Preliminary assessment of mating duration and prolonged post-copulatory associations in Arrenurus water mites (Actinotrichida: Parasitengonina: Hydrachnidiae)
Więcek, Mariusz  1
; Dabert, Jacek
1
; Dabert, Jacek  2
; Wilkinson, Karen3
and Proctor, Heather C.
2
; Wilkinson, Karen3
and Proctor, Heather C.  4
4
1✉ Department of Ecology, Faculty of Environmental Sciences, Czech University of Life Sciences Prague, Kamýcká 129, Prague - Suchdol, 165 00, Czech Republic & Department of Ecosystem Biology, Faculty of Science, University of South Bohemia, Branišovská 31, 37005 České Budějovice, Czech Republic.
2Department of Animal Morphology, Faculty of Biology, Adam Mickiewicz University, Uniwersytetu Poznańskiego 6, PL-61-614 Poznań, Poland.
3Toronto, Ontario, Canada.
4Department of Biological Sciences, University of Alberta, Edmonton, AB, T6G 2E9, Canada.
2022 - Volume: 62 Issue: 1 pages: 84-93
https://doi.org/10.24349/qrp6-e0naOriginal research
Keywords
Abstract
Introduction
Sexual selection theory was initially proposed to explain the existence of sexual dimorphism in species in which there is little ecological differentiation between the sexes (Darwin 1859). The originally described scenarios relate to situations in which males compete for access to females, either through combat or through courtship displays in which females choose the winner (Darwin 1871). However, there are situations where males show morphological, physiological and behavioural adaptations that appear to bypass female choice. Sexual conflict theory argues that in some species, male genitalia and behaviour evolved to force females to mate (= coercion). If this reduces female fitness, this should select for female resistance to male coercive morphology and behaviour (Arnqvist and Rowe 2005). Evolutionary conflicts of interest can apply to which sex controls mating rate, copulation duration, female remating propensity and ultimately fertilization of eggs (Edvardsson and Canal 2006; Tong et al. 2021).
Similarly, although females may benefit from mating with additional'higher quality' males after a first mating, it is in the first male's interest to prevent females from remating (Arnqvist and Rowe 2005; Firman et al. 2017). There are various adaptations to reduce polyandry including use of mating plugs by males, transferring manipulative seminal chemicals, and postcopulatory mate-guarding (Arnqvist and Rowe 2005; Firman et al. 2017). It has been experimentally shown for some species that prolonging the postcopulatory stage of mating increases probability of fertilization of eggs by sperm of a particular male, since more sperm and accessory ejaculate substances are moved into the female's reproductive tract over time (Eberhardt 1985; Arnqvist and Rowe 2005). In response to male adaptations to prolong mating duration, females have evolved counter-adaptations such as struggling in order to dislodge clinging males (Edvardsson and Canal 2006; Bergsten and Miller 2007).
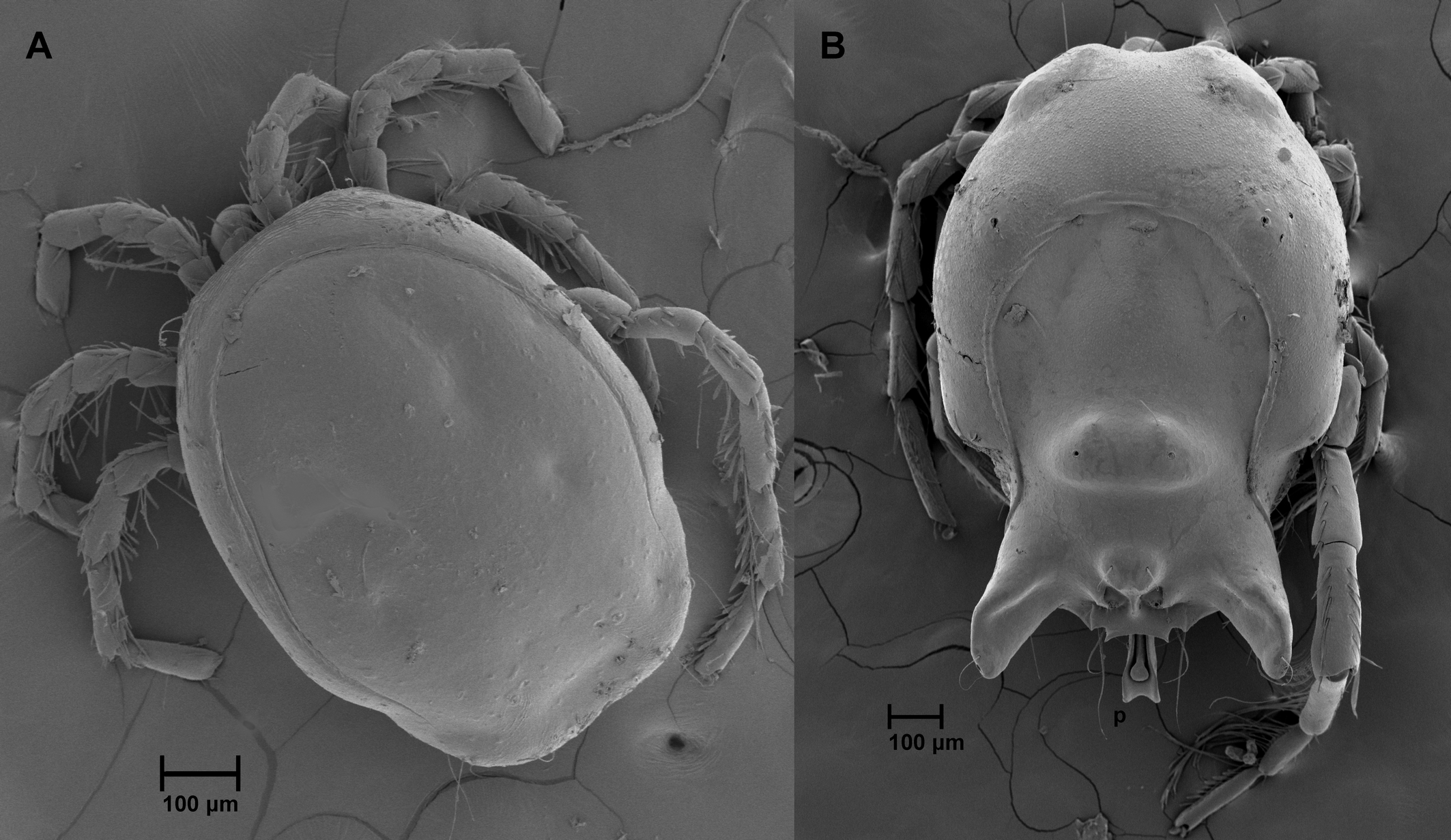
Sperm-transfer behaviour in water mites of the genus Arrenurus Dugès, 1834 (Actinotrichida: Parasitengonina: Hydrachnidiae) ranges from simple to very complex among around the ~1000 described species (Smit 2020; Więcek et al. 2021). In males, the hindbody (= cauda) may be simple and differ only slightly from the female's hindbody (Figure 1A), whereas in other species the males have elongated or complexly sculptured cauda. The most complex cauda are equipped with pygal lobes, various protrusions and an elaborate median intromittent organ (the petiole) (Figure 1B). The male's cauda and more anterior region of the hindbody have pairs of integumentary glands (= glandularia), some of which produce an adhesive secretion during mating that is used to glue the female to the male's hindbody (Jin et al. 1997). Moreover, males of some species have a spur-like extension on the fourth leg that is used to clasp the female's legs and hold her during the early stages of pairing (Jin et al. 1997; Proctor and Wilkinson 2001).
Among Arrenurus species there are two main modes of sperm transfer that appear to differ in the amount of control the female has over moving sperm into her genital tract (Figure 2). In one mode, males that lack an intromittent organ deposit spermatophores on the substrate and lower the female on top of the sperm packets on the spermatophores. The timing and mechanism of sperm uptake by the females of apetiolate species has not been definitively observed. In Arrenurus globator (O.F. Müller), clumps of sperm accumulate between the end of the male's cauda and the female's genital opening, so the female does not seem to have her genital flaps open at the time of spermatophore deposition (Böttger 1962). She may open them later while the male is engaged in post-transfer courtship, as suggested by Böttger (1962) for A. globator, or by grooming sperm into her genital opening after dismounting from the male, as Proctor (2003) suggested might be the case for A. manubriator Marshall. In the second mode, after depositing spermatophores, males gather the sperm on the tip of the petiole and push sperm into the female's genital tract with the petiole (e.g., Arrenurus valdiviensis K.O. Viets, Böttger 1965) (Figure 2D). Sexual conflict is likely more intense in species with males possessing well developed intromittent organs that appear to bypass female choice of sperm uptake, while female choice should be the stronger force of selection in species with males not equipped with such an organ (Proctor and Smith 1994; Proctor and Wilkinson 2001).
Both male and female Arrenurus of at least some species mate repeatedly in their lifespan, and therefore conflicts over mating rates and courtship duration may take place (Proctor and Wilkinson 2001; Proctor 2003). The duration of mating in different Arrenurus species seems to be largely controlled by males through various methods: the male clasping and manoeuvring the female before and during coupling with the use of a spur-like extension on the fourth leg, gluing the female to his hindbody, and (in some species) using the petiole to insert sperm into the female. Separation of male and female at the end of the mating sequence also sometimes seems to be controlled at least in part by the male pushing the female in a way that breaks the binding of the glue (Proctor and Wilkinson 2001). Here, we observed full mating sequences and examined mating durations of Arrenurus species differing in the presence of a well-developed petiole in the male. We predicted that males lacking well-developed petioles should spend more time on post-deposition courtship in order to'encourage' females to move sperm into their genital tracts. In contrast, we predicted that males equipped with petioles that forcefully push sperm into the female genital tract should spend less time on courtship following sperm transfer.





Materials and methods
Mite collection and maintenance
Arrenurus mites were collected by MW using a net with a mesh size of 250 µm in four locations in Poland: ponds at Adam Mickiewicz University (AMU) campus in Poznań (52°27′59.3″N 16°55′57.2″E), Bagnisko lake (53°29′55.7″N 16°28′42.3″E), Łagowo lake (52°19′33.6″N 15°17′16.8″E) and an unnamed pond near Zielona Góra (51°55′03.8″N 15°28′50.6″E), and one location in the Czech Republic in Milíčovský pond (50°01′33.3″N 14°32′22.5″E). Live specimens were sorted by MW under a stereomicroscope in the laboratory.
Adult mites were separated by species and sex, and maintained in tissue culture plates with diameter 5 cm and 3 cm deep at ambient temperatures (~20-25 °C) in the laboratory. Deutonymphs collected in the field were maintained individually in their own well-plates (2 cm diameter, 1 cm deep; hereafter, culture well) until they transformed into adults and could be sexed. Both adults and deutonymphs were fed with ostracods, cladocerans and copepods from laboratory colonies established from material collected in local water bodies. Adult mites were identified to species based on morphology (Gerecke et al. 2016).
Mating observations
Female and male mites (whenever possible, virgin specimens) were held individually in culture wells filled with aged tap water for 24 h before observation. The culture wells had diameter of 2 cm and depth of 1 cm with bottom etched to provide more stable footing for the mites during mating.
We observed entire mating sequences for eight species of Arrenurus: six with petiolate males (A. bicuspidator Berlese, A. bruzelii Koenike, A. claviger Koenike, A. cuspidator (O.F. Müller), A. cf. denticulatus Motaş and A. tricuspidator (O.F. Müller)) and two with apetiolate males (A. globator and A. stecki Koenike). Details of mating behaviour of these species are in Więcek (2016). A culture well with a single female was placed under an Olympus DP71 digital camera with CellD 2.8 software (Olympus Soft Imaging Solutions GmbH) connected to Zeiss Stemi 2000-C stereomicroscope with a moveable light source. Video recording began when a male was introduced to a culture well with a conspecific female. Recording was terminated upon physical separation of individuals after mating. The mating sequences of A. cf. denticulatus and A. globator (three pairings) were not recorded on video, and observations described in Results are based on notes made in real time by MW. All observations took place at ambient temperatures (~20-25 °C).
We divided mating sequences into the following categories, listed in temporal order: (a) pre-pairing stage prior to the female being glued to the male, (b) behaviours from initial pairing to the end of spermatophore deposition and sperm transfer and (c) post-deposition behaviour. In all categories except pre-pairing, the female was attached to the male. For statistical analysis we calculated the following: (a) pre-pairing duration = total time from introduction of a male into a female's well to the female being glued onto the male; (b) duration of behaviours from initial pairing to the end of sperm transfer (deposition of spermatophores was assumed based on male'dipping' movements); (c) post-deposition behaviour = time male and female were still attached to each other after spermatophore deposition/sperm transfer; (d) total duration of mating = total time that male and female were attached (b+c); Unlike in Lundblad (1929) and Więcek (2016), we treated the male behaviours of lateral waving and alternate bending of left and right legs as part of post-deposition behaviour.
SEM images of couples in copula were made in the Department of Earth and Atmospheric Sciences, University of Alberta. After dehydration through an alcohol-HMDS (hexamethyldisilazane) series, mites were mounted on stubs using double-sided tape, sputter coated with gold, and examined using a JEOL 630 I field emission scanning electron microscope (SEM). SEM photographs were edited in Photoshop 6.0.
Statistical analyses
Data were first tested for significant deviations from normal distribution with Shapiro-Wilk tests. The Fligner-Kileen test was applied to test for equal coefficient of variation in two samples. In each analysis, behavioural data did not fulfill at least one of the assumptions of a parametric test. Therefore, we applied non-parametric tests. Mann-Whitney U tests were used for comparing the two groups of species (apetiolate vs petiolate) for durations of particular stages of mating. A Kruskal-Wallis test was performed for each stage of mating, followed by Dunn's post hoc test in order to assess between which species pairs statistically signifficant differences are observed. For each stage of mating 55 Dunn's tests were run (results in Supplemental Tables S1-S4). The Bonferroni correction was not used here, as it is very conservative, and we view these results as a preliminary assessment for further investigation (Perneger 1998; Cabin and Mitchell 2000). P < 0.05 was used as the upper cutoff for significant differences. Data on differences in percentage of time spent on post-deposition behaviours were log transformed prior to analysis. For each species of Arrenurus we used 43 created box plots to show medians, 25-75 percent quartiles and ranges of minimal and maximal values. All analyses were run with the statistical software PAST 4.03 (Hammer et al. 2001).
In at least some species of Arrenurus, the duration of the pre-pairing stage of mating can be affected by female age and whether females had been previously exposed to males (HP, pers. obs.). Because mating status and age of mites were not standardized, we excluded pre-pairing duration from statistical tests and only present summary statistics. In order to obtain a larger sample size to test our predictions, the results were pooled with the data obtained by Proctor and Wilkinson (2001) for three North American species: petiolate A. sp. nr. reflexus Marshall and apetiolate A. manubriator and A. rufopyriformis Habeeb.
Results
In this study 60 pairings of eight Arrenurus species from Europe (A. bicuspidator, A. bruzelii, A. claviger, A. cuspidator, A. cf. denticulatus, A. globator, A. stecki, A. tricuspidator) and 3 species from Canada (A. manubriator, A. sp. nr. reflexus, A. rufopyriformis) were analysed (Table 1).



Box plots presenting durations of particular stages of mating are shown in Figures 3 and 4. Total mating duration ranged from 29.00 ± 5.00 minutes in apetiolate A. stecki to 630.00 ± 79.00 minutes in petiolate A. bicuspidator (Table 1). Total duration of mating in petiolate species was significantly longer (mean = 394.82 ± 22.02 min, N = 34 pairings) than in apetiolate species (mean = 94.04 ± 10.45 min, N = 26 pairings) (Mann-Whitney U Test, P < 0.0001). There was no significant difference in duration of behaviours from initial pairing to the end of spermatophore deposition and sperm transfer between petiolate (mean = 26.03 ± 2.48 min, N = 33 pairings) and apetiolate (mean = 46.11 ± 9.04 min, N = 26 pairings) species (Mann-Whitney U Test, P = 0.33). Petiolate species spent significantly more time on post-deposition behaviour (mean = 366.42 ± 22.08 min, N = 33 pairings) than apetiolate species (mean = 47.88 ± 6.67 min, N = 26 pairings) (Mann-Whitney U Test, P < 0.0001, both as measured in minutes and as a percentage of total mating time). Kruskal-Wallis tests showed overall significance (P < 0.05) in the differences between the examined species for the total duration of mating (Kruskal-Wallis H (10) = 54.23, P < 0.0001), duration of behaviours from initial pairing to the end of spermatophore deposition and sperm transfer (Kruskal-Wallis H (10) = 42.21, P < 0.0001) and duration of post-deposition behaviours (Kruskal-Wallis H (10) = 49.53, P < 0.0001). Post-hoc tests did not confirm significant differences (Dunn's test, P < 0.05) for most apetiolate-petiolate species pairs for the duration of behaviours from initial pairing to the end of spermatophore deposition and sperm transfer (Table S2). In contrast, significant differences were found between apetiolate-petiolate species pairs for the total duration of mating (except for A. globator - A. bruzelii (P = 0.062), A. manubriator - A. bruzelii (P = 0.085) and A. manubriator - A. sp. nr. reflexus (P = 0.053)) (Table S1). Moreover, clear differences throughout apetiolate-petiolate species pairs were found for duration of post-deposition behaviours with only a few exceptions: A. globator - A. tricuspidator (P = 0.055), A. globator - A. bruzelii (P = 0.14) and A. globator - A. sp. nr. reflexus (P = 0.086) (Table S3). For post-deposition behaviour expressed as percentage of the total time spent on mating all comparisons between apetiolate-petiolate species pairs were statistically significant (Dunn's test, P < 0.05) (Table S4).






Discussion
We had expected that Arrenurus males with a well-developed intromittent structure (the petiole) would spend less time in courtship behaviours after sperm transfer than apetiolate males, which appear to have less coercive control over whether sperm moves into the female's reproductive tract. Contrary to our prediction, males of petiolate species spent more time in post-deposition behaviours than males of apetiolate species, both absolutely (mean number of minutes) and relatively (as percentage of total time spent mating) (Table 1). Although both petiolate and apetiolate species exhibited prolonged post-deposition associations, in petiolate species post-deposition behaviour made up < 90% of total mating time in contrast to ~50% in apetiolate species. However, there was a considerable variation in time spent on post-transfer behaviours among apetiolate species (Table 1).
Why might males of both petiolate and apetiolate species show such long periods of attachment to females after transferring sperm? Protracted courtship after sperm transfer, or simply lengthy male attendance, occurs in many animal groups and is thought to reduce the probability of the female being inseminated by a subsequent male (mate guarding, Arnqvist and Rowe 2005). For example, Radwan and Siva-Jothy (1996) demonstrated that males of the terrestrial mite Rhizoglyphus robini Claparédè (Acaridae) increase fertilization of eggs by their own sperm by prolonging attachment to a female and preventing other males from mating with her. Male courtship and postmating behaviours may also encourage females to preferentially use sperm of a particular male and thus can be under selection by cryptic female choice (Firman et al. 2017). Conversely, in this stage of mating males can chemically manipulate a female's physiology by stimulating oviposition or decreasing production of sex pheromones attracting males (Arnqvist and Rowe 2005; Mazzi et al. 2009). We hypothesize that this could be an explanation of the functions of both the periods of motionlessness and the courtship behaviour after sperm transfer in the examined species of Arrenurus.
Why might petiolate males spend significantly more time in post-deposition behaviours than apetiolate ones? Males of apetiolate and petiolate species may differ in the amount of sperm competition they face, if, in addition to pushing sperm into the female's genital opening, the petiole also acts to remove or displace sperm deposited by previous males as do, e.g., the genitalia of male damselflies (Waage 1979). If so, the greater duration of post-transfer behaviour in petiolate species may reflect the lengths to which such males must go to increase the likelihood that females will use their sperm and not that of a previous or subsequent mate. To test this idea, the paternity of egg clutches could be assessed in matings in which the second male is allowed to complete the entire mating sequence or is interrupted at various times after sperm transfer.
Finally, although optimal copulation duration can be a subject of conflict between the sexes, which may be the case in the examined Arrenurus, it has been observed that females can sometimes benefit from prolonged copulations, e.g., if they receive larger ejaculates that can be used in part for nutrition (e.g. bruchid beetles, Edvardsson and Canal 2006). Therefore, further research should be done to determine if prolonged post-copulatory associations in Arrenurus mites influence female's lifetime fecundity as well as male's paternity.
The strength of our statistical interpretation of differences in mating durations was limited by sample size. Laboratory observations of mating behaviour in Arrenurus mites are affected by several factors. First, most Arrenurus species have a complex life cycle with larvae that parasitize insect hosts, which makes establishing laboratory colonies of mites challenging except for some rare cases of species that forego larval parasitism such as A. manubriator (see Smith and Hagman 2002). Second, field-collected deutonymphs raised to adults in captivity do not always show interest in mating (MW, pers. obs.), which may be caused by inappropriate food offered (prey species of water mites in general are largely unknown, Vasquez et al. 2021). Third, particular species collected in the field engage in mating in different periods of the year, in some cases only through a few weeks in season (MW, pers.obs). Finally, some species from springs and streams may not show interest in mating in standing water in laboratory conditions.
Acknowledgements
We thank Dr. G. Greczka, Poznan University of Medical Sciences, Poland, Samuel Dijoux, Department of Ecosystem Biology, University of South Bohemia in České Budějovice, Czechia, and Andrew Cook, University of Alberta, Edmonton, Canada, who provided statistical advice. The study is part of the International PhD Programme ''From Genome to Phenotype: A Multidisciplinary Approach to Functional Genomics'' (MPD/2010/3) funded by the Foundation for Polish Science (FNP). Additional funding was provided by a Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to HP, and the Internal Grant Agency of the Faculty of Environmental Sciences, Czech University of Life Sciences Prague (grant no. 42900/1312/3166).
Conflict of interest
The authors have no conflict of interest to declare.
Data statement
Raw data are available from the corresponding author upon request, with the provision that this article will be cited in any publication using the raw data.
Preprint version
A preprint version has been deposited in bioRxiv
https://www.biorxiv.org/content/10.1101/2021.05.23.445345v1 ![]()
References
- Arnqvist G., Rowe L. 2005. Sexual Conflict. Princeton: Princeton University Press. pp. 352. https://doi.org/10.1515/9781400850600-008
- Bergsten J., Miller K.B. 2007. Phylogeny of diving beetles reveals a coevolutionary arms race between the sexes. PLoS One, 2(6): e522. https://doi.org/10.1371/journal.pone.0000522
- Böttger K. 1962. Zur Biologie und Ethologie der einheimischen Wassemilben Arrenurus (Megaluracarus) globator (Mull.), 1776 Piona nodata nodata (Mull.), 1776 und Eylais infundibulifera meridionalis (Thon.), 1899 (Hydrachnellae, Acari). Zool. Jahrb. Abt. Syst. Geog. Biol. Tiere, 89: 501-584.
- Böttger K. 1965. Zur Ökologie und Fortpflanzungsbiologie von Arrenurus valdiviensis K. O. Viets 1964 (Hydrachnellae, Acari). [Ecology and reproductive biology of Arrenurus valdiviensis K. O. Viets 1964 (Hydrachnellae, Acari)]. Z. Morphol. Oekol. Tiere, 55: 115-141. https://doi.org/10.1007/BF00399510
- Cabin R.J., Mitchell R.J. 2000. To Bonferroni or not to Bonferroni: When and how are the questions. Bull. Ecol. Soc. Am., 81: 246-248.
- Darwin C. 1859. On the Origin of Species by Means of Natural Selection. 1st edition. Murray, London.
- Darwin C. 1871. The Descent of Man and Selection in Relation to Sex. 1st edition. Murray, London. https://doi.org/10.1037/12293-000
- Eberhardt W.G. 1985. Sexual Selection and Animal Genitalia. Cambridge. Mass.: Harvard University Press. https://doi.org/10.4159/harvard.9780674330702
- Edvardsson M., Canal D. 2006. The effects of copulation duration in the bruchid beetle Callosobruchus maculatus. Behav. Ecol., 17: 430-434. https://doi.org/10.1093/beheco/arj045
- Firman R.C., Gasparini C., Manier M.K., Pizzari T. 2017. Postmating female control: 20 years of cryptic female choice. Trends Ecol. Evol., 32: 368-382. https://doi.org/10.1016/j.tree.2017.02.010
- Gerecke R., Gledhill T., Pešić V., Smit H. 2016. Süßwasserfauna Von Mitteleuropa, Bd 7/2-3. Chelicerata: Acari III. Springer, Heidelberg. https://doi.org/10.1007/978-3-8274-2689-5
- Hammer Ø., Harper D.A.T., Ryan P.D. 2001. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron., 4: 1-9. Available from: https://palaeo-electronica.org/2001_1/past/issue1_01.htm

- Jin D., Li L., Wiles R. 1997. The structure and evolution of male cauda and petiole with a cladistic analysis of Chinese species of the genus Arrenurus (Acari: Arrenuridae). Syst. Appl. Acarol., 2: 195-209. https://doi.org/10.11158/saa.2.1.29
- Lundblad O. 1929. Über den Begattungsvorgang bei einigen Arrhenurus-Arten. [Mating behaviour of a few Arrenurus species]. Z. Morphol. Oekol. Tiere, 15: 705-722. https://doi.org/10.1007/BF00407388
- Mazzi D., Kesäniemi J., Hoikkala K., Klappert K. 2009. Sexual conflict over the duration of copulation in Drosophila montana: why is longer better? BMC Evol. Biol., 9: 132. https://doi.org/10.1186/1471-2148-9-132
- Perneger T.V. 1998. What's wrong with Bonferroni adjustments. BMJ, 316: 1236. https://doi.org/10.1136/bmj.316.7139.1236
- Proctor H. 2003. Male mating effort and female remating in the water mite Arrenurus manubriator (Acari: Arrenuridae). In: Smith I.M. (Eds.). An Acarological Tribute to Dave Cook. West Bloomfield: Indira Publishing House. ISBN 0-930337-18-2 (refereed). p. 211-221.
- Proctor H.C., Smith B.P. 1994. Mating behaviour of the water mite Arrenurus manubriator (Acari: Arrenuridae). J. Zool., 232: 473-483. https://doi.org/10.1111/j.1469-7998.1994.tb01588.x
- Proctor H., Wilkinson K. 2001. Coercion and deceit: water mites (Acari: Hydracarina) and the study of intersexual conflict. In: Halliday R.B., Walter D.E., Proctor H.C., Norton R.A. and Colloff M.J. (Eds.). Acarology: Proceedings of the 10th International Congress. Melbourne: CSIRO Publishing. p. 155-169.
- Radwan J., Siva-Jothy M.T. 1996. The function of post-insemination mate association in the bulb mite, Rhizoglyphus robini. Anim. Behav., 52: 651-657. https://doi.org/10.1006/anbe.1996.0209
- Smit H. 2020. Water mites of the world, with keys to the families, subfamilies, genera and subgenera (Acari: Hydrachnidia). Monogr. Nederl. Entomol. Ver., 12: 1-774.
- Smith, B.P., Hagman, J. 2002. Experimental evidence for a female sex pheromone in Arrenurus manubriator (Acari: Hydrachnida; Arrenuridae). Exp. Appl. Acarol., 27: 257-263. https://doi.org/10.1023/A:1023328428716
- Tong X., Wang P.-Y., Jia M.-Z., Thornhill R., Hua B.-Z. 2021. Traumatic mating increases anchorage of mating male and reduces female remating duration and fecundity in a scorpionfly species. Proc. R. Soc. London, Ser. B, 288: 20210235. https://doi.org/10.1098/rspb.2021.0235
- Vasquez A.A., Mohiddin O., Li Z., Bonnici B.L., Gurdziel K., Ram J.L. 2021. Molecular diet studies of water mites reveal prey biodiversity. PLoS One, 16(7): e0254598. https://doi.org/10.1371/journal.pone.0254598
- Waage J.K. 1979. Dual function of the damselfly penis: sperm removal and transfer. Science, 203: 916-918. https://doi.org/10.1126/science.203.4383.916
- Więcek M. 2016. Effects of the evolution of intromission on courtship complexity and male and female morphology: water mites of the genus Arrenurus (Acari; Hydrachnida) from Europe and North America [Phd Thesis]. Poznań, Poland: Adam Mickiewicz University. pp. 147.
- Więcek M., Broda Ł., Proctor H., Dabert M., Smith B.P., Dabert J. 2021. Species boundaries among extremely diverse and sexually dimorphic Arrenurus water mites (Acariformes: Hydrachnidiae: Arrenuridae). bioRxiv: https://doi.org/10.1101/2021.04.04.438411



2021-12-03
Date accepted:
2022-01-10
Date published:
2022-01-20
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Więcek, Mariusz; Dabert, Jacek; Wilkinson, Karen and Proctor, Heather C.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



