, 2010; Denizhan et al., 2015), Russia (Berezantsev, 1981).
Remarks — This mite was quite elongate and the genitalia were far away from the coxisternum II. According to Nalepa (1891), the mite caused smallpox-like galls on the leaves and many mites inhabited the space between the leaf mesophyll cells of the host.
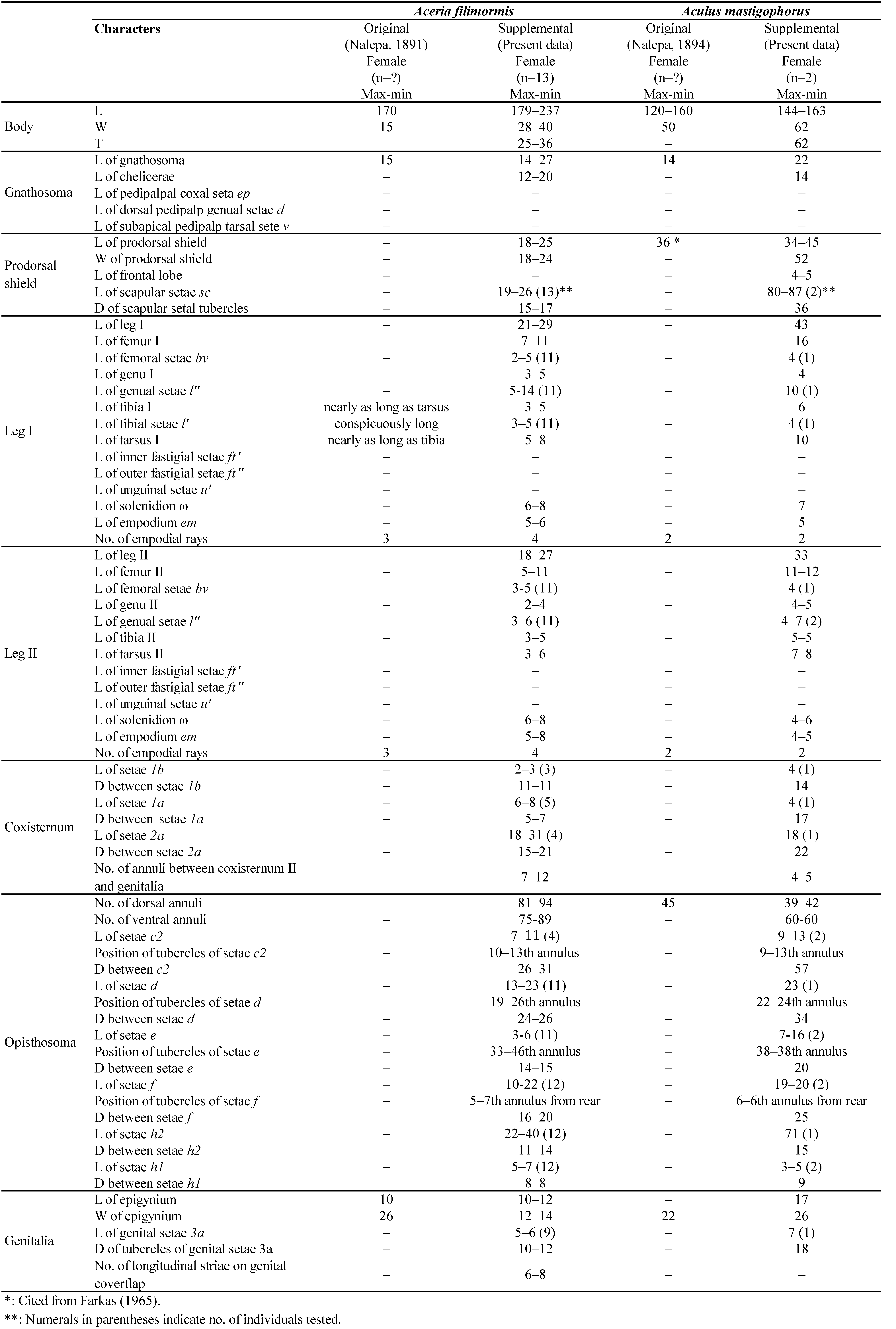


Aculus mastigophorus (Nalepa, 1890)
Phyllocoptes mastigophorus Nalepa, 1890 pp. 60, 67 no.44; Nalepa, 1894 pp. 308–309 pl. 3, f. 1–2 (Additional description); Liro & Roivainen, 1951 p. 189.
Vasates mastigophorus: Roivainen, 1950 p. 25.
Aculus mastigophorus: Amrine & Stasny, 1994 p. 136.
(Figs. 14, 15, Table 3)






Female (n=2) — Body fusiform, 153 (144-163), 62 (n=1) wide, 62 (n=1) thick.
Gnathosoma 22 (n=1), projecting obliquely downwards; chelicerae 14 (n=1).
Prodorsal shield semioval, 42 (34-45) (including frontal lobe), 52 (n=1) wide; frontal lobe 4-5, obtuse triangular with a blunt tip, with a median line complete, a little obscure, admedian lines complete, divergent to rear, 3 submedian lines in the lateral area of prodorsal shield; scapular tubercles 36 (n=1) apart, ahead of rear shield margin; scapular setae sc 84 (80-87), too long, directing forward.
Leg I 43 (n=1), femur 16 (n=1), femoral setae bv 4 (n=1) on anterior 1/2 from the base of femur; genu 4 (n=1), genual setae l″ 10 (n=1); tibia 6 (n=1), paraxial tibial setae l′ 4 (n=1) on anterior 1/2 from the base of tibia (Fig.15D); tarsus 10 (n=1), tarsal solenidion ω 7 (n=1), slightly curved with knob apically, tarsal empodium em 5 (n=1), simple 2–rayed.
Leg II 33 (n=1), femur 11 (11-12), femoral setae bv 4 (n=1) on anterior 1/2 from the base of femur; genu 4 (4-5), genual setae l″ 6 (4–7), tibia 5 (5-5); tarsus 7 (7-8), tarsal solenidion ω 5 (4-6), curved with knob apically, tarsal empodium em 5 (4-5), simple, 2–rayed.
Coxisternal plates smooth; prosternal apodeme contact at one point. Anterior setae on coxisternum I 1b 4 (n=1), 14 (n=1) apart, ahead of line through proximal setae 1a on coxisternum I; 1a 4 (n=1), 17 (n=1) apart, a little ahead of line through proximal setae 2a of coxisternum II, proximal seta 2a 18 (n=1), 22 (n=1) apart.
External genitalia 17 (n=1), 26 (n=1) wide, flat bowl-shaped; coverflap smooth; setae 3a 7 (n=1), 18 (n=1) apart. Coxigenital region with 4-5 semiannuli, microtubercles.
Opisthosoma with 39–42 dorsal, 60-60 ventral annuli. Dorsal annuli smooth, ventral annuli microtuberculate. Opisthosomal setae c2 11 (9-13), 57 (n=1) apart on 9–13th annulus; d 23 (n=1), 34 (n=1) apart on 22–24th annulus; e 11 (7-16), 20 (n=1) apart on 38-38th annulus; f 20 (19-20), 25 (n=1) apart on 6-6th annulus from rear; h1 4 (3-5), 9 (9-9) apart and h2 71 (n=1), 15 (n=1) apart.
Male — Not found.
Specimens examined — 2 females on 2 microscopic slides of inventory nos. NHMW29909/1-2 from vial no. 355 in Box TU from the Nalepa mite collection deposited in the NHMW.
Host plant — Ulmus campestris L.
Type locality — Not listed. Austria? (Nalepa Collection Locality. Unknown).
Distribution — Sweden (Roivainen, 1950).
Remarks — This species had very characteristic long scapular setae on the prodorsal shield. No seta was found on the tibial segment of the leg I of this mite (Nalepa, 1894) and if it was really absent, Amrine and Stasny (1994) suggested that a new genus might be created. However, our examination of the Nalepa specimen revealed that the mite apparently had a seta on the tibial segment of the leg I (arrow in Fig. 15D), so that it was not necessary to create a new genus.
Discussion
The specimens from the samples recovered from two vials (nos. 340 and 341) were belonged to the genera Aceria and Aculops based on the morphological characteristics of the genera, the former being identified as Aceria thomasi and the latter as Aculops thymi, based on the original description of Nalepa (1889). On the other hand, the measurements in the homologue symmetric structures other than the setae for A. thomasi and A. thymi are almost same between topotype specimens (present data) and specimens from Iceland (Szydło et al., 2010), but the setal lengths of topotype specimens was shorter than that of specimens from Iceland (Table 1). The mites that infect Japanese perilla with emaravirus was not morphologically difference from A. thymi. Therefore, we concluded that the Japanese pellira mite is correctly A. thymi.
We redescribed a mite in the vial collected from S. crenifolia (vial no. 117) as E. spiraeae. The spiraea gall mite, Eriophyes sp. collected from S. thunbergii Siebold ex Blume (Rosaceae) in Japan was considered to be morphologically closer to E. spiraeae. However, we found that there are some morphological differences between them; namely, Eriophyes sp. has median line on prodorsal shield, much more annuli in number in coxigenital region, and the design of lines on the coxisternum I and II. E. spiraeae causes malformation to flowers of S. crenifolia, while the mites of Eriophyes sp. cause edge–rolling damage to leaves of S. thunbergii, but do not cause malformation of flowers. Therefore, it is necessary to compare molecular information to clarify the taxonomic species status of the sample collected from S. thunbergii, as well as to conduct a more detailed morphological comparison of Eriophyes sp. and E. spiraeae.
Among the mites in the vial collected from U. campestris (vial no. 477), we redescribed two species, P. longirostris and S. ulmi. We also redescribed two eriophyid mites, A. filiformis and A. mastigophorus, in the vial no. 355 collected from U. campestris. However, the Japanese Rhyncaphytoptinae species collected from walnut, J. mandshurica, did not resemble any of the specimens in the Nalepa vials or other described Rhyncaphytoptinae species. Thus, the Rhyncaphytoptinae species needs to be described as a new species. In his original description of A. mastigophorus did not draw a paraxial tibial seta on the leg I. However, we confirmed that each A. mastigophorus individual in the Nalepa vial has one seta on the tibial segment of leg I (Fig. 15D, arrow).
Nalepa described 331 species, 42 varieties, and 28 subspecies of eriophyoid mites during his research period from 1887 to 1930 (Newkirk, 1984), but detailed taxonomic characters are not shown in his original descriptions. This may lead to misidentification of mite species or delay species identification by eriophyoid mite researchers. If all old mite collections including Keifer's and others could be restored, just as Chetverikov et al. (2016) could restore the old Nalepa's mite collections in their protocol, it would greatly contribute to the taxonomy of Eriophyoidea. In addition, recently, methods for extracting DNA without destroying mite specimens has been developed (Phillips and Simon, 1995; Ota et al., 2011; Sakamoto and Gotoh, 2017; Duarte et al., 2019). This would make it possible to compare the nucleotide sequence between the old mite type specimens and the current specimens. Therefore, we will try to extract DNA from the Nalepa mite specimens nondestructively and compare sequences to confirm whether Japanese mites and the Nalepa mites are conspecific or heterospecific. Furthermore, as many contemporary descriptions contain such data especially COI sequences, which are deposited to GenBank, the addition of molecular information to the traditional morphological traits would contribute greatly to the systematics of eriophyoid mite species.
Acknowledgments
We sincerely thank Dr. Peter Schausberger (University of Vienna) for arranging the collaboration with NHMW.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Amrine J.W. Jr., Manson D.C.M. 1996. Preparation, mounting and descriptive study of eriophyoid mites. In: Lindquist E.E., Sabelis M.W., Bruin J. (Eds.). Eriophyoid Mites. Their Biology, Natural Enemies and Control. World Crop Pests 6. Amsterdam, The Netherlands: Elsevier Science Publisher. pp. 383-396. https://doi.org/10.1016/S1572-4379(96)80023-6
- Amrine J.W. Jr., Stasny T.A.H. 1994. Catalog of the Eriophyoidea of the World. Michigan, USA: Indira Publishing House. pp. ix +798.
- Amrine J.W. Jr., Stasny T.A.H., Flechtmann C.H.W. 2003. Revised Keys to the World Genera of the Eriophyoidea (Acari: Prostigmata). West Bloomfield, Michigan, USA: Indira Publishing House. pp. ix + 244.
- Bagdasarian A.T. 1978. [A new genus of mites (Eriophyoidea)] Zool. Zh., 57: 936-939 (in Russian).
- Berezantsev A.Y. 1981. [New for the fauna of Primorye region and Kurile islands gall making Tetrapodili (Acarina)]. Entomol. Obozr., 60: 451-458 (in Russian).
- Boczek J., Chyczewski, J. 1978. Eriophyid mites (Acarina: Eriophyoidea) occurring on weed plants in Poland. Roczniki Nauk Rolniczych, Ser. E 7: 109-114.
- Boža P. 1983. Prilog poznavanju cecidofaune zeljastih biljaka Vojvodine. Matica srpska, Zbornik za prirodne nauke, 65: 131-140.
- Canestrini G. 1892. Prospetto dell'Acarofauna Italiana. Parte Va. Famiglia dei Phytoptini (Phytoptidae). Atti della Societá Veneto-Trentina di Scienze naturali, Padova, 1: 543-722 + plates 44-59.
- Chetverikov P.E., Fedorov, D.S., Letukhova, V.Y., Romanovich, A.E. 2021. Description of Cecidophyes fibigiae n. sp., new combinations, records, and DNA barcodes of eriophyid mites (Eriophyoidea, Eriophyidae) from Karadag Nature Reserve (Crimea). Syst. Appl. Acarol., 26: 818-828. https://doi.org/10.11158/saa.26.4.12
- Chetverikov P.E., Hörweg C., Kozlov M.I., Amrine J.W. Jr. 2016. Reconditioning of the Nalepa collection of eriophyoid mites (Acariformes, Eriophyoidea). Syst. Appl. Acarol., 21: 583-595. https://doi.org/10.11158/saa.21.5.3
- Cotte J. 1924. Les cécidies des Alpes Maritimes et leurs producteur. Mémoires de la Société Linnéenne de Marseille, 3: 1-56.
- de Lillo E., Craemer C., Amrine J.W. Jr., Nuzzaci G. 2010. Recommended procedures and techniques for morphological studies of Eriophyoidea (Acari: Prostigmata). Exp. & Appl. Acarol., 51: 283-307. https://doi.org/10.1007/s10493-009-9311-x
- Denizhan E., Çobanoglu, S. 2010. Van Gölü havzasında Ulmus campestris L. (Ulmaceae) üzerinde tespit edilen eriophyoid akarlar (Acari: Prostigmata: Eriophyoidea). Türk. Entomol. Derg., 34: 543-549.
- Denizhan E., Monfreda R., de Lillo E., Çobanoglu S. 2015. Eriophyoid mite fauna (Acari: Trombidiformes: Eriophyoidea) of Turkey: new species, new distribution reports and an updated catalogue. Zootaxa, 3991: 1-63. https://doi.org/10.11646/zootaxa.3991.1.1
- Duarte M.E., de Mendonça R.S., Skoracka A., Silva E.S., Navia D. 2019. Integrative taxonomy of Abacarus mites (Eriophyidae) associated with hybrid sugarcane plants, including description of a new species. Exp. Appl. Acarol., 78: 373-401. https://doi.org/10.1007/s10493-019-00388-y
- Farkas H. 1960. Über die Eriophyiden (Acarina) Ungarns I. Beschreibung neuer und wenig bekannter Arten. Acta Zool. Acad. Sci. Hung., 6: 315-339.
- Farkas H. 1965. Familie Eriophyidae, Gallmilben. In: Die Tierwelt Mitteleuropas, Bd. 3, Lief 3, Verlag von Quelle and Meyer, Leipzig. pp. 155.
- Hellrigl K. 2003. Faunistik der Gallmilben Südtirols (Acari; Eriophyoidea). Gredleriana, 3: 77-142. https://www.zobodat.at/pdf/Gredleriana_003_0077-0142.pdf

- Huang T. 1965. Five species of eriophyid mites of elm in Sapporo. J. Fac. Sci., Hokkaido Univ. Ser. VI, Zoology, 15: 608-617.
- Janežič T. 1982. Nekaj zoocecidijev na rastlinah Srbije. Zboruik Biotechniše fakultete Univerze v Ljubljani, 39: 147-171.
- Jočić I., Petanović P. 2012. Checklist of the eriophyoid mite fauna of Montenegro (Acari: Prostigmata: Eriophyoidea). Acta Entomologica Serbica, 17: 141-166. Available from http://aes.bio.bg.ac.rs/index.php/aes/article/view/75

- Keifer H.H. 1952. The Eriophyid Mites of California (Acarina, Eriophyidae). Bulletin of the California Insect Survey 2. Berkeley, USA: University California Press. pp. 123.
- Keifer H.H. 1962 Eriophyid Studies B-6. Sacramento: Bureau of Entomology California Department of Agriculture 20 pp.
- Kozłowski J. 1983. Eriophyid mites (Acarina: Eriophyoidea) appearing on some medical herbs. Zeszty Problemowe Postępow Nauk Rolniczych, 6(252): 81-87. (in Polish)
- Kubota K., Usugi T., Tomitaka Y., Shimomoto Y., Takeuchi S., Kadono F., Yanagisawa H., Chiaki Y., Tsuda S. 2020. Perilla mosaic virus is a highly divergent emaravirus transmitted by Shevtchenkella sp. (Acari: Eriophyidae). Phytopathology, 110: 1352-1361. https://doi.org/10.1094/PHYTO-01-20-0013-R
- Lindquist E.E., Amrine J.W. Jr. 1996. Systematics, diagnoses for major taxa, and keys to families and genera with species on plants of economic importance. In: Lindquist E.E., Sabelis, N.W., Bruin, J. (Eds.) Eriophyoid Mites: Their Biology, Natural Enemies and Control. World Crop Pests 6. Amsterdam, The Netherland: Elsevier Science Publisher. pp. 33-87. https://doi.org/10.1016/S1572-4379(96)80004-2
- Liro J.I. 1941. Über neue und seltene Eriophyiden (Acarina). Ann. Zool. Soc. Zool.-Bot. Fenn. Vanamo, 8(7): 1-53.
- Liro J.I., Roivainen H. 1951. Äkämäpunkit Eriophyidae. Suomen Eläimet- Animalia Fennica 6. Poorvo-Helsinki, W. Söderström Osakeyhtiö. pp. 281.
- Lotfollahi P., Irani-Nejad K.H., de Lillo E. 2014. Eight new records for the eriophyid (Trombidiformes Eriophyoidea Eriophyidae) mite fauna of Iran. Redia, 47: 51-61.
- Marinković S.M., Chetverikov P.E., Hörweg C., Petanović, R.U. 2018. Supplementary description of three species from the subfamily Cecidophyinae (Eriophyoidea: Eriophyidae) from the Nalepa collection. Sys. Appl. Acarol., 23: 838-859. https://doi.org/10.11158/saa.23.5.5
- Nalepa A. 1889. Beiträge zur Systematik der Phytopten. Sitz. kais. Akad. Wiss., Math. -natur. Kl., Wien, Abt. 1, 98(1): 112-156.
- Nalepa A. 1890. Zur Systematik der Gallmilben. Sitz. kais. Akad. Wiss., Math.-natur. Kl., Wien, Abt. 1, 99(2): 40-69.
- Nalepa A. 1891. Neue Gallmilben. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum. Verhandlungen der Kaiserlichen Leopoldinische-Carolinischen Deutschen Academie der Naturforscher (Hele), 55(6): 362-395. Taf.1-4.
- Nalepa A. 1893. Neue Gallmilben (7 Fortsetzung). Anzeiger kais. Akad. Wiss., Math. -natur. Kl., Wien, 30(12): 105.
- Nalepa A. 1894 Beitrage zur Kenntniss der Phyllocoptiden. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germanicae Naturae Curiosorum. Verhandlungen der Kaiserlichen Leopoldinische-Carolinischen Deutschen Academie der Naturforscher (Hele), 61: 308-309 pl.3, f.1-2
- Nalepa A. 1895. Beitrage zur Kenntniss der Gattungen Phytoptus Duj. und Monaulax Nal. Denkschr. Kais. Akad. Wiss., Math. -natur. Kl., Wien, 62: 627-640 Taf. I-IV.
- Nalepa A. 1898. Eriophyidae (Phytoptidae). Das Tierreich. Eine Zusammenstellung und Kennzeichnung der rezenten Tierformen. Berlin 4 Lief. Acarina. pp. ix + 74.
- Nalepa A. 1903. Neue Gallmilben (23. Fortsetzung). Anzeiger der kaiserlichen Akademie der Wissen-schaften. Mathematische-Naturwissenschaftliche Klasse (Vienna), 40(25): 292-294.
- Nalepa A. 1906. Ueber das Praeparieren und Konservieren der Gallmilben. Marcellia 5: 49-61.
- Nalepa A. 1922. Phyllocoptyches, eine neue Eriophyidengattung. Marcellia, 18(1-6): 190-194.
- Newkirk R.A. 1984. Eriophyid Mites of Alfred Nalepa. Entomological Society of America, College Park, Maryland USA: Thomas Say Foundtion Publications. 9, pp. 137.
- Newkirk R.A., Keifer H.H. 1971. Revision of types of Eriophyes and Phytoptus. p. 1-10 In: Keifer H.H. (Ed.) Eriophyid Studies C-5. U.S. Department of Agriculture, Agricultural Research Service. pp. 24.
- Ota A., Karasawa S., Nakamura T., Harada H., Shimano S. 2011. Non-destructive DNA extraction protocol for oribatid mites (Acari: Oribatida). Edaphologia, 89: 19-24.
- Phillips A.J., Simon C. 1995. Simple, efficient, and nondestructive DNA extraction protocol for arthropods. Ann. Entomol. Soc. Am., 88: 281-283. https://doi.org/10.1093/aesa/88.3.281
- Petanović R., Stanković S. 1999. Catalogue of Eriophyoidea (Acari: Prostigmata) of Serbia and Montenegro. Acta Entomologica Serbica. Special Issue, pp. 143.
- Ripka G., de Lillo E. 1997. New data to the knowledge on the eriophyoid fauna in Hungary (Aceri; Eriophyoidea). Folia Entomol. Hung., Series nova Rovartani Kozlemenyek, 58:147-157.
- Roivainen H. 1950. Eriophyid news from Sweden. Acta Ent. Fenn., 7: 1-51.
- Sakamoto H., Gotoh T. 2017. Non destructive direct polymerase chain reaction (direct PCR) greatly facilitates molecular identification of spider mites (Acari: Tetranychidae). 52: 661-665. Appl. Entomol. Zool., https://doi.org/10.1007/s13355-017-0512-1
- Skoracka A., Lewandowski M., Boczek J. 2005. A Catalogue of Eriophyoid Mites (Acari: Eriophyoidea) of Poland. Warszawa, Poland: Natura optima dux Foundation. pp. 199.
- Szydło W., Skaftaso J.F., Skoracka A. 2010. Eriophyoid mites (Prostigmata: Eriophyoidea: Eriophyidae) from Iceland: one new species, and three new mite records. Annales Zoologici (Warszawa), 60: 139-157. https://doi.org/10.3161/000345410X499623
- Takei M., Nakahira T., Okada T., Kagiwada S., Kadono F. 2019. Ecological characteristics of the perilla rust mite, Shevtchenkella sp., a serious pest species of Perilla frutescens. Acarol. Soc. Jpn., 28: 1-16 (in Japanese). https://doi.org/10.2300/acari.28.1
- Vaněčková-Skuhravá I. 1996. Eriophyid mites (Acari: Eriophyoidea) on trees and shrubs in the Czech Republic. Acta Soc. Zool. Bohem., 60: 223-246. https://doi.org/10.1007/BF01908435
- Xue X.-F., Guo J.-F., Hong X.-Y. 2013. Eriophyoid mites from Northeast China (Acari: Eriophyoidea). Zootaxa, 3689(1): 1-123. https://doi.org/10.11646/zootaxa.3689.1.1
- Xue X.-F., Sadeghi H., Hong X.-Y. 2009. Eriophyoid mites (Acari: Eriophyoidea) from Iran, with descriptions of three new species, one new record and a checklist. Internat. J. Acarol., 35: 461-483. https://doi.org/10.1080/01647950903427618



2021-02-17
Date accepted:
2022-02-17
Date published:
2022-02-21
Edited by:
Navia, Denise

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Kadono, Fujio; Takei, Madoka; Gotoh, Tetsuo; Kubota, Kenji; Hörweg, Christoph and Kagiwada, Satoshi
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



