A new species of Paragigagnathus Amitai & Grinberg (Mesostigmata: Phytoseiidae) from Iran
Arjmandi-Nezhad, Alireza1
; Kreiter, Serge  2
; Saboori, Alireza3
and Ravan, Sultan4
2
; Saboori, Alireza3
and Ravan, Sultan4
1Department of Plant Protection, College of Agriculture, University of Zabol, Zabol, Iran.
2Institut Agro (Montpellier SupAgro), UMR CBGP INRAE/ IRD/ CIRAD/ IA (SupAgro), Univ Montpellier, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France.
3Department of Plant Protection, Faculty of Agriculture, University of Tehran, Karaj, Iran.
4✉ Department of Plant Protection, College of Agriculture, University of Zabol, Zabol, Iran.
2022 - Volume: 62 Issue: 1 pages: 48-57
https://doi.org/10.24349/8dra-mc90ZooBank LSID: 5A740452-7CC1-4592-BF45-E936355E3787
Original research
Keywords
Abstract
Introduction
The family Phytoseiidae is widespread, present on all continents except Antarctica, and it consists of about 2,500 valid species in 94 genera and three subfamilies (Demite et al. 2021). They are active predators on phytophagous mites and small insects, like thrips and whiteflies, on cultivated plants and plants of the natural vegetation.
During 2018-2020, phytoseiid mite fauna of Sistan and Baluchestan province (Iran) was studied. The province of Sistan and Baluchestan (25°03'–31°27' N, 58°50'–63°21' E) is the second largest province in Iran with an area of 187, 502 km². It is in the southeast of the country, bordering South Khorasan province in the north, Kerman province and Hormozgan province in the west, the Gulf of Oman in the south, and Afghanistan and Pakistan in the east. The province of Sistan and Baluchestan is one of uninvestigated provinces of Iran with regard to phytoseiid mite fauna. It is necessary to mention, the territory of Iran is important from a zoogeographical point of view, as it straddles several major ecoregions of the world including the Palaearctic, Ethiopian and Oriental Realms. Iran is also part of the Irano-Anatolian hotspot of biodiversity with great floristic and faunistic diversities (Esmaeili et al. 2017).
The genus Paragigagnathus Amitai and Grinberg, 1971, consists of two species groups, desertorum and strunkovae, which based on the position of setae ST3 (on or off sternal shield, respectively), and include 12 species in total (Demite et al. 2021; Kreiter et al. 2021). The strunkovae species group is composed of seven species: P. strunkovae (Wainstein 1973), P. cataractus (Ueckermann and Loots 1988), P. molestus (Kolodochka 1989), P. bidentatus (Kuznetsov 1994), P. madinaensis Alatawi, Kamran and Basahih, in Alatawi et al. (2015), P. iraniensis Khanjani, Karimi, Asali-Fayaz and Ueckermann, in Khanjani et al. (2016), P. philippei Kreiter et al. 2021. The desertorum species groups composed of five species: P. tamaricis Amitai and Grinberg (1971), P. insuetus (Livshitz and Kuznetsov 1972), P. desertorum Amitai and Swirski (1978), P. amantis (Chaudhri, Akbar and Rasool, in Chaudhri et al. 1979), P. namibiaensis (Ueckermann and Loots 1988).
This study aims to describe a new species, Paragigagnathus sistaniensis Kreiter, Arjmandi-Nezhad and Saboori n. sp. based on the material collected from Tamarix sp. (Tamaricaceae) and Haloxylon sp. (Amaranthaceae) in southeastern Iran.
Material and methods
The material used in this study were collected by the senior author and the specimens were mounted in permanent slides. Species studied here are represented as adults. Foliage samples were taken from different regions, north of Sistan and Baluchestan province during 2018–2020. Mites were extracted from samples with two methods either by using Berlese-Tullgren funnels set over vials with 75% ethanol or washing method of Boller (1984). When the sampling sites were close to the laboratory, the foliage samples were transferred to plastic bags and immediately were moved to the laboratory whereas if they were located at a far distance, to protect the mites the washing processes of the host plants were performed in the sampling site. The collected mites were cleared in lactophenol fluid and permanently mounted on slides in Hoyer's medium (Walter and Krantz 2009). The slides were placed in an oven at 45 °C for two weeks. The specimens were examined under a phase-contrast microscope (BX51, Olympus, Japan) equipped with a drawing tube and digital camera (Olympus U-TVO.63XC). The setal nomenclature system adopted was that of Lindquist and Evans (1965) and Lindquist (1994) as adapted by Rowell et al. (1978) and Chant and Yoshida-Shaul (1989) for the dorsal idiosoma and by Chant and Yoshida-Shaul (1991) for the ventral idiosoma. Pore (solenostome) and poroid (lyrifissure) notations are those of Athias-Henriot (1975). Macrosetal notations (St = tarsal macroseta) are those of Muma and Denmark (1970). Types of spermatheca or insemination apparatus are those proposed by Denmark and Evans (2011). Numbers of teeth on the fixed and movable cheliceral digits do not include the respective apical teeth. Setae not referred to in results section should be considered as absent. The classification system follows Chant and McMurtry (2003 and 2007). All measurements are given in micrometers (μm). The average measurements are followed by ranges of the measurements in parentheses. We used the following abbreviations: dorsal length (Dsl); dorsal width (Dsw); solenostomes (gd); genital shield length (Gensl); genital shield width (Gensw); primary metapodal shield length (Lisl); primary metapodal shield width (Lisw); secondary metapodal shield length (Sisl); ventri-anal shield length (Vsl); ventri-anal shield width (Vsw); spermatheca calyx length (scl); spermatheca calyx width (scw); fixed digit length (Fdl); movable digit length (Mdl).
Results
Subfamily Amblyseiinae Muma
Amblyseiinae Muma 1961: 273.
Tribe Neoseiulini Chant & McMurtry
Neoseiulini Chant & McMurtry 2003: 6.
Paragigagnathus Amitai & Grinberg
Paragigagnathus Amitai & Grinberg 1971: 327, Chant & McMurtry 2003: 39, Moraes et al. 2004: 158.
Type species: Paragigagnathus tamaricis Amitai & Grinberg 1971: 327.
Paragigagnathus sistaniensis Kreiter, Arjmandi-Nezhad and Saboori n. sp.
ZOOBANK: BCDBA472-6B38-416F-8543-7631A27D2A3E ![]()
Diagnosis
All of dorsal setae set on prominent tubercles, except j1; dorsal setae short, setiform and subequal in length, J5 and Z5 serrate; setae ST3 and ST4 set on soft cuticle; movable digit of chelicera with one tooth, fixed digit of chelicera with two teeth; macroseta present on leg IV; anterior margin of ventrianal shield straight, with three pairs of preanal setae and preanal pores.
Description
Female (n = 10)
Figures 1 & 3.



Dorsal idiosoma — (Figure 1A & Figure 3B): Dorsal shield strongly sclerotized and ornamented, 320 (320–340) long and 190 (190–220) wide at level of setae Z1, constricted between Z1 and S2, with 17 pairs of short, smooth, sharp tipped and setiform setae, except J5 and Z5 serrated, all dorsal setae set on tubercles, except j1, with five pairs of solenostomes (gd1, gd2, gd6, gd8, gd9) and 13 pairs of visible poroids. Length of setae: j1 14 (13–15), j3 15 (15–17), j4 14 (14–15), j5 16 (14–16), j6 15 (14–16), J2 16 (16–18), J5 6 (6–8), z2 15 (15–17), z4 16 (16–18), z5 16 (14–16), Z1 17 (17–18), Z4 16 (16–18), Z5 20 (20–21), s4 18 (18–19), S2 17 (16–17), S4 17 (16–18), S5 19 (18–20), r3 14 (13–15), R1 13 (12–14).
Peritreme and peritremal shield — (Figure 1E & Figure 3C): extending to the level of setae j1, 174 (170–176) long; peritremal shield fused with dorsal shield at level of r3.
Ventral idiosoma — (Figure 1B): sternal shield smooth, convex anteriorly, with two pairs of subequal setae in length ST1 and ST2 12 (11–13) and one pair of pores (iv1); distances between ST1 – ST1 38 (35–40), ST2 – ST2 44 (39–45). Poroid iv2, iv3 and setae ST3, ST4 present on soft cuticle, 15(11–15) long. Genital shield 87 (88–90) long, 58 (53–59) wide at level setae ST5 and 40 (37–40) at posterior corners, distance between ST5 – ST5 55 (49–55), one pair of pores iv5 present laterally to genital shield at the level of setae st5 on soft cuticle. Ventrianal shield 105 (105–107) long, width at level ZV2 34 (34–35) and broadest at anus 55 (52–56), anterior margin straight, with three pairs of preanal setae JV1 12 (9–12), JV2 12 (9–12), ZV2 12 (9–12) and one pair of pores (gv3), distance between gv3 – gv3 10 (9–11). Four pairs of smooth, setiform and subequal setae [ZV1 12 (10–13), ZV3 12 (10–12), JV4 13 (12–15), JV5 12 (12–15)]. Two pairs of metapodal platelets; length of primary metapodal plate 47 (45–48) and length of secondary metapodal plate 22 (20–23), and five pairs of poroids ivo present on the membrane surrounding the ventrianal shield. No poroids ivp visible posteriorly to the ventrianal shield. Several cases of larvae inside the body of females were observed as viviparity phenomenon (Figure 3A), this phenomenon is common among Paragigagnathus species) Amitai & Grinberg 1971).
Spermatheca — (Figure 1C & Figure 3D): calyx dish-shaped; atrium not adjacent to the calyx and between of these considered as a neck, major duct thick, minor duct not visible.
Chelicera — (Figure 1D & Figure 3E): fixed digit 23 (23 – 25) long and with two small teeth and pilus dentilis; movable digit 20 (20 – 23) long with one tooth.
Legs — Chaetotaxy: Leg I: coxa 0 0/1 0/1 0, trochanter 1 1/2 0/1 0, femur 2 2/1 3/2 1, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 1/2 0/1 0, femur 2 2/1 3/0 2, genu 1 2/0 2/0 2, tibia 1 2/0 2/0 2. Leg III: coxa 0 0/1 0/1 0, trochanter 1 1/1 0/1 1, femur 1 2/1 0/1 1, genu 0 2/1 2/0 2 , tibia 0 2/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 1/2 0/1 0, femur 1 2/1 0/1 1, genu 1 2/0 2/1 1, tibia 1 2/0 2/1 1; all setae setiform and needle-like. Basitarsus of the leg IV with one thick and sharp tipped macroseta; StIV 21(21 – 22) long (Figure 1F & Figure 3I).
Male (n = 10).
Figures 2 & 3



Dorsal idiosoma — (Figure 2A): dorsal shield 260 (260–270) long and 160 (160–170) wide at level of R1, anteriorly lineate, and reticulated posteriorly, with five pairs of solenostomes (gd1, gd2, gd6, gd8 and gd9) and 13 visible pairs of poroids (id1, id2, id3, id4, id5, id6, idl1, idl2, idl4, idx, idm2, idm3, idm4) but probably more present. The dorsal shield bears 17 pairs of dorsal setae and two pairs of sub-lateral setae which are inserted on the dorsal shield: j1 11 (11–12), j3 13 (12–13), j4 11 (10–13), j5 11 (10–12), j6 11 (10–12), J2 12 (13–14), J5 4 (4–5), z2 13 (12–13), z4 13 (12–13), z5 11 (10–12), Z1 15 (13–15), Z4 14 (13–14), Z5 17 (17–19), s4 13 (12–13), S2 15 (13–15), S4 15 (13–15), S5 15 (13–15), r3 15 (14–15), R1 13 (13–14). All setae sharp-tipped and smooth, except J5 and Z5 slightly serrated.
Peritreme and peritremal shield — (Figure 2A): peritreme extending to level of setae j3; peritremal shield fused with dorsal shield at level of s4 – r3 level.
Ventral idiosoma — (Figure 2B): sternogenital shield smooth with five pairs of setae (ST1 to ST5) and three pairs of poroids iv1, iv2 and iv3. Distances between ST1 – ST1 52 (50–55), ST2 – ST2 55 (50–58), ST3 – ST3 55 (51–58), ST1 – ST5 114 (112–118), ST4 – ST4 36 (31–40), ST5 – ST5 31 (30–34). Ventrianal shield (Figure 3H) anteriorly striated with three pairs of pre-anal setae (JV1, JV2 and ZV2) and one pair of small crateriform pores gv3, posteromedian to JV2, one pair of iv5 (close to anterior margin of ventrianal shield). Ventrianal shield 100 (95–110) long, 120 (125–130) wide at level of anterior corners and 59 (50–65) wide at level of paranal setae. Soft cuticle surrounding ventrianal shield with three pair of pores, JV5 pointed and smooth 9 (9–11) long.
Chelicera — (Figure 2D & Figure 3F): fixed digit 18 (18 – 20) long, with two teeth and movable digit 16 (16 – 18) long with one tooth. Spermatodactyl L-shaped with toe slightly bulbous tip.
Legs — Chaetotaxy as in female; all setae setiform and needle-like. Basitarsus of the leg IV with one macroseta; StIV 17 (17 – 19) long.



Type material and depository
Holotype, female, Nimrooz city, Sistan region, Sistan and Baluchestan Province, Iran, 31°07′07ʺ N, 61°24′39ʺ E, alt. 480 m, 18 May, 10 September and 1 October 2018, on Tamarix sp. (Tamaricaceae). Paratypes (52): seven females and four males same data as holotype; three females and two males, Firouzehie Village, Hamoon City, 30°57′39ʺ N, 61°28′53ʺ E, alt. 480 m, 25 May 2020, on Haloxylon sp. (Amaranthaceae); seven females, Zahak City, 30°52′54ʺ N, 61°40′17ʺ E, alt. 491 m, 14 May, 2 July and 9 August 2018, on Tamarix sp.; five females, Khamak Village, Zabol City, 30°55′52ʺ N, 61°37′05ʺ E, alt. 487 m, 20 August 2018 and 15 June 2019, on Tamarix sp.; one female and one male, Zabol City, 31°05′08ʺ N, 61°34′00ʺ E, alt. 486 m, 20 June 2019, on Tamarix sp.; eight females and three males, Hamoon lake, 31°20′17ʺ N, 61°21′34ʺ E, alt. 475 m, 7 July 2018 and 11 August 2019, on Tamarix sp.; seven females and four males, Hirmand city, 31°08′ 41″ N, 61°47′27ʺ E, alt. 489 m, 26 July, 15 September and 7 May 2018, on Haloxylon sp. All specimens collected by A. Arjmandi-Nezhad.
The holotype, 10 paratypes (7 females and 3 males) are deposited in the Acarological Collection, Jalal Afshar Zoological Museum (JAZM), Department of Plant Protection, Faculty of Agriculture, University of Tehran, Karaj, Iran; 20 paratypes (17 females and 3 males) are deposited in the mite collections of Montpellier SupAgro conserved in UMR CBGP INRAE/ IRD/ CIRAD/ IA (SupAgro) and 23 paratypes (15 females and 8 males) are deposited in the collection of the Department of Plant Protection, College of Agriculture, Zabol University, Zabol, Iran.
Etymology
The specific epithet is derived from the type locality, Sistan region, Sistan and Baluchestan Province, Iran.
Discussion
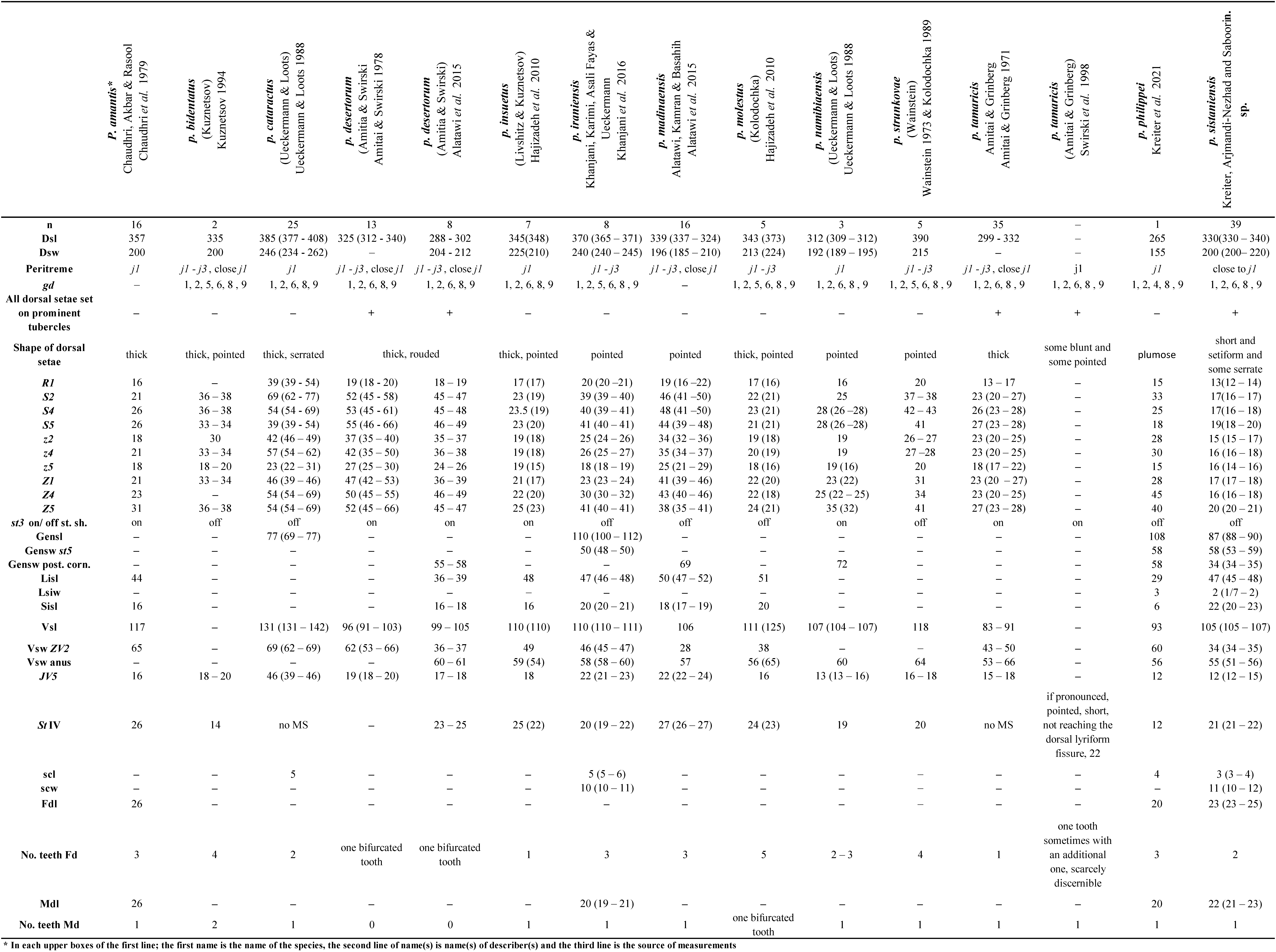


Paragigagnathus sistaniensis Kreiter, Arjmandi-Nezhad and Saboori n. sp. belongs to the strunkovae species group by insertion of seta ST3 off sternal shield and differ from all other species in this group by its all of dorsal setae set on prominent tubercles. This new species is morphologically very similar to P. desertorum Amitai and Swirski (1978) because both share the following characters: all of dorsal setae set on prominent tubercles, disk shaped calyx, three pairs of preanal setae, and ST4 set on soft cuticle. However it differs from P. desertorum by having: J5 and Z5 serrate, dorsal setae short and setiform, movable digit of chelicera with one tooth, fixed digit of chelicera with two teeth, presence of macroseta on the basitarsus of the leg IV and anterior margin of ventrianal shield straight. Paragigagnathus sistaniensis n. sp. also closely resembles to P. amantis (Chaudhri et al. 1979 in Honey et al. 2015) in having all of dorsal setae short/medium and subequal in length, disk shaped calyx, ST4 sets on soft cuticle, three pairs of preanal setae, anterior margin of ventrianal shield straight and macroseta on basitarsus of the leg IV present. However it differs from the later by following characters: setae ST3 sets on soft cuticle, setae Z5 and J5 serrated, post-anal seta equal to para-anal setae and fixed digit of chelicera with two teeth. Comparison of characters of the 12 species of Paragigagnathus is given in Table 1.
Acknowledgements
We express our sincere thanks to subject editor Dr. Farid Faraji (MITOX Consultants, Science Park 406, 1098 XH Amsterdam, The Netherlands) for his valuable comments and recommendations on the earlier version of the manuscript which highly improved the quality of the manuscript. We would like to thank the anonymous reviewers for their critical reading and valuable comments of the manuscript. This study was partly supported by University of Zabol, Zabol, Sistan and Baluchestan, Iran and partly from Department of Plant Protection, Faculty of Agriculture, University of Tehran, Karaj, Iran which is greatly appreciated.
References
- Alatawi F.J., Kamran M., Basahih J. 2015. First record of the genus Paragigagnathus Amitai and Grinberg, 1971 (Mesostigmata: Phytoseiidae) from Saudi Arabia with description of a new species. J. Nat. Hist., 41: 1-9. https://doi.org/10.1080/00222933.2015.1082656
- Amitai S., Grinberg T. 1971. Description of a new phytoseiid genus and species (Acarina: Mesostigmata) from Israel. Israel J. Entomol., 6: 327-335.
- Amitai S., Swirski E. 1978. A new genus and new records of phytoseiid mites (Mesostigmata: Phytoseiidae) from Israel. Israel J. Entomol., 12: 123-143.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. - Le relevé organotaxique de la face dorsale adulte (Gamasides, Protoadénique, Phytoseiidae). Acarologia, 17: 20-29.
- Berlese A. 1916. Centuria prima di Acari nuovi. Redia., 12: 19-66.
- Boller, E.F. 1984. Eine einfache Ausschwemm methode zur schellen Erfassung von Raumilben, Trips und anderen Kleinarthopoden im Weinbau.Zeitschr. Obstund Weinbau, 120: 249-255.
- Chant D.A., McMurtry J.A. 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part I. Neoseiulini new tribe. Internat. J. Acarol., 29: 3-46. https://doi.org/10.1080/01647950308684319
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acarina: Mesostigmata). Indira Publishing House, West Bloomfield, Michigan, USA, 220 pp.
- Chant D.A., Yoshida-Shaul E. 1989. Adult dorsal setal patterns in the family Phytoseiidae (Acari: Gamasina).Internat. J. Acarol., 15: 219-232. https://doi.org/10.1080/01647958908683852
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina).Intern. J. Acarol., 17(3): 187-199. https://doi.org/10.1080/01647959108683906
- Chaudhri W.M., Akbar S., Rasool A. 1979. Studies on the predatory leaf inhabiting mites of Pakistan. University of Agriculture, Faisalabad, Pakistan, 243 pp.
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R. de C. 2021. Phytoseiidae Database. [internet] [10 March 2021]. Available from: http://www.lea.esalq.usp.br/phytoseiidae
 .
. - Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, 451 pp.
- Esmaeili, H.R., Mehraban, H.R., Abbasi, K., Keivany, Y. Coad, B. 2017. Review and updated checklist of freshwater fishes of Iran: Taxonomy, distribution and conservation status. Iranian Soc. Ichthyol. 4(1): 1-114.
- Hajizadeh J., Faraji F., Rafatifard M., Kamranfard F. 2010. The genus Paragigagnathus Amitai and Grinberg (Acari: Phytoseiidae) in Iran, with a key to the known species. Syst. Appl. Acarol., 15: 222-227. https://doi.org/10.11158/saa.15.3.6
- Honey S.F., Bashir M.H., Khan B.S., Shahid M. 2015. On the identity of Paragigagnathus amantis (Chaudhri et al., 1979) (Acari: Phytoseiidae: Amblyseiinae) from Pakistan. Syst. Appl. Acarol., 20(4): 449-454. https://doi.org/10.11158/saa.20.4.10
- Khanjani M., Karimi M., Asali Fayaz B., Ueckermann E.A. 2016. Paragigagnathus iraniensis n. sp. (Acari: Phytoseiidae) from Western Iran. Acarologia 56: 195-201. https://doi.org/10.1051/acarologia/20162246
- Kolodochka L.A. 1989. A revision of the phytoseiid mites of the genus Pamiroseius Wain. (Parasitiformes: Phytoseiidae). Entomol. Obozr. Russia, 68: 221-229.
- KKreiter S., Payet R.-M., Abo-Shnaf R., Douin M. 2021, New Phytoseiidae (Acari: Mesostigmata) of Mascareignes and Comoros Archipelagos (Indian Ocean): one new record, three new species groups and description of six new species and of six unknown males. Acarologia 61(4): 845-889. https://doi.org/10.24349/Krky-e23s
- Kuznetsov N.N. 1994. Two new phytoseiid mite species (Parasitiformes, Phytoseiidae) from Armenia and Tadjikistan [in Russian]. Vestn. Zool., 2: 78- 81.
- Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35: 323-326.
- Lindquist E.E., Evans G.W. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina. Mem. Entomol. Soc. Canada, 47: 1-64. https://doi.org/10.4039/entm9747fv
- Livshitz I.Z., Kuznetsov N.N. 1972. Phytoseiid mites from Crimea (Parasitiformes: Phytoseiidae) [in Russian]. In: Pests and diseases of fruit and ornamental plants. Proceedings of The All-Union V. I. Lenin Academy of Agricultural Science, The State Nikita Botanical Gardens, Yalta, Ukraine, 61: 13-64.
- Moraes G.J.de, McMurtry J.A., Denmark H.A., Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. https://doi.org/10.11646/zootaxa.434.1.1
- Muma M.H. 1961. Subfamilies, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Fla St. Mus. Bul., 5(7): 267-302.
- Muma M.H., Denmark H.A. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighboring land areas. 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, USA, 150 pp.
- Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. https://doi.org/10.4039/Ent110859-8
- Swirski E., Chiara S.R., Tsolakis H. 1998. Keys to the Phytoseiid Mites (Parasitiformes, Phytoseiidae) of Israel. Phytophaga, Palermo, 8: 85-154.
- Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomol. Mem. Dept. Agric. Water Supply Rep. S. Africa, 73: 168 pp.
- Wainstein B.A. 1973. New genus and species of Phytoseiidae (Parasitiformes) [in Russian]. Zool. Zhurnal., Russia, 52: 953-955.
- Walter D.E., Krantz G.W. 2009. Collecting, rearing and preparing specimens. In: Krantz G.W., Walter D.E. (eds) A manual of acarology, 3rd ed. Texas Tech University Press, Lubbock. 807 pp.



2021-10-04
Date accepted:
2021-12-29
Date published:
2022-01-14
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Arjmandi-Nezhad, Alireza; Kreiter, Serge; Saboori, Alireza and Ravan, Sultan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



