The identity of Plesialges mimus Trouessart, 1919 and taxonomic notes on the feather mite genus Hemialges Trouessart, 1895 (Acariformes: Analgidae)
Mironov, Sergey V.1
1✉ Zoological Institute, Russian Academy of Sciences, Universitetskaya Embankment 1, 199034, Saint Petersburg, Russia.
2021 - Volume: 61 Issue: 3 pages: 626-640
https://doi.org/10.24349/Pejc-x0bNOriginal research
Keywords
Abstract
Introduction
The feather mite genus Plesialges Trouessart, 1919 (Analgidae: Analginae) was established by for a single species, Plesialges mimus Trouessart, 1919, collected from the museum skin of a White-browed Babbler, Pomatostomus superciliosus (Vigors & Horsfield) (Passeriformes: Pomatostomidae), from Australia (Trouessart 1919). It is necessary to note that this genus and species were described once more as new taxa in another paper, in an issue of ''Redia'' for 1919, which was actually published two years later (Trouessart and Berlese 1921). Both papers (Trouessart 1919; Trouessart and Berlese 1921) provided the same extremely short diagnoses saying only that the genus Plesialges resembles Analges Nitzsch, 1818 in the form of hyperthrophied legs III, but its male differs in having the pretarsus III in a form of bottle without a neck (l'ambulacre renflée en forme la bouteille depourvue de col), and the opisthosoma entire and much wider (l'abdomen entire et plus développé en largeur que chez Analges). Regarding the P. mimus female, Trouessart (1919) only indicated that it is similar to those of Analges. In the subsequent hundred years, this feather mite species has never been carefully redescribed or illustrated. Gaud and Atyeo (1982, 1996) included Plesialges in the genus Analges and treated it as a separate subgenus. In the review of the species content of the genus Analges, Mironov (2019) noted that P. mimus does not correspond to the criteria of this genus.
I re-examined the type specimens of P. mimus deposited in the collection of the Museum National d'Histoire Naturelle in Paris, one male and one female constituting the entire type series of this species, and have determined that the genus Plesialges corresponds to the taxonomic criteria of the genus Hemialges Trouessart, 1895 and should be included in this genus. The main mistake that led Trouessart to the suggestions that Plesialges is close to Analges was the incorrect interpretations of the structure of tarsus III in the male. Actually, a large claw on tarsus III in P. mimus is the apical process of the tarsal segment proper, which is one of the main diagnostic features of the genus Hemialges; in males of Analges, the apical ''claw'' of tarsus III is formed by the modified seta s (Gaud 1974; Mironov 2019). As for pretarsus of legs III in the male of P. mimus, the ambulacral stalk is thinned but retains a cylindrical form and a rudimentary ambulacral disc; in males of Analges, the entire pretarsus is modified into a very thin and transparent claw-like process, tightly adjacent to claw-like seta s. In addition, previous researchers did not pay attention to the structure of epimerites I in the female of P. mimus, which are fused into a Y, while in females of Analges, epimerites I are free.
In the present paper, Plesialges mimus is transferred to the genus Hemialges and redescribed. Additionally, I provide a new diagnosis for the genus Hemialges, comments on its taxonomy, and an updated checklist of species including host associations and localities.
Material and methods
The type specimens of Plesialges mimus were loaned from the Museum National d'Histoire Naturelle (Paris, France). Comparative materials representing various species of the genus Hemialges were examined in the feather mite collection of the Zoological Institute of the Russian Academy of Sciences (Saint Petersburg, Russia). Investigation and redescription of mite specimens were made with the aid of a Leica microscope (DM2500, Leica Microsystems, Inc.) equipped with differential interference contrast (DIC) and a camera lucida.
Descriptions of mite taxa follow the modern format proposed for genera and species of Analgidae (Mironov 2009, 2019; Dabert et al. 2018; Pedroso and Hernandes 2018). General morphological terminology and idiosomal chaetotaxy follow Gaud and Atyeo (1996) with minor corrections by Norton (1998), and the leg chaetotaxy is after Grandjean (1939). All measurements are in micrometres (μm). Classification and scientific names of birds follow Gill et al. (2021).
Systematics
Family Analgidae Trouessart & Mégnin, 1884
Subfamily Analginae Trouessart & Mégnin, 1884
Genus Hemialges Trouessart, 1895
Hemialges Trouessart, 1895: 32-33 (subgen.), 1899: 27 (subgen.), 57 (full genus); 1916: 218, 1920: 303; Gaud 1962: 34-36, 1970: 117; Gaud and Atyeo 1982: 303, 1991: 164, 1996: 53; Mironov and Galloway 2002: 186.
Plesialges Trouessart, 1919: 336-337; Trouessart and Berlese 1919: 4, syn. n.
Type species: Megninia pappus Trouessart & Neumann, 1888, by original designation.
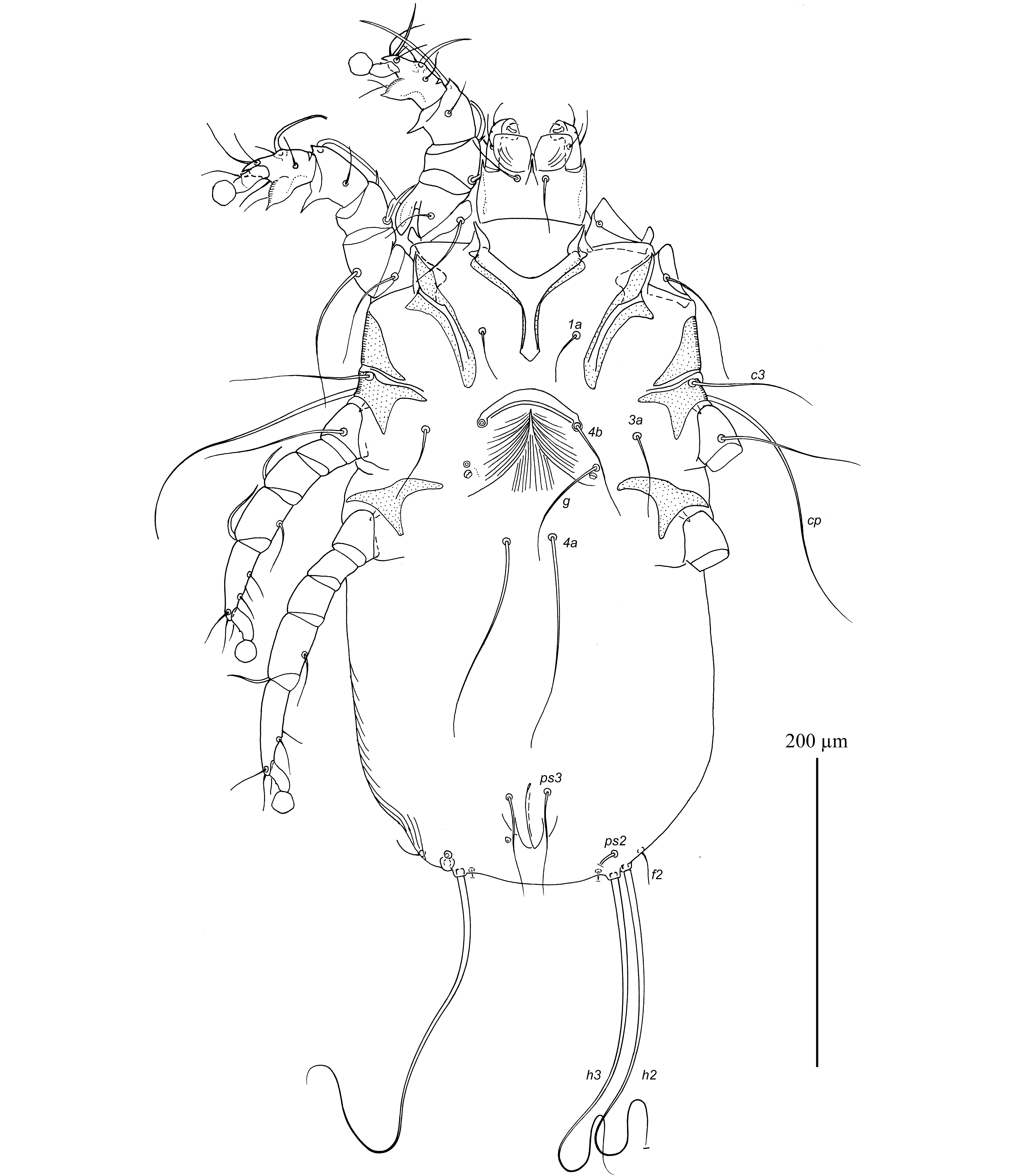
Description — Both sexes. Medium and large-sized analgines (up to 800 long). Prodorsal shield occupying median part of prodorsum, shaped as longitudinal trapezoid, a pair of longitudinal median ridges present or absent, a pair of acute suprategumental extensions on posterior margin present or absent, median area always with strong and dense punctuation (Figure 1). Scapular shields with suprategumental triangular or rounded extension on inner margin. Supracoxal setae scx setiform or absent. Dorsal hysteronotal setae c1, h1 absent. Vertical setae ve rudimentary, represented by alveoli. Epimerites I fused into a Y with long sternum (Figures 2, 4). Femur I with large hook-like lateral process rounded apically, trochanter I with tooth-like lateral process opposing to the femoral process; femur II without hook-like lateral process. Tarsi I, II, each with acute cuff-like ventral processes (''manchettes''), anterior margins of these processes usually with minute denticles and striation. Tibiae I, II, each with a spine-like hyaline ventral process. Tarsus I with 8 setae (ba, d, e, f, la, ra, wa, s); tarsus II with 7 setae (ventral seta wa absent) (Figure 5A, B).






Male. Strongly variable in general appearance among species and within species; intraspecific polymorphism mainly expressed in size and form of body and in structure of legs III. Idiosoma variable in shape, from roughly rhomboid (in heteromorph males of megamerus and pappus groups) to moderately ovate; lateral margins of opisthosoma gradually attenuate posteriorly or almost parallel-sided. Opisthosoma, in most species, with a pair of triangular opisthosomal lobes bearing terminal lamellae of various form (angular, tongue-shaped, leaf-like) and with interlobar membrane between lobes; rarely without distinct lobes, truncate or rounded, without lamellae (furcula group; homeomorph males of some species, e.g. H. effeminatus, H. hologaster, H. pilgrimi). Lateral margin of humeral shields with or without spine-like extensions. Lateral membranes of opisthosoma absent. Hysteronotal shield occupying median area of hysterosoma with anterior end usually extending to level of humeral shields or trochanters III; rarely developed only in posterior half of opisthosoma. Setae c2 situated on anterior ends of humeral shields and usually represented by macrosetae. Setae d2, e2 vary in size, represented by macrosetae or relatively short filiform setae; these pairs distant from each other, d2 situated at level of trochanters III, and e2 at midlength of opisthosoma. Setae ps2, h2 and h3 arranged in a short row on lateral margin of opisthosomal lobes or, when lobes not developed, on margin of opisthosoma. Setae ps1 short filiform. Supranal concavity present.
Coxal field III closed, completely sclerotized and fused with corresponding humeral shields and rarely with coxal fields II forming large combined shields on both sides of venter. Genital apparatus at level of anterior trochanters III. Epiandrum usually absent; paragenital apodemes flanking genital apparatus laterally present. Genital shield absent. Adanal shield represented by a pair of large longitudinal plates occupying area between genital apparatus and anal area; in some species, additional semicircular fragment of this shield partly encircles anal field (e.g. H. pilgrimi). Anal suckers circular, with smooth corolla. Cupules ih well developed in most species.
Legs III hypertrophied, much thicker and longer than legs IV; trochanter, femur, genu and tibia always enlarged, inner margin of femur III can bear 1-2 spine-like processes of various lengths and shapes. Tarsus III well developed, narrower than corresponding tibia, with large claw-like apical process (in some species half as long as entire segment), with or without basal spine-like extension on inner margin; all setae of tarsus III filiform; ambulacral stalk well developed as in other legs or slightly thinner (H. forcipes, H. spinicrus); ambulacral disc circular or reduced and shaped as small oval (H. mimus). Tarsus IV elongate, similar in length to tibia, with or without small apical process; modified setae d and e button-like; pretarsus IV developed as on legs I, II.
In heteromorph males, trochanter, femur, genu and tibia III strongly enlarged; inner margin of femur usually with spine- or claw-like process, rarely with 2 processes (furcula group); in cases of strongly developed claw-like process and relatively short genu, tibia and tarsus, whole leg III shaped as a chela (H. forcipes, H. mimus). In homeomorph males, trochanter, femur, genu and tibia III moderately enlarged, inner margin of femur with much smaller spines than in heteromorphs or lacking them.
Female. Hysteronotal shield absent, cuticle of hysterosoma finely striated. Setae e1, d1 represented by microsetae. Setae c2, d2 and e2 vary in size, represented by macrosetae or relatively short filiform setae, less than half the width of body. Setae ps1 present. Oviporus situated at level of trochanters III. Epigynum bow-shaped, free from epimerites III, distant from posterior tips of epimerites I, II. Lateral flaps of oviporus poorly sclerotized. Epimerites IIIa strongly reduced or absent. Anal opening ventral, copulatory opening posterior to anal opening.
Differential diagnosis — Combinations of characters differentiating Hemialges from remaining genera of Analginae. Both sexes. Epimerites I fused into a Y, ventral cuff-like processes of tarsi I, II in most species with minute denticles and striation on margin. Male. Pairs of setae d2 and e2 distant from each other, posterior end of opisthosoma with triangular opsthosomal lobes provided with variously shaped terminal lamellae, or opisthosoma can be without lobes and lamellae, truncate or rounded. Opisthosoma without lateral membranes. Paragenital apodemes small, flanking genital apparatus laterally. Adanal shield represented by a pair of longitudinal plates between genital apparatus and anal field. Legs III hypertrophied with trochanter, femur, genus and tibia enlarged. Tarsus III with large claw-like or spine-like apical process, all setae of tarsus simple filiform; ambulacral stalk well developed, almost as in other legs; ambulacral disc circular or ovate. Female. Hysteronotal shield absent, epigynum distant from posterior tips of epimerites I, II, setae ps1 present.
Remarks — Authority and date. The name Hemialges was established by Trouessart (1895) as a subgenus of the genus Megninia Berlese, 1881 for two species, Megninia pappus Trouessart and Neumann, 1888 (type species of the subgenus) and M. (Hemialges) magnifica Trouessart, 1895. In the next paper concerning this taxon, Trouessart (1899) described four more species in the content of this subgenus (in pages 27-28), but in the second part of his paper (Deuxième Note, page 57), he treated Hemialges as a full genus and described one more species, H. emarginata Trouessart, 1899. In his revision of feather mite genera, ''Sarcoptides plumicoles'', Trouessart (1916: 218) treated Hemialges as a full genus and established a new genus Hyperalges with a single species Hyperalges magnificus (Trouessart, 1895) transferred from Hemialges. It is interesting to note, in that general paper, Trouessart (1916) for the first time gave the erroneous date ''1888'' as the year of establishing the genus Hemialges.
Finally, in the monograph with revisions of the genera Hemialges and Hyperalges, Trouessart (1920: 302) made two erroneous statements: (a) that the taxon Hemialges was established in 1888, and (b) that it was elevated to the rank of full genus in 1916. Actually, Trouessart published only two papers in 1888, both co-authored by G. Neumann, and one of these papers (Trouessart and Neumann 1888b) included the description of Megninia pappus, but did not contain any mention of the taxon Hemialges. It is only possible to guess that Trouessart accidentally or deliberately applied the date 1888 to this genus, based on the year of the original description of M. pappus. The second paper by Trouessart and Neumann (1888a) dealt with skin-inhabiting feather mites, the families Dermationidae and Epidermoptidae in modern sense, and did not concern feather-inhabiting mites. It is also necessary to add that in his latest papers, Trouessart (1916, 1920) attributed only his name as the authority of M. pappus, although the original paper (Trouessart and Neumann 1888b) did not have any comments that he was the sole author of the new taxa.
These incorrect and conflicting statements in the papers by Trouessart entangled subsequent researchers. In the same paper but on different pages, Gaud and Atyeo (1982) applied to Hemialges the authorities ''Trouessart, 1888'' (p. 299) and ''Trouessart and Neumann, 1888'' (p. 303). In their world review of supraspecific taxa of feather mites, Gaud and Atyeo (1996) attributed to this genus two authors, Trouessart and Neumann, but in different pages provided different dates, 1888 (p. 53) and 1916 (p. 182).
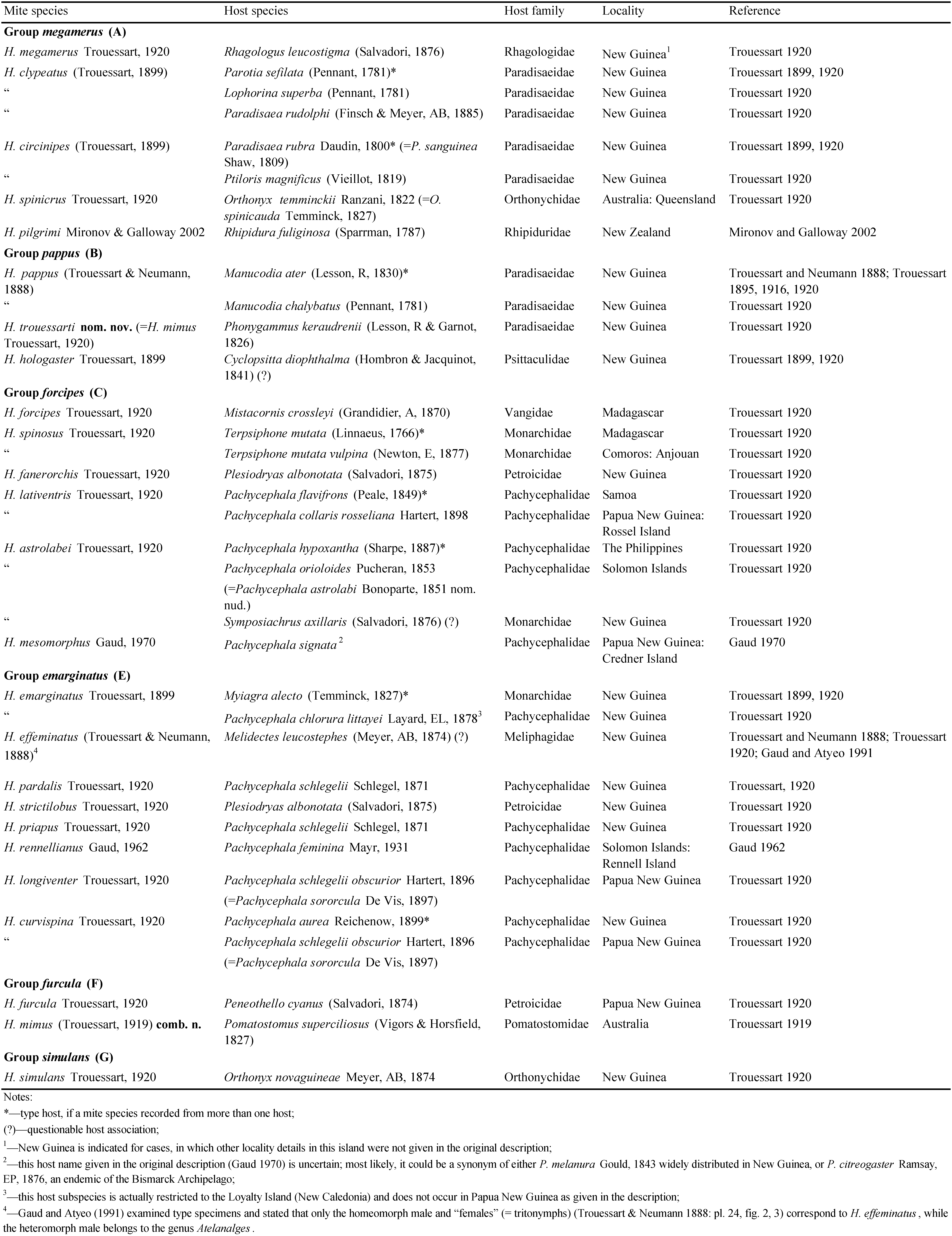
Species content. In the revision of the genus Hemialges, Trouessart (1920) considered 25 species, including 19 newly described, and arranged them in seven species groups designated in capital letters A–G. These groups were based on characters of heteromorph males, such as the general shape of the body, structure of the prodorsal shield, opisthosoma and legs III. In Table 1, all groups retained to date in the genus are provided with new names taken from the first species mentioned in corresponding groups of Trouessart, and the letter designations by Trouessart are shown in brackets. Species within each group are listed according to their sequences in the revision of Trouessart (1920).
During the past hundred years, many changes in the species content of the genus took place. Hemialges tumens (Trouessart, 1899) (ex group A) and H. humerosus Trouessart, 1920 (the sole species in group D) associated with broadbills (Passeriformes: Eurylaimidae) were arranged by Gaud and Atyeo (1967) in a new genus Therisalges Gaud & Atyeo, 1967. Further, these authors (Gaud and Atyeo 1991) moved the species H. attenuatus Trouessart, 1920 (ex group E) and H. laglaizeae (Trouessart, 1887) (ex group G) to monobasic genera, Atelanalges Gaud & Atyeo, 1991 and Euschizalges Gaud & Atyeo, 1991, respectively.
Berla (1960) described a new species Hemialges megapoda Berla, 1960 from a suboscine passerine host, the Sharpbill Oxyruncus cristatus (Swainson) (Oxyruncidae), in Brazil. However, this mite undoubtedly belongs to the genus Analges Nitzsch, 1818, because the description by Berla (1960: 97, fig. 8, 9) clearly shows that the tarsus of legs III is very small and bears a large claw-like seta s that is one of crucial generic characters of the genus Analges listed by Mironov (2019). Later on, two more Hemialges species were described from whistlers (Pachycephalidae) in the Solomon Islands and Bismarck Archipelago by Gaud (1962, 1970), and one species from a fan-tailed flycatcher (Rhipiduridae) in New Zealand (Mironov and Galloway 2002).
The transfer of Plesialges mimus Trouessart, 1919 to the genus Hemialges in the present work makes the species Hemialges mimus Trouessart, 1920 described from Phonygammus keraudrenii (Lesson, R & Garnot) (Paradisaeidae), a junior homonym. The latter mite species is given here a new valid name, Hemialges trouessarti nom. nov. Taking into account all aforementioned taxonomic changes, the genus Hemialges currently includes 25 species (Table 1).
Hosts — All presently known Hemialges species, except one, are associated with oscine passerines of the infraorder Corvida and distributed in the Old World, mainly in the Indo-Malayan and Australian realms (Table 1). The record of H. hologaster Trouessart, 1899 from the Double-eyed Fig Parrot, Cyclopsitta diophthalma (Psittaciformes: Psittaculidae), is most likely the result of accidental contamination, as was noted even by the author of this mite species (Trouessart 1899, 1820). It is interesting to note that this mite was described a new species in both cited papers of Trouessart. The majority of Hemialges species associated with oscine passerines were recorded from whistlers (Pachycephalidae), 9 species from 13 host species, and from birds-of-paradise (Paradisaeidae), 4 species from 8 host species; and a few or only one species were found of birds from the families Meliphagidae, Monarchidae, Orthonychidae, Petroicidae, Pomatostomidae, Rhagologidae, Rhipiduridae and Vangidae. Considering the wide distribution of the genus Hemialges on representatives of Corvida and the number of potential hosts from its families that have not been yet examined for feather mites, it would be reasonable to expect a vast number of undescribed species of this genus in the Indo-Malayan and Australian realms.
Hemialges mimus (Trouessart, 1919) comb. n.
(Figures 1-6)
Plesialges mimus Trouessart, 1919: 336-337; Trouessart and Berlese 1921: 4; Mironov 2019: 30.











Material examined — Male lectotype and female paralectotype (MNNH, Trouessart collection, # 30B1) from Pomatostomus superciliosus (Vigors & Horsfield, 1827) (Passeriformes: Pomatostomidae) [=Pomathorhinus superciliosus], Australia, no date, E. Trouessart. (Lectotype and paralectotype designated here).
Description — Heteromorph male (lectotype) (Figures 1, 2, 5, 6A, B). Idiosoma, length × width, 365 × 245, moderately widened, humeral areas blunt-angular. Hysterosoma length 265. Subcapitulum with lateral spines. Prodorsal shield: shaped as almost rectangular plate, posterior margin almost straight, posterior corners with roughly rounded extensions bearing setae se, median ridges not developed, posterior suprategumental processes absent, greatest length 82, width at posterior margin 88, surface of median area shaped as narrow trapezoid with dense and strongly pronounced punctuation, lateral areas almost smooth (Figure 1). Setae vi about 1.5 times longer than prodorsal shield. Setae se separated by 75, almost extending to posterior margin of opisthosoma; setae si not extending to level of setae d2. Supracoxal setae scx absent. Posteromesal corners of scapular shields with triangular suprategumental extensions. Humeral shields without lateral spines. Hysteronotal shield: roughly rectangular in shape, anterior margin straight, anterior angles acute, lateral margins posterior to level of trochanters IV slightly convex, greatest length of shield 215, width at anterior margin 87, anterior part with barely distinct transverse striae, remaining surface without ornamentation. Opisthosoma gradually attenuate posteriorly, posterior margin almost straight, opisthosomal lobes and lamellae not developed. Bases of macrosetae h2, h3 close to each other and situated in posterolateral corners of opisthosoma. Supranal concavity 43 long. Setae c2 situated in anteromesal extensions of humeral shields, extending beyond level of setae e2. Setae d2 in anterior angles of hysteronotal shield, extending beyond posterior margin of opisthosoma, setae e2 on lateral margins of this shield, with distal half extending beyond posterior margin of opisthosoma. Short setae d1 on striated tegument, near anterior margin of hysteronotal shield. Setae e1 and hysteronotal gland openings gl slightly anterior to level of setae e2. Setae f2 about 10 long, situated laterally at level of setae ps2. Setae ps1 about 10 long, slightly anterior to level of setae h2 and h3. Macrosetae h2 subequal in length to idiosoma, both macrosetae h3 in lectotype broken. Distances between dorsal setae and openings: c2:e2 62, d2:e2 93, e2:h3 105, d1:d2 20, e1:e2 15, e2:gl 10, h2:h2 75, h3:h3 58.
Epimerites I fused as a Y, length of sternum approximately equal to free parts of epimerites, posterior end of sternum acute (Figure 2). Coxal fields I sclerotized in anterolateral part, coxal fields II entirely sclerotized. Inner margins of coxo-humeral shields rounded. Genital apparatus 23 × 26; aedeagus narrow cone-shaped, half as long as genital apparatus. Epiandrum absent. Paragenital apodemes (sclerites) small, flanking genital apparatus laterally. Genital papillae on each side fused to each other, situated at midlevel of genital apparatus, off paragenital apodemes. Coxal setae 1a, 4a, 4b 35–40 long. Setae 4a at level of genital apparatus base; genital setae g minute, situated posterior to setae 4a; setae 3a and 4b at same transverse level. Adanal shield represented by a pair of small elongated sclerites with uneven margins, about 20 long (Figure 2, 6B). Adanal suckers with smooth corolla 20 in diameter. Cupules ih absent. Distances between ventral setae: 4b:g 38, 4b:4a 32, g:ps3 88, ps3:h3 70, ps3:ps3 15.
Ventral cuff-like processes of tarsi I, II with minute denticles and striations on margins. Femur II with rounded lateral margin (Figure 5B). Legs III strongly widened and roughly chela-shaped, femur especially strongly widened and bearing large bidentate extension on inner margin, genu and tibia shaped as truncated cones (frustums); tarsus with large claw-like apical process bearing setae e, d and f and with basal angular process on inner margin bearing setae w and s. All setae of tarsus III filiform, not longer than this segment; ambulacral stalk cylindrical, not extending beyond tip of apical claw; ambulacral disc reduced, small ovate and partly retracted into ambulacral stalk. Legs IV with distal part of tarsus extending beyond posterior margin of opisthosoma; tarsus IV 45 long, with small bidentate apical process; modified setae d and e small button-like, situated at midlength and apical process of this segment, respectively. Tibial solenidion φIII slightly shorter than tarsus III, solenidion φIV about 1.5 times longer than tarsus IV. Length of genual solenidia: σ2I 45, σII 10, σIII 35.
Female (paralectotype) (Figures 3, 4, 6C, D). Idiosoma, length × width, 435 × 220, length of hysterosoma 310. Prodorsal shield shaped almost as in male, except posterior margin slightly convex; greatest length 98, width at posterior margin 100. Setae se separated by 88, extending to level of setae e2. Scapular shields as in male, except suprategumental processes more rounded. Opisthosoma widely rounded posteriorly, nearly semicircular. Hysteronotal shield absent. Idiosomal setae c2, d2, and e2 represented by macrosetae. Setae c2 about 120, extending beyond bases of setae d2; setae d2 about 150, extending to midlength between bases of setae e2 and h2; setae e2 about 180, with distal one third extending past end of opisthosoma. Setae f2 about 15 long. Hysteronotal gland openings gl situated immediately anterior to bases of setae e2. Setae d1, e1 as microsetae. Distance between dorsal setae: c2:d2 70, d2:e2 95, e2:h3 105, d1:d2 30, e1:e2 35, h2:h2 110, h3:h3 98.
Epimerites I fused in a Y as in male. Coxal fields I, II without extensive sclerotization. Epigynum bow-shaped, without suprategumental processes, 25 long and 65 wide (Figure 4). Apodemes of oviporus not sclerotized. Genital papillae of each side fused to each other, situated posterior to setae g. Setae 1a short, not reaching epigynum. Setae 4b 55 long, situated on tips of epigynum, and extending to midlength between levels of setae g and 4a; setae g 55 long, situated at posterior ends of oviporus flaps extending slightly beyond level of setae 4a; setae 4a 120–130 long not extending to anterior end of anus. Setae ps3 about 60 long, extending slightly beyond posterior margin of opisthosoma. Distances between ventral setae: 4b:3a 17, 4b:g 42, g:4a 55. Copulatory opening situated asymmetrically, at level of posterior end of anus. Spermatheca and spermaducts in paralectotype specimen indistinct.
Legs I, II as in male. Legs IV with pretarsus not reaching posterior margin of opisthosoma. Tarsi III, IV 50 and 58 long, respectively (Figure 6C, D). Length of solenidia: σ2I 42, σII 10, σIII 35, φIII 43, φIV 28.


Differential diagnosis — Based on the subdivision of the genus Hemialges by Trouessart (1920) into species groups (Table 1), H. mimus (Trouessart, 1919) should be placed in the furcula group, the males of which are characterized in having femur III with two large spines and the posterior end of opisthosoma without distinct opisthosomal lobes. These two features clearly discriminate the furcula group from remaining species groups of Hemialges, always having well developed triangular lobes with variously shaped terminal lamellae, and femora III with one spine or without it. Hemialges furcula was described from a single heteromorph male without any illustrations and was the only species previously constituting the furcula group. The male of H. mimus differs from that of H. furcula in having the following features: in H. mimus, the large extension of the inner margin of femur III bears two relatively short spines similar in shape and size, and the posterior end of opisthosoma is trapezoidal in form, with almost straight posterior margin. According to the brief description of H. furcula, femur III bears two unequal spines situated one after another, among which the posterior one is smaller and shaped as a claw, and the posterior end of opisthosoma is slightly concave. Additionally, H. mimus is much smaller, with idiosoma 365 long; while H. furcula is a very large species, about 800 long (Trouessart 1920).
Acknowledgements
The author thanks Dr. Mark Judson (the Museum National d'Histoire Naturelle, Paris, France) for loaning the type material for the present study. The investigation was supported by the Ministry of Science and Higher Education of the Russian Federation (project No АААА-А19-119020790133-6).
References
- Berla H.F. 1960. Analgesoidea neotropicais. VII. Novas espécies de acarinos plumícolas. Anais da Academia Brasileira de Ciências, 32: 95-105.
- Dabert J., Mironov S.V., Janiga M. 2018. Two new species of the feather mite genus Analges Nitzsch, 1818 (Analgoidea: Analgidae) from accentors (Passeriformes: Prunellidae) - morphological descriptions with DNA barcode data. Syst. Appl. Acarol., 23: 2288-2303. https://doi.org/10.11158/saa.23.12.2
- Gaud J. 1962. Sarcoptiformes plumicoles (Analgesoidea) parasites d'oiseaux de l'Ile Rennell. Natur. Hist. Rennell Isl., British Solomon Isl., 4: 31-51.
- Gaud J. 1970. Sarcoptiformes plumicoles (Analgoidea) parasites d'oiseaux des Iles Rennell, New Britain et New Ireland (Troisième note). Natur. Hist. Rennell Isl., British Solomon Isl., 6: 115-138.
- Gaud J. (1973) 1974. Quelques espèces nouvelles de Sarcoptiformes plumicoles (Analgidae et Dermoglyphidae) parasites d'oiseaux d'Europe. Acarologia, 15: 727-758.
- Gaud J., Atyeo W.T. 1967. Cinq genres nouveaux de la famille des Analgidae, Trouessart & Mégnin. Acarologia, 9: 435-446.
- Gaud J., Atyeo W.T. 1982. The subfamilies of the Analgidae and Psoroptoididae (Acari: Analgoidea). J. Med. Entomol., 19: 299-305. https://doi.org/10.1093/jmedent/19.3.299
- Gaud J., Atyeo W.T., 1991. Huit genres nouveaux de la famille Analgidae (Acarina, Analgoidea). Acarologia, 32: 163-182.
- Gaud J., Atyeo W.T. 1996. Feather mites of the World (Acarina, Astigmata): the supraspecific taxa. Mus. Roy. Afr. Centr., Ann., Sci. Zool., 277: 1-193 (Pt. 1, text), 1-436 (Pt. 2, illustrations).
- Gill F., Donsker D., Rasmussen P. (Eds). 2021. IOC World Bird List (v. 11.1). Available from: http://www.worldbirdnames.org/
 (accessed 10 March 2021).
(accessed 10 March 2021). - Grandjean F. 1939. La chaetotaxie des pattes chez les Acaridiae. Bull. Soc. Zool. France, 64: 50-60.
- Mironov S.V. 2009. On identity of the type species of the feather mite genus Anhemialges Gaud 1958 (Astigmata: Analgidae), with the description of a new genus of the analgid subfamily Megniniinae. Acarina, 17: 89-100.
- Mironov S.V. 2019. A new species of the feather mite genus Analges Nitzsch, 1818 (Acariformes: Analgidae) from the Streaked Spiderhunter Arachnothera magna (Passeriformes: Nectariniidae), with a renewed diagnosis and world checklist to the genus. Acarina, 27: 19-43. https://doi.org/10.21684/0132-8077-2019-27-1-19-43
- Mironov S.V., Galloway T. D. 2002. New feather mite taxa (Acari: Analgoidea) and mites collected from native and introduced birds of New Zealand. Acarologia, 42: 185-201.
- Norton R. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol., 22: 559-594.
- Pedroso L.G.A., Hernandes F.A. 2018. Two new feather mite species of the family Analgidae (Acariformes: Analgoidea) from the Rufous-collared Sparrow Zonotrichia capensis (Müller, 1776) (Passeriformes: Passerellidae). Zootaxa, 4461 (2): 233-244. https://doi.org/10.11646/zootaxa.4461.2.4
- Trouessart E.L. (1886) 1887. Diagnoses d'espèces nouvelles de Sarcoptides plumicoles (Analgesinae). Bull. Soc. Étud. Sci. Angers, 16: 85-156.
- Trouessart E.L. 1895. Description de trois nouvelles espèces de grande taille du groupe des Sarcoptides plumicoles. Ann. Soc. Entomol. France, 64: CCCXI-CCCXIII.
- Trouessart E.L. (1898) 1899. Diagnoses préliminaires d'espèces nouvelles d'Acariens plumicoles. Additions et corrections à la sous-famille des Analgésinés. Bull. Soc. Étud. Sci. Angers, 28: 1-62.
- Trouessart E.L. (1915) 1916. Révision des genres de la sous-famille des Analgesinae, ou Sarcoptides plumicoles. Bull. Soc. Zool. France, 40: 207-223. (Séance du Déc. 14, 1915, publ. Mars 20, 1916)
- Trouessart E.L. 1919. Diagnose de genres nouveaux de Sarcoptides plumicoles (Analgesinae). Ann. Mag. Natural Hist., Ser. 9, 4: 336-338. https://doi.org/10.1080/00222931908673898
- Trouessart E.L. (1919) 1920. Monographie des genres Hemialges et Hyperalges (Sarcoptides plumicoles). Bull. Soc. Zool. France, 44: 302-321. (Séance du Oct. 28, 1919, publ. Feb. 28, 1920)
- Trouessart E.L., Berlese A. (1919) 1921. Generi nuovi di Acari. Redia, 14: 4.
- Trouessart E.L., Neumann G. (1887) 1888a. Types nouveaux de Sarcoptides épidermicoles et psoriques. Bull. Soc. Étud. Sci. Angers, 17:121-150 + pls. I-III.
- Trouessart E.L., Neumann G. 1888b. Diagnoses d'espèces nouvelles de Sarcoptides plumicoles (Analgesinae). Bull. Sci. France et Belgique, 19: 325-380 + pls. XXI-XXVII.



2021-06-07
Date accepted:
2021-08-02
Date published:
2021-09-14
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Mironov, Sergey V.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




