Contribution to the knowledge of the oribatid mite genus Gittella (Acari, Oribatida, Oppiidae), with description of a new species from Peru
Ermilov, Sergey G.1 ; Subias, Luis S.2 ; Shtanchaeva, Umukusum Ya.3 and Friedrich, Stefan4
1✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
2Complutense University, Madrid, Spain.
3Complutense University, Madrid, Spain.
4SNSB-Bavarian State Collection of Zoology, Munich, Germany.
2021 - Volume: 61 Issue: 4 pages: 1015-1022
https://doi.org/10.24349/z72f-jdlcZooBank LSID: 5467ED59-4E13-4A83-9516-9101F6813682
Original research
Keywords
Abstract
Introduction
The oribatid mite genus Gittella (Acari, Oribatida, Oppiidae) was proposed by Hammer (1961) with Gittella punctata Hammer, 1961 as type species. At present, the genus comprises seven species, which are distributed in the Neotropical region (Subías 2004, online version 2021). The main goals of the paper are: to describe and illustrate a new species of Gittella collected from Amazonian Peru; to summarize generic morphological traits; to provide an identification key and to present distribution and habitats of all known species of the genus.
Presently, two species of Gittella have been registered in Peruvian fauna (Hammer 1961; Ermilov and Gwiazdowicz 2015; Ermilov and Friedrich 2016, 2017): G. punctata Hammer, 1961; and G. variabilis Ermilov, Sandmann, Marian and Maraun, 2013.
Material and methods
Specimens — Substrate samples containing oribatid mites were collected from South America, Amazonian Peru, 09°37′S, 74°56′W, Huánuco Department, Puerto Inca Province, Yuyapichis District, Área de Conservación Privada, Panguana (biological field station), near Rio Yuyapichis (river), 230–260 m a.s.l., upper soil and leaf litter in the primary evergreen lowland rainforest, 23.IV.2016–09.V.2016 (S. Friedrich, F. Wachtel and D. Hauth). Mites were extracted from samples into 75% ethanol using Winkler's apparatus in laboratory conditions during 10 days.
Types are deposited in three institutions: the Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru (MUSM); the Tyumen State University Museum of Zoology, Tyumen, Russia (TSUMZ); and the SNSB-Bavarian State Collection of Zoology, Munich, Germany (ZSM).
Observation and documentation — Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the gastronotum. Notogastral width refers to the maximum width of the notogaster in dorsal view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu-tibia-tarsus. Drawings were made with a camera lucida using a Leica transmission light microscope ''Leica DM 2500''.
Terminology — Morphological terminology used in this paper follows that of F. Grandjean: see Travé & Vachon (1975) for references; Norton (1977) for leg setal nomenclature; and Norton & Behan-Pelletier (2009) for overview.
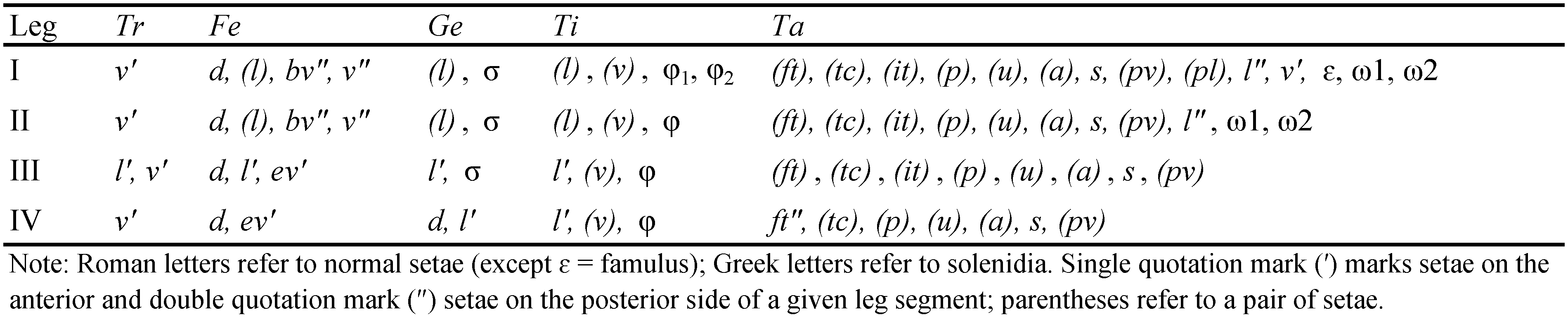
Abbreviations — Prodorsum: ro, le, in, bs, ex = rostral, lamellar, interlamellar, bothridial, and exobothridial seta, respectively; exv = vestige of the second exobothridial seta. Notogaster: c, da, dm, dp, la, lm, lp, h, p = setae; ia, im, ip, ih, ips = lyrifissures; gla = opisthonotal gland opening. Gnathosoma: a, m, h = subcapitular setae; or = adoral seta; d, l, v, cm, ul, su, vt, lt = palp setae; ω = palp solenidion; cha, chb = cheliceral setae; Tg = Trägårdh's organ. Epimeral and lateral podosomal regions: 1a–1c, 2a, 3a–3c, 4a–4c = epimeral setae; PdI = pedotectum I; dis = discidium. Anogenital region: g, ag, an, ad = genital, aggenital, anal, and adanal seta, respectively; iad = adanal lyrifissure; po = preanal organ. Legs: Tr, Fe, Ge, Ti, Ta = trochanter, femur, genu, tibia, tarsus, respectively; ω, φ, σ = solenidia; ɛ = famulus; d, l, v, bv, ev, ft, tc, it, p, u, a, s, pv, pl = setae; pa = porose area.
Taxonomy
Main generic traits of Gittella
Adult — Size. Length about 300–800. Integument. Notogaster and anogenital region smooth or microgranulate, sometimes notogaster and anogenital region foveolate. Prodorsum. Rostrum rounded. Costula present, often vestigial, or absent. Transcostula and lateral carina absent. Interbothridial and postbothridial tubercles present or absent. Interbothridial region with two or three pairs or without muscle sigillae. Rostral, lamellar and interlamellar setae well developed, setiform; le inserted closer to in than to ro. Bothridial seta pectinate. Notogaster. Without humeral tooth and crista. Thirteen pairs of setae, c represented by alveolus, others of medium-sized or long, setiform or flagellate. Gnathosoma. Subcapitulum diarthric. Adoral seta present, spiniform. Palp setation: 0-2-1-3-9(+1 solenidion). Palp solenidion very long, in anterior part attached to distal seta. Chelicera chelate-dentate. Epimeral and lateral podosomal regions. Epimeral border IV present. Epimeral setal formula: 3-1-3-3; all setae setiform. Ventrosejugal tubercle absent. Pedotectum I represented by small lamina. Discidium developed. Anogenital region. Five pairs of genital, one pair of aggenital, two pairs of anal, and three pairs of adanal setae, all setae setiform. Adanal seta ad1 posterolateral or lateral, ad2 lateral, ad3 anterolateral to anal plate, distance between ad3–ad3 longer than ag–ag and ad2–ad2. Adanal lyrifissure usually oblique, located close and lateral to anal aperture. Legs. Tarsus I with 20 setae (l″ and v′ present), tarsus II with 16 setae (l″ present). Tarsus II with two solenidia.
Juveniles — Unknown.
Description
Gittella kontschani n. sp.
ZOOBANK: xxxxxxxxxxx ![]()
(Figures 1, 2)






Diagnosis — Body size: 481–531 × 223–249. Body surface microgranulate; notogaster and anogenital region foveolate. Costula absent or vestigial. Rostral, lamellar, interlamellar, and exobothridial setae setiform, slightly barbed; relative length: ro ˃ le = in = ex. Bothridial seta with five to seven branches. Interbothridial region with two (rarely, with three) pairs of muscle sigillae. Interbothridial and postbothridial tubercles absent. Notogastral setae (except alveolar c) long, setiform, barbed. Epimeral and anogenital setae setiform, slightly barbed. Leg claws slightly barbed on dorsal side.
Description — Measurements – Body length: 531 (holotype, male), 481–531 (16 paratypes, 11 males and five females); body width: 223 (holotype), 223–249 (16 paratypes). No distinct difference between male and female in body size.
Integument – Body color light brown to brown. Body surface densely microgranulate (diameter of granule less than 1). Notogaster and anogenital region sparsely foveolate (diameter of foveola up to 8). Lateral part of body between bothridium and acetabula I-III densely tuberculate (diameter of tubercle up to 4).
Prodorsum — Rostrum broadly rounded. Costula absent, rarely vestigial, indistinct. Rostral (36–41), lamellar (28–32), interlamellar (28–32), and exobothridial (28–32) setae setiform, slightly barbed. Bothridial seta (82–86) with five to seven branches; of them, two or three distal branches shorter than others. Interbothridial region with two pairs of muscle sigillae, but sometimes anterior pair divided into two pairs (in these cases, three pairs of muscle sigillae present). Interbothridial tubercle absent, but sometimes indistinct thickening observed instead. Postbothridial tubercle absent. Longitudinal row, comprising several muscle sigillae, present in front of the bothridium.
Notogaster – Anterior border convex medially. Twelve pairs of notogastral setae (73–90) setiform, barbed; thirteenth pair (setae c) represented by alveoli. All notogastral lyrifissures and opisthonotal gland opening distinct.
Gnathosoma – Subcapitulum size: 118–127 × 90–94. Subcapitular setae (a: 18–20; m: 26–28; h: 22–24) setiform, slightly barbed. Adoral seta (4) spiniform, smooth. Palp length: 69–73. Postpalpal seta (4) spiniform, smooth. Chelicera length: 110–118. Cheliceral setae (cha: 34–36; chb: 20) setiform, barbed.
Epimeral and lateral podosomal regions – All epimeral setae (1a, 2a, 3a, 4b: 16; 1b, 1c, 3b, 4a: 20–22; 3c: 28–32; 4c: 32–36) setiform, slightly barbed. Discidium strong, triangular.
Anogenital region – Genital (g1: 16–18; others: 12–14), aggenital (16–18), adanal (36–41), and anal (16–18) setae setiform, slightly barbed. Adanal lyrifissure diagonal, close and lateral to anal aperture. Ovipositor elongated (159 × 28), blade (45) shorter than length of distal section (beyond middle fold; 114). Each of the three blades with four smooth setae, ψ1 ≈ τ1 (41) setiform, ψ2 ≈ τa ≈ τb ≈ τc (16) elongate thorn-like. Coronal setae not observed.
Legs – All leg claws with tubercle ventrobasally, slightly barbed on dorsal side. Porose area on femora I-IV and tibiae I, II slightly visible. Formulas of leg setation and solenidia: I (1-5-2-4-20) [1-2-2], II (1-5-2-4-16) [1-1-2], III (2-3-1-3-15) [1-1-0], IV (1-2-2-3-12) [0-1-0]; homology of setae and solenidia indicated in Table 1. Famulus of tarsus I erect, slightly swollen and blunted distally, inserted before solenidion ω1.


Type material — Holotype (male) and 16 paratypes (11 males and five females). The holotype is deposited in the collection of the MUSM; two paratypes are deposited in the collection of the ZSM; 14 paratypes are deposited in the collection of the TSUMZ. All specimens are preserved in 70% solution of ethanol with a drop of glycerol.
Etymology — The species name kontschani is given in honour of our friend and colleague, the well-known acarologist Dr. Jenő Kontschán (Budapest, Hungary), for his invaluable contributions to the knowledge of the mite fauna of ACP Panguana.
Remarks — Gittella kontschani n. sp. is morphologically most similar to Gittella variabilis Ermilov, Sandmann, Marian and Maraun, 2013 in having foveolate notogaster and anogenital region, but differs from the latter by the smaller body length (481–531 versus 647–680), the relative length of prodorsal setae (ro ˃ le = in = ex versus in ˃ le = ro ˃ ex), and the absence (versus presence) of interlamellar and postbothridial tubercles.
Distinctive characters of the new species compared with other members of Gittella can be found in the identification key below.
Key to known species of Gittella
1. Dorsal notogastral setae comparatively short, tip of dm distinctly not reaching insertion of dp; costula well developed; body length less than 400
...... 2
— Dorsal notogastral setae comparatively long, tip of dm reaching insertion of dp; costula absent or vestigial; body length more than 450
...... 3
2. All notogastral setae smooth; epimeral border IV straight; rostral seta longer than lamellar and interlamellar setae; body length: 360
...... Gittella punctata Hammer, 1961.
— All notogastral setae barbed; epimeral border IV arch-like; lamellar seta longer than rostral and interlamellar setae; body length: 311–328
...... Gittella minor Ermilov, Sandmann, Marian and Maraun, 2013.
3. Notogaster and anogenital region foveolate (well visible in lateral aspect)
...... 4
— Notogaster and anogenital region not foveolate
...... 5
4. Interbothridial and postbothridial tubercles present; interlamellar seta longer than rostral and lamellar setae; body length: 647–680
...... Gittella variabilis Ermilov, Sandmann, Marian and Maraun, 2013.
— Interbothridial and postbothridial tubercles absent; rostral seta longer than lamellar and interlamellar setae; body length: 481–531
...... Gittella kontschani n. sp.
5. Dorsal notogastral setae very long, flagellate, tip of dm reaching of insertion of lp; body length: 500–524
...... Gittella flagellata (Mahunka, 1983).
— Dorsal notogastral setae so not long, setiform, tip of dm distinctly not reaching of insertion of lp
...... 6
6. All notogastral setae smooth; interbothridial muscle sigillae not observed; body length: 630–796
...... Gittella ecuadoriensis Ermilov and Kalúz, 2012.
— All notogastral setae barbed; interbothridial muscle sigillae distinct
...... 7
7. Interlamellar seta longer than rostral and lamellar setae; pair of interbothridial tubercles absent; two pairs of interbothridial muscle sigillae; body length: 640
...... Gittella maxima (Balogh and Mahunka, 1981).
— Interlamellar seta not longer than rostral and lamellar setae; pair of interbothridial tubercles present; three pairs of interbothridial muscle sigillae; body length: 576–642
...... Gittella insularis Mahunka, 1998.
Distribution and habitat of Gittella
At present, all eight representatives of Gittella have been recorded only in the Neotropical region. Except for three species (G. insularis, G. maxima, G. variabilis), the other five species have a highly circumscribed geographic distribution, i.e. are endemic to a single country.
Three species were originally described from Ecuador: G. ecuadoriensis from litter (2000–2200 m a.s.l.) near San Francisco de las Pampas, Reserva de Bosque Integral Otonga, central Ecuador (Ermilov and Kalúz, 2012); G. minor and G. variabilis from the upper organic soil layer in mostly undisturbed rain forest (2000 m a.s.l.) near Estación Científica San Francisco, southern Ecuador (Ermilov et al. 2013). Later, G. variabilis was repeatedly recorded from litter (about 250 m a.s.l.) in the primary evergreen lowland rainforest near the river Río Yuyapichis, Panguana, central Peru (Ermilov and Gwiazdowicz 2015; Ermilov and Friedrich 2016, 2017); G. kontschani n. sp. was described from the same vicinities.
The species G. flagellata was originally described from soil in Assancao, northern Brazil (Mahunka 1983); G. insularis from very humid soil-litter (200-350 m a.s.l.) in rain forest in Quiless Reserve near Piton St. Esprit, Saint Lucia, Antilles (Mahunka 1998); G. maxima was originally described from wet soil and moss near Acaray waterfall in Ciudad del Este (as Puerto Presidente Stroessner), southeastern Paraguay (Balogh and Mahunka 1981); G. punctata from highly moist moss on the ground in Machu Picchu, eastern Cordillera, southern Peru (Hammer 1961). Also, G. insularis was recorded from litter in a rain forest at the border of Beni river in Maididi National Park, northwestern Bolivia (Ermilov and Niedbała 2013), and from leaf litter in a forest in Alejandro de Humboldt National Park, southeastern Cuba (Ermilov et al. 2016); G. maxima from tropical moist forest in Isla Coiba, Panama (Schatz 2007), and from fragments of semi-deciduous forests in Alter do Chão region, State of Pará, northern Brazil (Ferreira et al. 2012).
Acknowledgements
The authors thank Dr. Julia Baumann and two anonymous reviewers for valuable comments; Dr. Juliane Diller and Erich Diller for kindly inviting one of us (Stefan Friedrich) to Panguana; Franz Wachtel (Grünwald, Germany) and David Hauth (Marburg and Fürstenfeldbruck, Germany) for expertise and assistance in the field; Dr. Gerardo Lamas Müller and Dr. Diana Silva Dávila (both Museo de Historia Natural, Universidad Nacional Mayor de San Marcos, Lima, Peru) for cooperation; and the Servicio Nacional Forestal y de Fauna Silvestre (SERFOR) for issuing a collecting permit (# 007-2014-SERFOR-DGGSPFFS) and export permit (# 003052-SERFOR). This research was partially supported by the cooperative agreement No. FEWZ-2021-0004 from the Russian Ministry of Science and Higher Education.
References
- Balogh J., Mahunka S. 1981. New data to the knowledge of the oribatid fauna of the Neogaea, VI. (Acari). Acta Zool. Acad. Sci. Hung., 27(1-2): 49-102.
- Ermilov S.G., Friedrich S. 2016. Additions to the oppioid oribatid mite fauna of Peru (Acari, Oribatida, Oppioidea). Acarologia, 56(3): 379-391. https://doi.org/10.1051/acarologia/20162252
- Ermilov S.G., Friedrich S. 2017. New faunistic and taxonomic data on oribatid mites (Acari, Oribatida) of Peru. Acarina, 25(1): 3-13. https://doi.org/10.21684/0132-8077-2017-25-1-3-13
- Ermilov S.G., Gwiazdowicz D.J. 2015. Peruvian oribatid mites (Acari: Oribatida) from the German Biological Expedition, with description of a new species of the genus Pergalumna. ZooKeys, 487: 87-96. https://doi.org/10.3897/zookeys.487.9335
- Ermilov S.G., Kalúz S. 2012. Two new species of Oppiidae (Acari: Oribatida) from Ecuador. Int. J. Acarol., 38(6): 521-527. https://doi.org/10.1080/01647954.2012.687499
- Ermilov S.G., Niedbała W. 2013. Contribution to the knowledge of the oribatid mite fauna of Bolivia, Zambia, Cambodia and Vietnam, with descriptions of two new species (Acari, Oribatida). Spixiana, 36(1): 9-19. https://doi.org/10.11158/saa.19.3.12
- Ermilov S.G., Tolstikov A.V., Salavatulin V.M. 2016. Additions to the Cuban oribatid mite fauna (Acari, Oribatida), including new records and descriptions of two new species from the genera Eupelops (Phenopelopidae) and Malaconothrus (Malaconothridae). Acarologia, 56(1): 99-114. https://doi.org/10.1051/acarologia/20162195
- Ermilov S.G., Sandmann D., Marian F., Maraun M. 2013. Two new oribatid mite species of the genus Gittella from Ecuador (Acari, Oribatida, Oppiidae). Spixiana, 36(1): 1-8.
- Ferreira R.N.C., Franklin E., Souza J.L.P., Moraes J. 2012. Soil oribatid mite (Acari: Oribatida) diversity and composition in semi-deciduous forest fragments in eastern Amazonia and comparison with the surrounding savanna matrix. J. Nat. Hist., 46: 1-14. https://doi.org/10.1080/00222933.2012.707245
- Hammer M. 1961. Investigations on the oribatid fauna of the Andes Mountains. II. Peru. Det Kong. Dansk. Vidensk. Selsk. Biol. Skr., 13(1): 1-157.
- Mahunka S. 1983. Data to the knowledge of the oribatid fauna of Surinam and Brazil (Acari). Folia Ent. Hung., 44(2): 205-227.
- Mahunka S. 1998. New data on oribatids (Acari: Oribatida) from St. Lucia (Antilles). Acarologica Genevensia LXXXIX. Rev. suisse Zool., 105(4): 839-877. https://doi.org/10.5962/bhl.part.80061
- Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal D.L. (Ed.). Biology of oribatid mites. Syracuse: SUNY College of Environmental Science and Forestry. pp. 33-61.
- Norton R.A., Behan-Pelletier V.M. 2009. Oribatida. Chapter 15. In: Krantz G.W., Walter D.E. (Eds.). A Manual of Acarology. Lubbock: Texas Tech University Press. pp. 430-564.
- Schatz H. 2007. Biogeography of oribatid mites (Acari, Oribatida) from the Cordillera de Talamanca, Costa Rica and Panama. In: Morales-Malacara J.B., Behan-Pelletier V.M., Ueckermann E., Pérez T.M., Estrada-Venegas E.G., Badil, M. (Eds.). Acarology XI: Proceedings of the International Congress. México: Instituto de Biología and Facultad de Ciencias, Universidad Nacional Autónoma de México; Sociedad Latinoamericana de Acarología. pp. 151-167.
- Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes, Oribatida) del mundo (1758-2002). Graellsia, 60: 3-305. https://doi.org/10.3989/graellsia.2004.v60.iExtra.218
- Subías L.S. 2021. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles), 16ª actualización. 532 pp. Available from: http://bba.bioucm.es/cont/docs/RO_1.pdf
 (accessed March 2021).
(accessed March 2021). - Travé J., Vachon M. 1975. François Grandjean. 1882–1975 (Notice biographique et bibliographique). Acarologia, 17(1): 1–19.



2021-09-21
Date accepted:
2021-12-01
Date published:
2021-12-10
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Ermilov, Sergey G.; Subias, Luis S.; Shtanchaeva, Umukusum Ya. and Friedrich, Stefan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



