Hydrodroma angelieri (Acari, Hydrachnidia: Hydrodromidae) a new water mite species from Corsica based on morphological and DNA barcode evidence
Pešić, Vladimir1 and Smit, Harry2
1✉ Department of Biology, University of Montenegro, Cetinjski put b.b., 81000 Podgorica, Montenegro.
2Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, the Netherlands.
2022 - Volume: 62 Issue: 1 pages: 3-11
https://doi.org/10.24349/l06c-j0qmZooBank LSID: 5B105F82-EF77-4C18-B9DC-DF4B51123A54
Original research
Keywords
Abstract
Introduction
The family Hydrodromidae K. Viets, 1936 comprises of two genera, Oxopsis Nordenskiöld, 1905, known from a single specimen collected in Sudan (see Smit 2020) and the widely distributed Hydrodroma Koch, 1837, recorded from all continents except Antarctica. The representatives of the latter genus are often abundant both in lentic and lotic habitats. Important characters in hydrodromids include i) idiosoma integument completely soft, lacking muscle attachment sclerites, ii) the uppermost layer of the integument is characterized by dense papillosity, with papillae that differ in shape and which are species specific, iii) genital flaps bearing numerous small acetabula arranged in several rows along the medial edge; iv) legs rather uniform with interspecific differences in the absolute and relative size of claws (larger in stream dwelling species), and in number and arrangement of long, fine swimming setae; v) morphology of mouth-parts, with P-4 bearing a long and pointed dorsodistal extension reaching the tip of the slender and elongated P-5 (Gerecke 2017).
Currently, 31 species of the family Hydrodromidae are known worldwide (Zhi-Qiang et al. 2011; Pešić et al. 2021a, b), five of which are present in Europe, i.e. Hydrodroma despiciens (Müller, 1776), Hydrodroma pilosa Besseling, 1940, H. torrenticola (Walter, 1908), H. reinhardi Pešić, 2002, and H. cf. rheophila Cook, 1967. The latter species, originally described from India (Cook 1963), is known in Europe only from the Greek island of Lesbos (Pešić et al. 2010). Most of these species such as H. pilosa, H. reinhardi and H. torrenticola have a Western Palaearctic distribution. Hydrodroma despiciens was considered cosmopolitan (see Di Sabatino et al. 2010). Nevertheless, studies on extra-European populations of Hydrodroma, such as those on Australian populations (Pešić and Smit 2007a, b, 2011) have revealed the presence of several clearly distinct autochthonous species. Recently, Więcek et al. (2020) applied an integrative approach based on the DNA barcode of the mitochondrial cytochrome c oxidase subunit I (COI) gene sequence and morphology to delineate the status of some Hydrodroma species from North America and Europe.
In this paper we used morphological data and COI barcodes to describe one new species of the genus Hydrodroma from Corsica.
Material and methods
Water mites were collected by hand netting, sorted live in the field, and immediately preserved in 96% ethanol for the purpose of the molecular analyses. After DNA extraction, the holotype specimen was dissected and slide mounted in Faure's medium. Holotype and paratype of the new species are deposited in Naturalis Biodiversity Center in Leiden (RMNH).
All measurements are in µm. Morphological nomenclature follows Gerecke et al. (2016). The genital plates and number of acetabula were measured on both sides, and for and therefore their dimensions were given as a range. The following abbreviations are used: Ac = acetabula; Cx-I = first coxae; dL = dorsal length; H = height; I-L-4-6 = fourth-sixth segments of first leg; L = length; P-1-P-5 = palp segment 1-5; RMNH = Naturalis Biodiversity Center, Leiden; W = width.
DNA barcode analyses
Molecular analyses were conducted at the Canadian Centre for DNA Barcoding (Guelph, Ontario, Canada; (CCDB; http://ccdb.ca/)). In CCDB the specimens were sequenced for the barcode region of COI using standard invertebrate DNA extraction (Ivanova et al. 2007), amplification (Ivanova and Grainger 2007a) and sequencing protocols (Ivanova and Grainger 2007b). The DNA extracts were archived in −80 °C freezers at the Centre for Biodiversity Genomics (CBG; biodiversitygenomics.net), while the specimen vouchers were returned to the first author for morphological examination. In CCDB the chromatograms were assembled into consensus sequences for each specimen and uploaded to the barcode of life database (BOLD; https://www.boldsystems.org/ ![]() ). The sample identifiers in BOLD are given for each barcoded specimen.
). The sample identifiers in BOLD are given for each barcoded specimen.
Sequence comparisons were performed using MUSCLE alignment (Edgar 2004). Intra- and interspecific genetic distances were calculated based on the Kimura 2-parameter model (K2P; Kimura 1980), using MEGAX (Kumar et al. 2018) software. MEGAX software was used to calculate Neighbour-Joining (NJ) trees based on K2P distances (standard for barcoding studies) and pairwise deletion of missing data. The support for tree branches was calculated by the non-parametric bootstrap method (Felsenstein 1985) with 1000 replicates.
Results and discussion
Species delimitation using DNA-barcodes
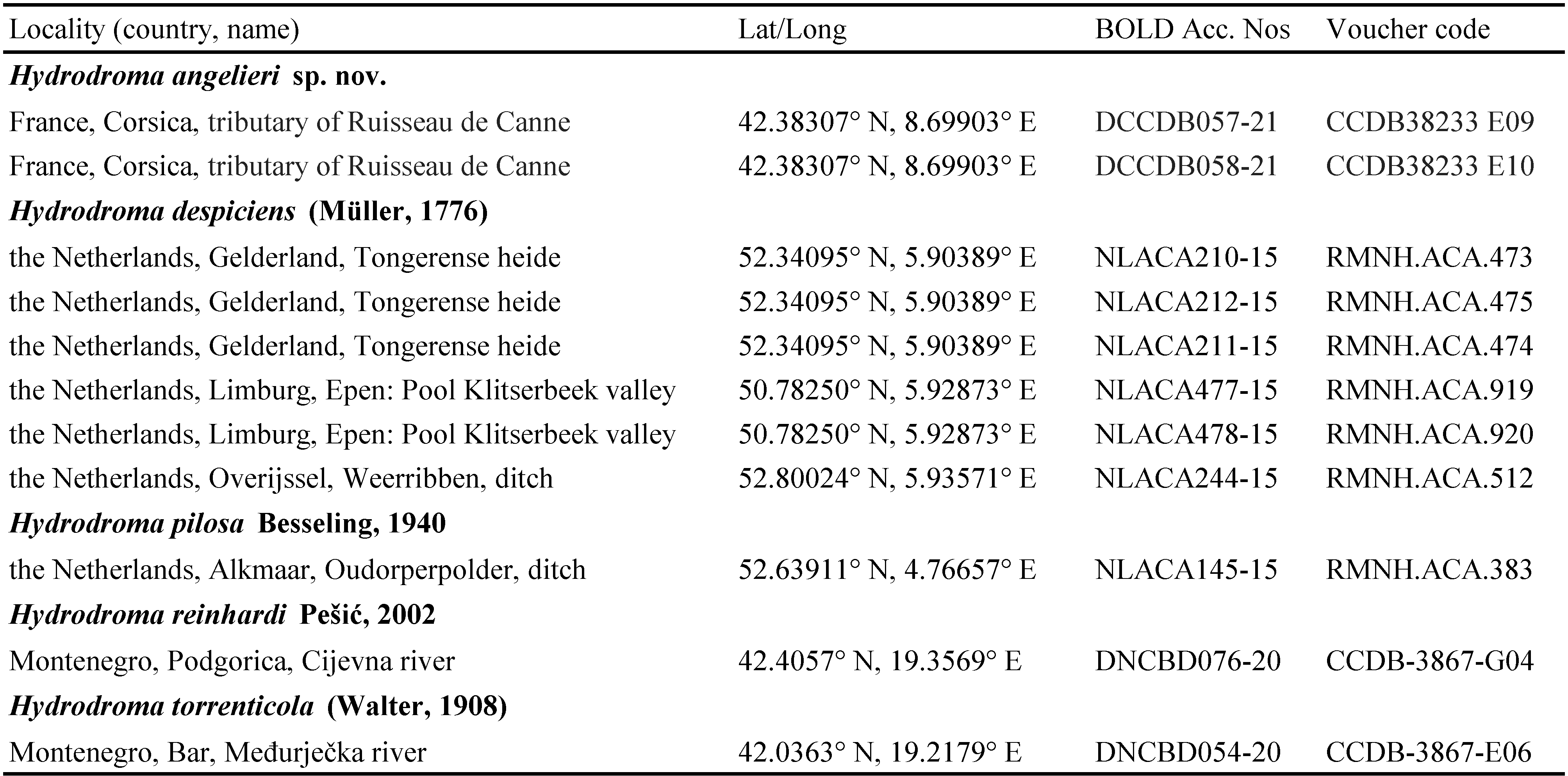
The final alignment for species delimitation using COI sequence data included 11 Hydrodroma specimens listed in Table 2 and one outgroup, Panisopsis thori (Walter, 1907) (BOLD DCBDJ086-21, Voucher code CCDB 38392 H02) from Germany to root the tree. The final alignment consisted of 658 nucleotide positions. The NJ tree is presented in Figure 3.
The sequences retrieved from two specimens of H. angelieri sp. nov. from Corsica appeared as a sister clade of the clade formed by H. despiciens and H. pilosa (Figure 3). The average K2P genetic distance between H. angelieri sp. nov. from Corsica and H. despiciens from the Netherlands was estimated 17.3±0.017 % (Table 3). The mean genetic distance between congeneric COI sequence groups recovered in the molecular analysis ranged from 13.1±0.015 % between H. despiciens and H. pilosa, to 22.2±0.021 % between H. torrenticola and H. pilosa (Table 3). The intraspecific distance of H. despiciens was 1% whereas H. angelieri sp. nov. showed no intraspecific variation.
Systematics
Family Hydrodromidae K. Viets, 1936
Diagnosis — Di Sabatino et al. 2010: 8.
Genus Hydrodroma Koch, 1837
Diagnosis — Di Sabatino et al. 2010: 9.
Hydrodroma angelieri Pešić & Smit sp. nov.
ZOOBANK: C279C2A2-E5E5-4C4D-8751-69B75F9BB0DD ![]()
Figs. 1A-F, 2C









Material examined — Holotype ♀ (RMHN.ACA.P.67573), France, Corsica, Tributary of Ruisseau de Canne, 14 Apr. 2015, leg. Smit (sequenced [DCCDB058-21], dissected and slide mounted [CCDB38233 E10]). Paratype: 1 ♀, same place and data as holotype (sequenced [DCCDB057-21]; preserved in Koenike fluid [CCDB38233 E09], RMNH.ACA.4508).
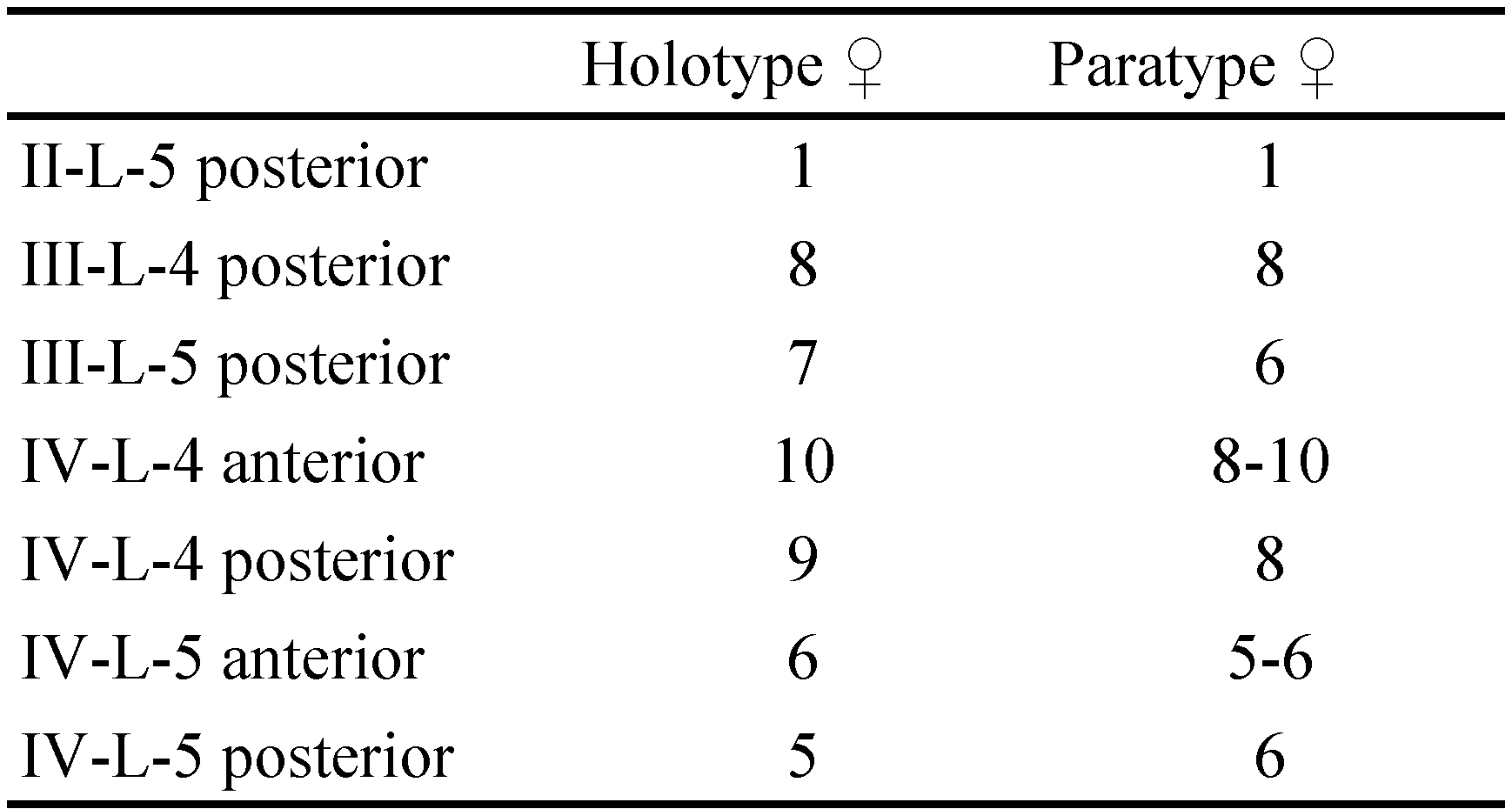
Diagnosis. Female (male unknown) — Idiosoma large (idiosoma L ˃ 1500, genital plates L ˃ 200 µm); integument papillae uniform, apically rounded. Genital plates with 69–80 pairs of Ac in at most 5 longitudinal rows. Legs with relatively short claws (L 42-47, ratio claw L/segment 5 L 11-14 %). Leg setae numbers: II-L-posterior 1; III-L-4 posterior 8; III-L-5 posterior 6-7; IV-L-4 anterior 10, posterior 9; IV-L-5 anterior 5-6, posterior 5-6.
Description — Female — Integument papillae distally rounded. Cx-I+II medially separated by a fine membranous line, with a row of long fine setae at medial margins of Cx-I, and posterior margins of Cx-II, -III, and –IV; a few most medial setae on Cx-I inserted on projections. Genital flaps with rounded lateral and concave medial margins. Excretory pore sclerotized. Leg claws without claw blade, with a dorsal clawlet. P-2 with pectinate mediodistal setae; P-4 with a long, pointed dorsodistal extension reaching tip of the slender and elongated P-5.
Measurements (holotype; in parentheses some measurements of the paratype specimen, n = 1): Idiosoma L 1615 (1550), W 1560 (1280). Coxal field L 731 (694); Cx-III W 994 (850); L Cx-I+II 375 (356); Cx-III+IV 400 (375); coxal setae numbers: Cx-I, 29-31 (24); Cx-II, 24 (21); Cx-III, 22 (21); Cx-IV 19 (20). Genital plate L 273 (244), on each plate Ac number 80 (69), with 45 setae, which in the anterior part are solid and flat, in the posterior part hollow. Egg maximum diameter 141-150, n = 3 (147, n = 2).
Gnathosoma (Fig. 1F) vL 322; chelicera (Fig. 1E) total L 392, L basal segment 320, claw 80, L ratio basal segment/claw 4.0. Palp (Figs. 1D-E) total L 561, dL/H, dL/H ratio: P-1, 52/56, 0.92; P-2, 103/70, 1.47; P-3, 55/75, 0.73; P-4, 241/57, 4.2; P-5, 110/23, 4.8; L ratio P-2/P-4, 0.43. dL I-L: 84, 122, 147, 244, 322, 272; ratio claw L/segment 5 L 13.9%. dL II-L-2-6: 153, 194, 331, 394, 322; ratio claw L/segment 5 L 11.9%. dL III-L-2-6: 166, 203, 327, 394, 344; ratio claw L/segment 5 L 11.2%. dL IV-L: 134, 209, 303, 448, 469, 409; ratio claw L/segment 5 L 11.1%.


Male — unknown.
Etymology — Named after the French acarologist Eugène Angelier in appreciation of his outstanding contribution to the study of water mite diversity of Corsica.
Discussion — Based on COI data the new species from Corsica seems to be most closely related to Hydrodroma despiciens (Müller, 1776) and H. pilosa Besseling, 1940. All three species are characterized by relatively small leg claws (L ratio claw/segment 5 < 14 %). Presence of only one short swimming seta on II-L-5 (II-L-5 posterior with 8-10 swimming setae in H. pilosa), makes the new species more similar to Hydrodroma despiciens, a species widely distributed in standing waters in Europe (Di Sabatino et al. 2010). The new species from Corsica can be distinguished from H. despiciens by the presence of anterior swimming setae from IV-L-5. The high genetic distance between the new species and H. despiciens (COI 17.3±0.017 K2P%) suggests a long independent history of the new species.





Two other species, H. torrenticolla and H. reinhardi, both reported from Corsica (see Angelier 1959; Santucci 1971 and Pešić 2002, respectively) differ from the new species among others in the morphology of legs with rather large claws (L ratio claw/segment 5 < 14%; see Figure 2A for comparison). Hydrodroma torrenticola, a species similar to H. angelieri sp. nov. in the presence of swimming setae on anterior IV-L-5, differs also in having bluntly pointed papillae and anterior face of IV-L-5 bearing 3-4 swimming setae; H. reinhardi differs in the absence of swimming setae from anterior IV-L-5 and a generally smaller measurements of idiosoma and gnathosoma (Pešić 2002; Gerecke 2017).
Distribution — France (Corsica).
Key to the European species of Hydrodroma Koch, 1837
1 Swimming setation strongly reduced (one short seta each on II-L-5, III-L-4/5, and IV-L-4/5
...... Hydrodroma cf. rheophila (In Europe reported only from the Greek island of Lesbos)
— Legs with more numerous swimming setae, at least on IV-L-4/5 located in rows
...... 2
2(1) II-L-5 with more than four swimming setae, leg claws relatively small (L ratio claw/segment 5, 7-12 %; after Gerecke 2017); integument papillae acutely pointed; genital flaps with 6-8 Ac lying along an imaginary transverse line crossing at maximum width
...... Hydrodroma pilosa
— II-L-5 with one swimming seta or without swimming setae; integument papillae various but not acutely pointed; genital flaps with 4-6 Ac lying along an imaginary transverse line crossing at maximum width; leg claws various
...... 3
3(2) IV-L-5 anteriorly without swimming setae
...... 4
— IV-L-5 anteriorly with swimming setae
...... 5
4(3) Number of swimming setae: III-L-4 posterior < 9, IV-L-4 anterior < 9; leg claws relatively small (L ratio claw/segment 5, 11-18 %; after Gerecke 2017)
...... Hydrodroma despiciens
— Number of swimming setae: III-L-4 posterior 2-4, IV-L-4 anterior 2-6; leg claws relatively large (L ratio claw/segment 5, 15-16 %; after Gerecke 2017)
...... Hydrodroma reinhardi
5(3) IV-L-5 anterior 2-4 swimming setae; leg claws relatively large (L ratio claw/segment 5, 11-18 %; after Gerecke 2017); integument papillae bluntly pointed
...... Hydrodroma torrenticola
— IV-L-5 anterior 5-6 swimming setae; leg claws relatively small (L ratio claw/segment 5, 11-14 %); integument papillae distally rounded
...... Hydrodroma angelieri sp. nov.
Acknowledgements
Special thanks to Milica Jovanović and Ana Manović (University of Podgorica) for their excellent laboratory work. HS would like to thank Truus van der Pal (Alkmaar) for her assistance with the field work on Corsica. This study is part of the ''DNA-Eco'' scientific project, supported by a grant of the Montenegrin Ministry of Science. We thank Joanna Mąkol (Wrocław) and two anonymous reviewers, whose constructive comments greatly improved this work.
References
- Angelier E. 1959. Acariens (Hydrachnellae et Porohalacaridae) des eaux superficielles.In: Angelier E. et collaborateurs, Hydrobiologie de la Corse. Vie et Milieu, Suppl., 8: 64-148.
- Cook D.R. 1967. Water mites from India. Mem. Am. Entomol. Inst., 9: 1-411.
- Di Sabatino A., Gerecke R., Gledhill T., Smit H. 2010. Chelicerata: Acari II. In: Gerecke R. (ed.) Chelicerata: Araneae, Acari I. Süßwasserfauna von Mitteleuropa, 7, 2-2: 1-134. München: Elsevier Spektrum Akademischer Verlag. https://doi.org/10.1007/978-3-8274-2266-8_1
- Edgar R.C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high 679 throughput. Nucleic acids Res 32(5): 1792-1797. https://doi.org/10.1093/nar/gkh340
- Gerecke R. 2017. The water mites of the genus Hydrodroma (Acari, Hydrachnidia, Hydrodromidae) in Europe and Africa. Ecol. Montenegrina, 13: 1-24. https://doi.org/10.37828/em.2017.13.1
- Gerecke R., Gledhill T., Pešić V., Smit H. 2016. Chelicerata: Acari III. In: Gerecke R. (ed.) Süßwasserfauna von Mitteleuropa, Bd. 7/2-3. Springer-Verlag Berlin, Heidelberg, pp. 1-429. https://doi.org/10.1007/978-3-8274-2689-5
- Felsenstein J. 1985. Confidence limits on phylogenies: An approach using the bootstrap. Evolution, 39: 783-791. https://doi.org/10.1111/j.1558-5646.1985.tb00420.x
- Ivanova N.V., de Waard J.R., Hebert P.D.N. 2007. CCDB protocols, glass fiber plate DNA extraction. http://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_DNA_Extraction.pdf

- Ivanova N.V., Grainger C.M. 2007a. CCDB protocols, COI amplification. Avialable at: http://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_Amplification.pdf

- Ivanova N.V., Grainger C.M. 2007b. CCDB protocols, sequencing. Avialable at: http://ccdb.ca/site/wp-content/uploads/2016/09/CCDB_Sequencing.pdf

- Kimura M. 1980. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol., 16: 111-120. https://doi.org/10.1007/BF01731581
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol., 35: 1547-1549. https://doi.org/10.1093/molbev/msy096
- Pešić, V. 2002. Hydrodroma reinhardi sp. n., a New Species of Water Mites (Acari, Actinedida, Hydrodromidae) from the Mediterranean Area. Aquat. Insects, 24: 317-323. https://doi.org/10.1076/aqin.24.4.317.8239
- Pešić V., Smit H. 2007a. Water mite species of the genus Hydrodroma Koch (Acari: Hydrachnidia, Hydrodromidae) from Australasia. Part I. Zootaxa, 1389: 31-44. https://doi.org/10.11646/zootaxa.1389.1.2
- Pešić V., Smit H. 2007b. Water mite species of the genus Hydrodroma Koch (Acari: Hydrachnidia, Hydrodromidae) from Australia. Part II. Zootaxa, 1509: 41-50. https://doi.org/10.11646/zootaxa.1509.1.4
- Pešić V., Smit H 2011. A new species of the genus Hydrodroma Koch, 1837 (Acari, Hydrachnidia, Hydrodromidae), with a key to the hitherto known six species of the genus in Australia. ZooKeys, 143: 13-22. https://doi.org/10.3897/zookeys.143.2115
- Pešić V., Smit H., Gerecke R., Di Sabatino A. 2010. The water mites (Acari: Hydrachnidia) of the Balkan peninsula, a revised survey with new records and descriptions of five new taxa. Zootaxa, 2586: 1-100. https://doi.org/10.11646/zootaxa.2586.1.1
- Pešić V., Smit H., Mary N.J. 2021a. Two new water mite species of the genus Hydrodroma Koch, 1837 from New Caledonia (Acari, Hydrachnidia: Hydrodromidae). Acarologia, 61: 581-590. https://doi.org/10.24349/aGHX-uIU1
- Pešić V., Zawal A., Saboori A., Smit H. 2021b. New records of water mites (Acari, Hydrachnidia) from Iran with the description of one new species based on morphology and DNA barcodes. Zootaxa, 5082: 425-440. https://doi.org/10.11646/zootaxa.5082.5.2
- Santucci J. 1971. Contribution à l'étude de la répartition des Hydracariens (Hydrachnellae) des eaux superficielles d'un torrent de Corse - Le Porto. Ann. Fac. Sci. Marseille, 45: 81-99.
- Smit H. 2020. Water mites of the world with keys to the families, subfamilies, genera and subgenera (Acari: Hydrachnidia). Monogr Ned. Entom. Ver., 12: 1-774.
- Więcek M., Szydło W., Dabert J., Proctor H. 2020. Delimiting species of water mites of the genus Hydrodroma (Acari: Hydrachnidiae: Hydrodromidae) from North America and Europe: Integrative evidence of species status from COI sequences and morphology. Zool. Anz., 284: 16-29. https://doi.org/10.1016/j.jcz.2019.11.004
- Zhang Z.-Q., Fan Q.-H., Pešić V., Smit H., Bochkov A.V., Khaustov A.A., Baker A., Wohltmann A., Wen T.H., Amrine J.W., Beron P., Lin J., Gabrys G., Husband R. 2011. Trombidiformes. In: Z.-Q. Zhang (ed.) Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 129-138. https://doi.org/10.11646/zootaxa.3148.1.24



2021-10-01
Date accepted:
2021-12-15
Date published:
2022-01-07
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Pešić, Vladimir and Smit, Harry
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



