New locality and host records of two chigger mite species (Acariformes: Trombiculidae) from Morocco
Stekolnikov, Alexandr A.1 ; Er-Rguibi, Omar2 ; Laghzaoui, El-Mustapha3 ; Aglagane, Abdessamad4 and El Mouden, El Hassan5
1✉ Laboratory of Parasitic Arthropods, Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
2Laboratory of Water, Biodiversity and Climate Change, Faculty of Sciences Semlalia, Department of Biology, Cadi Ayyad University, Marrakech, Morocco.
3Laboratory of Water, Biodiversity and Climate Change, Faculty of Sciences Semlalia, Department of Biology, Cadi Ayyad University, Marrakech, Morocco.
4Laboratory of Biodiversity and Ecosystem Functioning, Faculty of Sciences, Department of Biology, Ibn Zohr University, Agadir, Morocco.
5Laboratory of Water, Biodiversity and Climate Change, Faculty of Sciences Semlalia, Department of Biology, Cadi Ayyad University, Marrakech, Morocco.
2021 - Volume: 61 Issue: 3 pages: 538-547
https://doi.org/10.24349/acarologia/20214448Original research
Keywords
Abstract
Introduction
Morocco is one of the best-studied African countries as regards chigger mite fauna. Until the present, 23 localities have been sampled and 30 chigger species were recorded there (Stekolnikov 2018b). However, 28 of these 30 species are still known only from their original descriptions. Two species, Brunehaldia brunehaldi (Vercammen-Grandjean, 1956) and Schoutedenichia (Platytrichia) dipodilli Vercammen-Grandjean, 1958 were described from Morocco (Vercammen-Grandjean 1956, 1958) and later recorded, respectively, in Turkey and Egypt (Stekolnikov & Daniel 2012) and in Spain (Pereira-Lorenzo 1993). Straelensia variocula Brown, 2006 was described from both Morocco and Burkina Faso (Brown 2006), but is still known only from its type series. Moreover, the larger half of the chigger species known in Morocco (20 of 30) were collected only in Casablanca or its vicinities (Nfifikh forest, Oued Cherrat, and Tit Mellil). Thus, the knowledge of the chigger fauna in the country should be regarded as fragmentary.
During the recent survey of the parasites of geckos (Er-Rguibi et al. 2021), samples of the Trombiculidae were collected from two species of the genus Quedenfeldtia Boettger in the Agadir and Marrakech provinces of Morocco. In the present paper, we give results of their identification. In addition, reviews of some chigger collections performed recently by one of us (AAS) allowed a confirmation of our identifications by the comparison with type series, a correction of the literature data on the deposition of types, and resulted in the establishment of one new synonym.
Material and methods
The mites were collected in two localities, from two species of geckos. Oukaïmeden (31°12'19'' N, 7°51'44'' W; altitude 2700 m) is a ski resort located in the Atlas Mountains, in the Marrakech province of Morocco. It is characterized by fractured rock outcrops and stone walls with mountain vegetation consisting of plants of alpine and boreal origin (Alaoui Haroni et al. 2008). The region presents a Mediterranean climate, with mean annual rainfall around 500–600 mm/year. Mean temperature ranging from 22 °C in the warmest month (July) to −4 °C in the coldest one (January), with 82 to 139 days of frost per year (Alaoui Haroni et al. 2008). Snowfall occurs mainly between November and March in Oukaïmeden plateau (sometimes snow cover remains until the end of May) (Bouazza et al. 2016). A sample of chiggers was collected from adult geckos (16 males and 18 females) of Quedenfeldtia trachyblepharus (Boettger) (Squamata: Sphaerodactylidae) in May 2019. The groups of bright-orange mites were attached at front and hind legs, the back, the abdomen, the eyes, vent and tail of the hosts. The chiggers were collected gently with the use of tweezers and fixed in absolute ethanol.
Bigoudine town, Barrage Abdelmoumen (30°40'9'' N, 9°11'6'' W; altitude 625 m) is located in the Atlas Mountains, in the Agadir province of Morocco. The locality was characterized by mountain rock crevices, boulders and stone walls with cracks. The climate is typically Mediterranean, characterized by extreme variation in daily and monthly temperatures. Mean daily air temperatures ranged from 2 °C in winter to 32 °C in summer. Precipitation averaging 519 mm/year (M'Hirit et al. 1998).The vegetation is a sparse forest where argan trees (Argania spinosa), juniper trees (Juniperus phoenicea), and jujube trees (Zizyphus lotus) are accompanied with cultivated plants such as eucalyptus (Eucalyptus sp.), olive trees (Olea europaea) and almond trees (Prunus dulcis). In addition, glaucous tobacco (Nicotiana glauca) was the dominant tree around the barrage. Chiggers were collected from adult geckos (5 males and 2 females) of Quedenfeldtia moerens (Chabanaud) (Squamata: Sphaerodactylidae) in March 2019, as described above.
Mites were mounted on microscope slides using Berlese's medium (Walter and Krantz 2009). The slides were examined under a Leica DM 2500 compound microscope (Leica Microsystems GmbH, Wetzlar, Germany) using differential interference contrast (DIC). Microphotographs were taken by means of a Leica DMC 4500 digital camera, morphological drawings were prepared using a drawing tube. Measurements were taken using an ocular micrometer, on a MBI-3 microscope (LOMO plc, Saint Petersburg, Russia) with phase contrast optics.
The new material is deposited at the Zoological Institute RAS (ZIN, Saint Petersburg, Russia). Type materials were examined by AAS in Muséum d'Histoire Naturelle (MHNG, Geneva, Switzerland) and Muséum National d'Histoire Naturelle (MNHN, Paris, France), under microscopes Zeiss Axioskop (Carl Zeiss AG, Oberkochen, Germany) using DIC, ocular micrometer, and a drawing tube. A preliminary calibration by a stage micrometer was done for each of these microscopes.
We have used the specific chigger terminology summarized by Goff et al. (1982), which is prevailing in the taxonomy of these mites, easily comparable with the common terminology for Prostigmata, but has some advantages due to a greater conformity with the specific details of chigger morphology. The index DS is the number of dorsal idiosomal setae, which include humeral setae (shifted anteriad marginal setae of the row C) and setae of the posthumeral rows (C except humeral setae, D, E, F, etc.), but exclude setae situated on the scutum. The V is the number of ventral idiosomal setae excluding sternal and coxal. Measurements of legs (pa, pm, and pp) are being taken from the proximal end of coxa to the distal end of pretarsus, excluding claws. Other abbreviations are explained in the caption to the Table 1. Diagnostic formulas (SIF, fPp, and fD) are explained by Goff et al. (1982) and Stekolnikov (2018b).
Results
Ericotrombidium tarentolae (Vercammen-Grandjean & Langston, 1976) (Fig. 1A – C)


Leptotrombidium (Ericotrombidium) tarentolae Vercammen-Grandjean & Langston (1976) (original designation)
Ericotrombidium tarentolae Stekolnikov (2018b)
Material examined — Five larvae (ZIN 17114 – 17118) from Quedenfeldtia moerens (Chabanaud) (Squamata: Sphaerodactylidae), MOROCCO, Agadir province, Bigoudine town, Barrage Abdelmoumen, 30 Mar. 2019, collected by Omar Er-Rguibi, El-Mustapha Laghzaoui, and Abdessamad Aglagane.
Type deposition — According to the original description (Vercammen-Grandjean & Langston 1976), type series of this species was deposited in Musée Royal de l'Afrique Centrale (MRAC, Tervuren, Belgium). Currently, holotype and 53 paratypes are deposited in MHNG (Stekolnikov 2019), while only 11 non-type specimens from the type locality are deposited in MRAC (Stekolnikov 2018b).
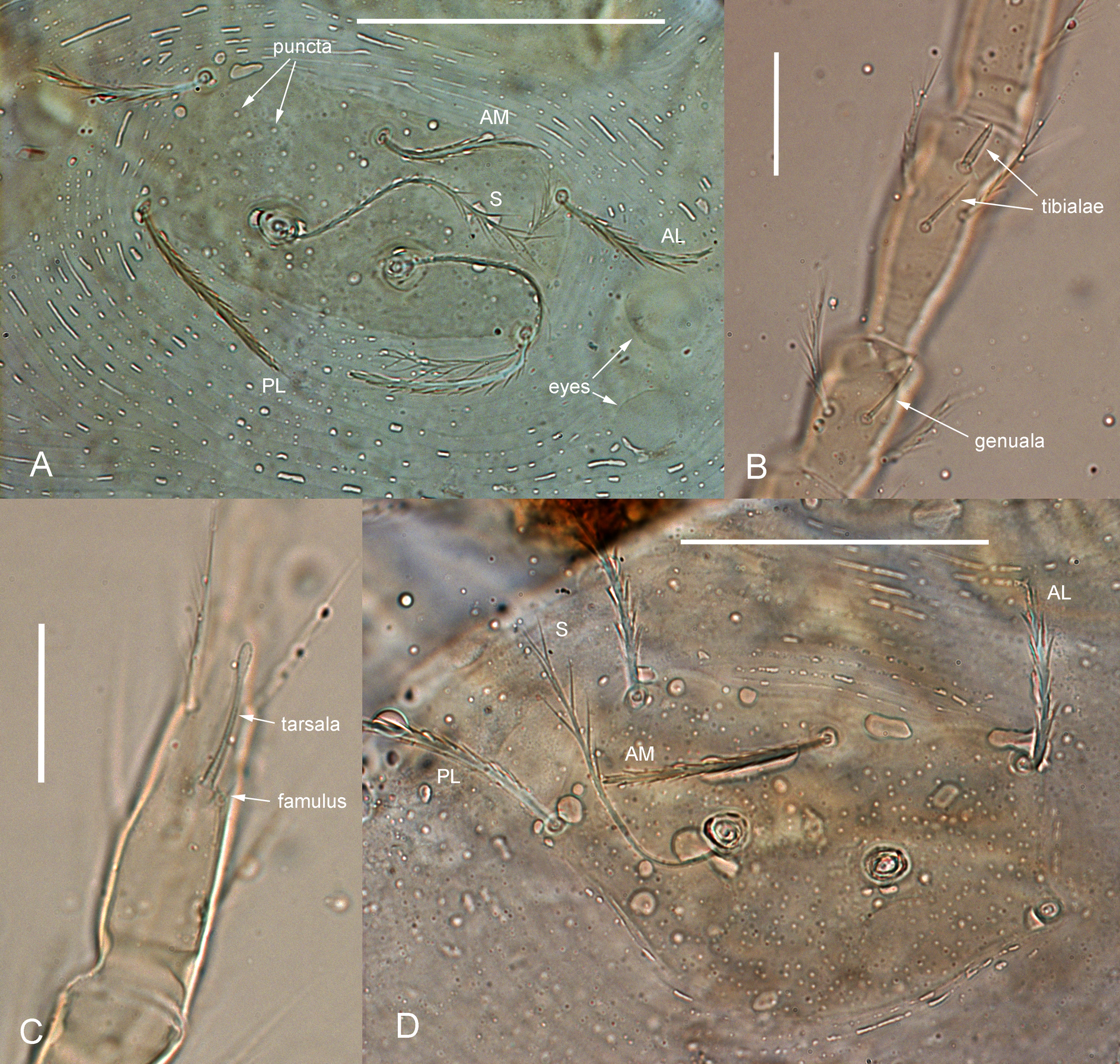
Diagnosis — SIF = 7BS-B-3-2111.0000; fPp = B/B/NNN(b); fD = 2H-8-6-6-4-2-(0-2); DS = 28 – 30; V = 24 – 26; NDV = 52 – 55; Ip = 846 – 893 (765 – 800 according to original description); scutum rectangular, with sparse large puncta; flagelliform sensilla covered with small cilia in proximal half and with 15 – 17 branches in distal half; sensillary (trichobothrial) bases at level or anterior to level of PL (by 3 – 5 µm); PL < AL < AM; marginal setae of 1st row slightly longer than humeral setae (36 – 40 vs. 31 – 34 µm); microtarsala I (ε) distal to tarsala I (ω); microtarsala II (ε) at level of tarsala II (ω); tarsala II thin, with inflated tip (bulbapex); distal tibiala II (φ) expanded, much thicker than proximal tibiala. Standard measurements given in Table 1.



Remarks — This species was known only from its type locality (Morocco, Casablanca) and the type host, Tarentola mauritanica (L.) (Squamata: Phyllodactylidae). Here, it is for the first time recorded in Agadir Province and on Quedenfeldtia moerens.
The difference by the lengths of legs between the new material and the type series according to the original description (846 – 893 vs. 765 – 800) could be an example of intraspecific variation or of a bias caused by differences in the mode of measuring, which can present specifically in the case of legs' length (Stekolnikov et al. 2019).
Noteworthy is the presence of the tarsala II having inflated tip in E. tarentolae. According to the observation of Loomis (1964), it is a characteristic of the chigger species from different genera parasitizing lizards. He suggested that the expanded tip of this chemoreceptor plays a role in the detection of the lizard hosts.
Neotrombicula orycti Taufflieb, 1960 (Figs 1D, 2)
Neotrombicula roubaudi var. orycti Taufflieb (1960a) (original designation)
Neotrombicula orycti Stekolnikov (2018b)
Neotrombicula roubaudi var. lemni Taufflieb (1960a) (original designation), n. syn.
Neotrombicula lemni Stekolnikov (2018b)
Type material examined — Holotype larva of N. orycti, MNHN 59D 11, ex Oryctolagus [cuniculus (L.)], Maroc (MOROCCO), [19]58; paratype larva of N. orycti, MHNG, ex O. cuniculus, Maroc, forêt de Nefifik (MOROCCO, Nfifikh forest), [19]58; holotype larva of N. lemni, MNHN 59E 16, ex Rattus rattus (L.), Cherrat, Maroc (MOROCCO, Oued Cherrat), [19]58; paratype larva of N. lemni, MHNG, Maroc, B. Cherrat (MOROCCO, Oued Cherrat), [19]59.
Additional material examined — Seven larvae (ZIN 17119 – 17125) from Q. trachyblepharus, MOROCCO, Marrakech province, Oukaïmeden, 28 May 2019, collected by Omar Er-Rguibi, El-Mustapha Laghzaoui, and Abdessamad Aglagane.
Type deposition — The original descriptions of N. orycti and N. lemni did not include an information on the type designation (Taufflieb 1960a). Currently, the holotype and 10 paratypes of N. orycti are deposited in MNHN (59D 1 – 11) and three paratypes are deposited in MHNG. The holotype and 11 paratypes of N. lemni are deposited in MNHN (59E 1, 5 – 8, 10 – 16) and three paratypes are deposited in MHNG.
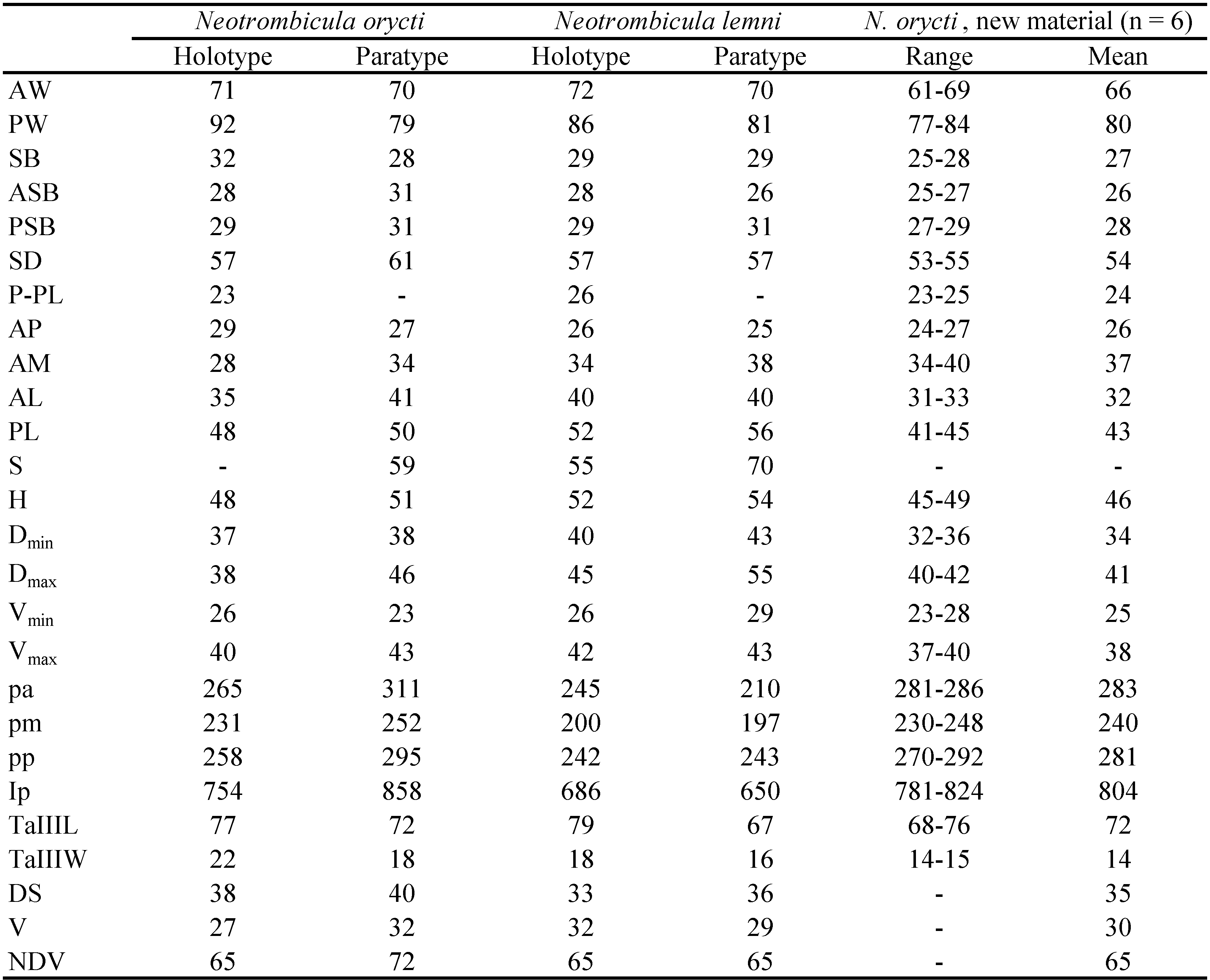
Diagnosis — SIF = 7BS-N-3-3111.1000; fPp = B/B/NNB; fD = 2H-(6 – 9)-(6 – 8)-(6 – 7)-(4 – 8)+(5 – 12); DS = 33 – 40; V =27 – 32 ; NDV = 65 – 72 (56 – 80 according to original descriptions of N. orycti and N. lemni); Ip = 650 – 858 (641 – 888 according to original descriptions); scutum pentagonal, with rounded posterior margin, with moderate number of small puncta; flagelliform sensilla (trichobothria) with ca. 7 branches in distal half; sensillary (trichobothrial) bases anterior to level of PL (by 3 – 6 µm); PL < AL < AM or PL < AM < AL; microtarsala I (ε) distal or proximal to tarsala I (ω); tarsala I longer than tarsala II (26 vs. 18 µm). Standard measurements given in Table 2.


Remarks — Neotrombicula roubaudi (Vercammen-Grandjean, 1956) was described from Tit Mellil and from Nfifikh forest (Morocco, Casablanca-Settat region), where it was collected from Apodemus sylvaticus (L.) (syn.: Sylvaemus sylvaticus hayi), Lemniscomys barbarus (L.), Mus spicilegus Petényi (Rodentia: Muridae), and Eliomys munbyanus (Pomel) (Rodentia: Gliridae). Later Taufflieb (1960a) described two varieties of N. roubaudi, which were raised to species by Stekolnikov (2018b) with their original author and date, according to ICZN Code, Art. 45.6.4 (ICZN 1999). All three species, N. roubaudi, N. orycti, and N. lemni were found in Nfifikh forest and in Oued Cherrat (a wadi located ca. 23 km NE of Nfifikh forest), on L. barbarus, Rattus rattus, Dipodillus campestris (Loche) (Rodentia: Muridae), Oryctolagus cuniculus (Lagomorpha: Leporidae), and on an inexactly identified passerine bird (''Rouge-gorge''). Both N. lemni and N. roubaudi were also recorded on E. munbianus, A. sylvaticus, Mus spretus Lataste (Rodentia: Muridae), and on Mustela nivalis numidica Pucheran (Carnivora: Mustelidae); the latter species was also recorded on another bird host (''Mésange'', i.e. Paridae gen. sp.).
Taufflieb (1960a) examined and measured rather large samples of these species. He took measurements of scutum for 32 N. lemni and 24 N. orycti specimens; legs were measured for 161 specimens of N. lemni, 58 N. orycti, and 115 N. roubaudi; the number of idiosomal setae was counted for 148 N. lemni and 76 N. orycti. Although ranges of quantitative characters were overlapped for the three species, the following two differences seem significant. The number of idiosomal setae is lower in N. roubaudi than in two other species (NDV = 50 – 58 vs. 56 – 74 in N. lemni and 64 – 80 in N. orycti). The legs are shorter in N. lemni than in two other species (Ip = 641 – 800 vs. 775 – 888 in N. orycti and 798 – 922 in N. roubaudi). Taufflieb also noted that the usual arrangement of setae in first two posthumeral rows is 6-6 in N. roubaudi, 6-8 in N. lemni, and 8-8 in N. orycti. In addition, the microtarsala (famulus, ε) of the leg I is situated at level or slightly distal to tarsala (ω) in N. roubaudi, distal to tarsala in N. lemni, and proximal to tarsala in N. orycti (Taufflieb 1960a).
We should note that using NDV and Ip allows almost exact discriminating of N. roubaudi from N. orycti + N. lemni: the combination of the latter two species is characterized by NDV < 58 or, if NDV = 56 – 58, then Ip ≤ 800. On the contrary, distinguishing between N. orycti and N. lemni by those characters seems unreliable. For example, the holotypes of N. orycti and N. lemni has the same arrangement of setae in first three rows, 6-8-6 (Fig. 2), and identical NDV (65); the only significant quantitative difference between them is Ip = 754 vs. 686 (Table 2). Meanwhile, as was demonstrated for Hirsutiella zachvatkini (Schluger, 1948), a difference in the length of legs can characterize intraspecific ecological groups of chiggers, associated with different host species (Moniuszko et al. 2015).



The position of microtarsala I in relation to the tarsala I is usually treated as a stable taxonomic character (Stekolnikov & González-Acuña 2012; Stekolnikov 2013). We suppose that in N. orycti it is variable. In the examined additional material, microtarsala I is situated distal to tarsala I, like in N. lemni, while all other characters (including Ip and the presence of eight setae in 1st posthumeral row) fit the diagnosis of N. orycti. Therefore, we conclude that N. lemni cannot be unambiguously discriminated from N. orycti, and synonymize the former species with the latter.
In the additional material, AM < AL, while in all four examined type specimens of N. orycti and N. lemni AL < AM. Since measurements of setae were not given in the original descriptions of these species, significance of this difference is unclear.
Here, N. orycti is for the first time recorded in Marrakech province and on a reptile host.
Discussion
The predominance of new species descriptions over finds of previously described species signifies a remarkable insufficiency in the taxonomy of chigger mites. The high rate of the Moroccan chigger species known only from their original descriptions (26 of 30, by the results of the present paper) corresponds to the similar data overall the African continent. Among 443 species known from Africa up to 2018, 73% have not been recorded outside their type localities (Stekolnikov 2018b). Although a taxonomic foundation for the identification of a larger half of African chiggers has been laid many decades ago by the works of Vercammen-Grandjean (1958, 1965, 1966), Vercammen-Grandjean & Langston (1976), and Taufflieb (1964, 1965, 1966), faunistic reports on African chiggers almost absent after 1970s, up to the paper of Stekolnikov (2018a), where 19 species have been recorded in new countries and/or on new hosts, and nine of them have been found outside their type localities for the first time. Our new data on the distribution and hosts for the species from the genera Ericotrombidium Vercammen-Grandjean and Neotrombicula Hirst is especially important, since some representatives of these genera can parasitize humans and domestic animals in Europe (Stekolnikov et al. 2014, 2016; di Meo et al. 2017; Areso Apesteguía et al. 2019; Ramilo et al. 2020), Africa (Taufflieb 1960b), and Western Asia (Stekolnikov & Kar 2015).
The ''varieties'' of N. roubaudi described by Taufflieb (1960a) represent an example of closely related sympatric forms of chigger mites that can be separate species or intraspecific ecological groups; Stekolnikov & Matthee (2019) discussed this phenomenon in some details. We suppose that N. orycti and N. lemni are rather indistinct forms of the same species, while N. roubaudi is possibly a separate species; however, this question requires further investigations.
Acknowledgements
We are grateful to Peter Schwendinger (curator of the collection of Arachnida at Muséum d'Histoire Naturelle, Geneva, Switzerland) and Mark Judson (curator of the collection of Arachnida at Muséum National d'Histoire Naturelle, Paris, France) for their valuable help during the visits of AAS in these museums. Field works were done with the permit of Haut Commissariat aux Eaux et Forêts et à la Lutte Contre la Désertification (HCEFLCD) of Morocco. Financial support for fieldwork was provided by Cadi Ayyad University (to OER and HEM). AAS was supported by the Ministry of Science and Higher Education of the Russian Federation (АААА-А19-119020790133-6).
References
- Alaoui Haroni S., Alifriqui M., Simonneaux V. 2008. Recent dynamics of the wet pastures at Oukaimeden plateau (High Atlas mountains, Morroco). Biodivers. Conserv., 18: 167-189. doi:10.1007/s10531-008-9465-6
- Areso Apesteguía M., Areso Portell J.B., Halaihel Kassab N., Gracia Salinas M.J. 2019. Severe trombiculiasis in hunting dogs infested with Neotrombicula inopinata (Acari: Trombiculidae). J. Med. Entomol., 56 (5): 1389-1394. doi:10.1093/jme/tjz071
- Bouazza A., Slimani T., El Mouden E.H., Blouin-Demers G., Lourdais O. 2016. Thermal constraints and the influence of reproduction on thermoregulation in a high-altitude gecko (Quedenfeldtia trachyblepharus). J. Zool., 300: 36-44. doi:10.1111/jzo.12353
- Brown W.A. 2006. Two new species of Apoloniinae (Acari: Trombiculoidea: Leeuwenhoekiidae) from African small mammals, with a key to the species of the world. Folia Parasitol., 53: 217-222. doi:10.14411/fp.2006.028
- di Meo N., Fadel M., Trevisan G. 2017. Pushing the edge of dermoscopy in new directions: entomodermoscopy of Trombicula autumnalis. Acta Dermatovenerol. APA, 26 (2): 45-46. doi:10.15570/actaapa.2017.14
- Er-Rguibi O., Laghzaoui E-M., Aglagane A., Kimdil L., Abbad A., El Mouden E.H. 2021. Determinants of prevalence and co-infestation by ecto- and endoparasites in the Atlas day gecko, Quedenfeldtia trachyblepharus, an endemic species of Morocco. Parasitol. Res. doi:10.1007/s00436-021-07120-z
- Goff M.L., Loomis R.B., Welbourn W.C., Wrenn W.J. 1982. A glossary of chigger terminology (Acari: Trombiculidae). J. Med. Entomol., 19 (3): 221-238. doi:10.1093/jmedent/19.3.221
- International Commission on Zoological Nomenclature. 1999. International Code of Zoological Nomenclature, 4th edition [Internet]. International Trust for Zoological Nomenclature. Available from: https://www.iczn.org/the-code/the-international-code-of-zoological-nomenclature/the-code-online/

- Loomis R.B. 1964. A new species of chigger (Acarina, Trombiculidae) from lizards of Western North America. Great Basin Nat., 24: 13-17. doi:10.5962/bhl.part.22776
- M'Hirit O., Benzyane M., Benchekroun F., El Yousfu S.M., Bendaanoun M. 1998. L'arganier - Une espèce fruitière-forestière à usages multiples. Mardaga, Sprimont. 150 pp.
- Moniuszko H., Zaleśny G., Mąkol J. 2015. Host-associated differences in morphometric traits of parasitic larvae Hirsutiella zachvatkini (Actinotrichida: Trombiculidae). Exp. Appl. Acarol., 67: 123-133. doi:10.1007/s10493-015-9925-0
- Pereira-Lorenzo A. 1993. Chiggers (Acarina: Trombiculidae) parasitizing small mammals in Galicia (NW Spain). Acarologia, 34: 323-329.
- Ramilo D.W., Costa P., Stekolnikov A.A., Cláudio J.M., Lourenço A.M., da Fonseca I.P., Cardoso L. 2020 (2021). First report of Ericotrombidium ibericense in domestic dogs. Acta Parasitol., 66 (1): 253-258. doi:10.1007/s11686-020-00247-6
- Stekolnikov A.A. 2013. Leptotrombidium (Acari: Trombiculidae) of the World. Zootaxa, 3728: 1-173. doi:10.11646/zootaxa.3728.1.1
- Stekolnikov A.A. 2018a. African chiggers (Acariformes: Trombiculidae) in the collection of Alex Fain, with a description of a new genus and three new species. Acarologia, 58 (2): 265-286. doi:10.24349/acarologia/20184240
- Stekolnikov A.A. 2018b. Taxonomy and distribution of African chiggers (Acariformes, Trombiculidae). Eur. J. Taxon., 395: 1-233. doi:10.5852/ejt.2018.395
- Stekolnikov A.A. 2019. A catalogue of the holotypes of chigger mites (Acariformes: Trombiculidae) at the Natural History Museum of Geneva. Zootaxa, 4620 (1): 1-71. doi:10.11646/zootaxa.4620.1.1
- Stekolnikov A.A., Daniel M. 2012. Chigger mites (Acari: Trombiculidae) of Turkey. Zootaxa, 3216: 1-104. doi:10.11646/zootaxa.3216.1.1
- Stekolnikov A.A., González-Acuña D. 2012. A revision of the chigger mite genus Paratrombicula Goff & Whitaker, 1984 (Acari: Trombiculidae), with the description of two new species. Syst. Parasitol., 83 (2): 105-115. doi:10.1007/s11230-012-9373-8
- Stekolnikov A.A., Kar S. 2015. A case of domestic goat parasitism by Neotrombicula heptneri (Acariformes: Trombiculidae) in Turkey. Acarologia, 55 (4): 355-359. doi:10.1051/acarologia/20152176
- Stekolnikov A.A., Matthee S. 2019. Six new and one little known species of chigger mites (Acariformes: Trombiculidae) from South Africa. Syst. Appl. Acarol., 24 (3): 435-466. doi:10.11158/saa.24.3.9
- Stekolnikov A.A., Saboori A., Shamsi M., Hakimitabar M. 2019. Chigger mites (Acariformes: Trombiculidae) of Iran. Zootaxa, 4549: 1-66. doi:10.11646/zootaxa.4549.1.1
- Stekolnikov A.A., Santibáñez P., Palomar A.M., Oteo J.A. 2014. Neotrombicula inopinata (Acari: Trombiculidae) - a possible causative agent of trombiculiasis in Europe. Parasit. Vectors, 7: 90. doi:10.1186/1756-3305-7-90
- Stekolnikov A.A., Waap H., Gomes J., Antunes T. 2016. Chigger mites of the genus Ericotrombidium (Acariformes: Trombiculidae) attacking pets in Europe. Vet. Parasitol., 221: 60-63. doi:10.1016/j.vetpar.2016.03.009
- Taufflieb R. 1960a. Contribution à l'étude des Trombiculidae Marocains. Description de nouvelles espèces et étude d'une population de Neotrombicula. Arch. Inst. Pasteur Maroc, 7 (1): 27-48.
- Taufflieb R. 1960b. Notes sur les Trombiculidae (Acarina) de la région de Brazzaville. Description de trois nouvelles espèces. Acarologia, 2 (4): 472-479.
- Taufflieb R. 1964. Les Schoengastiella (Acarina, Trombiculidae) de la Region Subsaharienne. Acarologia, 6 (3): 455-475.
- Taufflieb R. 1965. Le sous-genre Gahrliepia (Acarina, Trombiculidae) en Afrique Subsaharienne. Acarologia, 7 (3): 510-522.
- Taufflieb R. 1966. Deux nouvelles espèces de Neotrombicula et clé des espèces subsahariennes du genre. Acarologia, 8 (2): 296-301.
- Vercammen-Grandjean P.H. 1956. Les Heaslipia Ewing 1944 et les Neotrombicula Hirst 1915 sont-ils congénères? Description de cinq Trombiculidae originaires du Maroc. Arch. Inst. Pasteur Maroc, 5 (4): 75-86.
- Vercammen-Grandjean P.H. 1958. Revision du genre Schoutedenichia Jad. et Verc. Ann. Mus. Roy. Congo Belge, Sér. 8, 65: 1-103.
- Vercammen-Grandjean P.H. 1965. Revision of the genera: Eltonella Audy, 1956 and Microtrombicula Ewing, 1950, with descriptions of fifty new species and transferal of subgenus Chiroptella to genus Leptotrombidium (Acarina, Trombiculidae) - Acarologia, 7 (suppl.): 34-257.
- Vercammen-Grandjean P.H. 1966. Revision of the genus Herpetacarus Vercammen-Grandjean, 1960 (Trombiculidae - Acarina). Acarologia, 8: 631-674.
- Vercammen-Grandjean P.H., Langston R.L. 1976. The chigger mites of the world (Acarina: Trombiculidae et Leeuwenhoekiidae). III. Leptotrombidium complex. San Francisco: George Williams Hooper Foundation, University of California. pp. 1061.
- Walter D.E., Krantz G.W. 2009. Collecting, rearing, and preparing specimens, pp. 83-96. In Krantz G.W., Walter D.E. (eds.). A manual of acarology. Texas Tech University Press, Lubbock, TX.



2021-04-24
Date accepted:
2021-05-31
Date published:
2021-06-02
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Stekolnikov, Alexandr A.; Er-Rguibi, Omar; Laghzaoui, El-Mustapha; Aglagane, Abdessamad and El Mouden, El Hassan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



