Integrated taxonomy supports the identification of some species of Phytoseiidae (Acari: Mesostigmata) from Georgia
Tixier, Marie-Stephane  1
; Auger, Philippe2
; Migeon, Alain
1
; Auger, Philippe2
; Migeon, Alain  3
; Douin, Martial4
; Fossoud, Amandine5
; Navajas, Maria
3
; Douin, Martial4
; Fossoud, Amandine5
; Navajas, Maria  6
and Arabuli, Tea
6
and Arabuli, Tea  7
7
1✉ CBGP, Montpellier SupAgro, INRAE, CIRAD, IRD, Univ Montpellier, Montpellier, France, Campus International de Baillarguet, CS 30016, 34988 Montferrier-sur-Lez cedex, France.
2CBGP, INRAE, CIRAD, IRD, Montpellier SupAgro, Univ Montpellier, Montpellier, France, Campus International de Baillarguet, CS 30016, 34988 Montferrier-sur-Lez cedex, France.
3CBGP, INRAE, CIRAD, IRD, Montpellier SupAgro, Univ Montpellier, Montpellier, France, Campus International de Baillarguet, CS 30016, 34988 Montferrier-sur-Lez cedex, France.
4CBGP, Montpellier SupAgro, INRAE, CIRAD, IRD, Univ Montpellier, Montpellier, France, Campus International de Baillarguet, CS 30016, 34988 Montferrier-sur-Lez cedex, France.
5CBGP, IRD, INRAE, CIRAD, Montpellier SupAgro, Univ Montpellier, Montpellier, France, Campus Internation-al de Baillarguet, CS 30016, 34988 Montferrier-sur-Lez cedex, France.
6CBGP, INRAE, CIRAD, IRD, Montpellier SupAgro, Univ Montpellier, Montpellier, France, Campus International de Baillarguet, CS 30016, 34988 Montferrier-sur-Lez cedex, France.
7Institute of Zoology, Ilia State University, Kakutsa Cholokashvilli Ave 3/5, Tbilisi 0162, Georgia & Institute of Entomology, Agricultural University of Georgia, Kakha Bendukidze Campus, 240 David Aghamashenebeli Alley, Tbilisi 0131, Georgia.
2021 - Volume: 61 Issue: 4 pages: 824-844
https://doi.org/10.24349/m2Rp-WodGOriginal research
Keywords
Abstract
Introduction
Mites of the family Phytoseiidae are predators used for biological control of mite and small insect pests of various crops (McMurtry and Croft 1997; McMurtry et al. 2013; Knapp et al. 2018). The family Phytoseiidae, distributed worldwide, contains 2,521 valid species, with some biogeographic regions characterised by the highest number of species and associated reports, namely the Neotropic and Palearctic regions (Tixier et al. 2008b, 2012a; Demite et al. 2021). However, the species diversity and distribution of phytoseiids are only partially known and the fauna of some countries remains poorly investigated (Demite et al. 2021). Gaining in the knowledge on the distribution of Phytoseiidae is interesting for meta-analysis approaches aiming to characterise factors affecting diversity at local and global scales (i.e. Tixier et al. 2008b; Tixier and Kreiter 2009; Tixier 2018). Furthermore, this information is particularly relevant in biological control studies, by providing background data on the availability of natural enemy species / populations adapted to specific environmental conditions or pest availabilities.
The Phytoseiidae fauna in Georgia has been partially explored (Demite et al. 2021). As an additional contribution to the knowledge of this group of mites in the country, this paper presents results of surveys carried out in 2019. Among the 53 valid species of Phytoseiidae reported from Georgia, we were particularly interested in detecting Amblyseius swirskii Athias-Henriot, as this species is known for its interest in biocontrol (Knapp et al. 2018). It was introduced from the Middle-East in the 1960s to control Panonychus citri (McGregor) on citrus and according to Wainstein and Vartapetov (1973), it seemed to have successfully acclimated and was recovered three years later on citrus and Rubus spp. According to these objectives, surveys focused on regions where this species was previously reported in the country: Western coast of Adjara (Wainstein and Vartapetov 1973) and Eastern plains along Kura connected to Azerbaijan (Abbasova 1970). Eleven species were identified from our surveys, including seven that are new for the Georgian Fauna. While we did not find A. swirskii, a very closely related species, Transeius wainsteini (Gomelauri) was identified. Identifications were performed using an integrative taxonomy approach, including morphological analyses and molecular markers.
Material and methods
Field surveys were carried out in June 2019. Leaves and sprouts were carefully examined with a hand magnifier and when mites were detected, the plant parts were collected in paper or plastic bags for later examination in the laboratory. Mites were directly collected on leaves under a stereoscopic microscope, and transferred to vials: (i) filled with 70% ethanol for further morphological studies and (ii) filled with 100% ethanol for molecular analyses. Mites were mounted in Hoyer's medium and identified with a phase and interferential contrast microscope (Leica DLMB, Leica Microsystems) (400x magnification).
The generic classification proposed by Chant and McMurtry (2007) was used. The terminologies used for chaetotaxy were those proposed by Lindquist and Evans (1965) as adapted by Rowell et al. (1978) for dorsal idiosomal setae of Phytoseiidae and by Chant and Yoshida-Shaul (1991) for ventral idiosomal setae. Adenotaxy and poroidotaxy terminologies are those proposed by Athias-Henriot (1975). All measurements are given in micrometers (µm), average value provided first followed by minimum and maximum values into brackets. Specimens are deposited at the SupAgro-CBGP Acari collection at Montpellier, France.
For specimens preserved in 100% ethanol, DNA sequences of CytB, COI mtDNA and 12S rRNA markers were obtained for assisting morphological diagnosis (Tixier et al. 2012b, Dos Santos and Tixier 2017). DNA extraction and amplification follow the protocols well detailed by Kanouh et al. (2010) and Tixier et al. (2012b), respectively. The primers used are those proposed by Tixier et al. (2012b) for Cytb and COI mtDNA fragments and by Jeyaprakash and Hoy (2002) for the 12S rRNA fragment. After DNA extraction, voucher specimens were retrieved as described in Tixier et al. (2010b) to confirm molecular assignment. For the COI and CytB fragments, a preliminary analysis was conducted to check for the absence of stop codons. The sequences were analysed both strands (forward and reverse). The consensus sequences obtained were compared to those included in the NCBI GenBank database to detect possible contaminations. DNA sequences were aligned (using ClustalW) and analysed using MEGA 6.0.6® (Tamura et al. 2013). Genetic distances (using the Kimura 2 parameter) were calculated for comparing DNA sequences to references (sequences published in Genbank). Neighbour-Joining phylogenetic trees were built to assess relationships (i) between T. wainsteini, A. swirskii, and Amblyseius andersoni (Chant) and (ii) between Amblyseius eharai Amitai & Swirski, A. largoensis (Muma) and A. herbicolus (Chant). For all these phylogenetic trees, Euseius stipulatus (Athias-Henriot) was used as out-group.
Results
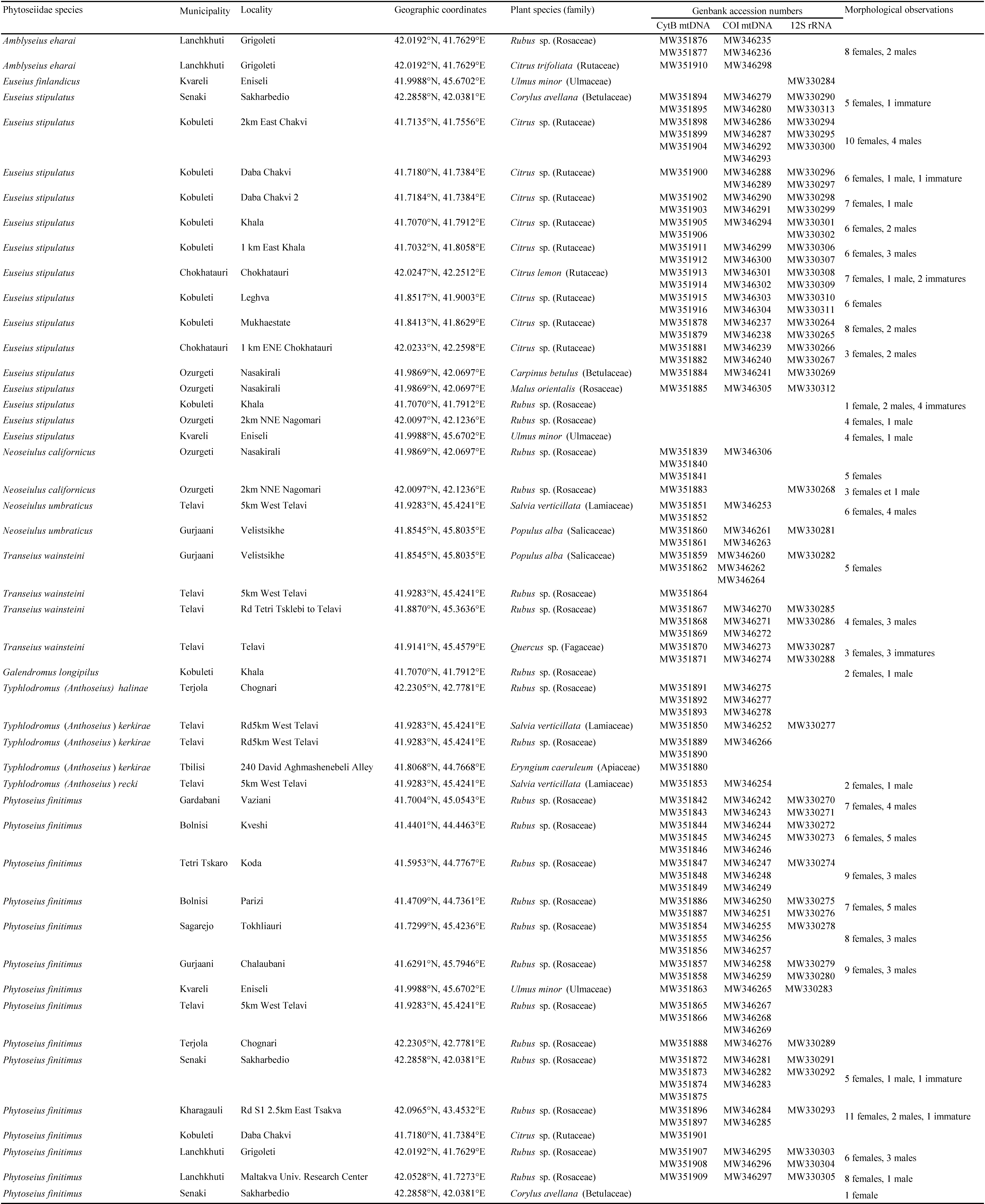
The Phytoseiidae species identified during the survey are listed below. Measurements of morphological characters are provided when they were necessary for species identification and comparison with morphologically close species. Table 1 presents the localities where species were retrieved and Genbank accession numbers of the DNA sequences obtained. When available, biological information is provided, especially for traits relevant to biological control. Data on the known distribution of the species is retrieved from the online Phytoseiidae catalogue (Demite et al. 2021).


Amblyseius eharai Amitai & Swirski
Amblyseius eharai Amitai & Swirski 1981: 60.
Specimens examined. At Lanchkhuti, Grigoleti (42.0192° N, 41.7629° E): 10 ♀♀ and 2 ♂♂ on Rubus sp. (Rosaceae), one ♀ on Citrus trifoliata (L.) Rafinesque (Rutaceae).
Previous records. China, Hong Kong, Japan, Malaysia, South Korea, Taiwan, Thailand.
Measurements of females (5 specimens)
Dorsum . Dorsal shield 362 (340–400) long and 199 (188–212) wide, smooth, with seven pairs of solenostomes (gd1, gd2, gd4, gd5 not well visible, gd6, gd8 and gd9), 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 35 (32–37), j3 44 (42–45), j4 4 (2–5), j5 4 (2–5), j6 4 (2–5), J2 4 (2–5), J5 4 (2–5), z2 7, Z1 5, z4 4 (2–5), z5 4 (2–5), Z4 104 (100–110), Z5 255 (250–262), s4 98 (97–100), S2 5, S4 7, S5 5, r3 11 (10–12) and R1 7 in length. All setae smooth.
Peritreme . Extending forwards to the bases of the setae j1.
Venter . Sternal shield with three pairs of setae and two pairs of poroids; one pair of sternal setae (st4) on small metasternal platelets; posterior margin with a truncated median projection. Distances between st1–st3 67 (65–70), st2–st2 70 (67–72), st5–st5 72 (70–77). Two pairs of metapodal plates, the largest one 19 (17–22) long and 6 (5–7) wide, the smallest one 8 (7–10) long and 2 wide. Ventrianal shield with three pairs of pre-anal setae JV1, JV2, ZV2 and pre-anal crescent pores (gv3) present, just under the setae JV2. Integument surrounding ventri-anal shield with four pairs of setae ZV1, ZV3, JV4 and JV5; ventri-anal shield 109 (100–130) long, 57 (55–62) wide at level of anterior corners, and 72 (67–77) wide at level of anus. JV5 64 (60–67) long.
Legs . Legs IV with three macrosetae: on the genu 126 (120–130), tibia 90 (85–95) and basitarsus 62 (60–65). SgeI 46 (42–47), SgeII 39 (37–42), SgeIII 47 (45–50), StiIII 36 (32–37). Genu II with seven setae (2–2/0, 2/0–1), Genu III with seven setae (2–2/0, 2/0–1).
Chelicera . Fixed digit 47, movable digit 42. Dentition not visible because the chelicerae are closed, but the fixed digit is clearly multidentate.
Spermatheca . Spermatheca with elongate cervix 21 (20–22) long, distal two-thirds gradually flaring, round atrium.
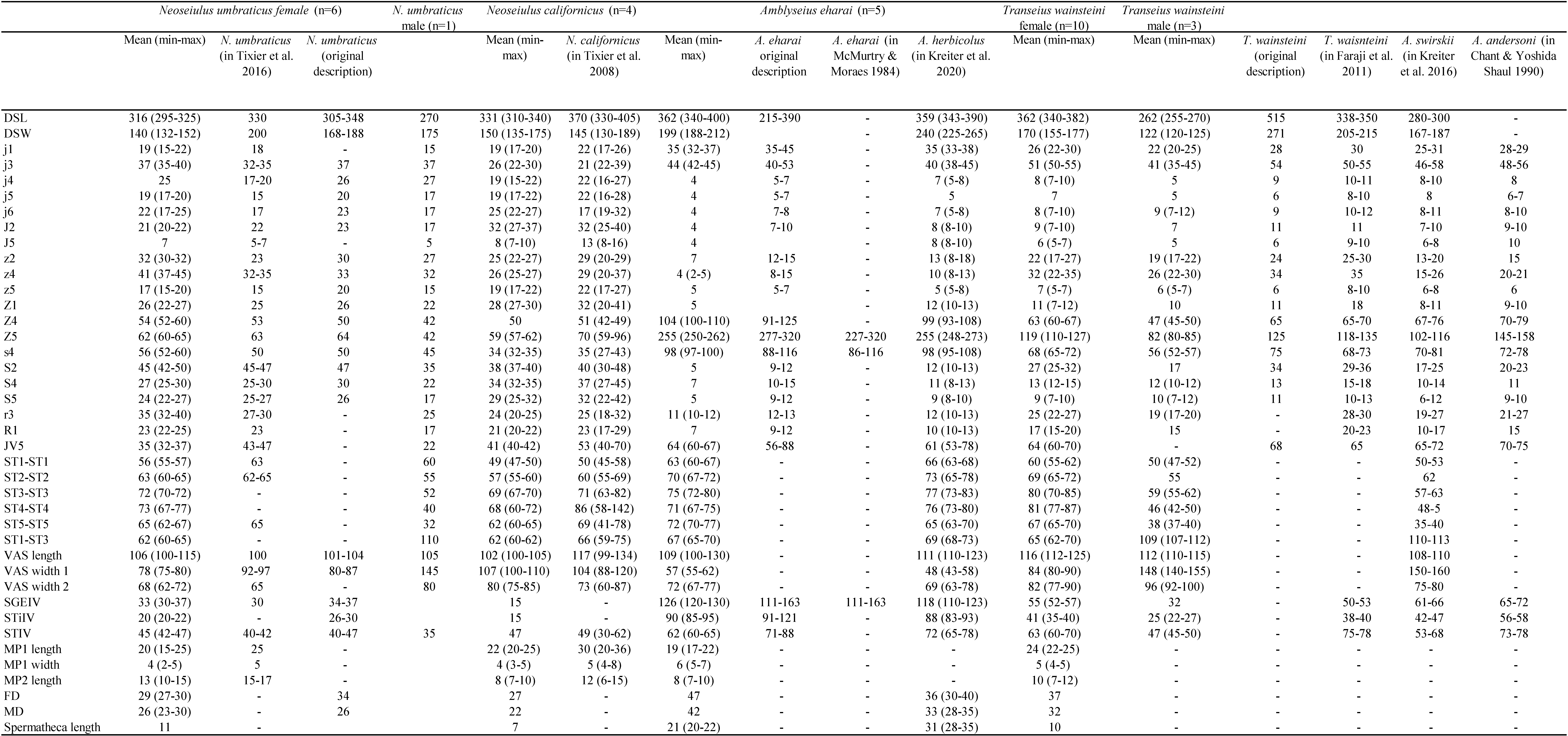
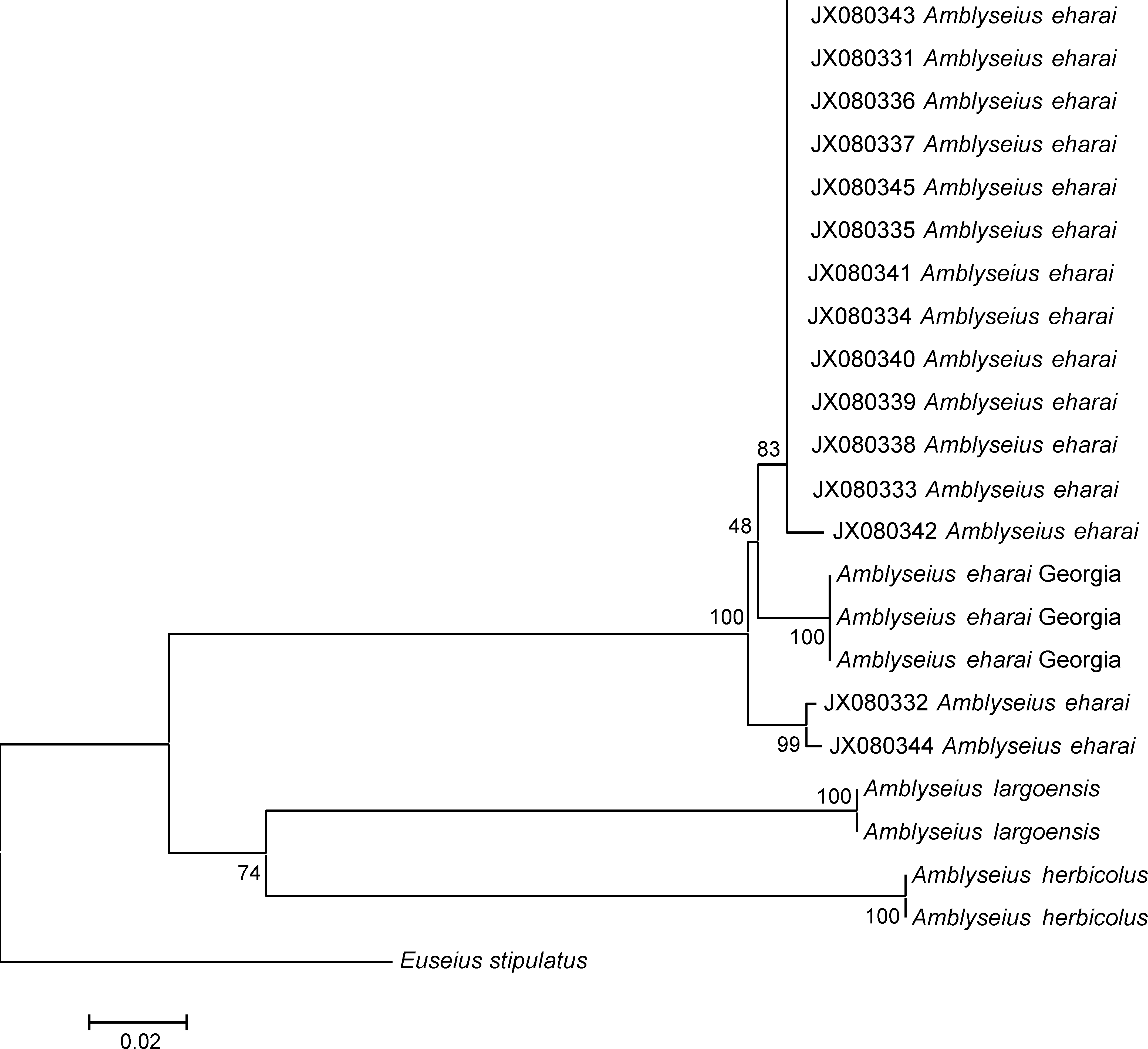
Remarks. Amblyseius eharai is morphologically close to A. herbicolus (Chant). Seta lengths are clearly overlapping and do not allow differentiating between these two species (Table 2). The only clear differences are the shape of the posterior border of the sternal shield (straight for A. herbicolus and with a truncated median projection for A. eharai) and the length and shape of the cervix of the spermatheca (long (23–29), distal two-thirds gradually flaring to 2–2.5 times basal diameter in A. herbicolus and short (18–24), flaring distally to 2–3 times narrowest diameter in A. eharai) (McMurtry and Moraes 1984). Because of these minor differences and because of the distribution of A. eharai only reported from Asia (whereas A. herbicolus is a cosmopolitan species), molecular markers were applied to assess further the identity of the Georgian specimens. Six DNA sequences (three sequences for CytB mtDNA, and three sequences for the COI mtDNA) were obtained from three specimens. The COI sequences were blasted in the Genbank database and were clearly assigned to A. eharai. Table 4a shows the COI genetic distances between the Georgian specimens and Amblyseius largoensis (Muma), A. herbicolus and A. eharai. The COI mtDNA sequences of the three Georgian specimens are identical (0%). They differ from A. largoensis and A. herbicolus sequences in Genbank by high genetic distances (27.2 and 29.2%, respectively), and from the 15 DNA fragments of A. eharai retrieved from Genbank by very low distances (2.1–3.2%), corresponding to intraspecific variation. The phylogenetic tree also illustrates that the Georgian specimens belong to the clade including the 15 DNA sequences retrieved from Genbank and assigned to A. eharai (JX080331–JX080345) (figure 1). No CytB mtDNA sequence of A. eharai is available in Genbank, whereas they are for A. herbicolus and A. largoensis (Supplementary Table S1b). The CytB genetic distances between the specimens herein considered and (i) A. herbicolus range between 39.1% and 40.3%, and (ii) A. largoensis range between 42.7% and 44.5%, clearly showing that Georgian specimens do not belong to these two latter species. Thus, based on morphological characteristics and molecular data, we conclude that the Georgian specimens belong to the species A. eharai.


It is the first time that this species is reported from this country and outside eastern Asia. In this survey, it was reported on Rubus sp. and Citrus trifoliata (L.) Rafinesque both at Lanchkhuti (near the Black Sea coast). Its unexpected presence in Georgia could be due to introduction from eastern Asia, as C. trifoliata is a species originating from Korea and north of China. Because A. eharai was also found on Rubus sp., it is possible that this species has adapted to new plants after its introduction into the region. Amblyseius eharai is considered to be an efficient natural enemy of mite pests and thrips in various crops, included citrus orchards (i.e. Ji et al. 2013, Park & Lee 2020).


Euseius stipulatus (Athias-Henriot)
Amblyseius stipulatus Athias-Henriot 1960: 294.
Amblyseius (Amblyseius) stipulates, Ueckermann & Loots 1988: 110.
Specimens examined. At Chokhatauri (42.0247° N, 42.2512° E): 9 ♀♀, 3 ♂♂ and 2 immatures on Citrus limon (L.) (Rutaceae), at Chokhatauri (1 km from Chokhatauri) (42.0233° N, 42.2598° E): 5 ♀♀ and 2 ♂♂ on Citrus sp. (Rutaceae), at Kobuleti (1 km East Khala) (41.7032° N, 41.8058° E): 8 ♀♀ and 3 ♂♂ on Citrus sp. (Rutaceae), at Kobuleti (2 kms from Esat Chakvi) (41.7135° N, 41.7556° E): 14 ♀♀ and 4 ♂♂ on Citrus sp. (Rutaceae), at Kobuleti (Daba Chakvi) (41.7180° N, 41.7384° E): 8 ♀♀ and 1 ♂ and 1 immature on Citrus sp. (Rutaceae), at Kobuleti (Daba Chakvi) (41.7184° N, 41.7384° E): 9 ♀♀ and 1 ♂ on Citrus sp. (Rutaceae), at Kobuleti (Khala) (41.7070° N, 41.7912° E): 8 ♀♀ and 2 ♂♂ on Citrus sp. (Rutaceae) and 1 ♀, 2 ♂♂ and 4 immatures on Rubus sp. (Rosaceae), Kobuleti (Leghva) (41.8517° N, 41.9003° E): 8 ♀♀ on Citrus sp. (Rutaceae), at Kobuleti (Mukhaestate) (41.8413° N, 41.8629° E): 10 ♀♀ and 2 ♂♂ on Citrus sp. (Rutaceae), at Kvareli (Eniseli) (41.9988° N, 45.6702° E): 4 ♀♀ and 1 ♂ on Ulmus minor Miller (Ulmaceae), at Ozurgeti (Nasakirali) (41.9869° N, 42.0697° E): 1 ♀ on Carpinus betulus L. (Betulaceae) and 1 ♀ on Malus orientalis Uglitzkich ex Iouzptchouk (Rosaceae), at Ozurgeti (2 kms from Nagomari) (42.0097° N, 42.1236° E): 4 ♀♀ and 1 ♂ on Rubus sp. (Rosaceae), at Senaki (Sakharbedio) (42.2858° N, 42.0381° E): 7 ♀♀ and 1 immature on Corylus avellana L. (Betulaceae)
Previous records. Algeria, Azores Island, Canary Island, France, Greece, Hungary, Italy, Iran, Madeira Islands, Montenegro, Morocco, Peru, Portugal, Slovenia, Spain, Syria, Tunisia, Turkey, USA.
Remarks. Euseius stipulatus was the second most frequent species found (36%), with most of the specimens retrieved from citrus (78% of E. stipulatus specimens). This species occurs in the south of the West Palearctic region especially around the Mediterranean basin especially on citrus orchards (Ferragut and Escudero 1997, Demite et al. 2021). Prior to this study only one species of Euseius, E. finlandicus (Oudemans) was known from Georgia. This is the first report of E. stipulatus for the Georgian mite fauna. Molecular sequences obtained (23 sequences for 12S rRNA, COI mtDNA markers and 22 sequences for CytB mtDNA fragment) were compared to those of the Euseius species reported from the West Palearctic region (E. stipulatus, E. scutalis (Athias-Henriot), E. finlandicus and E. gallicus Kreiter & Tixier). For all markers studied here, the sequences of the Georgian specimens are similar to the reference sequence of E. stipulatus (Supplementary Tables S2a, b, c). However, two specimens collected on Citrus limon at Chokhatauri are differentiated from the others and from the E. stipulatus references, by a distance of 11.1% – 12.3% for the CytB mtDNA, 2.8% for the 12S rRNA and 8.4% for the COI mtDNA (Supplementary Table S2a, b, c). Such high genetic distances in mitochondrial DNA have been already observed at the intraspecific level (up to 21.7% within Typhlodromus (Anthoseius) rhenanoides Athias-Henriot for the CytB mtDNA and up to 10.5% within Neoseiulus californicus (McGregor), for the COI mtDNA) (Okassa et al. 2011; Tixier et al. 2019). Furthermore, the genetic distances between these two specimens and E. gallicus, E. finlandicus and E. scutalis are high for all the DNA fragments considered, showing that these two specimens clearly do not belong to these three species (Supplementary Table S2a, b, c). We thus provisionally conclude that these two specimens belong to E. stipulatus and suggest that molecular difference might reflect some adaptations and different biological traits (i.e. Tixier et al. 2010a), but biological trials would be required to test this hypothesis.
Euseius finlandicus (Oudemans)
Seiulus finlandicus Oudemans 1915: 183.
Typhlodromus finlandicus, Oudemans 1930: 50.
Typhlodromus (Typhlodromus) finlandicus, Cunlife & Baker 1953: 19.
Amblyseius finlandicus, Athias-Henriot 1958: 34.
Typhlodromus (Amblyseius) finlandicus, Chant 1959: 67.
Typhlodromus (Typhlodromopsis) finlandicus, De Leon 1959: 113.
Amblyseius (Typhlodromalus) finlandicus, Muma (1961): 288.
Amblyseius (Amblyseius) finlandicus, Wainstein 1962: 15.
Amblyseius (Euseius) finlandicus, Arutunjan 1970: 11.
Typhlodromus pruni Oudemans 1929: 32 (synonymy according to Yoshida-Shaul & Chant 1995).
Specimens examined. At Kvareli (Eniseli) (41.9988° N, 45.6702° E): 1 ♀ on Ulmus minor Miller (Ulmaceae).
Previous records. Albania, Algeria, Angola, Argentina, Armenia, Austria, Azerbaijan, Belarus, Belgium, Bosnia and Herzegovina, Bulgaria, Canada, Caucasus Region, China, Croatia, Cyprus, Czech Republic, Denmark, England, Finland, France, Georgia, Germany, Greece, Hungary, India, Indonesia, Iran, Italy, Japan, Kazakhstan, Latvia, Lithuania, Macedonia, Mexico, Moldova, Montenegro, Netherlands, Nicaragua, Norway, Poland, Portugal, Russia, Scandinavia, Serbia, Slovakia, Slovenia, South Korea, Spain, Sweden, Switzerland, Tunisia, Turkey, Ukraine, USA.
Remarks. This species was reported from Georgia by Samsoniya (1972, 1977) and Wainstein and Vartapetov (1973) on tea, citrus trees and Prunus spp., especially in mountainous regions. It is reported to feed on eriophyid mites by Wainstein & Vartapetov (1973). One 12S rRNA sequence (from a specimen collected on Ulmus minor Miller) was obtained; it differs to the two DNA reference sequences of this species by 2.2 – 3.3% (Supplementary Table S2b).
Neoseiulus californicus (McGregor)
Typhlodromus californicus McGregor 1954: 89.
Amblyseius californicus, Schuster & Pritchard 1963: 271.
Cydnodromus californicus, Athias-Henriot 1977: 62.
Amblyseius (Amblyseius) californicus, Ueckermann & Loots 1988: 150; Ehara et al. 1994: 126.
Amblyseius (Neoseiulus) californicus, Ehara & Amano 1998: 33.
Specimens examined. at Ozurgeti (Nasakirali) (41.9869° N, 42.0697° E): 8 ♀♀ on Rubus sp. (Rosaceae), at Ozurgeti (2 kms from Nagomari) (42.0097° N, 42.1236° E): 4 ♀♀ and 1 ♂ on Rubus sp. (Rosaceae).
Previous records. Argentina, Azores, Brazil, Canada, Canary Islands, Chile, Colombia, Cuba, Cyprus, France, Greece, Guadeloupe, Guatemala, Italy, Japan, Madeira Islands (Kreiter et al. 2021), Mexico, Peru, Portugal, Reunion Island, Senegal, Serbia, Slovenia, South Africa, South Korea, Spain, Syria, Taiwan, Tunisia, Turkey, USA, Venezuela, Vietnam.
Measurements of females (4 specimens)
Dorsum . Dorsal shield 331 (310–340) long and 150 (135–175) wide, reticulated throughout, with three solenostomes (gd1, gd6 and gd9), 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 19 (17–20), j3 26 (22–30), j4 19 (15–22), j5 19 (17–22), j6 25 (22–27), J2 32 (27–37), J5 8 (7–10), z2 25 (22–27), Z1 28 (27–30), z4 26 (25–27), z5 19 (17–22), Z4 50, Z5 59 (57–62), s4 34 (32–35), S2 38 (37–40), S4 34 (32–35), S5 29 (25–32), r3 24 (20–25) and R1 21 (20–22) in length. All setae smooth except Z5 slightly barbed.
Peritreme . Extending forwards to the bases of the setae j1.
Venter . Sternal shield with three pairs of setae and two pairs of poroids; one pair of sternal setae (st4) on small metasternal platelets; posterior margin straight. Distances between st1–st3 62 (60–62), st2–st2 57 (55–60), st5–st5 62 (60–65). Two pairs of metapodal plates, the largest one 22 (20–25) long and 4 (3–5) wide, the smallest one 8 (7–10) long and 2 wide. Ventrianal shield with three pairs of pre-anal setae JV1, JV2, ZV2 and pre-anal crescent pores (gv3) present, posterior-paraxial to setae JV2. Integument surrounding ventrianal shield with four pairs of setae ZV1, ZV3, JV4 and JV5; ventrianal shield 102 (100–105) long, 107 (100–110) wide at level of anterior corners, and 80 (75–85) wide at level of anus. JV5 41 (40–42) long.
Legs . Legs IV with three macrosetae: on the genu 15, tibia 15 and basitarsus 47. Genu II with seven setae (2–2/0, 2/0–1) and Genu III with seven setae (1–2/1, 2/0–1).
Chelicera . Fixed digit 27, movable digit 22 (dentition not visible as the chelicerae are closed).
Spermatheca . Calyx cup-shaped 7–9 long and 7 in width, with a small atrium in base of the calyx.
Remarks. Neoseiulus californicus is commonly used in biological control. It is mass-released in crops, especially in vegetables for controlling Tetranychus urticae Koch, all over the World. This species can also naturally occur in vineyards and orchards (McMurtry and Croft 1997; Tixier et al. 2008a). The measurements of the Georgian specimens globally match with those reported in the re-description of Tixier et al. (2008a) (Table 2). Although the setae Z5 and JV5 are shorter on average in the Georgian specimens than in Tixier et al. (2008a, compiling 300 specimens from 10 populations), these differences are consistent with intraspecific variation range. Morphological identification was confirmed by DNA sequences obtained: one 12S rRNA, four cytB and one COI mtDNA. The CytB and the 12S rRNA sequences were compared to those reported in Okassa et al. (2011). The CytB and the 12S rRNA mean genetic distances between the Georgian and the commercial specimens (from different companies and those retrieved world-wide after commercial releases) are 0.04% and 0.1 %, respectively (Okassa et al. 2011). For COI sequences, the Georgian specimens are separated by distances ranging from 0.6 to 0.11% from specimens of N. californicus collected in apple orchards in France (Tixier et al. 2008a).
This is the first report of N. californicus collected in the wild in Georgia. Because of molecular similarity with the commercial specimens, we assume that the presence of N. californicus results from commercial releases and specimens herein collected on Rubus sp. might have dispersed from where they were released.
Neoseiulus umbraticus (Chant, 1956)
Typhlodromus umbraticus Chant 1956: 26.
Typhlodromus (Typhlodromus) umbraticus, Beglyarov 1958: 107.
Amblyseius umbraticus, Athias-Henriot 1959: 138.
Typhlodromus (Amblyseius) umbraticus, Chant 1959: 75.
Amblyseius (Typhlodromopsis) umbraticus, Muma 1961: 287.
Amblyseius (Amblyseius) umbraticus, Wainstein & Vartapetov 1973: 103.
Amblyseius (Neoseiulus) umbraticus, Karg 1991: 23.
Specimens examined. At Telavi (5 kms West from Telavi) (41.9283° N, 45.4241° E): 8 ♀♀ and 4 ♂♂ on Salvia verticillata L. (Lamiaceae), at Gurjaani (Velistsikhe) (41.8545° N, 45.8035° E): 3 ♀♀ on Populus alba L. (Salicaceae).
Previous records. Armenia, Azerbaijan, Azores Island, Belarus, Caucasus Region, Denmark, England, France, Georgia, Germany, Hungary, Iran, Italy, Jamaica, Latvia, Madeira Islands (Kreiter et al. 2021), Morocco, Mexico, Moldova, Montenegro, Norway, Poland, Russia, Slovakia, Slovenia, Spain, Switzerland, Turkey, Ukraine, USA.
Measurements of females (6 specimens)
Dorsum . Dorsal shield 316 (295–325) long and 140 (132–152) wide, slightly reticulated posteriorly, with five solenostomes (gd1, gd2, gd6, gd8 and gd9), 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 19 (15–22), j3 37 (35–40), j4 25, J5 19 (17–20), j6 22 (17–25), J2 21 (20–22), J5 7, z2 32 (30–32), Z1 26 (22–27), z4 41 (37–45), z5 17 (15–20), Z4 54 (52–60), Z5 62 (60–65), s4 56 (52–60), S2 45 (42–50), S4 27 (25–30), S5 24 (22–27), r3 35 (32–40) and R1 23 (22–25) in length. All setae smooth except Z5 slightly barbed.
Peritreme . Extending forwards to the bases of the setae j3.
Venter . Sternal shield with three pairs of setae and two pairs of poroids; one pair of sternal setae (st4) on small metasternal platelet; posterior margin straight. Distances between st1–st3 62 (60–65), st2–st2 63 (60–65), st5–st5 65 (62–67). Two pairs of metapodal plates, the largest one 20 (15–25) long and 4 (2–5) wide, the smallest one 13 (10–15) long and 2 wide. Ventrianal shield with three pairs of pre-anal setae JV1, JV2, ZV2 and pre-anal pores (gv3) present, posterior-paraxial to setae JV2. Integument surrounding ventrianal shield with four pairs of setae ZV1, ZV3, JV4 and JV5; ventrianal shield 106 (100–115) long, 78 (75–80) wide at level of anterior corners, and 68 (62–72) wide at level of anus. JV5 35 (32–37) long.
Legs . Legs IV with three macrosetae: on the genu 33 (30–37), tibia 20 (20–22) and basitarsus 45 (42–47). Genu II with eight setae (2–2/0, 2/1–1), Genu III with eight setae (2–2/0, 2/1–1).
Chelicera . Fixed digit 29 (27–30), movable digit 26 (23–30). Dentition not visible because the chelicerae are closed.
Spermatheca . Calyx cup-shaped 11 long and 11 in width, with an atrium well differentiated at the basis of the calyx.
Measurements on a male specimen are provided in the Table 2.
Remarks. This species was first described in England on Rubus fructicosus L. (Rosaceae) and then recorded mainly in the West Palearctic zone. It was reported from Georgia by Wainstein and Vartapetov (1973) on Rubus sp., Alnus sp. (Betulaceae), Ficus carica L. (Moraceae) and herbs. The measurements of specimens from Georgia fit with those provided by Tixier et al. (2016) for specimens collected from Morocco and with those of the original description (Table 2). The molecular distances range from 0 to 0.3 % between the four CytB mtDNA sequences and are null between the three COI mtDNA sequences. Only one 12S rRNA sequence was obtained. The eight DNA sequences for the three molecular fragments are now included in the Genbank database and will serve as references for further molecular identification of this species. Very few studies refer to the biology of N. umbraticus. This species seems able to feed on T. urticae and Thrips tabaci (Lindeman) (Sengonca and Dresher 2001, Kazak et al. 2002). Wainstein and Vartapetov (1973) reported that this species feeds on P. citri and T. urticae, and that it tends to prefer humid areas.
Transeius wainsteini (Gomelauri)
Amblyseius wainsteini Gomelauri 1968: 518.
Amblyseius (Amblyseius) wainsteini, Wainstein & Vartapetov 1973: 103.
Typhlodromips wainsteini, Rahmani et al. 2010: 498.
Transeius wainsteini, Chant & McMurtry 2004: 185,
Specimens examined. At Gurjaani (Velistsikhe) (41.8545° N, 45.8035° E): 8 ♀♀ on Populus alba L. (Salicaceae), at Telavi (5kms West from Telavi) (41.9283° N, 45.4241° E): 1 ♀ on Rubus sp. (Rosaceae), at Telavi (Rd Tetri Tsklebi to Telavi) (41.8870° N, 45.3636° E): 7 ♀♀ and 2 ♂♂ on Rubus sp. (Rosaceae), at Telavi (41.9141° N, 45.4579° E): 5 ♀♀ and 3 immatures on Quercus sp. (Fagaceae).
Previous records. Denmark, Georgia, Germany, Iran, Slovakia, Turkey.
Measurements of females (10 specimens)
Dorsum . Dorsal shield 362 (340–382) long and 170 (155–177) wide, smooth with small striation in the lateral posterial part, with seven solenostomes (gd1, gd2, gd4, gd5, gd6, gd8 and gd9), 17 pairs of dorsal setae and two pairs of sub-lateral setae: j1 26 (22–30), j3 51 (50–55), j4 8 (7–10), j5 7, j6 8 (7–10), J2 9 (7–10), J5 6 (5–7), z2 22 (17–27), z4 32 (22–35), z5 7 (5–7), Z1 11 (7–12), Z4 63 (60–67), Z5 119 (110–127), s4 68 (65–72), S2 27 (25–32), S4 13 (12–15), S5 9 (7–10), r3 25 (22–27) and R1 17 (15–20) in length. All setae smooth.
Peritreme . Extending forwards to the bases of the setae j1.
Venter . Sternal shield with three pairs of setae and two pairs of poroids; one pair of sternal setae (st4) on small metasternal platelets; posterior margin straight. Distances between st1–st3 65 (62–70), st2–st2 69 (65–72), st5–st5 67 (65–70). Two pairs of metapodal plates, the largest one 24 (22–25) long and 5 (4–5) wide, the smallest one 10 (7–12) long and 2 wide. Ventrianal shield with three pairs of pre-anal setae JV1, JV2, ZV2 and a pair of crescent pre-anal pores (gv3) present, slightly posterior-paraxial to setae JV2. Integument surrounding ventrianal shield with four pairs of setae ZV1, ZV3, JV4 and JV5; ventrianal shield 116 (112–125) long, 84 (80–90) wide at level of anterior corners, and 82 (77–90) wide at level of anus. JV5 64 (60–70) long.
Legs . Legs IV with three macrosetae: on the genu 55 (52–57), tibia 41 (35–40) and basitarsus 63 (60–70). SgeII 31 (30–35), SgeIII 32 (30–35), StiIII 26 (25–27). Genu II with seven setae (2–2/0, 2/0–1), Genu III with seven setae (1–2/1, 2/0–1).
Chelicera . Fixed digit 37 long, movable digit 32 long. Dentition not visible because the chelicerae are closed.
Spermatheca . Spermatheca with a cup-shaped calyx 8–10 long and 5 wide, with a well differentiated round and small atrium at the base of calyx.
The table 2 provides measurements of three males.
Remarks. This species was described from Georgia (Manglisi) on Corylus sp. (Betulaceae) and according to Wainstein and Vartapetov (1973), it is quite common across the country. These authors also noted that T. wainsteini feeds on P. citri and T. urticae. The measurements of the Georgian specimens are close to those reported in the original description and in the re-description of Faraji et al. (2011) (from Turkey), except for the setae STIV that were shorter in our specimens (60–70) than in those measured by Faraji et al. (2011) (75–78) (Table 2). However, the difference is minor and it is the only difference with T. wainsteini studied by Faraji et al. (2011); we therefore conclude that the specimens from Georgia belong to Transeius wainsteini (Table 2).
Differences between Amblyseius swirskii, Amblyseius andersoni (Chant) and T. wainsteini are minor. Amblyseius andersoni differs from A. swirskii and T. wainsteini by longer setae Z5 (Table 2). The main differences between A. swirskii and T. wainsteini are the measurements of the setae z2, z4, the ratio s4/S2 and the dentition of the chelicerae.
CytB (8), 12S (5) and COI (8) DNA sequences of Georgian specimens of T. wainsteini were obtained, respectively. Phylogenetic trees are presented in the figure 2. The mean genetic distances between these specimens are 1.6% (0–3.1%) for the CytB mtDNA marker, 0% for the 12S rRNA fragment, and 1.2% (0–3%) for the COI mtDNA marker. The mean genetic distances between T. wainsteini and A. swirskii, observed for the three molecular markers, support that these species are distinct taxa (CytB mtDNA marker: 44.2% (43.7% – 45.1%), 12S rRNA: 19.7%, COI mtDNA: 24.9% (23.8% – 25.9%)). Transeius wainsteini differs from A. andersoni by 26.3 % (25.5% – 27.2%) for the CytB mtDNA, 9.1% (8.8% – 9.4%) for the 12S rRNA marker and 19.9% (18.6% – 21.1%) for the COI mtDNA fragment (Supplementary Table S3a,b,c). These distances are clearly smaller than those observed between T. wainsteini and A. swirskii, suggesting that T. wainsteini is phylogenetically closer to A. andersoni than to A. swirskii. Interestingly, these genetic relationships do not reflect morphological similarities as (i) A. swirskii and A. andersoni are more similar to each other than to T. wainsteini and (ii) T. wainsteini is morphologically more similar to A. swirskii than to A. andersoni. The morphological similarities between T. wainsteini and A. swirskii suggest evolutionary convergence especially for the length of setae Z5. Further analyses would be interesting to carry out based, in particular, on the observation of spermatheca structures (atrium, calyx).



A very close relationship between A. andersoni and T. wainsteini is clearly supported by the 12S sequences (9.1%) (Supplementary Table S3b). In the absence of additional parameters (morphology and other molecular markers), this small distance could have wrongly lead to conclusion that they belong to the same species. The maximal intraspecific distance using the 12S rRNA marker for Phytoseiidae, was observed for the species Amblyseius largoensis (7.8% in Barbosa-Lima et al. 2018) and the minimal interspecific distances observed range between 9.5% and 12.5% (between Neoseiulus californicus and N. fallacis and N. californicus and N. idaeus, respectively) (Jeyaprakash and Hoy 2002; Okassa et al. 2011).
The phylogenetic closeness of A. andersoni and T. wainsteini questions the monophyly of the genus Amblyseius and the validity of the genus Transeius, as already stated by Tsolakis et al. (2012) who showed the proximity between A. andersoni, A. swirskii and Transeius montdorensis (Schicha). Further phylogenetic analyses would be required including additional Amblyseius and Transeius species, to conclude that Transeius is not a valid genus and that Amblyseius is paraphyletic.
Galendromus (Galendromus) longipilus (Nesbitt)
Typhlodromus longipilus Nesbitt 1951: 26.
Typhlodromus (Typhlodromus) longipilus, Cunliffe & Baker 1953: 17.
Typhlodromus (Typhlodromus) longipilis [sic], Chant 1959: 59.
Galendromus longipilis [sic], Muma 1961: 26.
Metaseiulus longipilus, Schuster 1966: 323.
Metaseiulus (Galendromus) longipilus, Wainstein 1973: 176.
Typhlodromus longipilis [sic], Ozman & Çobanoğlu 2001: 482.
Typhlodromus longipilis [sic], Çobanoğlu & Özman 2002: 92.
Galendromus longipilus, Kolodochka 2006: 171.
Metaseiulus longipilis [sic], Kulikova 2011: 59.
Specimens examined. At Kobuleti (Khala) (41.7070° N, 41.7912° E): 2 ♀♀ and 1 ♂ on Rubus sp. (Rosaceae).
Previous records. Austria, Bulgaria, Canada, Costa Rica, Cuba, Czech Republic, France, Galapagos, Germany, Hungary, Italy, Mexico, Moldova, Poland, Slovakia, Spain, Switzerland, Turkey, Ukraine, USA.
Measurements of females (2 specimens)
Dorsum . Dorsal shield 340 long and 155 wide, reticulated throughout, with two visible solenostomes (gd6 and gd9), 16 pairs of dorsal setae and one pair of sub-lateral setae inserted in the dorsal shield: j1 25, j3 65–67, j4 52–55, j5 60–62, j6 65–70, J2 75, J5 8, z2 67, z4 67–70, z5 62, Z4 70–75, Z5 60–65, s4 65–70, s6 80–82, S2 75–77, S5 62–70 and r3 55–57 in length. All setae smooth.
Peritreme . Extending slightly anteriorly to the bases of the setae z4.
Venter . Sternal shield with three pairs of setae and two pairs of poroid; one pair of sternal setae (st4) on small metasternal platelets; posterior margin straight. Distances between st1–st3 65–72, st2–st2 47–52, st5–st5 47. Two pairs of metapodal plates, the largest one 25 long and 4 wide, the smallest one 10 long and 2 wide. Ventrianal shield with four pairs of pre-anal setae JV1, JV2, JV3, ZV2 and a pair of small circular pre-anal pores (gv3) present, immediately posterior-mediad to JV3. Integument surrounding ventrianal shield with 3 pairs of setae ZV1, ZV3 and JV5; ventrianal shield 107 long, 50–55 wide at level of anterior corners, and 65 wide at level of anus. JV5 55–57 long.
Legs . Legs IV with one long setae on the basitarsus 35. Genu II with nine setae (2–2/1, 2/1–2), Genu III with seven setae (1–2/1, 2/0–1).
Chelicera . Fixed digit 22 long; and movable digit 20 long. Dentition not visible because chelicerae closed.
Spermatheca . Spermatheca with elongated and tubular cervix 37 long and 3 wide, with a small atrium inserted at the base of the cervix.
Remarks. The measurements of the Georgian specimens are close to those reported in the re-description of G. longipilus provided by Chant and Yoshida-Shaul (1984) (Table 3). Some differences are however observed in some seta lengths, which are slightly shorter in the specimens examined in this study.
Galendromus longipilus is morphologically very close to Galendromus occidentalis (Nesbitt). However, because of the peritreme length and because j6 is longer than the distance between j6 and J2, we conclude that the specimens herein examined belong to G. longipilus. No material preserved in 100% ethanol was available for DNA analysis; therefore, we could not strengthen the identification with molecular markers at this time.
This is the first report of G. longipilus from Georgia. This occurrence is however consistent with the reported distribution of this species from Turkey and Europe.



Typhlodromus (Anthoseius) recki (Wainstein)
Typhlodromus recki Wainstein 1958: 203.
Typhlodromus (Typhlodromus) recki, Chant 1959: 62.
Typhlodromella recki, Muma 1961: 299.
Amblydromella recki, Moraes et al. 1986: 171.
Amblydromella (Aphanoseia) recki, Denmark & Welbourn 2002: 308.
Specimens examined. At Telavi (5 kms West from Telavi) (41.9283° N, 45.4241° E): 3 ♀♀ and 1 ♂ on Salvia verticillata L. (Lamiaceae).
Previous records. Algeria, Armenia, Austria, Azerbaijan, Caucasus Region, Cyprus, France, Georgia, Greece, Hungary, Iran, Israel, Italy, Kazakhstan, Lebanon, Moldova, Morocco, Portugal, Russia, Slovenia, Spain (Ferragut 2018), Syria, Tunisia, Turkey, Ukraine.
Remarks. This species was known from Georgia, reported by Wainstein (1958) on Salvia nemorosa L. (Lamiaceae), and is commonly found in the West Palearctic region, especially on plants of the family Lamiaceae (Tixier et al. 2020a).
Two DNA sequences (one of the CytB and of the COI fragment) were obtained. CytB genetic distance between the Georgian specimen and the 54 specimens collected in South of France and Italy was 5.7% (4.7% – 14%) (Tixier et al. 2020b). Genetic distances among the COI sequences, ranged from 2 to 2.2% between the Georgian specimen and the four reported in Genbank (MT828361–364, from France and Italy). This differentiation can be due to population isolation or result from adaptation to climatic conditions (Tixier et al. 2020b; Queiroz et al. 2021).
Typhlodromus (Anthoseius) halinae (Wainstein & Kolodochka)
Anthoseius (Amblydromellus) halinae Wainstein & Kolodochka (1974): 629.
Anthoseius halinae, Rivnay & Swirski (1980): 177.
Amblydromella halinae, Moraes et al. (1986): 163.
Amblydromella (Amblydromella) halinae, Kolodochka (1998): 52.
Amblydromella (Aphanoseia) halinae, Denmark & Welbourn (2002): 308.
Specimens examined. At Terjola (Chognari) (42.2305° N, 42.7781° E): 3 ♀♀ on Rubus sp. (Rosaceae).
Previous records. Iran, Italy, Moldova, Norway, Russia, Slovakia, Ukraine.
Measurements of female. One specimen: voucher molecular specimen in the ''best'' state, the two other specimens are also voucher specimens and not all the characters can be measured.
Dorsum . Dorsal shield 310 long and 140 wide, reticulated throughout, five solenostomes not well visible (gd2, gd4, gd6, gd8 and gd9), 18 pairs of dorsal setae and two pairs of sub-lateral setae: j1 23, j3 20, j4 15, j5 13, j6 18, J2 20, J5 5, z2 20, z3 25, z4 20, z5 13, Z4 25, Z5 43, s4 25, s6 25, S2 30, S4 25, S5 20, r3 23 and R1 23 in length. All setae smooth.
Peritreme . Extending forwards between the bases of the setae j3 and j1.
Venter . Sternal shield with three pairs of setae and two pairs of poroids; one pair of sternal setae (st4) on small metasternal platelets; posterior margin straight. Distances between st1–st3 50, st2–st2 55, st5–st5 53. Two pairs of metapodal plates, the largest one 25 long and 3 wide, the smallest one 13 long and 2 wide. Ventrianal shield with four pairs of pre-anal setae JV1, JV2, JV3, ZV2 and a pair of small circular pre-anal pores (gv3) present (horizontally aligned with JV3 and vertically aligned with JV2). Integument surrounding ventrianal shield with three pairs of setae ZV1, ZV3 and JV5; ventrianal shield 88 long, 88 wide at level of anterior corners, and 75 wide at level of anus. JV5 33 long.
Legs . Legs IV with one macroseta on the basitarsus (23), and setae (not macrosetae) on the genu (18), tarsus (20). Genu II with seven setae (2–2/0, 2/0–1), Genu III with seven setae (1–2/0, 2/1–1).
Chelicera . Fixed digit 25 long; and movable digit 23 long. Dentition not visible because chelicerae closed.
Spermatheca . Spermatheca with cervix 15–18 long (on the two sides) and 10 wide, with an atrium inserted in the cervix.
Remarks. The specimen studied is morphologically close to Typhlodromus (Anthoseius) rhenanus (Oudemans), even if some differences are observed in spermatheca shape (Table 3). Molecular comparisons with specimens from our own database show high CytB distances between the sequences herein obtained and those referring to T. (A.) rhenanus (19.6% – 20.3%). Similar high distances have been previously observed at the intraspecific level (i.e. Tixier et al. 2017, 2019). However, for the COI fragment, 22% divergence was observed between T. (A.) rhenanus and our specimens. Such large divergences indicate that these specimens do not belong to T. (A.) rhenanus, but to a morphologically similar species.
They are also close to T. (A.) georgicus Wainstein, but difference in spermatheca shape and S5 length (30 for T. (A.) georgicus and 20 for the presently examined specimen) (Hajizadeh and Mortazavi 2015) seem to show that the specimen observed do not belong to this latter species. It is difficult to assign a single specimen to a species. Tixier (2013) tried to provide some decision rules and proposed based on statistical analysis, that a difference of 11 microns between two specimens would be sufficient to conclude that these specimens might belong to different species. We see that in the present case the difference between the specimen examined and T. (A.) georgicus (10 microns) would be just included in this interspecific variation. However, because of this slight difference, we can have still some doubts. Considering other species, especially Typhlodromus (Anthoseius) halinae, it seems that the specimen observed is much closer to this latter species than to T. (A.) georgicus. However, the specimens were also morphologically very close to Typhlodromus (Anthoseius) salviae (Kolodochka) but unfortunately we did not find in the description of this latter species, information on the differentiation with T. (A.) halinae (Table 3).
Even if some doubts exist between an identification assigned to T. (A.) halinae or to T. (A.) georgicus, because of closer morphological traits with the former species, we considered that the specimen herein collected belong to T. (A.) halinae. Molecular sequences would help in assisting the diagnosis of these morphological close species in the future, as well as in clarifying the fact that some authors stated that differentiation between some Typhlodromus (Anthoseius) species is only possible based on male observation (Kolodochka 1978). This would be the first report of T. (A.) halinae from Georgia. Because of its current distribution in Eastern Europe and Middle East, the report of the species in Georgia is not surprising.
Typhlodromus (Anthoseius) kerkirae Swirski & Ragusa
Typhlodromus kerkirae Swirski & Ragusa 1976: 101.
Anthoseius kerkirae, Rivnay & Swirski 1980: 177.
Typhlodromus kerkyrae [sic], Papaioannou-Souliotis 1981: 41.
Amblydromella kerkirae, Moraes et al. 1986: 165.
Amblydromella (Aphanoseia) kerkirae, Denmark & Welbourn 2002: 308.
Specimens examined. At Telavi (5 kms from West Telavi) (41.9283° N, 45.4241° E): 1 ♀ on Salvia verticillata L. (Lamiaceae) and 2 ♀♀ on Rubus sp. (Rosaceae), at Tbilisi (240 David Aghmashenebeli Alley) (41.8068° N, 44.7668° E): 1 ♀ on Eryngium caeruleum L. (Apiaceae).
Previous records. Croatia, France, Greece, Iran, Italy, Spain, Turkey.
Measurements of female (1 specimen: voucher molecular specimen in ''best'' state)
Dorsum . Dorsal shield 315 long and 135 wide, reticulated throughout, with six solenostomes (gd2, gd4, gd6, gd8 and gd9), 18 pairs of dorsal setae and two pairs of sub-lateral setae: j1 20, j3 22, j4 15, j5 15, j6 18, J2 23, J5 8, z2 20, z3 25, z4 20, z5 18, Z4 32, Z5 52, s4 25, s6 30, S2 30, S4 30, S5 27, r3 18 and R1 18 in length. All setae smooth except Z5 lightly barbed.
Peritreme . Eextending nearly reaching the bases of the setae j1.
Venter . Sternal shield with three pairs of setae and two pairs of poroids; one pair of sternal setae (st 4) on small metasternal platelets; posterior margin straight. Distances between st1–st3 not visible, st2–st2 not visible, st5–st5 63. Metapodal shields not visible due to mountings (DNA voucher specimen). Ventrianal shield with four pairs of pre-anal setae JV1, JV2, JV3, ZV2 and a pair of small circular pre-anal pores (gv3) present, at level of setae JV3, posterior or slightly postero-paraxial to setae JV2. Integument surrounding ventrianal shield with four pairs of setae ZV1, ZV3, JV4 and JV5; ventrianal shield 98 long, 75 wide at level of anterior corners, and 63 wide at level of anus. JV5 33 long.
Legs . Legs IV with one macrosetae on the basitarsus 27. Genu II with seven setae (2–2/0, 2/0–1), chaetotaxy of the Genu III not clearly visible (leg folded).
Chelicera . Fixed digit 28 long; and movable digit 23 long. Dentition not visible because chelicerae closed.
Spermatheca . Spermatheca with campanulate calyx 13 long and 8 wide, with an atrium incorporated at the basis of the cervix.
Remarks. The morphological features reported above are in line with those reported by Tixier et al. (2019) for T. (A.) kerkirae (Table 3). Four DNA sequences were obtained for CytB, one for 12S and two for the COI fragments. The Genbank database only includes CytB sequences for T. (A.) kerkirae (accession number: MK014094), which have 15.4% – 16.1% divergence with sequences from our specimens from Georgia. In contrast, the mean distance between the four Georgian specimens is 0.25%. Although the genetic distance between the French and Georgian specimens is high, it is lower than the intraspecific variation already observed for species of the sub-family Typhlodrominae and the genus Typhlodromus (Anthoseius) (i.e. 21.7% for T. (A.) rhenanoides in Tixier et al. 2019). The COI and 12S rRNA sequences newly included in the Genbank database will serve as references for further molecular identification of this species.
Phytoseius finitimus Ribaga
Phytoseius finitimus Ribaga 1904: 178.
Phytoseius (Dubininellus) finitimus, Wainstein 1959: 1365.
Phytoseius (Pennaseius) finitimus, Pritchard & Baker 1962: 223.
Pennaseius finitimus, Schuster & Pritchard 1963: 279.
Phytoseius (Phytoseius) finitimus, Denmark 1966: 16.
Phytoseius dubinini Beglyarov 1958: 116 (synonymy according to Pritchard & Baker 1962).
Specimens examined. At Bolnisi (Kveshi) (41.4401° N, 44.4463° E): 9 ♀♀ and 5 ♂♂ on Rubus sp. (Rosaceae), at Bolnisi (Parizi) (41.4709° N, 44.7361° E): 9 ♀♀ and 5 ♂♂ on Rubus sp. (Rosaceae), at Gardabani (Vaziani) (41.7004° N, 45.0543° E): 9 ♀♀ and 4 ♂♂ on Rubus sp. (Rosaceae), at Gurjaani (Chalaubani) (41.6291° N, 45.7946° E): 11 ♀♀ and 3 ♂♂ on Rubus sp. (Rosaceae), at Kharagauli (Rd S1 2.5km East of Tsakva) (42.0965° N, 43.4532° E): 13 ♀♀, 2 ♂♂ and 1 immature on Rubus sp. (Rosaceae), at Kobuleti (Daba Chakvi) (41.7180° N, 41.7384° E): 1 ♀ on Citrus sp. (Rutaceae), at Kvareli (Eniseli) (41.9988° N, 45.6702° E): 2 ♀♀ on Ulmus minor (Ulmaceae), at Lanchkhuti (Grigoleti) (42.0192° N, 41.7629° E): 8 ♀♀ and 3 ♂♂ on Rubus sp. (Rosaceae), at Lanchkhuti (Maltakva Univ. Research Center) (42.0528° N, 41.7273° E): 9 ♀♀ and 1 ♂ on Rubus sp. (Rosaceae), at Sagarejo (Tokhliauri) (41.7299° N, 45.4236° E): 11 ♀♀ and 3 ♂♂ on Rubus sp. (Rosaceae), at Senaki (Sakharbedio) (42.2858° N, 42.0381° E): 9 ♀♀, 1 ♂ and 1 immature on Rubus sp. (Rosaceae) and 1 ♀ on Corylus avellana (Betulaceae), at Telavi (5 kms West from Telavi) (41.9283° N, 45.4241° E): 3 ♀♀ on Rubus sp. (Rosaceae), at Terjola (Chognari) (42.2305° N, 42.7781° E): 1 ♀ on Rubus sp. (Rosaceae), at Tetri Tskaro (Koda) (41.5953° N, 44.7767° E): 12 ♀♀, and 3 ♂♂ on Rubus sp. (Rosaceae).
Previous records. Algeria, Azores, Egypt, France, Greece, Iran, Israel, Italy, Montenegro, Morocco, Portugal, Slovenia, Spain, Syria, Tunisia, Turkey, USA.
Remarks. According to the world database of Demite et al. (2021), P. finitimus is not reported from Georgia. However, Phytoseius plumifer (Canestrini and Fanzago) has been reported several times from this country. Because of its long history of misidentification with P. finitimus (Duso and Fontana 2002), we can wonder about the specimens reported under the name of P. plumifer from Georgia.
Phytoseius finitimus was the most frequent species retrieved herein (41% of the specimens collected).
CytB (29), COI (28) and 12S (18) sequences were obtained and compared to those of Tixier et al. (2017) for specimens collected on Viburnum tinus (Adoxaceae), Vitis vinifera (Vitaceae) from Italy and Actinidia deliciosa (Actinidiacae) from France. The supplementary table S4 shows the genetic distances obtained. Low intraspecific variation was observed for the Georgian specimens. The Georgian specimens are molecularly closer to those from A. deliciosa and V. vinifera than to those from V. tinus, whatever the samples considered (locations and plants). It is worth to note that for the three molecular fragments, a high genetic distance is observed between a specimen collected from Rubus sp. at Bolnisi-Kveshi and all the others (Supplementary Table S4).
Conclusion
Eleven species of Phytoseiidae were identified during this survey, and among them seven are new for the Georgia Fauna. Results show that despite the reduced number of host plant species sampled, new occurrences were revealed, emphasizing knowledge gaps on Phytoseiidae distribution. Main features resulting from the survey include (i) the occurrence of common East European species, already retrieved from this country and neighbouring countries, and (ii) the unexpected occurrence of some species, which could be explained by exotic introductions (accidental or for biological control purposes). The fact that E. stipulatus and P. finitimus were observed for the first time in Georgia, while they were the two most abundant species retrieved, is quite unexpected. Fauna modification due for instance to climate change (especially for E. stipulatus as this species is mainly reported from the Mediterranean coast climate) and/or misidentifications (especially for P. finitimus because of repeated misidentification with P. plumifer) are hypotheses that can be put forward to explain these results. The study also illustrates the utility of integrative taxonomy for diagnosis purposes. The observation of the lowest intraspecific distance never detected for the 12S rRNA marker and the validity of the genus Transeius are the main taxonomic issues pinpointed.
Acknowledgements
This work was supported by the European Union's Horizon 2020 research and innovation program [grant 773 902-SuperPests]. We are very grateful to the three reviewers for their important comments on an early version of this manuscript.
acarologia_4471-supplementary-tables.xlsx
References
- Abbasova E. D. 1972. Phytoseiid mites (Parasitiformes: Phytoseiidae) of Azerbaijan. Avtoreferat Dissertatsii na Soiskanie Uchenoy Stepeni Kandidata Biologicheskikh Nauk. Akadrmiya Nauk Azerbaydzhanskoy SSR, Institut Zoologii, Baku, Azerbaijan, 34 pp.
- Amitai S., Swirski E. 1981. A new species of Amblyseius (Acarina: Phytoseiidae) from the Far East. Isr. J. Entomol., 15: 59-66.
- Arutunjan E.S. 1970. Phytoseiid mites (Phytoseiidae) on agricultural crops in the Armenian SSR. Akademii Nauk Armyanskoi SSR, Otdelenie Biologicheskikh Nauk, Dissertatsii na Soiskanie Uchenoi Stepeni Candidata Biologrcheskikh Nauk, Zooliya, 97, 31 pp.
- Athias-Henriot C. 1958. Phytoseiidae et Aceosejidae (Acarina: Gamasina) d'Algérie. II. Phytoseiidae. Clé des genres Amblyseius Berlese (Suite) et Seiulus Berlese. Bull. Soc. Hist. Nat. Afrique du Nord, 49: 23-43.
- Athias-Henriot C. 1959. Acariens planticoles d'Algérie. I. 5e contribution au genre Amblyseius Berlese (Phytoseiidae). II. Première liste d'Actinochitinosi (Cheyletidae, Caligonellidae, Hemisarcoptidae). Bull. Acad. Roy. Belgique, Sciences (Ser. 5), 45: 130-153.
- Athias-Henriot C. 1960. Nouveaux Amblyseius d'Algérie (Parasitiformes, Phytoseiidae). Acarologia, 2: 288-299.
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides protoadéniques, Phytoseiidae). Acarologia, 17(1): 20-29.
- Athias-Henriot C. 1977. Nouvelles notes sur les Amblyseiini. III. Sur le genre Cydnodromus: Redéfinition, composition (Parasitiformes, Phytoseiidae). Entomophaga, 22: 61-73. https://doi.org/10.1007/BF02372991
- Barbosa Lima D., Rezende-Puker D., Santos de Mendonça R., Tixier M.-S., Guedes Correia Gondim Junior M., Wagner da Silva Melo J., Chiaradia Oliveira D., Navia D. 2018. Molecular and morphological characterization of the predatory mite Amblyseius largoensis (Acari: Phytoseiidae). Surprising similarity between an Asian and American populations. Exp. Appl. Acarol., 76: 287-310. https://doi.org/10.1007/s10493-018-0308-1
- Beglyarov G.A. 1958. Species of Phytoseiidae (Parasititormes: Gamasoidea) predatory upon tetranychid mites in orchards of the Krasnodar region. Trudy Vsesoiuznogo Institut Zashchity Rastenii, Leningrad, Russia, 10: 98-124.
- Chant D.A. 1956. Some mites of the subfamily Phytoseiinae (Acarina: Laelaptidae) from southeastern England, with descriptions of new species. Can. Entomol., 88: 26-37. https://doi.org/10.4039/Ent8826-1
- Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. https://doi.org/10.4039/entm9112fv
- Chant D.A., Yoshida-Shaul E. 1984. A world review of the occidentalis species group in the genus Typhlodromus Scheuten (Acarina: Phytoseiidae). Can. J. Zool., 62: 1860-1871. https://doi.org/10.1139/z84-272
- Chant D.A., Yoshida Shaul E. 1990. The identities of Amblyseius andersoni (Chant) and A. potentillae (Garman) in the family Phytoseiidae (Acari: Gamasina). Int. J. Acarol., 16(1): 5-12. https://doi.org/10.1080/01647959008683857
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Int. J. Acarol., 17: 187-199. https://doi.org/10.1080/01647959108683906
- Chant D.A., McMurtry J.A. 2004. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part III. The tribe Amblyseiini Wainstein, subtribe Amblyseina n. subtribe. Int. J. Acarol., 30(3): 171-228. https://doi.org/10.1080/01647950408684388
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and sub-genera of the Phytoseiidae of the World. Indira Publishing House, 220 pp.
- Cunliffe F., Baker E.W. 1953. A guide to the predatory phytoseiid mites of the United States. Pinellas Biology Laboratory, Inc., 1, 28 pp.
- De Leon D. 1959. Two new genera of Phytoseiid mites with notes on Proprioseius meridionalis Chant (Acarina: Phytoseiidae). Entomol. News, 70: 257-262.
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2021. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae
 (accessed 20/XII/2020)
(accessed 20/XII/2020) - Denmark H.A. 1966. Revision of the genus Phytoseius Ribaga, 1904 (Acarina: Phytoseiidae). Fla. Dep. Agric. Bul., 6: 1-105.
- Denmark H.A., Welbourn W.C. 2002. Revision of the genera Amblydromella Muma and Anthoseius De Leon (Acari: Phytoseiidae). Int. J. Acarol., 28(4): 291-316. https://doi.org/10.1080/01647950208684308
- Dos Santos V., Tixier M.-S. 2017. Molecular markers for analysing phylogenetic relationships within the mite family Phytoseiidae (Acari: Mesostigmata). Cladistics 28(5): 1-16. https://doi.org/10.1111/cla.12166
- Duso C., Fontana P. 2002. On the identity of Phytoseius plumifer (Canestrini & Fanzago, 1876) (Acari: Phytoseiidae). Acarologia, 42 (2) : 127-136.
- Ehara S., Amano H. 1998. A revision of the mite family Phytoseiidae in Japan (Acari: Gamasina), with remarks on its biology. Species Divers., 3(1): 25-73. https://doi.org/10.12782/specdiv.3.25
- Ehara S., Okada Y., Kato H. 1994. Contribution to the knowledge of the mite family Phytoseiidae in Japan (Acari: Gamasina). J. Fac. Educ. Tottori University Nat. Sc., 42(2): 119-160.
- Faraji F., Cobanoglu S., Cakmak I. 2011. A checklist and a key for the Phytoseiidae species of turkey with two new species records (Acari: Mesostigmata). Int. J. Acarol., 37 (suppl. 1): 221-243. https://doi.org/10.1080/01647954.2011.558851
- Ferragut, F., Escudero, A. 1997. Taxonomy and distribution of predatory mites belonging to the genus Euseius Wainstein 1962, in Spain (Acari, Phytoseiidae). Bol. San. Veg. Plagas, 23(2): 227-235.
- Ferragut F. 2018. New records of phytoseiid mites of the subfamilies Typhlodrominae and Phytoseiinae (Acari: Phytoseiidae) from Spain, with description of a new species and re-description of four species of Typhlodromus Scheuten. Syst. Appl. Acarol., 23(5): 883-910. https://doi.org/10.11158/saa.23.5.8
- Gomelauri L.A. 1968. Three new species of mites of the family Phytoseiidae in southern Georgia [in Russian]. Bulletin of the Academy of Sciences of the Georgian SSR, Zoology and Parasitology, 52(2): 515-520.
- Hajizadeh J., Mortazavi S. 2015. The genus Euseius Wainstein (Acari: Phytoseiidae) in Iran, with a revised key to Iranian phytoseiid mites. Int. J. Acarol., 41 (1) : 53-66. https://doi.org/10.1080/01647954.2014.985712
- Jeyaprakash A., Hoy M.A. 2002. Mitochondrial 12S rRNA sequences used to design a molecular ladder assay to identify six commercially available phytoseiids (Acari: Phytoseiidae). Biol. Control, 25: 136-142. https://doi.org/10.1016/S1049-9644(02)00056-7
- Ji J., Lin T., Zhang Y., Lin J., Li L., Chen X. 2013. A comparison between Amblyseius (Typhlodromips) swirskii and Amblyseius eharai with Panonychus citri (Acari: Tetranychidae) as prey: developmental duration, life table and predation. Syst. Appl. Acarol., 18(2): 123-129. https://doi.org/10.11158/saa.18.2.4
- Kanouh, M., Tixier, M.-S., Guichou, S., Cheval, B. & Kreiter, S. 2010. Two synonymy cases within the genus Neoseiulella (Acari: Phytoseiidae): is the molecular evidence so evident? Biol. J. Linn. Soc., 101: 323-344. https://doi.org/10.1111/j.1095-8312.2010.01516.x
- Karg W. 1971. Acari (Acarina), Milben, Unterordnung Anactinochaeta (Parasitiformes): Die freilebenden Gamasina (Gamasides), Raubmilben. Die Tierwelt Deutschlands und der angrenzenden Meeresteile, 59. Teil, VEB Gustav Fischer Verlag, Jena, 475 pp.
- Karg W. 1991. Die Raubmilbenarten der Phytoseiidae Berlese (Acarina) Mitteleuropas sowie angrenzender Gebiete. Zool. Jahrb. Syst., 118(1): 1-64.
- Knapp M., Van Houten, Y., Van Baala E., Groot T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia 58(Suppl): 72-82. https://doi.org/10.24349/acarologia/20184275
- Kazak C., Yildiz S., Sekeroglu E. 2002. Biological characteristics and life tables of Neoseiulus umbraticus Chant (Acari, Phytoseiidae) at three constant temperatures. J. Pest Sci., 75: 118-121. https://doi.org/10.1046/j.1472-8206.2002.02034.x
- Kolodochka L.A. 1978. Manual for the identification of plant-inhabiting phytoseiid mites. Akademii Nauk Ukrainian SSR, Instituta Zoologii, Naukova Dumka , Kiev, 79 pp.
- Kolodochka L.A. 2006. Phytoseiid mites of the Palaearctic Region (Parasitiformes, Phytoseiidae): faunistic, taxonomy, ecomorphology, evolution. Vest. Zool., suppl. 21, 250pp.
- Kreiter S., Douin M., Tixier M.-S. 2021. New records of phytoseiid mites (Acari: Mesostigmata) from Madeira Island. Acarologia 61 (2): 217-240. https://doi.org/10.24349/acarologia/20214428
- Kreiter S., Payet R.-M., Douin M., Fontaine O., Fillatre J., Lebellec F. 2020. Phytoseiidae of La Réunion Island (Acari: Mesostigmata): three new species and two males described, new synonymies, and new records. Acarologia, 60(1) : 111-195. https://doi.org/10.24349/acarologia/20204361
- Kreiter S., Dos Santos Vicente V., Tixier M.-S., Fontaine O. 2016. An unexpected occurrence of Amblyseius swirskii Athias-Henriot in La Réunion Island (Acari: Phytoseiidae). Acarologia, 56(2): 175-181. https://doi.org/10.1051/acarologia/20162254
- Kulikova L. 2011. Mites of fruit plantations of the Republic of Moldova. Muzeul Olteniei Craiova. Oltenia. Studii şi comunicări. Ştiinţele Naturii, 27(1): 55-62.
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol. Biol. Evol., 35: 1547-1549. https://doi.org/10.1093/molbev/msy096
- Lindquist E., Evans G.W. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina Acarina: Mesostigmata. Mem. Entomol. Soc. Canada, 47, 1-64. https://doi.org/10.4039/entm9747fv
- McGregor E.A. 1954. Two new mites in the genus Typhlodromus (Acarina: Phytoseiidae). South. Calif. Acad. Sc. Bul., 53: 89-92.
- McMurtry JA, Croft BA 1997. Life-styles of phytoseiid mites and their roles in biological control. Ann. Rev. Entomol., 42: 291-321. https://doi.org/10.1146/annurev.ento.42.1.291
- McMurtry J.A., Moraes G.J.de 1984. Some phytoseiid mites from the South Pacific, with descriptions of new species and a definition of the Amblyseius largoensis species group. Int. J. Acarol., 10: 27-37. https://doi.org/10.1080/01647958408683347
- McMurtry J.A., Moraes G.J. de, Sourasso N.F. 2013. Revision of the life styles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A catalog of the mite family Phytoseiidae. References to taxonomy, synonymy, distribution and habitat. EMBRAPA - DDT, Brasilia, Brazil, 353 pp.
- Muma M.H. 1961. Subfamiles, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Fla. St. Mus. Bull., 5(7): 267-302.
- Okassa M., Kreiter S., Guichou S., Tixier M.-S. 2011. Molecular and morphological boundaries of the predator Neoseiulus californicus McGregor (Acari: Phytoseiidae). Biol. J. Linn. Soc., 104: 393-406. https://doi.org/10.1111/j.1095-8312.2011.01717.x
- Oudemans A.C. 1915. Acarologische Aanteekeningen. LVI. Entomol. Berichten, 4: 180-188. https://doi.org/10.5962/bhl.part.1128
- Oudemans A.C. 1929. Acarologische Aanteekeningen. C. Entomol. Berichten, 8: 28-36.
- Oudemans A.C. 1930. Acarologische Aanteekeningen. CI. Entomol. Berichten, 8: 48-53.
- Papaioannou-Souliotis P. 1981. Predacious mites (Phytoseiidae) observed on various plants in Greece. Annales de l'Institut Phytopathologique Benaki, 13: 36-58.
- Park Y., Joon-Ho Lee J.H. 2020. Temperature-dependent development and oviposition models and life history characteristics of Amblyseius eharai (Amitai et Swirski) (Acari: Phytoseiidae) preying on Tetranychus urticae (Koch) (Acari: Tetranychidae). J. Asia-Pac. Entomol., 23 (4): 869-878. https://doi.org/10.1016/j.aspen.2020.07.021
- Pritchard A.E., Baker E.W. 1962. Mites of the family Phytoseiidae from Central Africa, with remarks on the genera of the world. Hilgardia, 33(7): 205-309. https://doi.org/10.3733/hilg.v33n07p205
- Queiroz M.C., Douin M., Marques de Souza S., Sato E., Tixier M.-S. 2021. Molecular variations of the Cytochrome b DNA and protein sequences in Phytoseiulus macropilis Banks (Acari: Phytoseiidae) and P. persimilis (Athias-Henriot) (Acari: Phytoseiidae) reflect population structuration. Exp. Appl. Acarol., 84(4):687-701. https://doi.org/10.1007/s10493-021-00648-w
- Rahmani H., Kamali K., Faraji F. 2010. Predatory mite fauna of Phytoseiidae of northwest Iran (Acari: Mesostigmata). Turk. J. Zool., 34: 497-508. https://doi:10.3906/zoo-0902-23
- Ribaga, C. 1904 (1902) Gamasidi planticoli. Riv. Patol. Veg., 10, 175-178.
- Rivnay T., Swirski E. 1980. Four new species of phytoseiid mites (Acarina: Mesostigmata) from Israel. Phytoparasitica, 8: 173-187. https://doi.org/10.1007/BF03158314
- Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae. Can. Entomol., 110: 859-876. https://doi.org/10.4039/Ent110859-8
- Samsoniya T.I. 1972. Species composition of predatory mites (Parasitiformes: Phytoseiidae) on stonefruit plants in eastern Georgia. Bull. Acad. Sci. of Georgian SSR, 65(1): 193-196.
- Samsoniya T.I. 1977. Zonal-vertical distribution of Phytoseiidae in eastern Georgia on pip fruit culture. Bull. Acad. Sci. of Georgian SSR, 87(1): 181-183.
- Schuster R.O., Pritchard A.E. 1963. Phytoseiid mites of California. Hilgardia, 34: 191-285. https://doi.org/10.3733/hilg.v34n07p191
- Sengonca C., Drescher K. 2001. Laboratory studies on the suitability of Thrips tabaci Lindeman (Thysanoptera, Thripidae) as prey for the development, longevity, reproduction and predation of four predatory mite species of the genus Amblyseius (Acari, Phytoseiidae). Z. PflKrankh. PflSchutz., 108: 66-76.
- Swirski E., Ragusa S. 1976. Notes on predacious mites of Greece, with a description of five new species (Mesostigmata: Phytoseiidae). Phytoparasitica, 4: 101-122. https://doi.org/10.1007/BF02980341
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. 2013. MEGA6: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Mol. Biol. Evol., 30: 2725-2729. https://doi.org/10.1093/molbev/mst197
- Tixier M.-S. 2018. Predatory mites (Acari: Phytoseiidae) in agro-ecosystems and conservation biological control: a review and explorative approach for forecasting plant-predatory mite interactions and mite dispersal. Frontiers in Ecology & Evolution, 6: 192. https://doi.org/10.3389/fevo.2018.00192
- Tixier M.-S., Allam L., Douin M., Kreiter S. 2016. Phytoseiidae (Acari: Mesostigmata) of Morocco: new records, descriptions of five new species, re-descriptions of two species, and key for identification. Zootaxa, 4067(5): 501-551. https://doi.org/10.11646/zootaxa.4067.5.1
- Tixier M.-S., Guichou S., Kreiter S. 2008a. Morphological variation in the biological control agent Neoseiulus californicus (McGregor) (Acari: Phytoseiidae): consequences for diagnostic reliability and synonymies. Invert. Syst., 22: 453-469. https://doi.org/10.1071/IS07052
- Tixier M.-S., Kreiter S. 2009. Arthropods in biodiversity hotspots: the case of the Phytoseiidae (Acari: Mesos-tigmata) Biodivers. Conserv., 18: 507-527. https://doi.org/10.1007/s10531-008-9517-y
- Tixier M.-S., Principato D., Douin M., Kreiter S. 2019. Mites of the genus Typhlodromus (Acari: Phytoseiidae) from Southern France: combined morphological and molecular approaches for species identification. Zootaxa, 4604(2): 242-280. https://doi.org/10.11646/zootaxa.4604.2.2
- Tixier M.-S., Ferrero M., Okassa M., Guichou S., Kreiter S. 2010a. On the specific identity of specimens of Phytoseiulus longipes Evans (Mesostigmata: Phytoseiidae) showing different feeding behaviours: morphological and molecular analyses. Bull. Entomol. Res., 100: 569-579. https://doi.org/10.1017/S0007485309990617
- Tixier M.-S., Okassa M., Liguori M.L., Poinso A., Salerno B., Kreiter S. 2010b. Voucher specimens for DNA sequences of Phytoseiid mites (Acari: Mesostigmata). Acarologia, 50: 487-494. https://doi.org/10.1051/acarologia/20101984
- Tixier M.-S., Dos Santos V., Douin M., Duso C., Kreiter S. 2017. Great molecular variation questions the status of the species Phytoseius finitimus (Acari: Phytoseiidae) and the barcoding decision diagnosis. Acarologia, 57(3): 493-515. https://doi.org/10.24349/acarologia/20174168
- Tixier M.-S., Kreiter S., Moraes G.J. 2008b. Biogeographic distribution of the mites of the family Phytoseiidae (Acari: Mesostigmata). Biol. J. Linn. Soc., 93: 845-856. https://doi.org/10.1111/j.1095-8312.2007.00937.x
- Tixier M.-S., Kreiter S., Douin M., Moraes G.J. 2012a. Rates of description of Phytoseiidae (Acari: Mesostigmata): space, time and body size variations. Biodiv. Conserv.,*21: 993-1013. https://doi.org/10.1007/s10531-012-0235-0
- Tixier M.-S., Okassa M., Kreiter S. 2012b. An integrative morphological and molecular diagnostics for Typhlodromus pyri (Acari: Phytoseiidae). Zool. Scr., 41: 68-78. https://doi.org/10.1111/j.1463-6409.2011.00504.x
- Tixier M.-S., Perez Martinez S., Douin M. 2020a. Markers for life traits: the example of variations in morphology, molecular and amino acid sequences within the species Typhlodromus (Anthoseius) recki Wainstein (Acari: Mesostigmata: Phytoseiidae). Biol. J. Linn. Soc. 103, online first. https://doi.org/10.1093/biolinnean/blaa103
- Tixier M.-S., Douin M., Oliva R., Gonzalez L., Pount B., Kreiter S. 2020. Distribution and biological features of the species Typhlodromus (Anthoseius) recki (Acari: Phytoseiidae) on Tetranychus urticae, T. evansi (Acari: Tetranychidae) and Aculops lycopersici (Acari: Eriophyidae). Acarologia, 60(4): 684-697. https://doi.org/10.24349/acarologia/20204396
- Tsolakis H., Tixier M.-S., Kreiter S., Ragusa S. 2012. The genus concept within the family Phytoseiidae (Acari: Parasitiformes). Historical review and phylogenetic analyses of the genus Neoseiulus Hughes. Zool. J. Linn. Soc., 165: 253-273. https://doi.org/10.1111/j.1096-3642.2011.00809.x
- Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomol. Mem., Dep. Agric. Water Supply, Rep. South Africa 73, 168 pp.
- Wainstein B.A. 1959. New subgenus and species of the genus Phytoseius Ribaga, 1902 (Phytoseiidae: Parasitiformes). Zool. Zhur., 38: 1361-1365.
- Wainstein B.A. 1962. Révision du genre Typhlodromus Scheuten, 1857 et systématique de la famille des Phytoseiidae (Berlese 1916) (Acarina: Parasitiformes). Acarologia, 4: 5-30.
- Wainstein B.A., Vartapetov S.G. 1973. Predatory mites of the family Phytoseiidae (Parasitiformes) of Adzharskaya ASSR. Akademiya Nauk Armyanskoy SSR, Biologicheskiy Zhurnal Armenii, 26(2): 102-105.
- Wainstein B.A. 1958. New species of mites of the genus Typhlodromus (Parasitiformes: Phytoseiidae) from Georgia. Soobshcheniya Akademii Nauk Gruzinskoy SSR, 21(2): 201-207.
- Yoshida-Shaul E., Chant D.A. 1995. A review of the species of Phytoseiidae (Acari: Gamasina) described by A. C. Oudemans. Acarologia, 36(1): 3-19.



2021-01-19
Date accepted:
2021-09-24
Date published:
2021-10-12
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Tixier, Marie-Stephane; Auger, Philippe; Migeon, Alain; Douin, Martial; Fossoud, Amandine; Navajas, Maria and Arabuli, Tea
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



