Water mites from West Africa (Acari: Hydrachnidia)
Smit, Harry1
1✉ Naturalis Biodiversity Center, P.O. Box 9517, 2300 RA Leiden, the Netherlands.
2021 - Volume: 61 Issue: 3 pages: 700-746
https://doi.org/10.24349/5d6U-DX5NZooBank LSID: 96CFD924-75DE-4BCE-81B7-E5D35AAD1920
Original research
Keywords
Abstract
Introduction
Research on water mites from Western Africa started at the beginning of the 20th century with a series of publications of Karl Viets (1911, 1912, 1913/1914, 1916, 1925). All the papers of Viets were dealing with water mites from Cameroon. Cook (1966) published one of the most comprehensive studies with the water mites from Liberia. A review on all African papers on water was given by K. Viets (1953) and K.O. Viets (1970). Since then, relative few papers have been published on the water mites from West Afrika (see Table 1). A number of publications are dealing with a wider range in Africa but include material from West Africa (Table 2).



This paper deals with water mites from Ghana and the Gambia not treated in any of the papers from Table 1 or 2. Not included are most specimens of the genera Eylais Latreille (current state of knowledge of the genus insufficient) and Hydrodroma, Atractides and Torrenticolidae (these will be published in separate papers). Despite a long history in the study of water mites from West Africa, our knowledge is still poor. The aim of this study is, therefore, to increase the knowledge of water mites from West Africa.



Material and methods
The material described in this paper has been collected in 1998 (the Gambia), 2011 and 2013 (Ghana). All material from this study is collected by the author, unless stated otherwise. Holotypes, paratypes and all non-type material will be lodged in Naturalis Biodiversity Center, Leiden (RMNH). The following abbreviations are used: a.s.l. – altitude above sea level; Cx-I–IV – first to fourth coxae; Cxgl-1-42 – coxoglandularia 1–4; Dgl-1–4 – dorsoglandularia 1–4; IV-leg-6 – sixth segment of fourth leg; L – length; NHRS – Swedish Museum of Natural History, Stockholm; NP – National Park; suture line Cx-III/IV –suture line between third and fourth coxae; P1–5 – palp segments 1–5; SMF – Senckenberg Museum, Frankfurt a. Main; Vgl-1-3 – ventroglandularia 1-3. All measurements are in μm, measurements of palp and leg segments are of the dorsal margins, measurements of paratypes are given in parentheses. Ventral length is measured from the tip of Cx-I till posterior idiosoma margin. Numbers are given as male/female/deutonymph or adult/deutonymph. All coordinates are taken with a GPS. Coordinates given as degrees, minutes and seconds are taken from Google Earth and are by approximation. Data on the world distribution are taken from Smit (2020).
Taxonomy
Family Eylaidae Leach
Genus Eylais Latreille, 1796
Eylais degenerata Koenike, 1897
New record. The Gambia. 0/0/1, pond ± 5 km W of Basse, along road Basse-Bansang, Upper River Division, 12 Feb. 1998.
Distribution. A widespread species, known from Europe, Asia and Africa.
Family Limnocharidae Grube
Subfamily Limnocharinae Grube
Genus Limnochares Latreille, 1796
Limnochares (Limnochares) expansipalpis Cook, 1966
New record. Ghana. 1/0/0, Apkonu stream, downstream of falls, Logba Tota, 6°53.054′ N 0°28.024′ E, 362 m a.s.l., 21 Feb. 2013.
Distribution. Liberia (Cook 1966), Ghana (this study).
Family Hydrachnidae Leach
Genus Hydrachna Müller, 1776
Hydrachna mirifica (Koenike, 1893)
New records. Ghana. 1/0/0, lake near Mankessim, 5°16′3.93″ N 1°0″11.74″ W, 3 Jan. 2010, leg. P. Wondergem; 1/0/2, Lake Kokrobite, Weija, 5°33′7.90″ N 0°22′34.69″ W, 7 Jan. 2010, leg. P. Wondergem; 2/1/2, drinking water reservoir, W of Accra, 5°33.133′ N 0°22.147′ W, 14 m a.s.l., 21 Feb. 2011; 2/1/1, Inlet Volta River at Kpong, 6°09.183′ N 0° 03.709′ E, 25 m a.s.l., 10 Mar. 2011; 1/5/5, Fist pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013; 2/1/2, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Hydrachna spinosa Koenike, 1893
New records. Ghana. 1/1/0, Fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011.
Remarks. From Niger H. spinosa subtilis Walter, 1931 has been reported (Walter 1935). I follow Lundblad (1969), who synonymized this subspecies with the nominate taxon.
Distribution. Widespread in Africa south of the Sahara.
Hydrachna tchadensis Smit, 1994
New records. The Gambia. 1/0/0, pond ± 5 km W of Basse, along road Basse-Bansang, Upper River Division, 12 Feb. 1998.
Distribution. Cameroon (Smit 1994), the Gambia (this study).
Family Hydryphantidae Piersig
Subfamily Diplodontinae K. Viets
Genus Diplodontus Dugès, 1834
A widespread genus, with several species known from Europe, Asia, Africa and Australia.
Diplodontus schaubi (Koenike, 1893)
New records. Ghana. 0/2/2/, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 Mar. 2011; 1/0/0, pool Nsu Appiah, near Kakum NP, 5°22.895′ N 1°25.012′ W, 159 m a.s.l., 12 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Subfamily Hydryphantinae Piersig
Hydryphantes (Polyhydryphantes) incertus Koenike, 1893
New records. Ghana. 1/0/1, pool 1 Ankasa NP, 5°17.407′ N 2°38.378′ W, 59 m a.s.l., 26 Feb. 2011; 0/1/0, Amansuri Wetland, 5°00.398′ N 2°35.162′ W, 12 m a.s.l., 26 Feb. 2011; 1/0/0, Fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 0/1/1, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 Mar. 2011; 1/0/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l, 9 Feb. 2013; 3/11/2, pool Nsu Appiah, near Kakum NP, 5°22.895′ N 1°25.012′ W, 159 m a.s.l., 12 Feb. 2013; 0/1/1, First pond Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013; 0/1/0, Second pond Ankasa NP, 5°17.424′ N 2°38.286′ W, 58 m a.s.l, 14 Feb. 2013; 1/0/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 1/2/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Subfamily Mamersinae K. Viets
Genus Mamersa Koenike, 1898
A genus with several species known from the Afrotropical, Oriental and Australasian regions.
Mamersa expansa Cook, 1979 – nov. stat.
New records. Ghana. 1/1/0, lake near Mankessim, 5°16′3.93″ N 1°0′11.74″ W, 3 Jan. 2010, leg. P. Wondergem; 3/1/2, roadside pool along road Axim-Elubo, 4°58.162 N 2°24.815 W, 9 m a.s.l., 25 Feb. 2011; 3/1/0, Amansuri Wetland, 5°00.398′ N 2°35.162′ W, 12 m a.s.l., 26 Feb. 2011; 4/2/0, shallow part Amansuri Wetland, 4°59.635′ N 2°35.417′ W, 26 Feb. 2011; 1/0/0, First pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013; 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. Cook (1979) described this taxon as a subspecies of M. testudinata Koenike, 1898. Now the nominate taxon and its subspecies have been found on the same locality, they cannot belong to the same species. Moreover, the differences between the two taxa are quite large. Cook (1979) mentioned already the separation of Cxgl-1 and -2 by the genital plates. Mamersa expansa is much longer than M. testudinata and the genital plates are much longer (see Table 1). Consequently, the number of acetabula is much larger in M. expansa.
For differences between males and females see under M. testudinata.
Distribution. Uganda (Cook 1979), Ghana (this study).
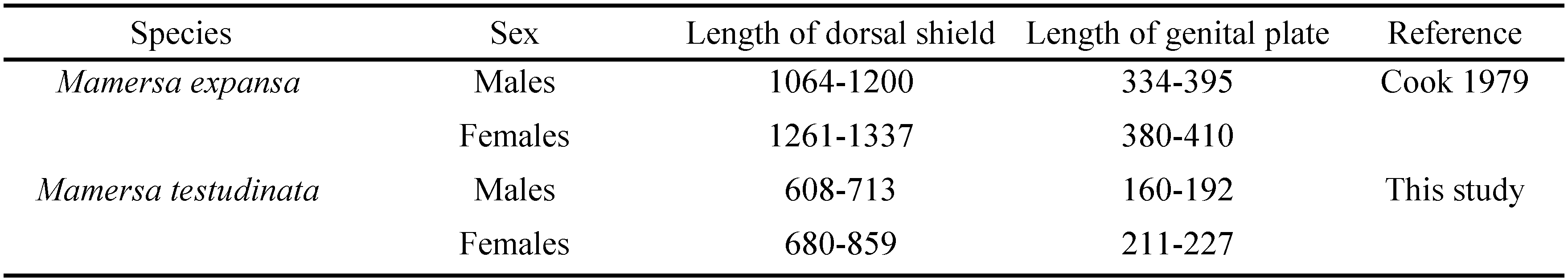


Mamersa testudinata Koenike, 1898
New records. Ghana. 1/0/0, lake near Mankessim, 5°16′3.93″ N 1°0′11.74″ W, 3 Jan. 2010, leg. P. Wondergem; 3/1/0, pool 2 Ankasa NP, 5°17.250′ N 2°38.092′ W, 66 m a.s.l., 27 Feb. 2011; 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 10 Mar. 2011; 1/0/0, Second pond, Ankasa NP, 5°17.424′ N 2°38.286′ W, 58 m a.s.l., 14 Feb. 2013; 0/0/1, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 2/6/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. Females have a wider pre-genital sclerite than males, in the latter the pre-genital sclerite is more triangular. Moreover, the genital plates have a short anteromedial extension in the females, which is absent in the males.
Distribution. Widespread in Africa south of the Sahara.
Family Lebertiidae Thor
Genus Lebertia Neuman, 1880
A genus with numerous species known, but from the Afrotropical region only two species have been described, one from South Africa and one from Liberia (Cook 1966).
Lebertia (Lebertia) liberiensis Cook, 1966
(Figure 1)



New record. Ghana. 4/2/3, Supon stream near Asiakwa, Atewa Hills, 6°15.530′ N 0°30.642′ W, 250 m a.s.l., 7 Mar. 2011.
Remarks. Cook (1966) illustrated the female only, but the male is more characteristic with the long setae on the posterior part of the genital flaps (Figure 1). This species has many swimming setae, also on II-leg-5 (one). III-leg-5 has up to seven swimming setae. The integument is lineated.
Distribution. Liberia (Cook 1966), Ghana (this study).
Family Oxidae K. Viets
Genus Africoxus Cook, 1966
Africoxus (Africoxus) curvisetus (K. Viets, 1916)
New record. The Gambia. 1/0, small pond Kampanti, Western Division, 7 Feb. 1998.
Distribution. Cameroon (K. Viets 1916), Liberia (Cook 1966), Ethiopia (Lundblad 1942), Congo (Lundblad 1949), Kenya (Lundblad 1952), the Gambia (this study).
Genus Oxus Kramer, 1877
Oxus (Oxus) stuhlmanni (Koenike, 1895)
New record. Ghana. 1/0, pool Axim Beach Hotel, Axim, 4°51.405′ N 2°14.103′ W, 25 Feb. 2011; 2/0, roadside pool along road Axim-Elubo, 4°58.162′ N 2°24.815′ W, 9 m a.s.l., 25 Feb. 2011; 1/0, shallow roadside pond along road Axim-Elubo, 5°09.080′ N 2°37.354′ W, 46 m a.s.l., 27 Feb. 2011; 1/0, roadside pond W of entrance to Ankasa NP, 5°10.277′ N 2°39.357′ W, 37 m a.s.l., 14 Feb. 2013; 1/0, Second pond, Ankasa NP, 5°17.424′ N 2°38.286′ W, 58 m a.s.l., 14 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Family Limnesiidae Thor
Subfamily Limnesiinae Thor
Genus Limnesia Koch, 1836
Limnesia (Limnesia) longidens magnipora Cook, 1966
New records. Ghana. 2/3/2, Pool 1 Ankasa NP, 5°17.407′ N 2°38.378′ W, 59 m a.s.l., 26 Feb. 2011; 1/2/2, First pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013.
Remarks. In all but one aspects similar to the description given by Cook (1966). The posterodorsal platelet of the males of this study has a straight anterior margin instead of a rounded anterior margin.
Distribution. Liberia (Cook 1966), Congo (K.O. Viets 1973), Ghana (this study).
Limnesia (Limnesia) maglioi Cook, 1966
New records. Ghana. 1/1/0, roadside pool along road Axim-Elubo, 4°58.162′ N 2°24.815′ W, 9 m a.s.l., 25 Feb. 2011.
Remarks. See under L. monodi.
Distribution. Liberia (Cook 1966), Ghana (Cook 1979, this study).
Limnesia (Limnesia) monodi Walter, 1946
(Figure 2)



New records. The Gambia. 1/1/1, pond Chamois Bridge, Basse, Upper River Division, 11 Feb. 1998; 8/2/1, pool along Prufu Bolon stream, Basse, Upper River Division, 12 Feb. 1998. Ghana. 0/1/0, Lake Agbo near Dahwenya, 5°46′44.80″ N 0°3′4.36″ E, 27 Dec. 2009, leg. P. Wondergem; 2/15/2, Black Volta River at Buipe, 8°45.674′ N 1°27.320′ W, 76 m a.s.l., 6 Mar. 2011; 0/5/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 10 Mar. 2011; 0/8/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l., 9 Feb. 2013; 13/19/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 0/3/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. The male matches the description of Walter (1946) and Lundblad (1949), but almost all female specimens have the Cx-I separated (Figure 2), unlike the descriptions of the latter authors. In a few females of this study Cx-I are near-fused. Lundblad (1949) described the subspecies L. monodi processifera with Cx-I separated, but this subspecies has smaller acetabula. Other subspecies have Cx-I fused, or the female is unknown. For the time being I assigned the specimens from this study to the nominate taxon.
Males of L. maglioi and L. monodi are easy to separate in the large (monodi) or small (maglioi) ventral extension of P4, but females of L. monodi with Cx-I separated are very similar to L. maglioi. The only differences are the more slender palp of maglioi, and possibly Cxgl-4 are closer to the associated seta in maglioi.
Distribution. Widespread in Africa south of the Sahara.
Limnesia (Limnesia) paracampanulata Cook, 1966
New records. Ghana. 4/0/0, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013; 16/5/0, Ankasa Exploration Base trail stream, Ankasa NP, 5°16.415′ N 2°38.751′ W, 80 m a.s.l., 14 Feb. 2013; 2/2/0, Ankasa Exploration Base trail stream, upstream, Ankasa NP, 5°16.376′ N 2°38.655′ W, 94 m a.s.l., 14 Feb. 2013; 3/0/0, Amedzofe Falls, 6°50.656′ N 0°26.868′ E, 599 m a.s.l., 20 Feb. 2013.
Distribution. Liberia (Cook 1966), the Gambia (Smit 1994), Ghana (this study).
Limnesia (Limnesia) stagnalis n. sp.
ZOOBANK: F7999793-634A-42EF-B557-906500A8FF83 ![]()
(Figure 3A-D)



Material examined. Holotype male, shallow part Amansuri wetland, Ghana, 4°59.635′ N 2°35.417′ W, 26 Feb. 2011 (RMNH). Paratypes: one male, one female [young], Amansuri wetland, Ghana, 5°00.398′ N 2°35.162′ W, 12 m a.s.l., 26 Feb. 2011 (RMNH).
Diagnosis. P2 ventrally with a setal tubercle, seta longer than tubercle; Cx-I fused medially, Cxgl-4 close to accompanying seta.
Description. Male: Integument smooth. Idiosoma dorsally 583 (632) long and 510 (575) wide, ventrally 616 (656) long. Dorsum posteriorly with a pair of small platelets, 30 and 36 long, respectively. Cx-I fused medially, apodemes of anterior coxae short. Cx-III + IV medially with a short secondary sclerotization. Cxgl-4 located close to accompanying seta (Figure 3A). Genital field with three pairs of acetabula, 160 long and 186 wide; gonopore 106 long. Second and third pair of acetabula closer to each other than to first pair. Length of P1–5: 26, 105, 72, 160, 44. P2 ventrally with a setal tubercle, and a seta directed anteriorly, this seta longer than the setal tubercle. P4 ventral margin undulating, with 2+1 setal tubercles (Figure 3B). Length of I-leg-4–6: 104, 122, 118. Length of IV-leg-4–6: 172, 176, 172; length of terminal seta 73; IV-leg-6 geniculated (Figure 4C), but not in the paratype. Swimming setae: III-leg-5 five, IV-leg-4 four or five, IV-leg-5 six. Excretory pore not sclerotized.
Female: Idiosoma dorsally 583 long and 130 wide, ventrally 624 long. Coxae as in male. Genital field 200 long, pregenital sclerite 102 wide. Genital field with three pairs of acetabula, second and third pair closer to each other than to first pair (Figure 3D). Length of P1-5: 28, 122, 96, 208, 55. Palp as in male. Length of I-leg-4-6: 114, 134, 112. Length of IV-leg-4-6: 211, 227, 219; terminal seta of IV-leg-6 78 long. Swimming setae: III-leg-6 six, IV-leg-4 five, IV-leg-5 seven. Excretory pore not sclerotized.
Etymology. Named for its occurrence in a wetland.
Remarks. The new species is close to L. lacustris K.O. Viets, 1973 from Kivu Lake, Congo. It differs in the following characters (in parentheses L. lacustris): Cxgl-4 close to accompanying seta (widely separated), genital field of male rounded (with an indistinct to distinct posterior extension), seta of P2 longer than setal tubercle (about as long as setal tubercle). Moreover, the palp of the new species is much longer, e.g., P4 of the male measures 160 in the new species and 100-110 in lacustris (K.O. Viets 1973). The new species has the fourth leg with more swimming setae than in lacustris.
Limnesia (Limnesia) walteri Migot, 1926
New records. Ghana. 1/0/0, Kue River, Kyabobo NP, 8°31.087′ N 0°36.049′ E, 208 m a.s.l., 25 Feb. 2013.
Distribution. Mediterranean countries, Mauretania (Walter 1946), Kenya (Lundblad 1942), South Africa (K.O. Viets 1964), Togo (Bader 1981), Ghana (this study).
Genus Tubophora Walter, 1935
A genus with two species known from the Afrotropical region.
Tubophora limnesioides Walter, 1935
(Figure 4)
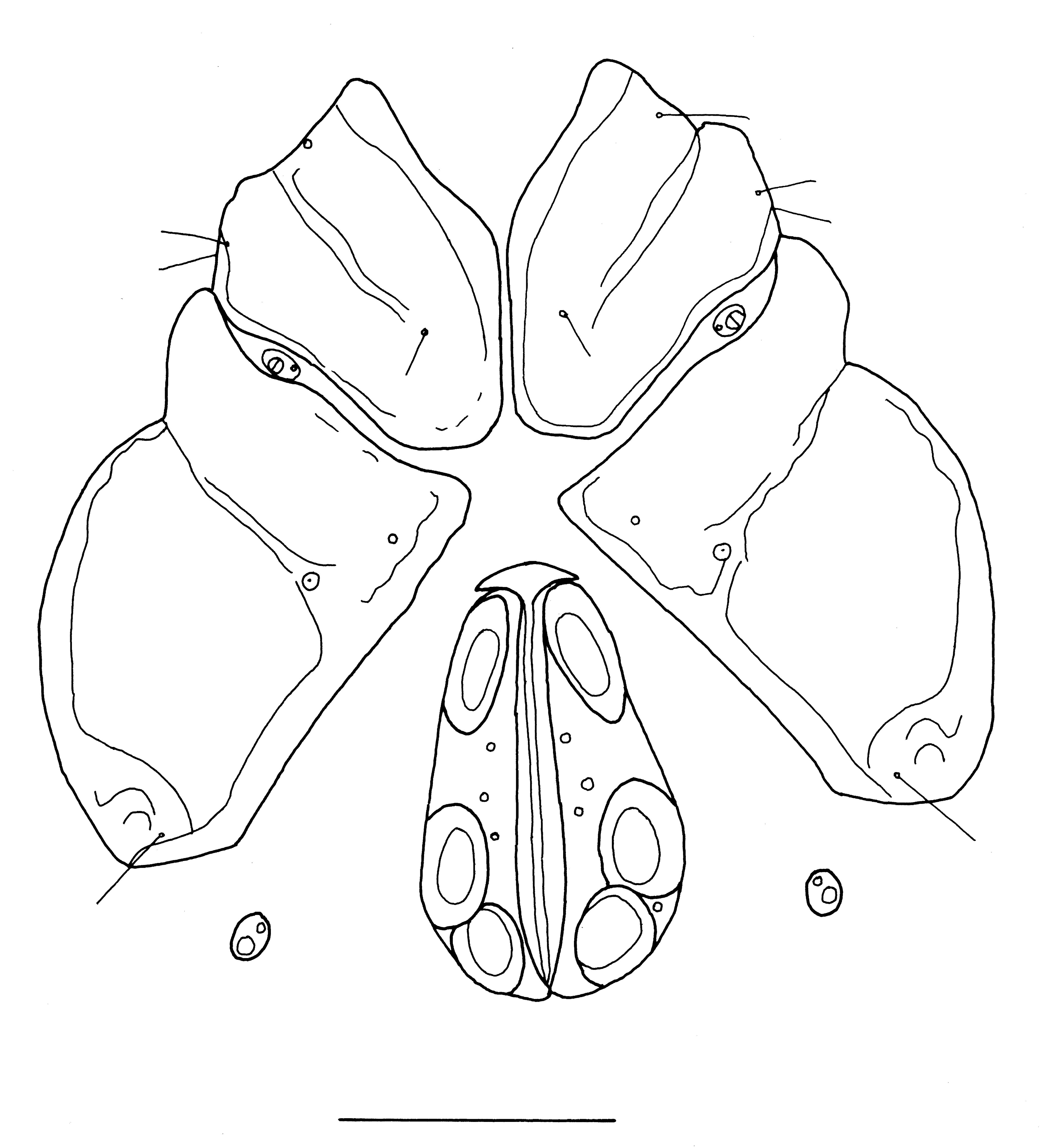


New records. Ghana. 1/0/0, small rainforest stream upstream Sagyimase, Atewa Hills, 6°13.964′ N 0°33.116′ W, 654 m a.s.l., 8 Mar. 2011; 2/3/0, Apkonu stream, downstream of falls, at Logba Tota, 6°53.054′ N 0°28.024′ E, 362 m a.s.l., 21 Feb. 2013.
Description. Female: Idiosoma dorsally 616 long and 478 wide, ventrally 632 long. Gnathosoma attached to a protrusable tube. Cx-I+II medially separated. Cx-III medially bluntly pointed, Cxgl-4 well separated from associated seta (Figure 4). Genital field 158 long, with three pairs of acetabula, second and third pair closer to each other than to first pair. Length of P1–5: 24, 52, 48, 72, 38. Length of I-leg-4–6: 96, 96, 80. Length of IV-leg-4–6: -, 116, 136; terminal seta of IV-leg-6 66 long. Legs without swimming setae.
Remarks. Thus far, known only from the male holotype, and the female is described here for the first time.
Distribution. Ivory Coast (Walter 1935), Ghana (this study).
Family Sperchontidae Thor
Genus Sperchon Kramer, 1877
Sperchon (Hispidosperchon) biscutatus Lundblad, 1941
New records. Ghana. 1/0/1, Tagbo River downstream of falls, 7°00.708′ N 0°34.326′ E, 394 m a.s.l., 23 Feb. 2013; 0/1/0, Kue River, Kyabobo NP, 8°31.087′ N 0°36.049′ E, 208 m a.s.l., 25 Feb. 2013.
Distribution. Kenya (Lundblad 1952), South Africa (K.O. Viets 1964), Congo (K.O. Viets & Böttger (1974), Ghana (this study).
Sperchon (Hispidosperchon) mutatus K.O. Viets, 1981
New records. Ghana. 9/12/2, Apkonu stream, downstream of falls, at Logba Tota, 6°53.054′ N 0°28.024′ E, 362 m a.s.l., 21 Feb. 2013; 0/1/0, Agumatsa River at first bridge, Agumatsa Wildlife Sanctuary, 7°06.830′ N 0°35.760′ E, 253 m a.s.l., 22 Feb. 2013; 1/0/0, Tagbo River, downstream, 7°00.974′ N 0°33.471′ E, 255 m a.s.l., 23 Feb. 2013; 0/1/0, Laboun River downstream of falls, Kyabobo NP, 8°19.836′ N 0°35.487′ E, 342 m a.s.l., 24 Feb. 2013.
Remarks. The male from Tagbo River, downstream is intermediate between the two species. Palp and venter are as described for S. mutatus. The dorsum, however, lacks the ornamentation of mutatus and is similar to biscutatus. A hybrid form?
Distribution. South Afrika (K.O. Viets 1981), Ghana (this study).
Family Hygrobatidae Koch
Genus Atractides Koch, 1837
Atractides (Atractides) vietsi (Cook, 1966)
(Figure 5A-E)



New record. Ghana. 0/1 [palp + I-leg-5–6 mounted]/0, small rainforest stream upstream Sagyimase, Atewa Hills, 6°13.964′ N 0°33.116′ W, 654 m a.s.l., 8 Mar. 2011.
Material for comparison. Atractides rectipes (K. Viets, 1924), holotype male, Múke-Fluß, Kamerun, 15 Jan. 1916 (slide 45938, SMF). Atractides vietsi (Cook, 1966), paratype male, stream at Bridge No 19 on Bomi Hills Road, Liberia, 23 Jan. 1957 (sign. 36, leg. Cook) (slide 53005, SMF; paratype female, stream at Bridge No 19 on Bomi Hills Road, Liberia, 23 Jan. 1957 (sign. 36, leg. Cook) (slide 53006, SMF).
Remarks. According to Cook (1966), the differences between males of A. rectipes and A. vietsi are the single dorsal platelet in the former and the paired dorsal platelets in the latter. Unfortunately, the female of A. rectipes is unknown. The female of vietsi has a pair of dorsal platelets, as in the male (Figure 5A). A character not discussed by Cook (1966), is the shape of the two distal setae of I-leg-5. These are slightly curved in both species, but rounded at its tip in rectipes, and truncated in vietsi (and not pointed as illustrated by Cook 1966) (Figure 5E). Given these differences, the female of this study must be assigned to A. vietsi.
Distribution. Liberia (Cook 1966).
Genus Hygrobates Koch, 1837
Hygrobates (Hygrobatomegapus) coriaceus (Lundblad. 1952) – nov. stat.
New records. Ghana. 1/0/0, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013; 0/1/0, Tagbo River, downstream, 7°00.974′ N 0°33.471′ E, 255 m a.s.l., 23 Feb. 2013.
Remarks. Initially, this taxon was described as a subspecies of H. spathuliferus (Lundblad, 1927). However, Lundblad (1952) reported H. coriaceus from the same locality as the nominate taxon, and therefore, they cannot belong to the same species. Also, in the Supon stream I collected both taxa. They are easily separated by the shape of the projection of P2: relatively short in coriaceus, and long in spathuliferus. Males of coriaceus have a ventral shield, but this can be absent in young specimens. More characters to separate the two species can be found in K.O. Viets (1963).
Distribution. Uganda (Lundblad 1952), South Africa (K.O. Viets 1963), Ghana (this study).
Hygrobates (Hygrobates) dentipalpis n. sp.
ZOOBANK: 6229E1E4-B4C7-4E31-B803-60971D506602 ![]()
(Figure 6A-C)



Material examined. Holotype male, stream Asukuma, Kakum National Park, Ghana, 5°26.995′ N 1°25.071′ W, 131 m a.s.l., 12 Feb. 2013 (RMNH). Paratypes: One male, same data as holotype (RMNH); 1/0/0, Supon stream near Asiakwa, Atewa Hills, Ghana, 6°15.530′ N 0°30.642′ W, 250 m a.s.l., 7 Mar. 2011 (RMNH); 1/0/0, River Tordzi, 6°32.819′ N 0°41.445′ E, 79 m a.s.l., 19 Feb. 2013; 1/0/0, Kue River, Kyabobo NP, Ghana, 8°31.087′ N 0°36.049′ E, 208 m a.s.l., 25 Feb. 2013 (RMNH).
Material for comparison. Hygrobates sudafricanus K.O. Viets, 1963: male, Klein Vaal River near source, Südafrika, Val. 857, Chütter leg., 11 Nov. 1959 (slide 51656, SMF); female, Klein Vaal River near source, Südafrika, Val. 857, Chütter leg., 11 Nov. 1959 (slide 51657, SMF); male, Klein Vaal River near source, Südafrika, Val. 857, Chütter leg., 11 Nov. 1959 (slide 51658, SMF); female, Klein Vaal River near source, Südafrika, Val. 857, Chütter leg., 11 Nov. 1959 (slide 51660, SMF).
Diagnosis. Anterior coxae only slightly tapering posteriorly; genital field with three pairs of acetabula; P2 with an anteroventral extension with several denticles, P3 with several relatively large denticles.
Description. Male. Integument finely lineated, only visible at higher magnification. Idiosoma dorsally 446 (360-413) long and 369 (300-356) wide, ventrally 486 (405-454) long. Dorsum without platelets. Anterior coxae only slightly tapering posteriorly. Cx-I fused medially. Suture line Cx-III/IV incomplete, Cxgl-4 located near this suture line, Cx-IV rounded medially (Figure 6A). Genital field 102 long and 112 wide, with three pairs of acetabula in a triangle; gonopore 64 long. Length of P1–5: 20, 78, 50, 116, 41. P2 with an anteroventral extension with several small denticles, a few denticles present posterior to this extension, but these only visible in ventral view. P3 ventrally with four relatively large denticles, medially with a few more denticles. P4 slender (Figure 6B). Length of I-leg-4–6: 103, 104, 72. I-leg-5 distally with two setae, one of these slightly bowed (Figure 6C). Length of IV-leg-4-6: 136, 154, 124. Legs without swimming setae.
Female: Unknown.
Etymology. Named for the presence of relatively large denticles on P3.
Remarks. The new species is similar to H. niloticus Walter, 1922 in the presence of an anteroventral extension of P2, with several denticles on this extension. However, H. niloticus has strongly tapering anterior coxae. Males of Hygrobates sudafricanus K.O. Viets, 1963 from South Africa, have P3 with 5–6 smaller denticles, P2 has a less extending anteroventral extension with larger and fewer denticles (about 2–3), in the new species there are about 6–7 small denticles on the anteroventral extension. Females of H. sudafricanus have P2 with a larger anteroventral extension and P3 with larger denticles, but the female of the new species is unknown.
Hygrobates (Hygrobates) laceratus Lundblad, 1927
New records. Ghana.2/1/0, Kintampo Falls, 8°05.413′ N 1°41.881′ W, 235 m a.s.l., 3 Mar. 2011; 3/4/0, Fuller Falls, 8°04.975′ N 1°47.842′ W, 189 m a.s.l., 6 Mar. 2011; 6/10/0, small rainforest stream upstream Sagyimase, Atewa Hills, 6°13.964′ N 0°33.116′ W, 654 m a.s.l., 8 Mar. 2011; 13/16/0, Akaa Falls, 6°10.516′ N 0°11.723′ W, 180 m a.s.l., 9 Mar. 2011; 0/4/0, Namini stream, Kakum National Park, 5°23.396′ N 1°23.294´ W, 185 m a.s.l., 12 Feb. 2013; 1/0/0, Ankasa Exploration Base stream, Ankasa NP, 5°16.413′ N 2°38.810′ W, 81 m a.s.l., 14 Feb. 2013; 13/10/0, plunge pool Kulugu River, 6°51.365′ N 0°25.101′ E, 388 m a.s.l., 19 Feb. 2013; 2/5/0, Kulugu River, 6°51.365′ N 0°25.101′ E, 388 m a.s.l., 19 Feb. 2013; 0/1/0, Kulugu River, upstream, hyporheic, N of Biakpa, 6°51.223′ N 0°25.141′ E, 410 m a.s.l., 20 Feb. 2013; 5/5/0, Amedzofe Falls, 6°50.656′ N 0°26.868′ E, 599 m a.s.l., 20 Feb. 2013; 8/14/0, Kulugu River, upstream, N of Biakpa, 6°51.223′ N 0°25.141′ E, 410 m a.s.l., 20 Feb. 2013; 1/0/0, Apkonu stream, downstream of falls, at Logba Tota, 6°53.054′ N 0°28.024′ E, 362 m a.s.l., 21 Feb. 2013; 5/2/0, plunge pool Tsatsudo Falls, 7°07.390′ N 0°23.365′ E, 179 m a.s.l., 22 Feb. 2013; 6/1/2, Tagbo River downstream of falls, 7°00.708′ N 0°34.326′ E, 394 m a.s.l., 23 Feb. 2013; 11/4/1, Laboun River downstream of falls, Kyabobo NP, 8°19.836′ N 0°35.487′ E, 342 m a.s.l., 24 Feb. 2013; 3/4/0, Kue River, Kyabobo NP, 8°31.087′ N 0°36.049′ E, 208 m a.s.l., 25 Feb. 2013; 41/27/0, unnamed stream between Apepem and Koju Amu, Atewa Hills, 6°10.252′ N 0°36.520′ W, 424 m a.s.l., 27 Feb. 2013; 7/2/0, unnamed stream upstream Sagyimase, Atewa Hills, 6°13.966′ N 0°33.114′ W, 671 m a.s.l., 28 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Hygrobates (Hygrobates) liberiensis Cook, 1966
New records. Ghana. 0/1/0, Ankasa Exploration Base trail stream, upstream, Ankasa NP, 5°16.376′ N 2°38.655′ W, 94 m a.s.l., 14 Feb. 2013.
Distribution. Liberia (Cook 1966), Ghana (this study).
Hygrobates (Hygrobates) niloticus Walter, 1922
(Figure 7A-C)



New record. Ghana. 0/1/0, unnamed stream crossing road to Ankasa NP, 5°11.435′ N 2°39.429′ W, 20 m a.s.l., 27 Feb. 2011.
Description. Female. Integument finely lineated, only visible at higher magnification. Idiosoma dorsally 591 long and 462 wide, ventrally 640 long. Cx-I fused medially. Anterior coxae tapering and extending far posteriorly, reaching almost posterior margin of Cx-IV. Posterior to anterior coxae a small area of secondary sclerotization. Suture line Cx-III/IV incomplete, Cxgl-4 located near this suture line. Posterior to Cx-IV an area of secondary sclerotization, not incorporating Vgl-1. Genital field 144 long and 259 wide, with three pairs of acetabula. Pregenital sclerite small, surrounded by a large area of secondary sclerotization. Length of P1–5: 28, 118, 76, 104, 40. P2 anteroventrally with several denticles, ventral margin of P3 with about five relatively large denticles. Length of I-leg-4–6: 118, 104, 74. First leg segments rather stocky, I-leg-5 anteriorly without stout setae.
Distribution. Sudan (Walter 1922), Ghana (this study).
Hygrobates (Hygrobates) pseudoniloticus n. sp.
ZOOBANK: 0F6BBBE8-EF76-4B8C-88C6-17D4A7EE3754 ![]()
(Figure 8A-C)



Material examined. Holotype female, Ankasa Exploration Base stream, Ankasa NP, Ghana, 5°16.413′ N 2°38.810′ W, 81 m a.s.l., 14 Feb. 2013 (RMNH). Paratype: One female, same data as holotype (RMNH).
Diagnosis. Female: Anterior coxae tapering, extending far posteriorly; P2 with an anteroventral extension, with several denticles, P2 with a few, relatively large denticles; first leg segments slender.
Description. Female: Integument finely lineated, only visible at higher magnification. Idiosoma dorsally 612 long and 462 wide, ventrally 608 long. Dorsum without platelets. Gnathosoma fused with Cx-I. Cx-I fused medially, extending far posteriorly, reaching middle of Cx-IV. Suture line of Cx-III/IV incomplete; Cxgl-4 located near suture line Cx-III/IV. Genital field 114 long and 196 wide, with three pairs of acetabula in a triangle. Pre-genital sclerite 82 wide. Length of P1–5: 28, 82, 62, 116, 48. P2 with a short anteroventral extension, covered with a few denticles. Ventral margin of P3 with about five denticles. P4 slender, near ventral margin two fine setae. Length of I-leg-4–6: 118, 140, 136 (till tip of segments). First leg segments slender. Length of IV-leg-4–6: 168, 190, 172 (till tip of segment). Legs without swimming setae.
Male: Unknown.
Etymlogy. Named for its similarity with H. niloticus.
Remarks. Hygrobates pseudoniloticus n. sp. is similar to H. niloticus in long and tapering anterior coxae. Unfortunately, H. niloticus is insufficiently described. The figures provided by Walter (1922) are sketchy and lack details, and only one male was known to Walter. The holotype is apparently lost. It is, therefore, difficult to decide which of the two species with long and tapering anterior coxae of this study belongs to H. niloticus. The latter species is quite large (the male is 700 long), and the anterior coxae are extremely tapering. Therefore, I assigned the largest of the two species, with the longest and most tapering anterior coxae to H. niloticus.
Hygrobates (Hygrobates) soari K. Viets, 1911
New records. Ghana. 4/0/2, unnamed stream crossing road to Ankasa NP, 5°11.435′N 2°39.429′ W, 20 m a.s.l., 27 Feb. 2011; 0/1/0, Black Volta River at Buipe, 8°45.674′ N 1°27.320′ W, 76 m a.s.l., 6 Mar. 2011; 1/0/0, small rainforest stream upstream Sagyimase, Atewa Hills, 6°13.964′ N 0°33.116′ W, 654 m a.s.l., 8 Mar. 2011; 0/7/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l., 9 Feb. 2013; 1/1/1, stream at Boti Falls, 6°11.508′ N 0°13.010′ W, 273 m a.s.l., 9 Mar. 2011; 13/4/0, Afiaso stream, Kakum NP, 5°30.087′ N 1°26.373′ W, 114 m a.s.l., 12 Feb. 2013; 3/2/0, stream Asukuma, Kakum NP, 5°26.995′ N 1°25.071′ W, 131 m a.s.l., 12 Feb. 2013; 5/4/0, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013; 2/0/0, Ankasa Exploration Base trail stream, upstream, Ankasa NP, 5°16.376′ N 2°38.655′ W, 94 m a.s.l., 14 Feb. 2013; 3/5/0, tributary of Oguntwe, Ankasa NP, 5°16.563′ N 2°38.733′ W, 78 m a.s.l., 14 Feb. 2013; 0/1/2, Nubui River, Agumatsa Wildlife Sanctuary, 7°06.986′ N 0°35.548′ E, 254 m a.s.l., 22 Feb. 2013; 1/0/0, plunge pool Tsatsudo Falls, 7°07.390′ N 0°23.365′ E, 179 m a.s.l., 22 Feb. 2013; 1/3/0, unnamed stream upstream Sagyimase, Atewa Hills, 6°13.966′ N 0°33.114′ W, 671 m a.s.l., 28 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Hygrobates (Hygrobatomegapus) spathuliferus (Lundblad, 1927)
New records. Ghana. 0/1/0, Kintampo Falls, 8°05.413′ N 1°41.881′ W, 235 m a.s.l., 3 Mar. 2011; 16/8/2, Kulugu River, 6°51.365′ N 0°25.101′ E, 388 m a.s.l., 19 Feb. 2013; 26/26/13, Kulugu River, upstream, N of Biakpa, 6°51.223′ N 0°25.141′ E, 410 m a.s.l., 20 Feb. 2013; 6/5/3, Apkonu stream, downstream of falls, at Logba Tota, 6°53.054′ N 0°28.024′ E, 362 m a.s.l., 21 Feb. 2013; 2/0/0, Tagbo River, downstream of falls, Ghana, 7°00.708′ N 0°34.326′ E, 394 m a.s.l., 23 Feb. 2013; 2/0/0, Laboun River downstream of falls, Kyabobo NP, 8°19.836′ N 0°35.487′ E, 342 m a.s.l., 24 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Genus Hygrobatopsis K. Viets, 1924
A genus with three species known thus far, one from Cameroon (K. Viets 1925) and two from South Africa (Cook 2005).
Hygrobatopsis (?) convexipalpis n. sp.
ZOOBANK: A728411D-45EA-479D-A935-EC4DB3846D2A ![]()
(Figure 9A-D)
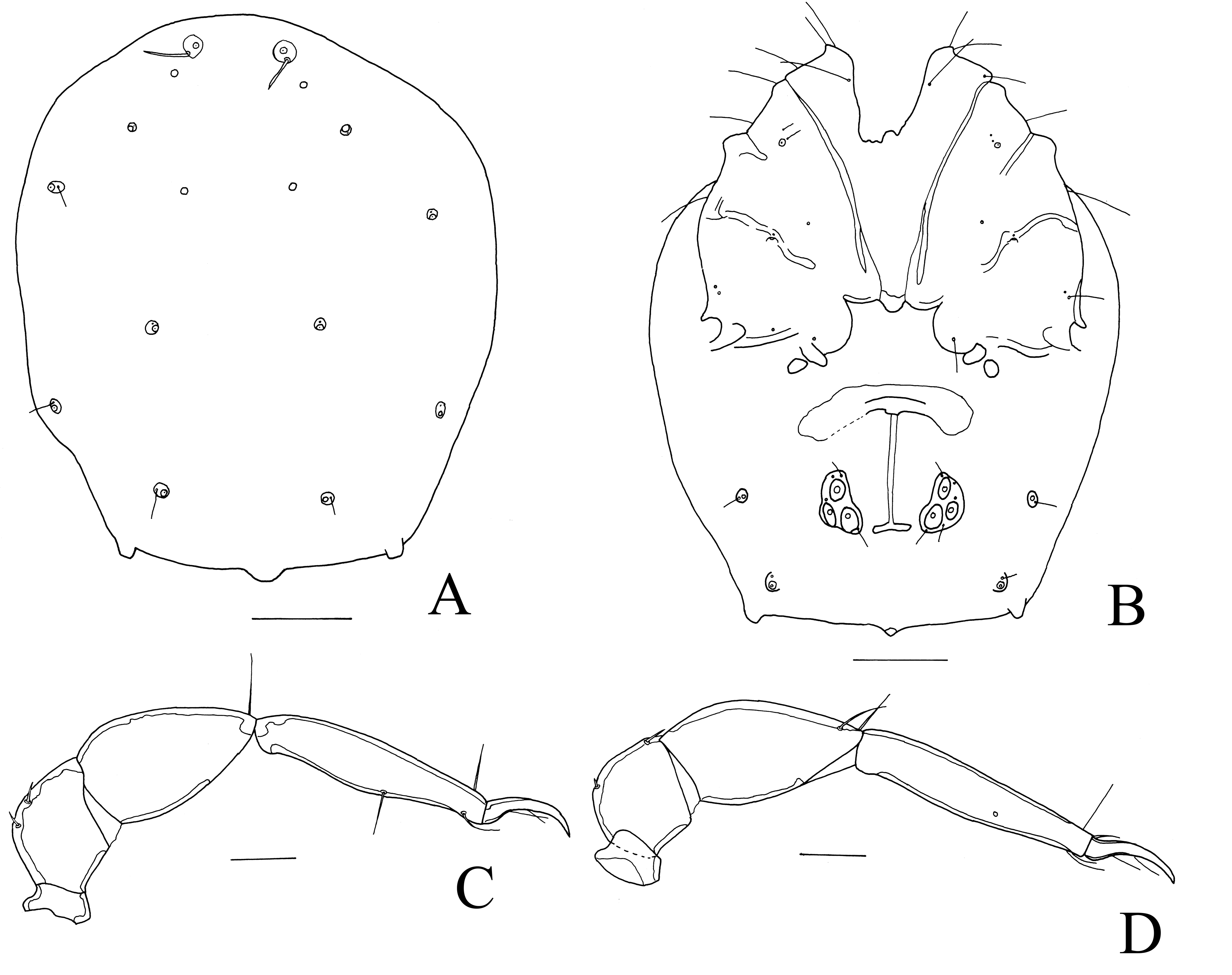





Material examined. Holotype female, stream Asukuma, Kakum NP, Ghana, 5°26.995′ N 1°25.071′ W, 131 m a.s.l., 12 Feb. 2013 (RMNH). Paratype: one female, same data as holotype (RMNH).
Material for comparison. Hygrobatopsis levipalpis (K. Viets, 1924). One female (photograph), stream Koptawelil, Kenya, Feb. 1925, leg. F. Bryk (NHRS-GULI000073414); one female (photograph), stream Koptawelil, Kenya, 29 Jun. 1925, leg. F. Bryk (NHRS-GULI000073415).
Diagnosis. Dgl-1 not on sclerites, gonopore relatively short, P3 with a convex ventral margin.
Description. Female: Idiosoma dorsally 543 (583) long and 478 (486) wide, ventrally 599 (624) long. Dgl-1 not on sclerites (Figure 9A). Gnathosoma lightly fused with Cx-I. Suture lines of Cx-III/IV incomplete, Cxgl-4 near the latter suture line. Cx-IV posteromedially with a short projection (Figure 9B). Genital field 144 long and 148 wide, with three pairs of acetabula. Pregenital sclerite appearing large due secondary sclerotization lateral and anterior to the pregenital sclerite, but this sclerite itself small. Excretory pore near posterior idiosoma margin. Idiosoma posterolaterally with a pair of large glandularia tubercles. Length of P1–5: 23, 99, 146, 186, 68. P3 with a convex ventral margin (Figure 9C-D). Length of I-leg-4–6: 134, 155, 108. I-leg-5 without downturned seta. Length of IV-leg-4–6: 180, 210, 124. Legs without swimming setae.
Male: Unknown.
Etymology. Named for the convex P3.
Remarks. The new species is close to Hygrobatopsis levipalpis. The female of this species is not well-described. In the original description (K. Viets 1924), only the male has been described. Lundblad (1927) reported the female of this species, but in his material no males were present. I examined his specimens with the help of some photographs. P3 of Lundblad's specimens have a more or less straight ventral margin (like the male), P4 has a more distinct ventral bulge (Figure 10A-B), Dgl-1 are on small sclerites and the gonopore is relatively longer. Moreover, P3 and P4 of Lundblad's specimens measure 93 and 129, respectively, much shorter than the specimens from this study.
Hygrobatopsis (Hygrobatopsella) inflata (K. Viets, 1925)
(Figures 11A-E, 12A-D)






New records. Ghana. 0/1/0, unnamed stream crossing road to Ankasa NP, 5°11.435′ N 2°39.429′ W, 20 m a.s.l., 27 Feb. 2011; 0/1/1, stream Asukuma, Kakum NP, 5°26.995′ N 1°25.071′ W, 131 m a.s.l., 12 Feb. 2013; 1/2/0, Kulugu River, upstream, N of Biakpa, 6°51.223′ N 0°25.141′ E, 410 m a.s.l., 20 Feb. 2013.
Description. Male: Idiosoma dorsally 332 long and 200 wide, ventrally 348 long. Dorsum with a 4-part dorsal shield, consisting of a large anterior plate, 180 long and 194 wide, with the postocularia and one pair of glandularia, a smaller posterior plate with one pair of glandularia and two small platelets flanking the posteromedial projection, each with a glandularium (Figure 11A). Gnathosoma narrowly fused with Cx-I. Coxae incorporated in a ventral shield, not including the genital field. All suture lines of coxae incomplete. Cxgl-4 located near suture line Cx-III/IV (Figure 11B). Genital field terminal, 100 wide, with three pairs of acetabula on a pair of separate genital plates; medial and posterior margin with a row of setae (Figure 11C). Length of P1–5: 18, 70, 72, 100, 36. P2 anteroventrally with a large projection, P4 ventrally bulging, P5 slender (Figure 11D). Length of I-leg-4–6: 92, 102, 74. I-leg-5 anteriorly with a few fine setae. Length of IV-leg-4–6: 120, 132, 102. Fourth leg much heavier than first three legs, claw relatively large. IV-leg-5 and IV-leg-4 medially with three pectinate setae, IV-leg-4 anteromedially with one pectinate seta (Figure 11E). Swimming setae absent.
Female: Idiosoma dorsally 567-583 long and 470-518 wide, ventrally 575-616 long. Dgl-1 on a large sclerite (Figure 12A). A small area of integument posterior to the preocularia sclerite reticulated, but this reticulation absent in the rest of the integument. Posterior dorsal and ventral glandularia on large tubercles. Gnathosoma narrowly fused with Cx-I. Cxgl-4 near suture line Cx-III/IV. Posterior to Cx-IV a large area of secondary sclerotization. Also anterior to genital field a broad sickle-shaped area of secondary sclerotization (Figure 12B). Genital field 160 long, genital plates compact, 74 long, with three pairs of acetabula. Excretory pore on a large terminal tubercle. Length of P1–5: 30, 104, 118, 158, 56. Anteroventral projection of P2 more slender than in male, tip with a few minute denticles. Ventral margin of P4 less bulging than in male (Figure 12C). Length of I-leg-4–6: 130, 142, 103. I-leg-4 anteroventrally with two blunt stout setae, I-leg-5 without downturned seta (Figure 12D). Length of IV-leg-4–6: 176, 191, 132. Swimming setae absent.
Remarks. K. Viets (1925) described H. inflata based on the deutonymph only. Thus far, the adults of this species were unknown. The palp of the deutonymph of H. inflata as described by Viets agrees well with the palp of the adults and deutonymph collected in Ghana, especially the bulging ventral margin of P4 in combination with P2 with a ventral projection is characteristic.
The male of the new species is remarkably similar to the South African H. bella Cook, 2005, given the distance between the two countries. The anterior dorsal plate of H. bella is narrower and more elongated, and the posterior dorsal plate of bella is more or less as long as wide (wider than long in inflata). The anteroventral projection of P2 is less slender compared to bella. The female is well-characterized by its large glandularia tubercles and the large sclerite with Dgl-1.
Distribution. Cameroon (K. Viets 1925), Ghana (this study).
Hygrobatopsis (Hygrobatopsis) pauciglandulosa n. sp.
ZOOBANK: 84196F88-4DD0-4450-972E-98D1D3345DF3 ![]()
(Figures 13A-E, 14A-B)






Material examined. Holotype male, Ankasa Exploration Base stream, Ankasa NP Ghana, 5°16.413′ N 2°38.810′ W, 81 m a.s.l., 14 Feb. 2013 (RMNH). Paratype: one female, Ankasa River, Ankasa NP, Ghana, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013 (RMNH).
Diagnosis. Both sexes: legs with swimming setae. Male: Dorsum with a dorsal shield, with the postocularia and one pair of glandularia; Vgl-3 fused with ventral shield.
Description. Male: Idiosoma dorsally 296 long and 251 wide, ventrally 340 long. Dorsum with a large dorsal shield, 259 long and 206 wide, with the postocularia and posteriorly with one pair of glandularia. Dorsal furrow with three pairs of glandularia (Figure 13A). Gnathosoma lightly fused with Cx-I. Venter with a ventral shield incorporating the coxae and Vgl-3. Genital field 92 wide, lying in a deep genital bay (Figure 13B); gonopore 38 long. Genital field with three pairs of acetabula, posterior to the acetabula numerous setae. From the gonopore extends a hyaline, open tube-like structure. Near posterior idiosoma margin a pair of large tubercles. Excretory pore fused with dorsal shield. Length of P1–5: 17, 50, 56, 96, 32. P2 without a ventral projection, P4 slender, ventrally slightly bulging, P5 slender (Figure 13C). Length of I-leg-4–6: 79, 90, 68. I-leg-5 anteriorly with a few fine setae, one of these elongated (but lost due to mounting), no downturned seta present (Figure 13D). Length of IV-leg-4–6: 88, 112, 88. Fourth legs much heavier than other three legs (Figure 13E). Swimming setae: III-leg-5 and IV-leg-5 with two swimming setae, II-leg-5 with a short swimming seta.
Female: Idiosoma dorsally 441 long and 365 wide, ventrally 470 long. Integument finely lineated. Dgl-1 each on a small sclerite (Figure 14A). Gnathosoma lightly fused with Cx-I, but fusion more extensive than in male. Posterior margin of Cx-IV with short projections. Genital field 122 long, with three pairs of acetabula in a somewhat triangular configuration (Figure 14B). Genital plates 57 long, pregenital sclerite 82 wide. Excretory pore on a tubercle near posterior idiosoma margin. Idiosoma posterolaterally with a pair of large glandularia tubercles. Length of P1–5: 22, 80, 86. 152, 48. Palp as in male. Length of I-leg-4–6: 92, 93, 70. I-leg-5 anteriorly with a few fine setae, one of these elongated. Length of IV-leg-4–6: 120, 148, 110. Swimming setae: II-leg-5, III-leg-5 and IV-leg-5 with two swimming setae.
Etymology. Named for the presence of few glandularia on the dorsal shield of the male.
Remarks. Thus far, two species were known of the nominate subgenus. The male of Hygrobatopsis levipalpis K. Viets, 1925 (Cameroon) has a more shallow genital bay, Vgl-3 is not fused with the ventral shield and P4 is more bulging ventrally. The male of Hygrobatopsis sudafricana Cook, 2005 (South Africa) has the dorsal shield with three pairs of glandularia and the genital bay is shallow too. Both these species lack swimming setae. The presence of swimming setae will separate the female from other species of the genus.
Genus Tetrabates Thor, 1922
A genus with three or four species known from the Afrotropical region.
Tetrabates (Liberiobates) bomiensis (Cook, 1966)
New records. Ghana. 0/1/0, Kintampo Falls, 8°05.413′ N 1°41.881′ W, 235 m a.s.l., 3 Mar. 2011; 0/1/3, Supon stream near Asiakwa, Atewa Hills, 6°15.530′ N 0°30.642′ W, 250 m a.s.l., 7 Mar. 2011; 0/1/0, Ankasa Exploration Base stream, Ankasa NP, 5°16.413′ N 2°38.810′ W, 81 m a.s.l, 14 Feb. 2103; 1/0/0, Ankasa Exploration Base trail stream, upstream, Ankasa NP, 5°16.376′ N 2°38.655′ W, 94 m a.s.l., 14 Feb. 2013.
Distribution. Liberia (Cook 1966), Ghana (this study).
Tetrabates (Tetrabates) szalayi (Lundblad, 1949)
New records. Ghana. 0/2/0, Ankasa Exploration Base stream, Ankasa NP, 5°16.413′ N 2°38.810′ W, 81 m a.s.l, 14 Feb. 2103.
Distribution. Congo (Lundblad 1949), Liberia (Cook 1966), Ghana (this study).
Family Pionidae Thor
Subfamily Pioninae Thor
Genus Piona Koch, 1842
Piona africana K. Viets, 1940
New record. Ghana. 9/4/3, Amansuri Wetland, 5°00.398′ N 2°35.162′ W, 12 m a.s.l., 26 Feb. 2011; 3/0/0, shallow part Amansuri Wetland, 4°59.635′ N 2°35.417′ W, 26 Feb. 2011.
Distribution. Cameroon, Tanzania (Koenike 1895), Liberia (Cook 1966), Uganda (Cook 1979), Ghana (this study).
Piona angulata K. Viets, 1921
(Figure 15)
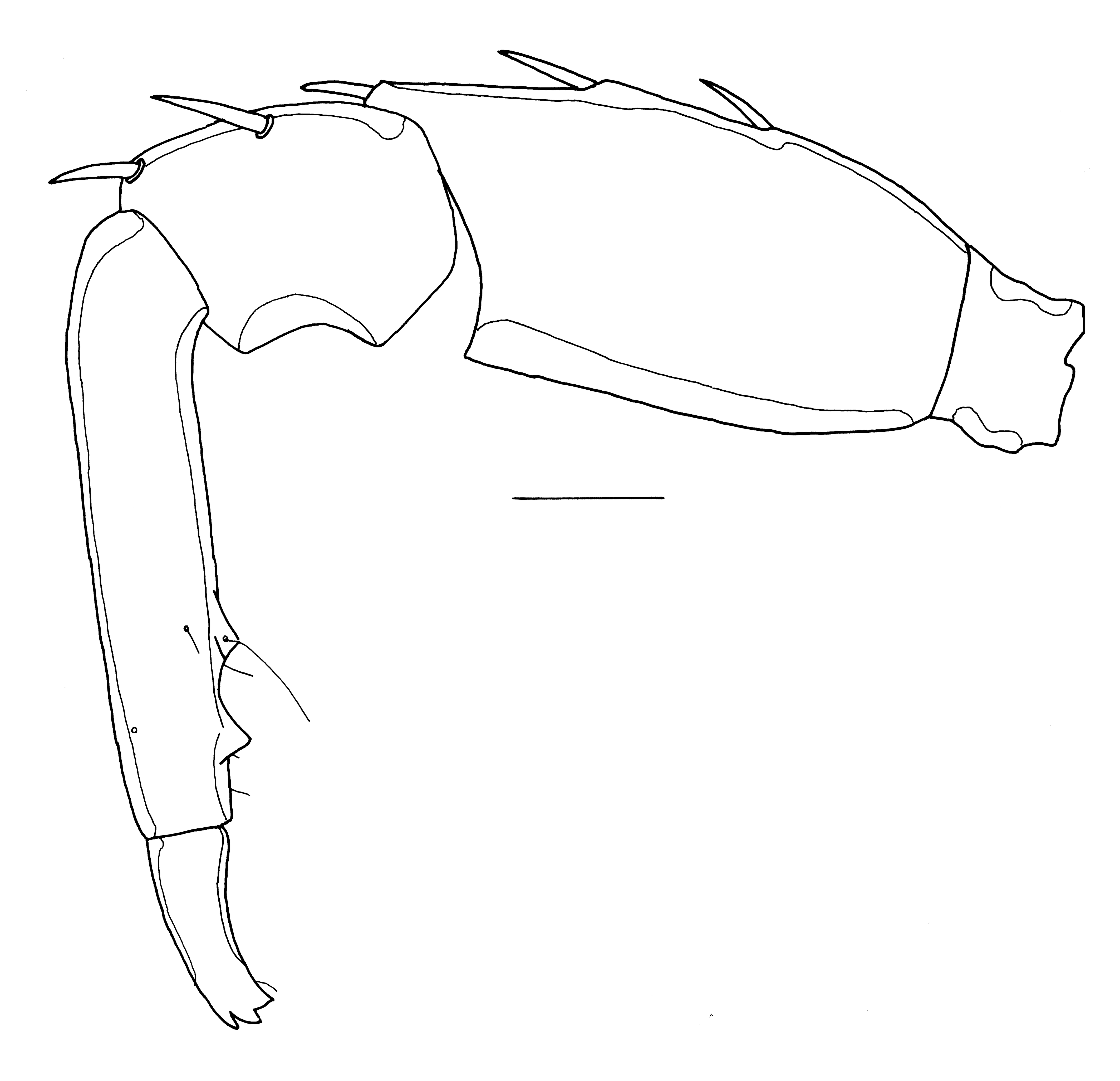


New records. The Gambia. 1[mounted]/0/0, pond Chamois Bridge, Basse, Upper River Division, 11 Feb. 1998. Ghana. 1/1/0, pool 2 Ankasa NP, 5°17.250′ N 2°38.092′ W, 66 m a.s.l., 26 Feb. 2011; 0/1/1, Second pond, Ankasa NP, Ghana, 5°17.424′ N 2°38.286′ W, 58 m a.s.l., 14 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Piona angulata multiacetabulata Cook, 1966
New records. Ghana. 5/3/3, pool 1 Ankasa NP, 5°17.407′ N 2°38.378′ W, 59 m a.s.l., 26 Feb. 2011; 4/4/2, fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 4/2/0, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 Mar. 2011; 5/1/2, First pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013.
Distribution. Liberia (Cook 1966), Ghana (this study).
Piona damasiella Cook, 1966
(Figure 16A-B)



New record. Ghana. 0/1/0, lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l., 9 Feb. 2013; 1/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Description. Male: Idiosoma of the male of this study not fully sclerotized, dorsally 680 long and 547 wide, ventrally 688 long. Apodemes of anterior coxae short. Cx-III+IV fused with genital field. Genital field with numerous acetabula, extending laterally beyond posterolateral corner of Cx-IV (Figure 16A). Excretory pore probably fused with genital field. Length of P1–5: 30, 176, 64, 179, 64. P2 with a slightly convex ventral margin, P4 ventrally with three small setal tubercles (Figure 16B). Length of I-leg-4–6: 192, 215, 178. Length of IV-leg-4–6: 219, 235, 162. III-leg-6 with one of the claws elongated. Swimming setae: II-leg-5 one, III-leg-4 four, III-leg-5 two, IV-leg-4 three, IV-leg-5 five.
Remarks. The male of this species has not been described previously. Compared to P. damasi Lundblad, 1949, the genital plates are longer and extend more laterally. As in the female, the ventral margin of P2 is slightly convex, but straight in P. damasi.
Distribution. Liberia (Cook 1966), Nigeria (Cook 1979), Ghana (this study).
Piona seyrigi continentalis Lundblad, 1952
New records. Ghana. 5/0/1, fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 1/3/1, shallow roadside pond along road Axim-Elubo, 5°09.080′ N 2°37.354′ W, 46 m a.s.l., 27 Feb. 2011; 1/0/0, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 Mar. 2011; 2/2/1, Second pond, Ankasa NP, Ghana, 5°17.424′ N 2°38.286′ W, 58 m a.s.l., 14 Feb. 2013.
Remarks. One of the males from the fish pond along road Elubo-Axim has around 35 pairs of acetabula, all other males have less than 30 pairs of acetabula. According to Cook (1966) the only reliable character to separate the nominate form and continentalis is the number of acetabula, 30-38 in the seyrigi seyrigi and 16-26 in seyrigi continentalis. This study shows there can be an overlap in the number of acetabula, and that this character is not always reliable.
Distribution. Uganda (Lundblad 1952), Liberia (Cook 1966), Ghana (this study).
Family Unionicolidae Oudemans
Subfamily Encentridophorinae K. Viets
Genus Encentridophorus Piersig, 1897
Encentridophorus (Encentridophorus) tumidus Walter, 1937
New records. Ghana. 2/14/0, Amansuri Wetland, 5°00.398′ N 2°35.162′ W, 12 m a.s.l., 26 Feb. 2013; 0/6/0, shallow part Amansuri Wetland, 4°59.635′ N 2°35.417′ W, 26 Feb. 2011; 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. The assignment of this species is uncertain. Cook (1966) tentatively assigned specimens from Liberia to this species, which was described by Walter (1937) based on the female only. The specimens from Ghana match the description of Cook (1966) well.
Distribution. Angola (Walter 1937), Liberia (Cook 1966), Ghana (this study).
Encentridophorus (Encentridophorus) vietsi Bader, 1981
New records. The Gambia. 0/3/0, small pond Kampanti, Western Division, 7 Feb. 1998; 0/8/0, pool Prufu Swamp, Basse, Upper River Division, 10 Feb. 1998; 1/0/0, pool along Prufu Bolon stream, Basse, Upper River Division, 12 Feb. 1998. Ghana. 3/4/2, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 Mar. 2011; 1/13/0, Black Volta River at Buipe, 8°45.674′ N 1°27.320′ W, 76 m a.s.l., 6 Mar. 2011; 0/8/0, rock pools upstream Fuller Falls, 8°04.958′ N 1°47.749′ W, 188 m a.s.l., 6 Mar. 2011; 0/2/0, lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l., 9 Feb. 2013; 1/10/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013.
Remarks. Viets (1916) assigned specimens from Cameroon to E. spinifer (Koenike, 1893), a species described initially based on the female sex only. However, Encentridophorus females show few species-specific characters, and therefore I agree with Bader (1981) that the assignment of Viets (1916) is questionable. Bader (1981) described his specimens from Cameroon as E. vietsi Bader, 1981, and postulated that the specimens of Viets might belong to this species. My male from the Gambia has the posterior idiosoma margin with 4+1 (in the holotype 5+1) pairs of stout setae, but is otherwise similar to E. vietsi. In my opinion the male from the Gambia and the specimens from Viets (1916) belong all to E. vietsi. The number of stout setae at the posterior idiosoma margin is, therefore, 4–6 pairs of setae + a separated seta on each side. Bader (1981) was the opinion that the specimens reported by Walter 1939) from Chad do not belong to E. vietsi, but belong possibly to a new species. Bader based this opinion on the measurements of leg segments and the number of swimming setae. I disagree with Bader, and I don′t see any marked differences between the specimens from Chad and Cameroon.
Distribution. West Africa, previously reported from Cameroon (Viets 1916; Bader 1981), Chad (Walter 1939), the Gambia, Ghana (this study).
Subfamily Pionatacinae K. Viets
Genus Neumania Lebert, 1879
Neumania (Alloneumania) marginata (K. Viets, 1916) – nov. comb.
Ecpolus dorsofenestratus Lundblad, 1949 – new syn.
New records. The Gambia. 0/5/0, pool Prufu Swamp, Basse, Upper River Division, 10 Feb. 1998; 0/1/0, pond ± 5 km W of Basse, along road Basse-Bansang, Upper River Division, 12 Feb. 1998. Ghana. 1/1/0, pool 2 Ankasa NP, 5°17.250′ N 2°38.092′ W, 66 m a.s.l., 26 Feb. 2011; 0/1[young]/0, shallow roadside pond, along road Axim-Elubo, 5°09.080′ N 2°37.354′ W, 46 m a.s.l., 27 Feb. 2011; 0/7/2, First pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013; 0/3/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 0/2/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. Cook (1966) suggested that Neumania marginata K. Viets, 1916 might belong to the subgenus Alloneumania. Males of Neumania dorsofenestrata (Lundblad) and females of N. marginata have been collected together in this study, and I consider them belonging to the same species (both with hook-like lateral extensions of coxae and extensive secondary sclerotization). Therefore, the suggestion of Cook (1966) was correct. I do not see any differences between the males from Ghana and Congo, and therefore I propose to synonymize N. dorsofenestrata with N. marginata. Young males do not have a dorsal shield.
Walter (1935) reported this species from Niger and Burkina Faso. His illustration of the female shows a narrow strip of secondary sclerotization along Cx-IV. However, N. marginata has very broad and distinct secondary sclerotization along Cx-IV, almost completely filling the medial space between Cx-IV. In my opinion the specimens of Walter (1935) must be assigned to N. separata Cook, 1966. The female of this species has a genital field which is very similar to that of N. marginata, but lacks the broad secondary sclerotization. Walter & Bader (1952) reported N. marginata from Kenya, but they illustrated the deutonymph only. Therefore, it is not clear to which species their material belongs.
Distribution. Cameroon (Viets 1916), the Gambia, Ghana (this study).
Neumania (Allolemienia) falcifera Cook, 1974
(Figure 17A-C)
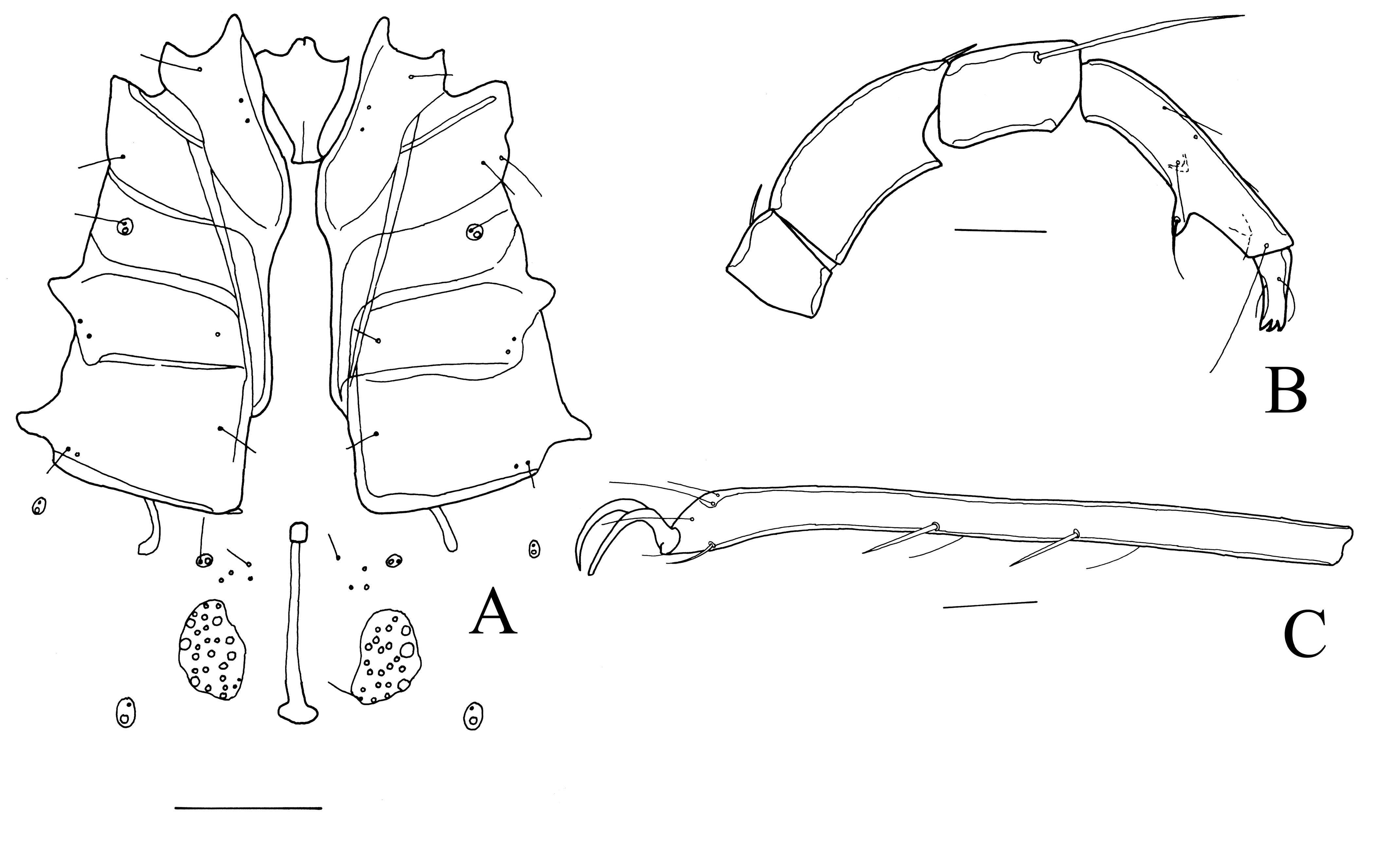


New records. The Gambia. 0/1[mounted]/0, pool Prufu Swamp, Upper River Division, 10 Feb. 1998; 1/0/0, pool along Prufu Bolon stream, Basse, Upper River Division, 12 Feb. 1998. Ghana. 16/18/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l., 9 Feb. 2013.
Description. Female: Integument smooth, idiosoma dorsally 884-1226 long and 648-995 wide, ventrally 810-1045 long. Apodemes of anterior coxae reaching to anterior part of Cx-IV. Posterior margin of Cx-IV with relatively long apodemes (Figure 17A), lateral extensions rounded. Venter without secondary sclerotization. Gonopore 267 long. Genital plates rounded, 130 long, somewhat longer than wide, each plate with 27-29 acetabula. Between Cxgl-4 and genital field four pairs of setae lying free in the integument. Length of P1–5: 42, 124, 72, 148, 46. P4 with a large ventral setal tubercle, more medially two more smaller setal tubercles present (Figure 17B). Length of I-leg-4–6: 243, 356, 352; grooved setae very long. Length of IV-leg-4–6: 356, 360, 324. Claws of legs relatively large, e.g., claw of IV-leg-6 50 long (Figure 17C). IV-leg-5 with one trifurcate seta, other trifurcate setae lost. Swimming setae lost, but probably IV-leg-5 and IV-leg-4 with four swimming setae.
Remarks. The male specimen from the Gambia differs somewhat from the holotype and only known specimen thus far. The large claw of II-leg-6 is 86-94 long (121 in the holotype), and IV-leg-3 and IV-leg-4 have only one trifurcate ventral seta (versus IV-leg-3 with two or three and IV-leg-4 with three in the holotype). As nothing is known about the variation in these characters, I assign the specimen from West Africa for the time being to falcifera. The female has not been described previously.
Distribution. Uganda (Cook 1974), the Gambia, Ghana (this study).
Neumania (Soarella) fusiformis n. sp.
ZOOBANK: 0B11B4E8-7C64-47CF-85C5-90CC92994AF4 ![]()
(Figure 18A-C)



Material examined. Holotype female, plunge pool Kulugu River, Ghana, 6°51.365′ N 0°25.101′ E, 388 m a.s.l., 19 Feb. 2013 (RMNH).
Diagnosis. Apodemes of anterior coxae short, extending to Cx-III; post-genital sclerite large; excretory pore not on a tubercle.
Description. Female: Integument with relatively large spiny setae. Idiosoma dorsally 486 long and 381 wide, ventrally 470 long. Idiosoma with large spindle-shaped tubercles. Dorsum with a pair of small platelets (Figure 18A). Coxal field small, apodemes of anterior coxae short, extending to Cx-III. Genital field 158 wide, genital plates 58 long with seven pairs of acetabula. Pre-genital sclerite much smaller the post-genital sclerite, the latter relatively large, 114 wide (Figure 18B). Excretory pore not on a tubercle. Length of P1-5: 20, 69, 31, 56, 38. P4 anteroventrally with a short, blunt seta (Figure 18C). Length of I-leg-4–6: 60, 70, 74. Length of IV-leg-4–6: 107, 122, 96. IV-leg-3–6 ventrally with 2, 2, 3, 1 pectinate setae, respectively. Swimming setae: III-leg-4 and -5 with three, III-leg-3 with one, IV-leg-5 with two, IV-leg-4 with three, IV-leg-4 with one.
Male: Unknown.
Etymology. Named for the large spindle-shaped tubercles.
Remarks. The new species is somewhat similar to N. tuberculata Cook, 1966 and N. ecphyma Cook, 1996, but differs in the short apodemes of the anterior coxae, and the excretory pore is not on a large tubercle.
Neumania (Soarella) ghanaensis n. sp.
ZOOBANK: 70EA4C7E-81B6-462A-9550-0F87411A49A8 ![]()
(Figure 19A-D)



Material examined. Holotype male, Apkonu stream, downstream of falls, Logba Tota, Ghana, 6°53.054′ N 0°28.024′ E, 362 m a.s.l., 21 Feb. 2013 (RMNH). Paratypes (all RMNH): one female, same data as holotype; one female, tributary of Oguntwe, Ankasa NP, 5°16.563 N 2°38.733 W, 78 m a.s.l., 14 Feb. 2013; six females ([one young], unnamed stream between Apepem and Koju Amu, Atewa Hills, Ghana, 6°10.252′ N 0°36.520′ W, 424 m a.s.l., 27 Feb. 2013; one female, one deutonymph, Tagbo River, downstream of falls, Ghana, 7°00.708′ N 0°34.326′ E, 394 m a.s.l., 23 Feb. 2013.
Diagnosis. Male: Acetabula in two distinctly separated groups, anteriorly 4-5 pairs, posteriorly 11-14 pairs; one of the claws of P5 large. Female: Acetabula as in male, but genital field with a lateral extension.
Description. Integument covered with long, spiny setae. Glandularia, postocularia and excretory pore on relatively short tubercles (Figure 19A). Apodemes of anterior coxae extending to anterior part of Cx-IV. Posterior margin of Cx-IV with a short extension. Cx-III anterolaterally with an extension. Venter without secondary sclerotization. Male: Idiosoma 522 long and 429 wide, ventrally 514 long. Dorsum without platelets. Gonopore 60 long and 34 wide, genital field 206 wide. Acetabula in two groups, a group of 4or 5 anterior acetabula distinctly separated from 11-14 posterior pairs (Figure 19B). Length of P1–5: 22, 92, 38, 82, 28. P2 relatively long, P4 relatively stocky, the latter segment anteroventrally with a blunt seta, dorsally with two long, curved setae. P5 with one large claw (Figure 19C). Length of I-leg-4–6: 124, 146, 148. Grooved or fluted setae of first leg larger than those of second and third legs. Length of IV-leg-4–6: 138, 186, 118. IV-leg-3, -4 and -5 ventrally with two, three and four pectinate setae, respectively. Swimming setae: IV-leg-4 and -4 three, IV-leg-3 one, III-leg-3 one (relatively short), III-leg-4 four, III-leg-5 three. I-leg-5 and II-leg-5 with one and two short swimming setae, respectively.
Female: Idiosoma dorsally 575 (518-624) long and 486 (462-563) wide, ventrally 567 (543-591) long. Dorsum with two small platelets, 16 in diameter. Genital field with two (fused) groups of acetabula, anteriorly 3-4 pairs of acetabula well separated from the posterior acetabula, posteriorly 10-11 pairs of acetabula; genital field with a lateral extension with three pairs of acetabula (Figure 19D). Length of P1–5: 22, 88, 42, 72, 26. P2 slender as in male, but P5 with the large claw. Length of I-leg-4–6: 139, 152, 134. Length of IV-leg-4–6: 162, 202, 158. Legs as in male, but IV-leg-5 with two or three swimming setae.
Etymology. Named after the country where the new species was collected.
Remarks. The new species is somewhat similar to N. ecphyma Cook, 1966 from Liberia. The new species has smaller tubercles, and three of the dorsal tubercles are located medially (in ecphyma all tubercles are located near the lateral idiosoma margin). Moreover, the lateral extension of the genital field is absent in N. ecphyma.
Neumania (Neumania) liberiensis Cook, 1966
(Figure 20)
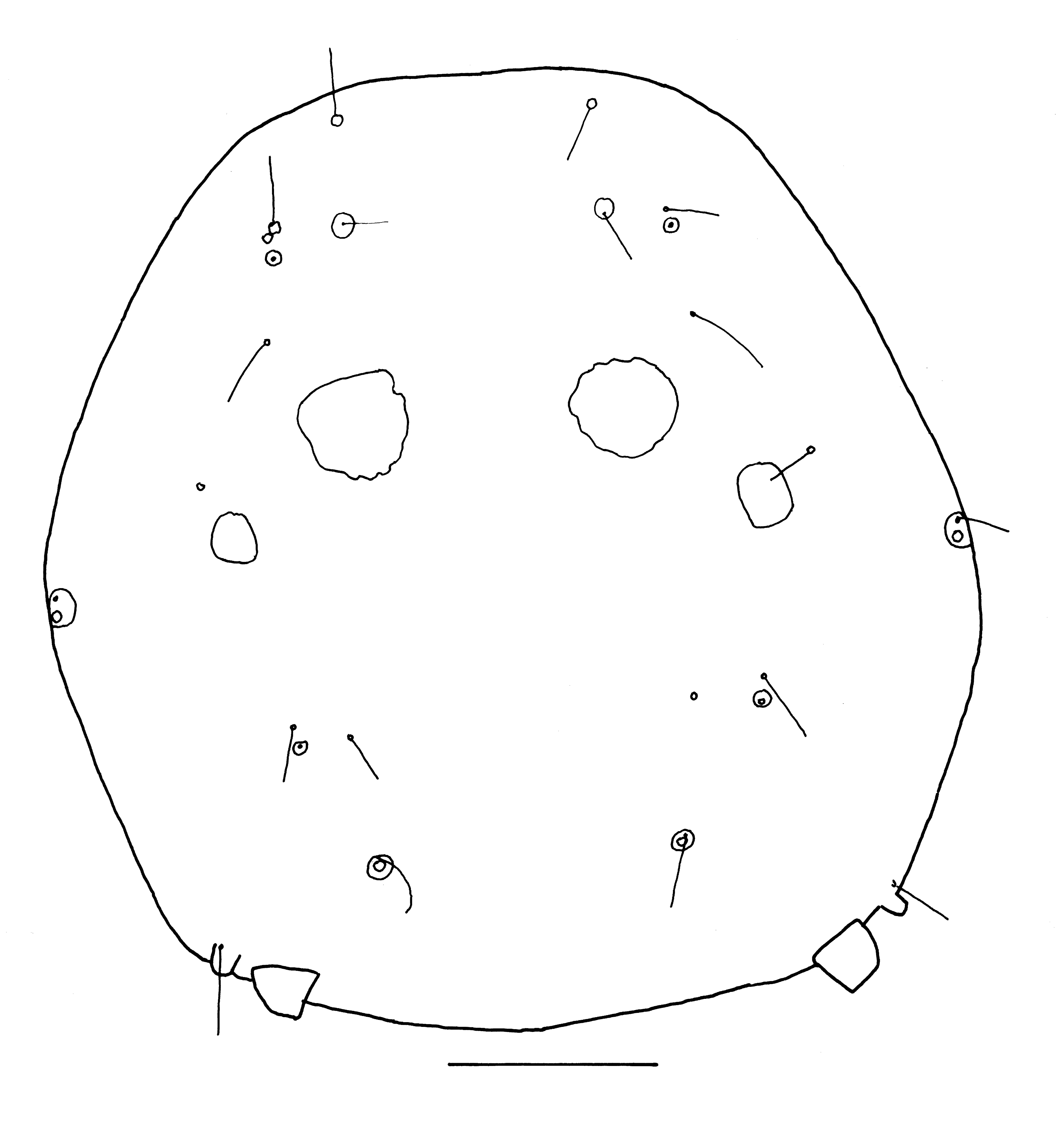


New record. Ghana. 3/2/1, Afiaso stream, Kakum NP, 5°30.087′ N 1°26.373′ W, 114 m a.s.l., 12 Feb. 2013.
Remarks. The male matches the description of Cook (1966), apart from the apodemes of the anterior coxae. According to Cook, they extend to the anterior part of Cx-IV, the specimens from Ghana have longer apodemes, extending to the middle of Cx-IV. Cook (1966) was uncertain about the assignment of the female, but in my opinion this is correct. Females have the venter with less secondary sclerotization compared to the male, and the apodemes of the anterior coxae extend to the middle of Cx-IV. Some measurements are given below. Males: Integument smooth. Idiosoma dorsally 437-454 long and 421-441 wide, ventrally 429-470 long. Females: Idiosoma dorsally 514-591 long and 454-531 wide, ventrally 535-591 long.
Distribution. Liberia (Cook 1966), Ghana (this study).
Neumania (Leptopterotrichophorus) manokensis K. Viets, 1942
New records. Ghana. 1/0/0, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013.
Distribution. Cameroon (K. Viets 1914), Liberia (Cook 1966), Ghana (this study).
Neumania (Neumania) marginata K. Viets, 1916
New records. The Gambia. 0/5/0, pool Prufu Swamp, Basse, Upper River Division, 10 Feb. 1998; 0/1/0, pond ± 5 km W of Basse, along road Basse-Bansang, Upper River Division, 12 Feb. 1998. Ghana. 0/1[young]/0, shallow roadside pond, along road Axim-Elubo, 5°09.080′ N 2°37.354′ W, 46 m a.s.l., 27 Feb. 2011; 0/1/0, fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 0/7/2, First pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, alt. 89 m a.s.l., 14 Feb. 2013; 0/3/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 0/2/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. Walter (1935) reported this species from Niger and Burkina Faso. His illustration of the female shows a narrow strip of secondary sclerotization along Cx-IV. However, N. marginata has very broad and distinct secondary sclerotization along Cx-IV, almost completely filling the medial space between Cx-IV. In my opinion the specimens of Walter (1935) must be assigned to N. separata Cook, 1966. The female of this species has a genital field which is very similar to that of N. marginata, but lacks the broad secondary sclerotization. Walter & Bader (1952) reported N. marginata from Kenya, but they illustrated the deutonymph only. Therefore, it is not clear to which species their material belongs.
Distribution. Cameroon (Viets 1916), South Africa (K.O. Viets 1962), Ruanda (Bader 1968), Burundi (Bader 1968), the Gambia, Ghana (this study).
Neumania (Neumania) paucipora (Koenike, 1895)
New records. Ghana. 0/1/0, Ankasa Exploration Base stream, Ankasa NP, 5°16.413′ N 2°38.810′ W, 81 m a.s.l., 14 Feb. 2013.
Distribution. Tanzania (Koenike 1895, Daday 1910), Cameroon (Viets 1913/1914), Liberia (Cook 1966), Ghana (this study).
Neumania (Neumania) reticulata K. Viets, 1913
New records. Ghana. 0/3/1, Black Volta River at Buipe, 8°45.674′ N 1°27.320′ W, 76 m a.s.l., 6 Mar. 2011; 0/1/0, Namini stream, Kakum NP, 5°23.396′ N 1°23.294′ W, 185 m a.s.l., 12 Feb. 2013; 2/1/1, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013; 2/0/0, tributary of Ankasa River, Ankasa NP, 5°13.130′ N 2°39.119′ W, 96 m a.s.l., 13 Feb. 2013.
Distribution. Cameroon (Viets 1913/1914) , Liberia (Cook 1966), Ghana (this study).
Neumania (Lemienia) separata Cook, 1966
New records. Ghana. 12/7/8, shallow roadside pond, along road Axim-Elubo, 5°09.080′ N 2°37.354′ W, 46 m a.s.l., 27 Feb. 2011; 0/1/0, Fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 0/2/3, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 mar. 2011; 0/1/0, roadside pond W of entrance to Ankasa NP, 5°10.277′ N 2°39.357′ W, 37 m a.s.l., 14 Feb. 2013; 0/1/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Liberia (Cook 1966), Niger, Burkina Faso (Walter 1935), Ghana (this study).
Neumania (Soarella) thori Viets, 1913
New records. Ghana. 0/2/0, Amedzofe Falls, 6°50.656′ N 0°26.868′ E, 599 m a.s.l., 20 Feb. 2013; 0/1/0, plunge pool Tsatsudo Falls, 7°07.390′ N 0°23.365′ E, 179 m a.s.l., 22 Feb. 2013; 0/1/0, Laboun River downstream of falls, Kyabobo NP, 8°19.836′ N 0°35.487′ E, 342 m a.s.l., 24 Feb. 2013.
Distribution. Cameroon (Viets 1913/1914; Bader 1981), Ghana (this study).
Genus Nyangalla K. Viets, 1935
Nyangalla (Ecpolopella) acuticaudata K. Viets, 1916 – nov. stat.
(Figure 21)



New records. The Gambia. 1//0/0, pool Prufu Swamp, Basse, Upper River Division, 10 Feb. 1998; 0/1/0, pond ± 5 km W of Basse, along road Basse-Bansang, Upper River Division, 12 Feb. 1998. Ghana. 1/0/0, pool 2 Ankasa NP, 5°17.250′ N 2°38.092′ W, 66 m a.s.l., 26 Feb. 2011; 0/1/0, shallow part Amansuri Wetland, 4°59.635′ N 2°35.417′ W, 26 Feb. 2011; 1/0/0, Fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 0/1/0, pool Damango, 9°03.983′ N 1°48.419′ W, 185 m a.s.l., 4 Mar. 2011; 2/0/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Description. Female: Idiosoma brownish, dorsally 705 long and 632 wide, ventrally 689 long. Dorsal shield consisting of four pairs of platelets and an unpaired central platelet, flanked by four pairs of smaller lateral platelets and one pair of very small platelets. Glandularia are present on the four pairs of lateral platelets, the central platelet and the pair posterior to the central platelet. The unpaired central platelet is 243 long. Venter, palp and legs as in N. tesselata (Daday, 1910). Male: Posterior margin of cauda with spiny setae.
Remarks. Previously this taxon was considered a subspecies of N. tesselata. As already mentioned by Viets (1916), the two taxa can be found on the same locality, and therefore, they cannot belong to the same species. Also, in Ghana the two taxa co-occur. Moreover, there are distinct differences between the two taxa. The female of N. acuticaudata has not been described previously. The female of the N. tesselata has a larger central platelet with two pairs of glandularia and the two pairs of platelets posterolateral and posterior to the central platelet are absent. The male of N. tesselata has posterior to the central platelet a separate platelet with a truncated posterior extension, in N. acuticaudata there is a no such separate platelet, but there is a pointed extension posterior to the central platelet. Given the large differences between the two taxa, in males as well as in females, and the co-occurrence of the two taxa, I propose to consider N. acuticaudata a full species.
Distribution. Cameroon (K. Viets 1916), Liberia (Cook 1966), the Gambia, Ghana (this study).
Nyangalla (Ecpolopella) peltophora (K. Viets, 1916)
New records. Ghana. 0/2/0, Ankasa Exploration Base Stream, Ankasa NP, 5°16.413′ N 2°38.810′ W, 81 m a.s.l., 14 Feb. 2013.
Distribution. Cameroon (K. Viets 1916), Liberia (Cook 1966), Ghana (this study).
Nyangalla (Ecpolopella) tesselata (Daday, 1908)
New records. Ghana. 0/1/0, pool 1 Ankasa NP, 5°17.407′ N 2°38.378′ W, 59 m a.s.l., 26 Feb. 2011; 0/2/0, pool 2 Ankasa NP, 5°17.250′ N 2°38.092′ W, 66 m a.s.l., 26 Feb. 2011; 1/1/0, Amansuri Wetland, 5°00.398′ N 2°35.162′ W, 12 m a.s.l., 26 Feb. 2011; 5/3/0, Fish pond along road Elubo-Axim, E of entrance Ankasa NP, 5°09.637′ N 2°38.682′ W, 36 m a.s.l., 27 Feb. 2011; 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 10 Mar. 2011; 6/13/0, same location, 27 Feb. 2013; 2/0/0, First pond, Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013; 0/2/0, roadside pond W of entrance to Ankasa NP, 5°10.277′ N 2°39.357′ W, 37 m a.s.l., 14 Feb. 2013; 1/0/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013.
Description. The species has been described by Daday (1908) and Viets (1916). Therefore, I give only some measurements and additional characters. Male: Idiosoma 478-522 long (measured from anterior idiosoma margin till posterior margin of cauda), 445-478 wide. Posterior margin of cauda with spiny setae. Female: Idiosoma 656-810 long and 583-721 wide. Medial area between Cx-IV almost filled up completely by secondary sclerotization.
Distribution. Tanzania (Daday 1910), Cameroon (Viets 1916), Congo (Bader 1956), Liberia (Cook 1966), Ghana (this study).
Subfamily Unionicolinae Oudemans
Genus Unionicola Haldeman, 1842
Unionicola cooki Bader, 1981
New records. Ghana. 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 10 Mar. 2011.
Distribution. Cameroon (Bader 1981, Smit 1994), Ivory Coast (Hevers 2010), Ghana (this study).
Unionicola heversi n. sp.
ZOOBANK: AA0F66D6-6FE2-48D7-8447-A149AD276899 ![]()
(Figure 22A-D)



Material examined. Holotype female, pool 1 Ankasa NP, Ghana, 5°17.407′ N 2°38.378′ W, 59 m a.s.l., 26 Feb. 2011 (RMNH).
Diagnosis. Cx-II and Cx-III with anterolaterally with a pointed extension; palp stocky, especially P4, this segment with two setal tubercles and anteromedially a stout pointed seta, P5 with large claws, ventrally with two claws but only visible in a skew position.
Description. Female: Idiosoma dorsally 745 long and 656 wide, ventrally 689 long. Anterior coxae with short apodemes, extending to Cx-III. Cx-II anterolaterally with a pointed triangular extension, Cx-III anterolaterally with a broader, pointed extension (Figure 22A). Posterior margin of Cx-IV with a short projection. Genital field with five pairs of acetabula on two pairs of platelets, all platelets with long posterior setae. Anterior platelets with two acetabula each, posterior pair with three acetabula each. Unfortunately, the genital field is distorted in the slide. Palp stocky, especially P4, the latter segment with two setal tubercles and a short, stout pointed seta anteromedially. P5 with large claws, ventrally with two claws but only visible in a skew position as these are lying behind each other (Figure 22B-C). Length of I-leg-4–6: 203, 227, 158. Long setae of first leg not fluted or grooved (Figure 22D). Length of IV-leg-4–6: 251, 316, 284. IV-leg-5 with three, IV-leg-4 with two swimming setae.
Male: Unknown.
Etymology. Named after Jürgen Hevers for his excellent work on the genus Unionicola.
Remarks. The palp with its large claws is somewhat similar to palps of U. dentifera Cook, 1966 and U. cooki Bader, 1981, but these species have the coxae with very large anterolateral extensions.
Unionicola koenikei K. Viets, 1913
New records. Ghana. 0/2/0, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013.
Distribution. Cameroon (K. Viets 1913/1914), Burkina Faso (Walter 1935), Angola (Walter 1937), Liberia (Cook 1966), Ivory Coast (Hevers 2020), Ghana (this study). Outside Africa reported from Israel (Por 1968).
Unionicola latilaminata K. Viets, 1911
New records. Ghana. 7/1/0, Afiaso stream, Kakum NP, 5°30.087′ N 1°26.373′ W, 114 m a.s.l., 12 Feb. 2013; 1/0/0, Ankasa Exploration Base trail stream, Ankasa NP, 5°16.415′ N 2°38.751′ W, 80 m a.s.l., 14 Feb. 2013; 0/1/0, Amedzofe Falls, 6°50.656′ N 0°26.868′ E, 599 m a.s.l., 20 Feb. 2013; 0/2/0, unnamed stream between Apepem and Kojo Amu, Atewa Hills, 6°10.252′ N 0°36.520′ W, 424 m a.s.l., 27 Feb. 2013; 1/1/0, unnamed stream upstream Sagyimase, Atewa Hills, 6°13.966′ N 0°33.114′ W, 671 m a.s.l., 28 Feb. 2913.
Distribution. Cameroon (K. Viets 1911, 1912), Liberia (Cook 1966), Ivory Coast (Hevers 2010), Ghana (this study). Outside Africa reported from Israel (Por 1968).
Unionicola megalopsis K. Viets, 1925
New records. Ghana. 0/1/0, Afiaso stream, Kakum NP, 5°30.087′ N 1°26.373′ W, 114 m a.s.l., 12 Feb. 2013.
Distribution. Cameroon (K. Viets 1925), Ivory Coast (Hevers 2010), Ghana (this study).
Unionicola minuta K. Viets, 1916
New records. Ghana. 0/1/0, Ankasa River, Ankasa NP, 5°13.011′ N 2°39.126′ W, 60 m a.s.l., 13 Feb. 2013.
Distribution. Cameroon (K. Viets 1916), Burkina Faso (Walter 1935), Ivory Coast (Hevers 2010), Ghana (this study).
Unionicola pollicigera K. Viets, 1921
New records. Ghana. 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 10 Mar. 2011.
Distribution. Congo (K. Viets 1921), Ivory Coast (Hevers 2010), Ghana (this study). Some doubtful records published by Walter (1935, 1937), based on deutonymphs only.
Family Arrenuridae Thor
Genus Arrenurus Dugès, 1834
Arrenurus (Arrenurus) capensis Thor, 1902
New record. Ghana. 11/4/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l, 9 Feb. 2013; 0/3/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara.
Arrenurus (Arrenurus) chappuisi Walter, 1922
New records. Ghana. 3/0/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Remarks. Arrenurus chappuisi is close to A. okavango Smit, 2012. I separated the two species based on the presence or absence of a two-lobed dorsal shield, and in lateral view the petiole is straight or upturned. With new material available of A. chappuisi, the slender idiosoma, and the more slender and longer petiole (both characters in dorsal view), are better characters to separate these two species
Distribution. Sudan, Congo (Bader 1968), Ghana (this study).
Arrenurus (Megaluracarus) chutteri ankasa n. sp.
ZOOBANK: 13821AE5-C70B-4DE4-BAF2-2758B7386E92 ![]()
(Figure 23A-D)
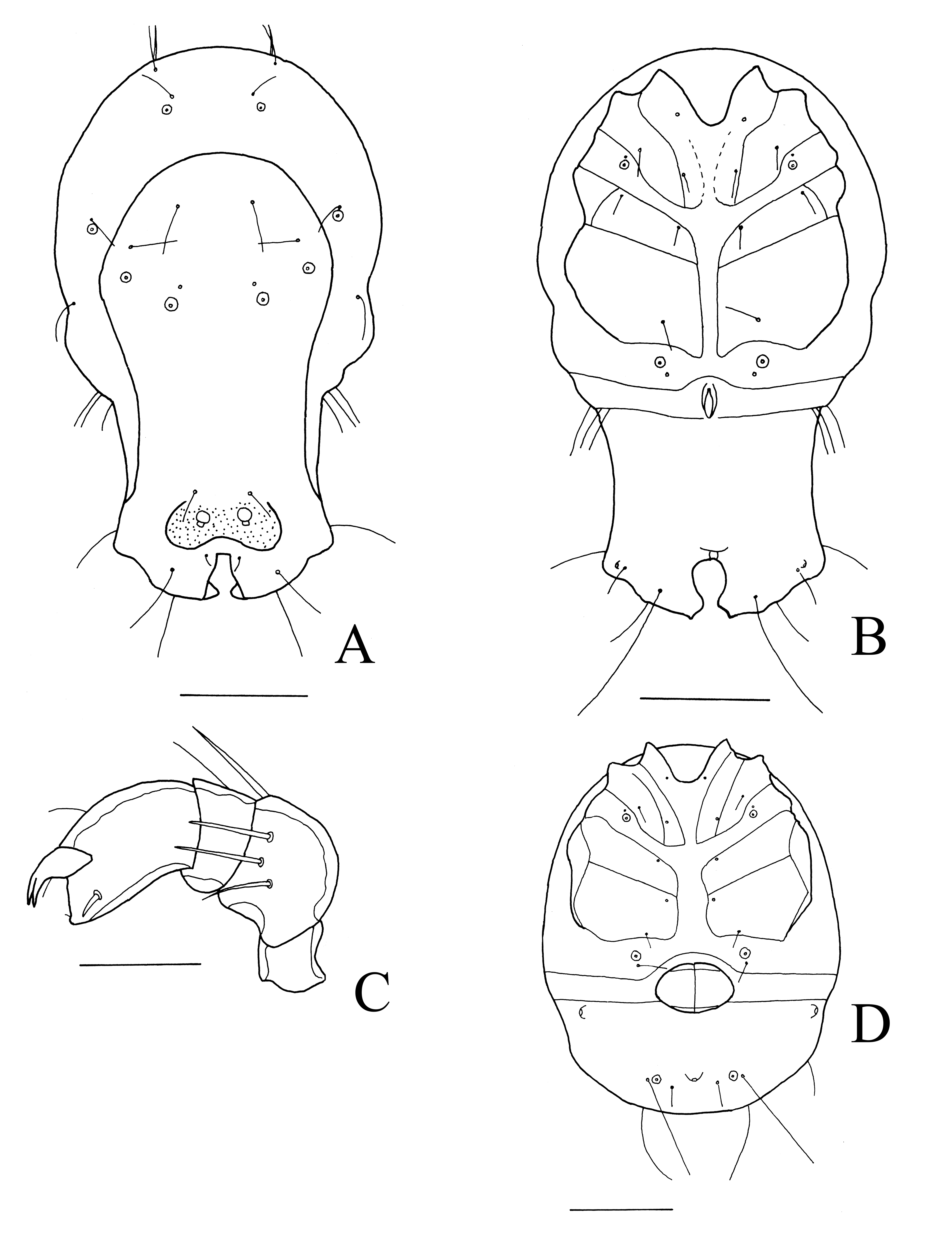


Material examined. Holotype male, Second pond, Ankasa NP, Ghana, 5°17.424′ N 2°38.286′ W, 58 m a.s.l., 14 Feb. 2013 (RMNH). Paratypes: one male, one female, same data as holotype (RMNH).
Diagnosis. Cauda of male expanding laterally, posterior margin of cauda with a relatively wide median cleft which is widening posteriorly.
Description. Male: Idiosoma greenish, dorsally 838 (867) long and 502 (494) wide, ventrally 838 (851) long. Dorsal shield 360 wide. Anterior idiosoma margin convex. Lateral idiosoma margin bulging anterior to the position where the genital plates extend onto the lateral part of the idiosoma. Cauda expanding posterolaterally, in dorsal view lateral margins of cauda concave (Figure 23A). Posterior margin of cauda with a relatively wide median cleft which is widening posteriorly; lateral to cleft a pair of small setae. Dgl-4 lying in an area with spiny setae. Gonopore 52 long. Genital plates long, extending onto lateral margins of idiosoma (Figure 23B). Length of P1–5: 24, 56, 30, 60, 30. P2 medially with three setae (Figure 23C). Length of I-leg-4–6: 108, 110, 112. Length of IV-leg-4–6: 170, 138, 146. IV-leg-4 with a short spur, IV-leg-6 slightly bowed. Third and fourth legs with numerous swimming setae.
Female: Idiosoma dorsally 713 long and 567 wide, ventrally 721 long. Dorsal shield complete, 543 long and 413 wide. Anterior coxae extending beyond anterior idiosoma margin. Medial margin of Cx-IV longer than medial margin of Cx-III. Medial distance of Cx-IV smaller than width of one genital valve. Gonopore field 148 wide, with anteriorly and posteriorly a small band of sclerotized patches; gonopore 92 long. Genital plates long and narrow, extending to lateral idiosoma margin (Figure 23D). Length of P1–5: 27, 56, 26, 66, 34. P2 medially with four setae. Length of I-leg-4–6: 104, 104, 92. Length of IV-lg-4–6: 130, 140, 118. Third and fourth legs with numerous swimming setae.
Etymology. Named after Ankasa National Park, the name is a noun in apposition.
Remarks. The male of the new species differs from A. chutteri K.O. Viets, 1966 and A. chutteri longipes Smit, 2012 in a much more laterally expanding posterior part of the cauda. The female of A. chutteri has shorter genital plates, not extending to the lateral idiosoma margin. The female of A. chutteri longipes has undulating genital plates.
Arrenurus (Megaluracarus) chutteri longipes Smit, 2012
New records. Ghana. 2/0/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Botswana (Smit 2012), Ghana (this study).
Arrenurus (Micruracarus) circulodorsalis n. sp.
ZOOBANK: 6B1E9954-8274-4089-B1DA-00EFEF6F02AE ![]()
(Figure 24A-D)



Material examined. Holotype male, Lake Dahwenya, Ghana, 5°46.760′ N 0°03.049′ E, 24 m a.s.l., 9 Feb. 2013 (RMNH). Paratypes: four females (one not sclerotized), same data as holotype (RMNH). Other material. The Gambia. 0/1/0, pond ± 5 km W of Basse, along road Basse – Bansang, Upper River Division, 12 Feb. 1998.
Diagnosis. Male: Dorsum with a small, rounded dorsal shield, idiosoma posteriorly with a large hollow, posterior idiosoma margin with a closed cleft, gonopore not visible in ventral or posteromedial view. Female: Genital plates extending halfway gonopore field and lateral idiosoma margin and widening laterally.
Description. Male: Idiosoma yellowish, dorsally 648 long and 583 wide, ventrally 624 long. Anterior idiosoma margin straight. Dorsal shield almost circular, 235 long and 251 wide, with the postocularia and Dgl-2. Dgl-1 shifted posteriorly, somewhat posterior to level of Dgl-2 (Figure 24A). No petiole present, although the structure illustrated at the posterior margin might suggest this, but this is just an interruption of the idiosoma pores. Idiosoma with a large hollow posteriorly, but this can be examined only in posteromedial view. Gonopore not visible, not in ventral view, nor in posteromedial view. Posterior idiosoma margin straight, with a closed cleft, which widens anteriorly (Figure 24B). Length of P1–5: 26, 60, 46, 72, 44. P2 medially with three setae (Figure 24C). Length of I-leg-4–6: 101, 114, 132. Length of IV-leg-6: 144, 98, 96. I- and II-leg-6 with numerous small setae. Legs with many swimming setae.
Female: Idiosoma dorsally 907 (932-948) long and 802 (794-810) wide, ventrally 899 (923-956) long. Dorsal shield complete, 656 (644-680) long and 506 (498-526) wide. Anterior idiosoma margin straight, posterolateral corners of idiosoma absent. Anterior coxae extending to or just beyond anterior idiosoma margin. Medial margin of Cx-IV longer than medial margin of Cx-III. Medial distance of Cx-IV longer than width of one genital valve. Gonopore field 166 wide and without sclerotized patches, gonopore 105 long. Genital field 567 wide, genital plates widening laterally, extending halfway gonopore field and lateral idiosoma margin (Figure 24D). Excretory pore lying halfway gonopore field and posterior idiosoma margin. Length of P1–5: 32, 72, 58, 80, 50. P3 medially with one seta. Length of I-leg-4–6: 110, 124, 122. I- and II-leg-6 with less small setae compared to male. Length of IV-leg-4–6: 170, 162, 136. Legs with many swimming setae.
Etymology. Named for the rounded dorsal shield of the male.
Remarks. The male is well characterized by its rounded dorsal shield, the absence of a petiole and the gonopore not visible in ventral or posteromedial view. No other Afrotropical Arrenurus species shares this combination of characters. The female is well characterized by the shape of the genital plates.
Arrenurus (Micruracarus) damkoehleri K. Viets, 1914
New records. Ghana. 2/0/0, Second pond, Ankasa NP, 5°17.424′ N 2°38.286′ W, 58 m a.s.l., 14 Feb. 2013; 1/1/0, roadside pond W of entrance to Ankasa NP, 5°10.277′ N 2°39.357′ W, 37 m a.s.l., 14 Feb. 2013; 32/31/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Cameroon (K. Viets 1913/1914, K.O. Viets 1972, Mali, Burkina Faso (Walter 1935), Liberia (as A. haliki, Cook 1966), Ghana (Smit 2012).
Arrenurus (Megaluracarus) geniculatus Koenike, 1898
(Figure 25A-B)



New record. Ghana. 3/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Description. Female: Idiosoma colour greenish. Anterior idiosoma margin slightly concave, posterolateral corners of idiosoma indistinct. Idiosoma dorsally 778 long and 660 wide, ventrally 782 long. Dorsal shield incomplete, 429 wide. Anterior coxae extending beyond anterior idiosoma margin. Medial margin of Cx-IV slightly longer than medial margin of Cx-III. Medial distance of Cx-IV shorter than width of gonopore field. Genital field 421 wide, genital plates relatively broad, extending more or less halfway lateral idiosoma margin (Figure 25A). Length of P1–5: 20, 60, 34, 68, 33. P2 medially with three short setae; P4 stocky, anteroventrally rounded, anterior margin with a small extension (Figure 25B). Length of I-leg-4–6: 128, 118, 124. Length of IV-leg-4–6: 122, 130, 148. Legs with numerous swimming setae.
Remarks. The female of this species has not been described before. The small extension of the anterior margin of P4 is also present in the males of this study.
Distribution. Madagascar, Ghana (this study).
Arrenurus (Brevicaudaturus) ghanaensis Cook, 1979
New records. Ghana. 0/1/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l, 9 Feb. 2013; 0/1/0, pool Nsu Appiah, near Kakum NP, 5°22.895′ N 1°25.012′ W, 159 m a.s.l., 12 Feb. 2013; 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Ghana (Cook 1979, this study).
Arrenurus (Brevicaudaturus) neolaticodulus Cook, 1966
New records. Ghana. 1/2/0, First pond Ankasa NP, 5°17.415′ N 2°38.384′ W, 89 m a.s.l., 14 Feb. 2013.
Distribution. Liberia (Cook 1966), Ghana (Smit 2012).
Arrenurus (Brevicaudaturus) odonatophilus Münchberg, 1958
New records. Ghana. 0/2/0, Second pond Ankasa NP, 5°17.424′ N 2°38.286′ W, 58 m a.s.l, 14 Feb. 2013; 5/8/0, roadside pond W of entrance to Ankasa NP, 5°10.277′ N 2°39.357′ W, 37 m a.s.l., 14 Feb. 2013.
Distribution. Congo (Münchberg 1958), Liberia (Cook 1966), Ghana (Smit 2012).
Arrenurus (Brevicaudaturus) oldhami Cook, 1979
New records. Ghana. 0/1/0, roadside pond W of entrance to Ankasa NP, 5°10.277′ N 2°39.357′ W, 37 m a.s.l., 14 Feb. 2013.
Distribution. Nigeria, Ghana (Cook 1979, this study).
Arrenurus (Rhinophoracarus) praeacutus (K. Viets, 1916)
New records. Ghana. 0/1/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Cameroon, Ghana (Cook 1979, this study).
Arrenurus (Micruracarus) tubifer Walter, 1922
New records. Ghana. 1/0/0, Lake Dahwenya, 5°46.760′ N 0°03.049′ E, 24 m a.s.l, 9 Feb. 2013; 1/2/0, Avu Lagoon, 5°59.244′ N 0°42.476′ E, 13 m a.s.l., 18 Feb. 2013; 2/2/0, Inlet Volta River at Kpong, 6°09.183′ N 0°03.709′ E, 25 m a.s.l., 27 Feb. 2013.
Distribution. Widespread in Africa south of the Sahara. First records for Ghana.
Genus Thoracophoracarus K. Viets, 1914
A genus with nine species known from Africa (Gerecke 2009). Furthermore, one species known from Chile and one from Asia (Smit 2020).
Thoracophoracarus (Thoracophoracarus) kuehnei K. Viets, 1916
New records. Ghana. 0/1/0, plunge pool Tsatsudo Falls, 7°07.390′ N 0°23.365′ E, 179 m a.s.l., 22 Feb. 2013.
Remarks. With a straight posterior margin, a convex posterior margin and P2 with two setae, the specimen from Ghana matches the description given by Gerecke (2009) well.
Distribution. Cameroon (K.O. Viets 1972), Ghana (this study).
Thoracophoracarus (Thoracophorurus) petioluriger K. Viets, 1925
(Figure 26A-C)



New record. Ghana. 0/1/0, unnamed stream upstream Sagyimase, Atewa Hills, 6°13.966′ N 0°33.114′ W, 671 m a.s.l., 28 Feb. 2013.
Description. Female: Idiosoma colour brownish. Idiosoma dorsally 514 long and 381 wide, ventrally 502 long. Anterior idiosoma margin strongly concave, excretory pore terminal lying in a concave posterior idiosoma margin. Glandularia tubercles in posterior half of dorsum large (Figure 26A). All suture lines of the coxae indistinct. Gonopore field 90 long, genital plates very indistinct and hardly discernible (Figure 26B). Length of P1–5: 18, 42, 30, 50, 22. P2 medially with three setae, P4 anteroventrally pointed (Figure 26C). Length of I-leg-4–6: 104, 104, 92. Length of IV-leg-4–6: 80, 84, 70. Swimming setae: III-leg-3 two, III-leg-4 four, III-leg-5 four, IV-leg-2 and -3 one, IV-leg-4 four, IV-leg-5 four.
Remarks. As stated by Gerecke (2009), attribution of males and females in arrenurids can be problematic due to the strong sexual dimorphism. This applies to the specimen of this study also. Based on the strong concave anterior margin, assignment to T. whartoni Cook, 1966 would be most likely. However, due to the relative slender P4 and the large humps of the dorsum, I assigned this specimen to A. petioluriger. Females of the latter species, originally described as T. mammosus K. Viets, 1925, are known from two specimens only collected in Cameroon. Moreover, there are some differences with the description given by Gerecke (2009), i.e., the anterior margin is stronger concave and the dorsum has two more pairs of glandularia. Therefore, an emended description is given of the female of petioluriger.
Distribution. Cameroon (K. Viets 1925), Ghana (this study).
Family Mideopsidae Koenike
Genus Djeboa K. Viets, 1911
A genus with numerous species known from the Afrotropical region.
Djeboa expansipalpis (Cook, 1966)
New records. Ghana. 1[mounted]/0/0, stream Asikuma, Kakum NP, 5°26.995′ N, 1°25.071′ W, 12 Feb. 2013.
Distribution. Liberia (Cook 1966), Ivory Coast (Pešić et al. 2013), Ghana (this study).
Djeboa ferruginea K. Viets, 1914
New records. Ghana. 0/6[one mounted]/0, stream Asikuma, Kakum NP, 5°26.995′ N 1°25.071′ W, 12 Feb. 2013.
Distribution. Cameroon, Ghana, Ivory Coast, Zimbabwe (Pešić et al. 2013).
Djeboa unimaculata (Cook, 1966)
New records. Ghana. 1/0/0, stream Asikuma, Kakum NP, 5°26.995′ N, 1°25.071′ W, 12 Feb. 2013.
Distribution. Liberia (Cook 1966), Ivory Coast, Ghana (Pešić et al. 2013).
Family Momoniidae K. Viets
Subfamily Momonidinae Lundblad
Genus Momonides Lundblad, 1941
A genus with a disjunct distribution, with two species known from the Oriental region, one from the Palaearctic region and one from the Afrotropical region.
Momonides ghanaensis Smit, 2012
(Figure 27)



New records. Ghana. 1/1/0, unnamed stream between Apepem and Kojo Amu, Atewa Hills, 6°10.252′ N 0°36.520′ W, 424 m a.s.l., 27 Feb. 2013; 1/0/0, unnamed stream upstream Sagyimase, Atewa Hills, 6°13.966′ N 0°33.114′ W, 671 m a.s.l., 28 Feb. 2013.
Description. Female. Idiosoma dorsally 413 long and 405 wide, ventrally 429 long. Integument and lobed sclerites as described for male. Genital field with two genital plates, 88 long. Acetabula in three indistinct groups, with ± 25 pairs of acetabula (Figure 27). Pre-genital sclerite 44 wide, post-genital sclerite 56 wide, more slender than pre-genital sclerite. Length of P1–5: 30, 50, 31, 44, 36. Palp as in male. Length of I-leg-4–6: 88, 185, 98. Length of IV-leg-4–6: 118, 140, 122. Legs as in male. Male: Idiosoma dorsally 369-377 long and 365-369 wide, ventrally 421-429 long.
Remarks. Thus far known only from the holotype male, and the female is described here for the first time.
Distribution. Atewa Hills, Ghana (Smit 2012).
Acknowledgements
I am indebted to the Wildlife Division of the Forestry Commission (Accra) for their permission to collect in the national parks and nature reserves, to Peter Wondergem (Accra) and Truus van der Pal (Alkmaar) for their help and/or assistance during the fieldwork, to Vladimir Pešić (Podgorica) for identifying the Djeboa species and to Peter Jäger and Julia Altmann (SMF) for loan of material from the Viets collection. Gunvi Lindberg (NHRS) made the photographs of Hygrobatopis levipalpis.
References
- Bader C. 1956. Neue Fundorte von Wasssermilben aus dem Belgisch-Kongo. Rev. Zool. Bot. Afr., 53: 281-286.
- Bader C. 1968. Wassermilben aus Zentralafrika. Mus. R. Afr. Centr., Tervuren. Ann. Ser. IN 8, Sci. zool., 163: 1-50.
- Bader C. 1981. Wassermilben aus Westafrika (Acari, Prostigmata, Hydrachnellae). Rev. zool. Afric., 95: 927-953.
- Cook D.R. 1974. Water mite genera and subgenera. Mem. Amer. Entom. Inst., 21: 1-860.
- Cook D.R. 1979. New water mite species from tropical Africa (Acari: Hydracarina). Int. J. Acarol., 5: 199-211. https://doi.org/10.1080/01647957908683150
- Cook D.R. 2005. A new subgenus and two new species of Hygrobatopsis (Acari: Hydrachnida: Hygrobatidae) from South Africa. Int. J. Acarol., 31: 249-254. https://doi.org/10.1080/01647950508684428
- Daday E. von 1910. Untersuchungen über die Süsswasser-Mikrofauna Deutsch-Ost-Afrikas. Zoologica, Stuttgart, 23:1-314. https://doi.org/10.5962/bhl.title.11655
- Gerecke R. 2004. Taxonomy and phylogeny in African water mites of the genus Diplodontus Dugès, 1834 (Acari, Hydrachnidia, Hydryphantidae). Ann. Limnol. - Int. J. Lim., 40: 71-85. https://doi.org/10.1051/limn/2004007
- Gerecke R. 2006. Revisional study on water mites of the family Harpagopalpidae K. Viets, 1924 (Acari: Hydrachnidia), with descriptions of new species from Cameroon and Madagascar. Ann. Limnol. - Int. J. Lim., 42: 109-125. https://doi.org/10.1051/limn/2006008
- Gerecke R. 2009. Revisional studies on Thoracophoracarus K. Viets, 1914 (Arachnida, Acari, Hydrachnidia, Arrenuridae). Zoosystema, 39: 127-145. https://doi.org/10.5252/z2009n1a7
- Gerecke R. 2017. The water mites of the genus Hydrodroma (Acari, Hydrachnidia, Hydrodromidae) in Europe and Africa. Ecol. Monten., 13: 1-24. https://doi.org/10.37828/em.2017.13.1
- Green J., Corbet S.A., Betney E. 1974. Ecological studies on crater lakes in West Cameroon. Debundsha Lake. J. Zool., London, 173: 199-223. https://doi.org/10.1111/j.1469-7998.1974.tb03126.x
- Gledhill T. 2003. A new genus and species of water mite (Acari: Hydrachnidia: Hygrobatidae) from the prosobranch gastropod Lanistes libycus (Morelet) (Streptoneura: Ampullariidae) in Nigeria. In: Smith, I.M. (ed.), An acarological tribute to David R. Cook (From Yankee Springs to Wheeny Creek): 75-82. Indira Publishing House, West Bloomfield.
- Gledhill T., Agbolade O. 2006. A new species of water mite (Acari: Hydrachnidia: Hygrobatidae) from the mantle cavity of the prosobranch Potadoma moerchi (Reeve)(Streptoneura: Thiaridae) in Nigeria. Zootaxa, 1160: 37-47. https://doi.org/10.11646/zootaxa.1160.1.4
- Gledhill T., Vidrine M.F. 2002. Two new sympatric water-mites (Acari: Hydrachnidia: Unionicolidae) from the mutelid bivalve Aspatharia sinuata (von Martens) in Nigeria with some data on unionicoline - bivalve relationships. J. Nat. Hist., 36: 1351-1381. https://doi.org/10.1080/00222930110051734
- Hevers J. 2010. Die Unionicola-Arten (Acari: Hydrachnidia) des temporären Flüsses N\$'\$Zi in der Elfenbeinküste, Westafrika. Stuttg. Beitr. Naturkd. A, Neue Serie, 3: 33-78.
- Koenike F. 1895. Die Hydrachniden Ostafrikas. In: Stuhlmann F. (ed.), Deutsch-Ost-Afrika, Band 4. Die Thierwelt Ost-Afrikas. Wirbellose Thiere, 6: 1-18.
- Lundblad O. 1942. Afrikanische Hydracarinen. Ent. Tidskr., 63: 155-209.
- Lundblad O. 1949. Hydrachnellae. In: Explor. Park National Albert. 2. Miss. H. Damas (1935-1936), 18: 1-87.
- Lundblad O. 1952. Hydracarinen von den ostafrikanischen Gebirgen. Ark. Zool., (Ser. 2), 3: 391-525.
- Lundblad O. 1969. Indische Wassermilben, hauptsächlich von Hinterindien. Ark. Zool., 22: 289-443.
- Pešić V., Cook D., Gerecke R., Smit H. 2013. The water mite family Mideopsidae (Acari: Hydrachnidia): a contribution to the diversity in the Afrotropical region and taxonomic changes above species level. Zootaxa, 3720: 1-75. https://doi.org/10.11646/zootaxa.3720.1.1
- Pešić V., Smit H. 2014. Torrenticolid water mites (Acari: Hydrachnidia: Torrenticolidae) from Ghana. Zootaxa, 3820: 1-80. https://doi.org/10.11646/zootaxa.3820.1.1
- Pešić V., Smit H. 2015. Water mites of the genus Atractides Koch, 1837 (Acari: Hydrachnidia: Hygrobatidae) from Ghana. Zootaxa, 3911: 343-356. https://doi.org/10.11646/zootaxa.3911.3.2
- Por F.D. 1968. The invertebrate fauna of lake Tiberias. I. Qualitative aspects. Israel J. Zool., 17: 51-79.
- Smit H. 1994. New species of water mites from West Africa (Acari, Hydrachnellae), including a checklist of species recorded from Cameroon. Storkia, 3: 5-11.
- Smit H. 2012a. First record of the water mite genus Momonides Lundblad from the Afrotropical region (Acari: Hydrachnidia: Momoniidae). Syst. Appl. Acarol., 17: 346-348. https://doi.org/10.11158/saa.17.3.13
- Smit H. 2012b. New records of the water mite family Arrenuridae from the Afrotropical region, with the description of 11 new species and two new subspecies. Zootaxa, 3187: 1-31. https://doi.org/10.11646/zootaxa.3187.1.1
- Smit H. 2014. The first hyporheic water mites from the Afrotropical region (Acari: Hydrachnidia), with new species of the genera Kawamuracarus Uchida and Bharatohydracarus Cook. Acarologia, 54: 39-45. https://doi.org/10.1051/acarologia/20142112
- Smit H. 2016. The water mite family Aturidae Thor (Acari: Hydrachnidia) from Ghana, with the description of twelve new species. Zootaxa, 4158: 523-543. https://doi.org/10.11646/zootaxa.4158.4.5
- Smit H. 2020. Water mites of the world, with keys to the families, subfamilies, genera and subgenera. Monogr. Ned. Ent. Ver., 12: 1-774.
- Viets, K. 1911. Neue Wassermilben aus Kamerun. Zool. Anz., 38: 492-495.
- Viets K. 1912. Hydracarinen aus Kamerun. Arch. Hydrobiol., 8: 156-178.
- Viets K. 1913/1914. Hydracarinen-Fauna von Kamerun. Arch. Hydrobiol., 9: 1-52, 177-225, 331-338.
- Viets K. 1914. Diagnosen neuere Hydracarinen. Abh. naturw. Ver. Bremen, 22: 221-240.
- Viets K. 1916. Ergänzung zur Hydracarinen-Fauna von Kamerun. Arch. Hydrobiol., 11: 241-305, 335-403.
- Viets K. 1924. Diagnosen einiger neuer Hydracarinen. Zool. Anz., 58: 103-106.
- Viets K. 1925. Nachträge zur Hydracarinen-Fauna von Kamerun. Arch. Hydrobiol., 16: 197-242.
- Viets K. 1953. Die aus Afrika bekannten Wassermilben (Hydrachnellae, Acari). August Thienemann zum 70. Geburtstage7. September 1952. Hydrobiologia, 5: 1-178. https://doi.org/10.1007/BF00023587
- Viets K.O. 1962. Diagnosen neuer und seltener Neumania-Arten (Acari, Hydrachnellae) aus Südafrika. Acarologia, 4: 605-622.
- Viets K.O. 1963. Hygrobatidae (Acari, Hydrachnellae) aus Südafrika. Arch. Hydrobiol., 59: 467-485.
- Viets K.O. 1964. Neufunde und Taxonomie afrikanischer Hydrachnellae. Acarologia, 6: 129-162.
- Viets K.O. 1970. Unser Zuwachs an Kenntnissen über die aus Afrika bekannten Wassermilben (Hydrachnellae, Acari) (mit Anhang: Limohalacaridae). Hydrobiologia, 35: 65-126. https://doi.org/10.1007/BF00143304
- Viets, K.O. 1972. Revision alter Diagnosen von Wassermilben-Arten aus Kamerun. Zool. Anz., 187: 406-422.
- Viets K.O. 1973. Wassermilben (Hydrachnellae, Acari) aus dem Gebiet der Kivu-Sees, Zaire (Kongo). Acarologia, 15: 324-336.
- Viets K.O. 1981. Neue Wassermilben (Hydrachnellae, Acari) aus Südafrika. Acarologia, 22: 373-384.
- Viets K.O., Böttger K. 1974. Zur Systematik und Ökologie rheophiler Hydrachnellae (Acari) Zentralafrikas. Acarologia, 16: 106-159.
- Walter C. 1922. Hydracarina. Zoologische Resultate der Reise von Dr. P.A. Chappuis an den oberen Nil. II. Hydracarina. Rev. Suisse Zool., 30: 63-86.
- Walter, C. 1935. Hydracarina. Voyage de Ch. Alluaud et P.A. Chappuis en Afrique occidentale française. Arch. Hydrobiol., 28: 69-136.
- Walter C., 1937. Contribution à l'étude du Plancton d'eau douce d'Angola. Arch. Hydrobiol., 32: 503-509.
- Walter C. 1939. Hydracariens récoltés par M. Murat dans la région du Tchad. Bull. Soc. Hist. natur. Afrique Nord, 30: 246-252.
- Walter C. 1946. Hydracariens de Mauritanie. Bull. Inst. franç. Afrique noire, 2: 416-422.
- Walter C., Bader C. 1952. Mission scientifique de l'Omo. Hydracarina. Mém. Mus. Hist. natur., Paris, (n.s.), (ser. A), Zool., 4: 87-236.



2021-07-14
Date accepted:
2021-09-09
Date published:
2021-09-27
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Smit, Harry
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



