Podapolipoides chorthippus n. sp. (Acari: Prostigmata: Podapolipidae), an ectoparasite of Chorthippus sp. (Orthoptera: Acrididae) from southern Iran
Majidi, Maryam1 ; Hajiqanbar, Hamidreza2 and Saboori, Alireza3
1Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, 14115-336, Tehran, Iran.
2✉ Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, 14115-336, Tehran, Iran.
3✉ Department of Plant Protection, Faculty of Agriculture, University of Tehran, Karaj, Iran.
2022 - Volume: 62 Issue: 1 pages: 120-129
https://doi.org/10.24349/38vh-n9rcZooBank LSID: A4CEBBCB-0059-4259-A824-8FBCB4408D0D
Original research
Keywords
Abstract
This article is dedicated to the memory of Hamidreza Hajiqanbar who unfortunately passed away after submitting the manuscript, on 14 October 2021 in a tragic car crash.
Introduction
Symbiotic relationships, including parasitism, with arthropods have been frequently evolved among different groups of mites. One of the most sophisticated ones is Prostigmata (Acari: Acariformes) which is comprised of species that occupy various habitats (Walter et al. 2009). Some species of this lineage are phoretic or parasitic on insects, mostly classified in Parasitengona and Heterostigmata cohorts, yet some other families like Cheyletidae and Dytiscacaridae have evolved parasitism (Walter et al. 2009; Mortazavi et al. 2018). Within Heterostigmata, the family Podapolipidae Ewing, 1922 with 32 genera and more than 290 described species, is one of the largest families in Prostigmata (Majidi et al. 2019). Members of this family are highly specialized obligate parasites of insects of five orders namely, Coleoptera, Orthoptera, Blattodea, Heteroptera and Hymenoptera (Husband & OConnor 2014; Seeman 2021).
Five genera of podapolipids parasitise orthopterans: Locustacarus Ewing, 1924 with four species (Rahmatzaei et al. 2021); Podapolipus Rovelli & Grassi, 1888 with 31 species (Lindquist & Sidorchuk 2019); Podapolipoides Regenfuss, 1968 with 31 species (Hajiqanbar & Joharchi 2011); Orthapolipus Husband & Li, 1993 with 3 species (Husband et al. 2005) and monobasic Wetapolipus Husband & Zhang, 2002 (Husband & Zhang 2002). The genus Podapolipoides is confined to grasshopper of the families Acrididae (mostly) and Pyrgomorphidae (Husband 1995). So far, P. grassii (Berlese, 1897) on Locusta migratoria L. and different acridids and P. anacridii Hajiqanbar & Joharchi, 2011 on Anacridium sp. have been reported from Iran (Ostovan & Saboori 1999; Hajiqanbar & Joharchi 2011). In this study, we described a new species of Podapolipoides recovered from the acridid grasshopper, Chorthippus sp., from southern Iran and compared it with morphologically closely related species.
Materials and Methods
Collected grasshoppers were preserved in vials with 96% ethanol. Subsequently, grasshoppers and alcohol sediments from the vials were examined for parasitic mites. Host grasshoppers were collected from Latifi region, located in Fars province, Southern Iran in 2017. Colonies of mites were separated from pronotum and near wing bases of acridid grasshoppers, Chorthippus sp. (Orthoptera: Acrididae: Gomphocerinae) (Figure 1). Mite specimens were cleared in Nesbitt's solution and mounted in Hoyer's medium. Morphology of the mites was studied using a compound microscope (Olympus BX51, Tokyo, Japan) equipped with phase contrast illumination. Photographs were taken with the aid of Samsung i70 digital camera. All measurements in the descriptions are given in micrometers for the holotype and five paratypes (in parentheses). The terminology follows Lindquist (1986). Details of geographical position have been recorded using GPS mobile device. Setae with no longer than the diameters of setal acetabulae are considered as microsetae and those with only acetabulae and no setal remnants are assigned as vestigial setae. The host insect was identified with the help of Dr. Mustafa Ünal (Abant İzzet Baysal Üniversitesi, Turkey).



Results
Family Podapolipidae Ewing, 1922
Genus Podapolipoides Regenfuss, 1968
Type-species: Podapolipoides (Podapolipus) grassii (Berlese, 1897), by original designation.
Podapolipoides chorthippus Majidi and Hajiqanbar n. sp.
ZOOBANK: 6EC39A40-9645-4C0F-8570-153EFE16AD3F ![]()
Diagnosis
Larval females with dorsal setae d (7–8) short, simple and pointed; f serrate; h2 simple, pointed and slightly thickened; v1 (2–4), v2 (5–10); 1a m, 2a m, 3b m (m-4). Males with dorsal setae sc2 86 and c2 118 long, sc2 extending beyond bases of setae c2 .
Type-host
Chorthippus sp. (Orthoptera: Acrididae: Gomphocerinae). Attachment site on host: On pronotum and near wing bases (Figure 1).
Type-material
Holotype female (MM20170303) and paratypes (9 adult females, 1 male, 22 larval females) found in , Fars Province, Southern Iran, 27°41ʹN, 54°24ʹ E and altitude 799 m, 3 III 2017; collected by Maryam Majidi; deposited in the Acarological Collection, Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, Tehran, Iran. One adult female and one larval female are deposited in Zoological Institute, University of Hamburg, Hamburg, Germany.
Description
Adult female — (Figure 2)



Gnathosoma (Figure 2A) — Gnathosoma length 62 (52–62), width 55 (53–60); cheliceral stylets robust, length 16 (16–27); pharynx oval and well developed, length 41 (37–45), width 33 (24–34).
Idiosoma (Figure 2A) — Idiosoma ovoid and smooth, length 620 (458–670), width 482 (451–517), with bi-lobed protuberances anterolaterally and genital opening posteroventrally; prodorsal shield poorly sclerotised. Stigmata conspicuous and situated at anterior border of prodorsal shield, each with an elongate broad atrium with 4 distal tracheal branches, length of tracheal atrium about 2 times length of pharynx before enlarging and getting off multiple tracheal branches.
Legs — (Figure 2B) Only single pair of anterior legs; tarsus with one subunguinal seta (s) modified as terminal claw and both spine-like tectal setae tc′ and tc″ blunt; tibia with 1 spine-like seta l′; genu nude; femur with one prominent seta l′ 18 (18–20).
Adult Male — (Figures 3–4)





Gnathosoma (Figure 3A) — length 33, width 29, dorsally with one pair of cheliceral seta ch 3 and ventrally with one pair of subcapitular seta su 5; cheliceral stylets 14; pharynx length 17, width 13; distances between gnathosomal setae: ch-ch 19, su-su 13.
Idiosomal dorsum (Figure 3A) — length 180, width 140; prodorsal plate (PrS) subtriangular, with smooth and pointed setae v1 4, v2 5 and attenuated sc2 86. Genital capsule placed in mid-dorsal idiosoma; pointed setae c2 118; distances between dorsal idiosomal setae: v1-v1 24, v2-v2 43, sc2-sc2 56, c2-c2 102.
Idiosomal venter (Figure 3B) — with apodemes I, II and presternal apodeme (appr) developed; apodemes II (ap2) not reaching to presternal apodeme; coxal fields I without setae 1a; coxal fields II with one pair of smooth and pointed setae 2a 6; coxal fields III fused and bearing only one pair of smooth and pointed setae 3b 16.
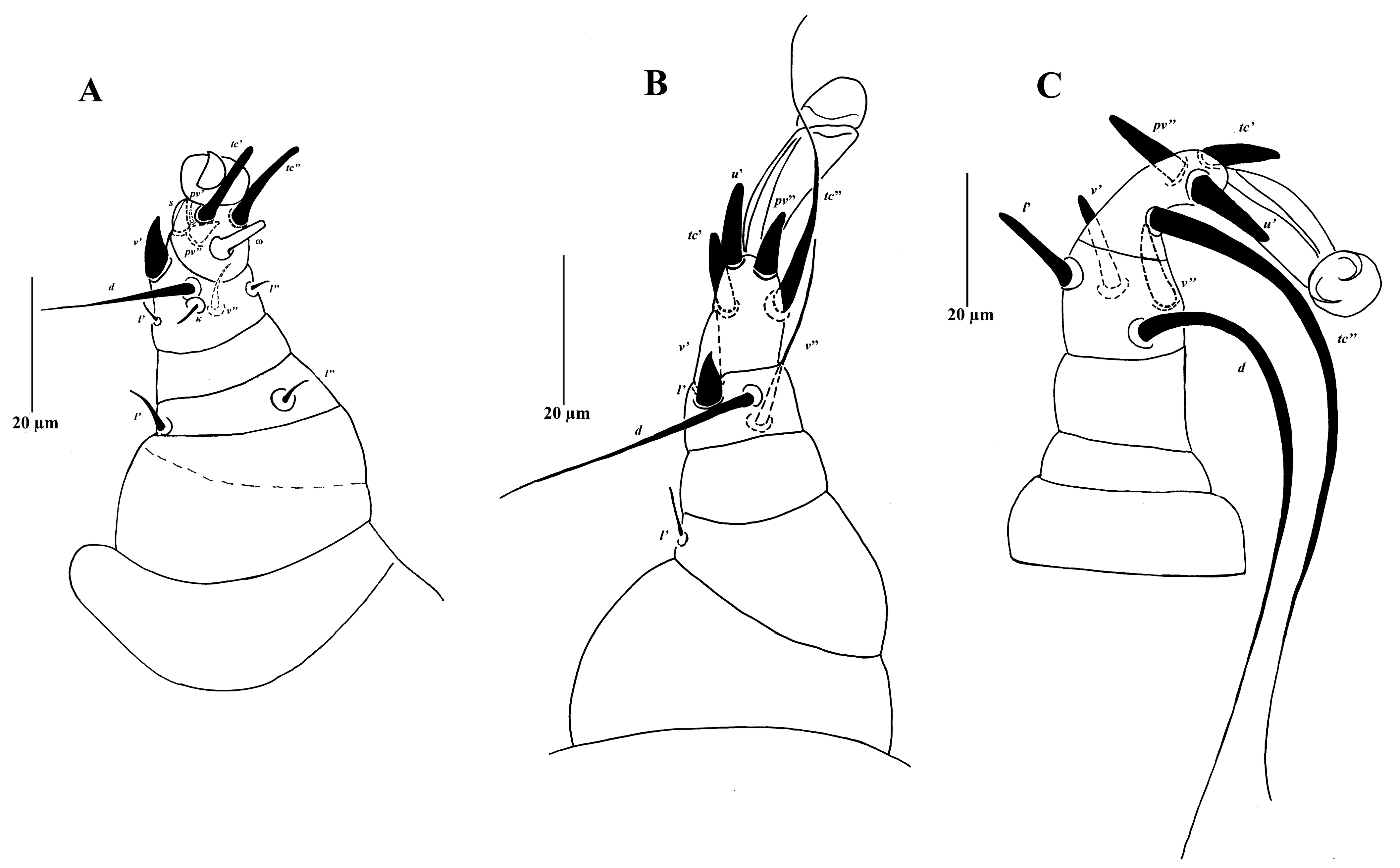
Legs (Figure 4). Leg I (Figure 4A) thicker and shorter than other legs; setal formula (number of solenidia in parentheses): 0-2-0-6-5(1); ambulacrum I with single small claw in sucker-like pad; tarsus with 2 distinctly blunt-ended eupathidial setae tc′ and tc″, solenidion ω 7, prominent and digitiform, setae s and pv″ spine-like; tibia with one spine-like seta v′ 9 and 5 setiform setae, eupathidion k 4; femur with two setae, seta l″ 5. Leg II (Figure 4B) with setal formula: 0-1-0-4-4; ambulacrum II with no claw but empodium well developed; tarsus with 3 spine-like setae tc′, u′ and pv″, seta tc″ attenuated, 42 long; tibia with two spine-like setae v′ 11 and l′ 8, seta d longer than v″; femur with one seta l′. Leg III (Figure 4C) with setal formula: 0-0-0-4-4; ambulacrum III without claw, empodium well developed; tarsus with 3 spine-like setae tc′, u′ and pv″, seta tc″ attenuate, 103 long; tibia with three spine-like setae v′ 14, v″ 13 and l′ 14, seta d attenuate, 99 long.
Larval female — (Figures 5–6)






Gnathosoma (Figure 5A) — length 48 (58–60), width 70 (60–77); dorsally with one pair of cheliceral seta ch 16 (38–43) and ventrally with one pair of subcapitular seta su 8 (8–9); palpal setae dFe 12 (9–14); dGe 11 (11–16), all setae ch, su, dFe, dFe smooth and pointed; cheliceral stylets 63 (60–86); pharynx oval, length 17 (16–18), width 17 (14–19); distances between gnathosomal setae: ch-ch 34 (37–43), dFe-dFe 17 (17–23), dGe-dGe 8 (8–12), su-su 17 (17–23).
Idiosomal dorsum (Figure 5A) — oval, length 154 (154– 182), width 136 (132–155); prodorsal plate (PrS) subrectangular, wider than long, smooth and pointed setae v1 4 (2–4), v2 7 (5–10), and attenuated setae sc2 93 (85–111); setae sc2 extending beyond posterior margin of tergite D; tergite C surrounding anterior and lateral margins of tergite D, with only one pair of setae c2 18 (12–22); tergite D almost sub-circular, with one pair of needle-like setae d 7 (7–8); tergite EF subtriangular, with one pair of setae f 9 (9–18), indistinctly serrate (left side seta detached); tergite H with two pairs of setae, h1 207 (207–314) and h2 10 (10–19); setae h1 whip-like and attenuated, h2 slightly thick; distance between idiosomal setae: v1-v1 58 (52–62), v2-v2 89 (81–98), sc2-sc2 52 (50–63), c2-c2 125 (134–148), d-d 23 (22–32), f-f 47 (34–47), h2-h2 14 (12–14).
Idiosomal venter (Figure 5B) — apodemes I (ap1) and II (ap2) lightly sclerotized but ap2 not reaching presternal apodeme (appr); coxal fields I and II each bearing one pair of microsetae 1a and 2a, respectively; coxal fields III separated from each other and from coxal fields II; setae 3b m (m-4), with striation between and around coxal fields III.
Legs — (Figure 6), Leg I (Figure 6A) thicker than other legs; setal formula (number of solenidia in parentheses): 0-3-1-6-7(1); ambulacrum I with bifid small claw in sucker-like pad; tarsus with two distinctly blunt-ended eupathidial setae tc′ and tc″, solenidion ω 7 (6–8) prominent and digitiform, seta s spine-like; tibia with eupathidion k 6 (6–9) adjacent to long seta d 36 (34–46), setae v″ and l″ subequal; genu with one seta v′; femur with three setae, seta l′ needle-like, seta l″ 31 (34–46) extending beyond tip of leg and distinctly longer than seta v″ 26 (31–35). Leg II (Figure 6B) with setal formula: 0-1-1-4-4; ambulacrum II without claw, empodium well-developed; tarsus with two spine-like setae tc′ and u′, seta pv″ needle-like; tibia with seta l′ needle-like, rest of setae of the segment pointed; seta d 28 (27–39) subequal with seta v″ 27 (21–27); genu and femur each with one seta v′ and l′, respectively, l′ needle-like. Leg III (Figure 6C) with setal formula: 0-0-1-4-4; ambulacrum III without claw, empodium well- developed; tarsus with two spine-like setae tc′ and u′, seta pv″ needle-like; tibia with seta d 30 (45–50), all setae of segment pointed; genu with one needle-like seta v′.
Differential diagnosis
The male of new species belongs to a cluster of species sharing setae c2 100-125 µm long (see the following key for related these species): Podapolipoides faini Husband, 1995, P. locustanus Lavoipierre, 1941, P. alatus Husband, 1990, P. cochisensis Husband, 1993 and P. anacridii Hajiqanbar and Joharchi, 2011. According to length of setae sc2 (80-96), P. chorthippus (86) is similar to P. faini (80) and P. cochisensis (96) but differ from from P. cochisensis by the length of setae 3b 16 (vs vestigial in P. cochisensis) and P. faini in the length of cheliceral stylets 14 (vs 8 in P. faini) and coxal seta 3b shorter than gnathosomal width (vs equal to gnathosomal width in P. faini) also coxal seta 2a shorter than 1/3 gnathosomal width (vs about 1/3 gnathosomal width in P. faini), and in P. chorthippus larvae setae c2 is longer than d (vs c2 is subequal to d in P. faini). Regarding to setae 1a, P. chorthippus n. sp. with no setae 1a is similar to P. alatus but differs from it by setae sc2 reaching to beyond bases of setae c2 (vs not reaching to bases of setae c2 in P. alatus). Based on apodemes II, P. chorthippus n. sp. resembles P. anacridii, P. faini and P. locustanus but differs from P. anacridii by ap1 reaching appr (vs not reaching appr in P. anacridii) and differs from P. locustanus by setae sc2 reaching beyond bases of setae c2 (vs not reaching beyond bases of setae c2 in P. locustanus). Among the congeners found in neighboring countries, it is similar to P. jordani Husband, 1992. However, male of the new species differs from that of P. jordani by sc2 reaching beyond bases of setae c2 , ap2 not reaching appr, and length of tc″ 103 (vs sc2 not reaching beyond bases of setae c2 , ap2 reaching appr, tc″ 156 in P. jordani). The remainder of the comparative measurements is summarized in Table 1.



Based on length of setae v1 and v2 (v1 < v2 ), larval females of the P. chorthippus n. sp. can be easily distinguished from all other species of the genus except P. flechtmanni Husband, 1993. Based on longer setae v2 than v1 , and almost the same length of setae h1, sc2 and f, larval females of P. chorthippus are similar to those of P. flechtmanni. However, they differ in shape of setae d, f, h2 . In P. chorthippus, setae d are thin and pointed, f serrate, and h2 slightly thickened (vs d, f, h2 thick and minutely spine-like in P. flechtmanni); setae c2 12–22 at least two times longer in P. chorthippus n. sp. (vs 6 in P. flechtmanni); and coxal setae I and II microsetae (vs 2 in P. flechtmanni).
Etymology
The new species is named based on the genus name of the grasshopper host (Chorthippus).
Key to males of Podapolipoides spp. with setae c2 100-125 µm
1. Seta 3b vestigial
...... P. cochisensis
— Seta 3b not vestigial, setiform
...... 2
2. Seta sc2 reaching bases of seta c2 or beyond
...... 3
— Seta sc2 never reaching bases of seta c2
...... 5
3. Coxal fields I without setae 1a
...... P. chorthippus n. sp.
— Coxal fields I with one pair of vestigial setae 1a
...... 4
4. Femur I seta v″ equal to or longer than seta l″
...... P. locustanus
— Femur I seta v″ about half of the length of seta l″
...... P. fiani
5. Setae 2a microsetae or vestigial
...... P. alatus
— Setae 2a not microseta or vestigial, setiform
...... P. anacridii
Discussion
The genus Chorthippus is a new host record for parasitic mites of the genus Podapolipoides. It is a member of the subfamily Gomphocerinae (Orthoptera: Acrididae). According to the prevalence of Podapolipoides mites on orthopterans provided by Hajiqanbar and Joharchi (2011), 23 genera of Acrididae (22) and Pyrgomorphidae (1) are parasitized by this mite genus. Sarangi et al. (2012) described a new Podapolipoides from Oxya (Acrididae: Oxyinae), a grasshopper genus previously recorded as host of three species of Podapolipoides: P. andrei, P. hopperae and P. mohanasundarami (see Husband 1995; Ramaraju & Mohanasundaram 1996; Ramaraju & Suresh 1999). Hitherto, only one genus of the subfamily Gomphocerinae was recorded as a host of Podapolipoides, i.e. Orphulella (Feldman-Muhsam and Havivi, 1973). Including the current study, Gomphocerinae includes two host genera (Orphulella and Chorthippus) of Podapolipoides. This extend the number of orthopteran host genera of Podapolipoides to 24 (23 belonging to Acrididae). Therefore, the prevalence of Podapolipoides mites on acridids and pyrgomorphids subfamilies is updated as follows: Oedipodinae (38.6%), Cyrtacanthacridinae (27.2%), Acridinae (11.4%), Oxyinae (9.1%), and Melanoplinae, Gomphocerinae, and Pyrgomorphinae each with 4.5%.
Acknowledgments
We are grateful to Dr. Mustafa Ünal (Abant İzzet Baysal Üniversitesi, Turkey) for help in identifying the host grasshopper. Also, we are thankful to anonymous reviewers for their useful comments and suggestions which highly improved the paper.
References
- Feldman-Muhsam B., Havivi Y. 1973. Podapolipus (Podapolipoides) peruvensis, a new species from Peru (Acarina, Podapolipodidae). Acarologia, 15: 716-723.
- Hajiqanbar H., Joharchi O. 2011. World distribution and host range of Podapolipoides spp. (Acari: Heterostigmatina: Podapolipidae), with the description of a new species. Syst. Parasitol., 78(2): 151-162. https://doi.org/10.1007/s11230-010-9284-5
- Husband R.W. 1995. Four new species of Podapolipoides (Acari: Podapolipidae), ectoparasitic on grasshoppers (Orthoptera: Acrididae) from Africa. Int. J. Acarol., 21(1): 47-61. https://doi.org/10.1080/01647959508684042
- Husband R.W., OConnor B.M. 2014. A new genus and species of Podapolipidae (Acari: Heterostigmata) parasitic on Physonota alutacea (Boheman) (Coleoptera: Chrysomelidae; Cassidinae) in Mexico and Central America. Syst. Appl. Acarol., 19(4): 435-446. https://doi.org/10.11158/saa.19.4.7
- Husband R.W., Zhang Z.-Q. 2002. Wetapolipus jamiesoni gen. nov., spec. nov. (Acari: Podapolipidae), an ectoparasite of the mountain stone weta, Hemideina maori (Orthoptera: Anostostomatidae) from New Zealand. Zootaxa, 125(1): 1-12. https://doi.org/10.11646/zootaxa.125.1.1
- Husband R.W., OConnor B.M., Ochoa R. 2005. Two new species of Orthapolipus (Acari: Podapolipidae), parasites of Central American Tettigoniidae (Hexapoda: Orthoptera), with a rediagnosis of the genus. Int. J. Acarol., 31: 355-362. https://doi.org/10.1080/01647950508683675
- Lindquist E.E. 1986. The world genera of Tarsonemidae (Acari: Heterostigmata): a morphological, phylogenetic, and systematic revision, with a reclassification of family-group taxa in the Heterostigmata. Mem. Ent. Soc. Can., 118(S136): 1-517. https://doi.org/10.4039/entm118136fv
- Lindquist E.E., Sidorchuk E.A. 2019. A new species of Podapolipus (Acari: Heterostigmata: Podapolipidae) from an Australian gryllacridid cricket (Orthoptera), with keys to orthopteran-associated species of the genus. Zootaxa, 4647(1): 115-133. https://doi.org/10.11646/zootaxa.4647.1.11
- Majidi M., Hajiqanbar H., Saboori A. 2019. A new species of Podapolipus (Acari: Podapolipidae) from Iran: the first host record of a podapolipid mite parasitizing the widespread beetle Trachyderma hispida (Coleoptera: Tenebrionidae). Biologia, 74(6): 675-682. https://doi.org/10.2478/s11756-019-00206-1
- Mortazavi A., Hajiqanbar H., Lindquist E.E. 2018. A new family of mites (Acari: Prostigmata: Raphignathina), highly specialized subelytral parasites of dytiscid water beetles (Coleoptera: Dytiscidae: Dytiscinae). Zool. J. Lin. Soc., 184(3): 695-749. https://doi.org/10.1093/zoolinnean/zlx113
- Ostovan H., Saboori A. 1999. Some mites of the families Podapolipidae, Acarophenacidae and Podocinidae in Iran. J. Agric. Sci. Islam. Azad Univ., 5: 81-90.
- Rahmatzaei B., Hajiqanbar H., Mortazavi A., Husemann M. 2021. Global distribution and host range of the endoparasitic mite genus Locustacarus (Acari: Podapolipidae) with description of a new species from Iran parasitizing grasshoppers (Orthoptera: Acrididae). Syst. Parasitol., 98(4): 487-501. https://doi.org/10.1007/s11230-021-09991-3
- Ramaraju K., Mohanasundaram M. 1996. New species of Podapolipus, Podapolipoides and Tarsopolipus (Acari: Podapolipidae) from South India. Int. J. Acarol., 22: 33-41. https://doi.org/10.1080/01647959608684079
- Ramaraju K., Suresh S. 1999. A new species of Podapolipoides (Acari: Podapolipidae) from Tamil Nadu, India. Int. J. Acarol., 25: 195-199. https://doi.org/10.1080/01647959908684153
- Sarangi P., Biswas H., Gupta S.K., Saha G.K. 2012. Description of two new species of ectoparasitic mites of Podapolipus and Podapolipoides (Acari: Podapolipidae) on Oxya sp. from West Bengal, India. J. Asia. Pac. Entomol., 15: 192-195. https://doi.org/10.1016/j.aspen.2011.10.007
- Seeman O.D. 2021. Contrasting species diversification of Eutarsopolipus (Acariformes: Podapolipidae) on Castelnaudia and Notonomus (Coleoptera: Carabidae). Zootaxa, 4971(1): 1-74. https://doi.org/10.11646/zootaxa.4971.1.1
- Walter D.E., Lindquist E.E., Smith I.M., Cook D.R., Krantz G.W. 2009. Order Trombidiformes. In: Krantz G.W., Walter D.E, (Eds.), A manual of acarology (3rd ed.), Lubbock: Texas Tech University Press. p. 233-420.



2021-09-05
Date accepted:
2022-01-19
Date published:
2022-01-28
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Majidi, Maryam; Hajiqanbar, Hamidreza and Saboori, Alireza
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



