Morphological ontogeny and molecular analyses of geographic strains of two closely related Neoseiulus species (Acari: Phytoseiidae)
Negm, Mohamed W.  1
; Matsuda, Tomoko
1
; Matsuda, Tomoko  2
; Kayukawa, Takumi3
; Ho, Chyi-Chen4
; Hsu, Yu-Tzu
2
; Kayukawa, Takumi3
; Ho, Chyi-Chen4
; Hsu, Yu-Tzu  5
; Kongchuensin, Manita6
; Konvipasruang, Ploychompoo7
and Gotoh, Tetsuo
5
; Kongchuensin, Manita6
; Konvipasruang, Ploychompoo7
and Gotoh, Tetsuo  8
8
1Laboratory of Applied Entomology and Zoology, Faculty of Agriculture, Ibaraki University, Ami, Ibaraki 300-0393, Japan & Department of Plant Protection, Faculty of Agriculture, Assiut University, Assiut 71526, Egypt.
2Nihon BioData Corporation, Kawasaki, Kanagawa 213-0012, Japan.
3Institute of Agrobiological Sciences, National Agriculture and Food Research Organization, Ohwashi, Tsukuba, Ibaraki 305‑8634, Japan.
4Taiwan Agricultural Research and Extension Station, Council of Agriculture, Taichung 41362, Taiwan (retired)..
5Taitung District Agricultural Research and Extension Station, Council of Agriculture, Taitung 95055, Taiwan.
6Plant Protection Research and Development Office, Department of Agriculture, Bangkok 10900, Thailand.
7Plant Protection Research and Development Office, Department of Agriculture, Bangkok 10900, Thailand.
8✉ Laboratory of Applied Entomology and Zoology, Faculty of Agriculture, Ibaraki University, Ami, Ibaraki 300-0393, Japan & Faculty of Economics, Ryutsu Keizai University, Ryugasaki, Ibaraki 301-8555, Japan.
2021 - Volume: 61 Issue: 2 pages: 432-452
https://doi.org/10.24349/acarologia/20214440Original research
Keywords
Abstract
Introduction
The Phytoseiidae Berlese is one of the most important families in the Acari that includes predatory mites used to control spider mites (Tetranychidae), eriophyoid mites (Eriophyoidea) and small pest insects (McMurtry and Croft 1997). Neoseiulus Hughes is one of the largest genera in the family Phytoseiidae, with about 415 species, including synonyms (Demite et al. 2020). Chant and McMurtry (2003) classified Neoseiulus species into 10 species groups mainly based on the presence or absence of setae J1 and ZV3, seta ST3 situated on or off the sternal shield, the shape of female ventrianal shield and spermatheca, and relative lengths of dorsocentral setae. Neoseiulus longispinosus (Evans, 1952) and N. womersleyi (Schicha, 1975) belong to the womersleyi species subgroup of the barkeri species group. Because very few morphological features are known to separate N. longispinosus from N. womersleyi, and sterile female offspring were obtained from crosses between N. longispinosus females and N. womersleyi males (Ullah et al. 2017), we described all life stages to find out whether there might be new morphological characters that could be used to separate the species.
DNA sequences for the genes that encode proteins such as the cytochrome c oxidase subunit I (COI) and 12S rRNA of mitochondrial DNA (mtDNA), and the internal transcribed spacer (ITS) and 28S regions of nuclear ribosomal DNA (nrDNA) have been widely used to identify species and resolve phylogenetic relationships of phytoseiid mites (Okassa et al. 2009, 2020; Sonoda et al. 2012; Vicente dos Santos and Tixier 2017, 2018; Nguyen et al. 2019; Inak et al. 2020). We also examined whether the two closely related species (N. longispinosus and N. womersleyi) could be distinguished from each other as well as from other species using the 28S region of nrDNA.
Material and methods
Mite samples
Mite species and strains used in the morphological and DNA analyses are listed in Table 1. Laboratory stocks were separately reared on detached leaves of common bean, Phaseolus vulgaris L. (Fabaceae) placed on water-saturated polyurethane mats in plastic dishes (90-mm diameter, 20-mm depth) at 25±1˚C under a 16:8 h light:dark photoperiod. The two-spotted spider mite, Tetranychus urticae Koch (Tetranychidae) was provided as prey.



Morphological analyses
Adult females, males and immatures for certain strains were mounted on permanent slides using Hoyer's medium. The specimens were examined using a BX53® (Olympus, Tokyo, Japan) differential interference contrast microscope equipped with a DP72® digital camera (Olympus). Illustrations were done with Adobe Illustrator (Adobe Systems Incorporated, San Jose, CA, USA) and body parts and setae were measured using the imaging software Sensiv Measure1 ver. 2.6.0. All measurements are given in micrometers (μm) as minimum and maximum values from individuals examined. Body length measurements represent the distance between the anterior and posterior margins of dorsal shield. The setal nomenclature used for dorsal and ventral sides follows that of Lindquist and Evans (1965) as adapted by Rowell et al. (1978) and Chant and Yoshida-Shaul (1991), respectively. The notation for gland pores (solenostomes) or lyrifissures (poroids) follows Athias-Henriot (1975). The generic classification is according to the definitions given by Chant and McMurtry (2007). Voucher specimens of the redescribed species were deposited in the Laboratory of Applied Entomology and Zoology, Ibaraki University (AEZIU), Japan, under the serial voucher specimen numbers.
Molecular analyses
Adult females from each strain/species were arbitrarily selected and used for molecular analyses. Total DNA was extracted from the whole body of each female by crushing with a toothpick in 20 μl of 2x PCR Buffer for KOD FX Neo (Toyobo, Osaka, Japan). To amplify a fragment of the 28S region, the primers 43F (5′- GCTGCGAGTGAACTGGAATCAAGCCT -3′) and 929R (5′- AGGTCACCATCTTTCGGGTC -3′) (Dowling and OConnor 2010) were used. Polymerase chain reaction (PCR) was performed in a total of 20 μl of reaction solution containing 5 μl of DNA template, 0.4 μl of KOD FX Neo (1 U/μl, Toyobo), 0.6 μl of each primer (10 pmol/μl each), 4 μl of 2 mM dNTPs (Toyobo), 5 μl of 2x PCR Buffer for KOD FX Neo (Toyobo) and 4.4 μl of distilled water. PCR cycling conditions were 3 min at 94 °C, followed by 42 cycles of 10 sec at 98 °C, 30 sec at 52-58 °C and 1 min at 68 °C, and a final extension at 68 °C for 30 sec. PCR products were visualized by electrophoresis on an agarose gel. The PCR products showing a single band were purified using a Sephacryl S-300 HR column (GE Healthcare, Chicago, IL, USA) and directly sequenced. If the PCR products showed multiple bands, the bands of the expected size were gel purified using Wizard SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) and directly sequenced. The sequencing was carried out in both directions using the amplifying primers with BigDye Terminator Cycle Sequencing Kit v.3.1 (Applied Biosystems, Foster City, CA, USA) and on an ABI 3130xl Genetic Analyzer (Applied Biosystems).
All obtained sequence data were deposited in DDBJ/EMBL/GenBank International Nucleotide Sequence databases under the accession numbers LC591988–LC592035. The 28S sequences of outgroup taxon (Hypoaspis miles Berlese; Laelapidae; accession number: KU318163) were obtained from previously published data (Vicente dos Santos and Tixier 2017). Obtained sequences were aligned using CLUSTAL W and the numbers of parsimony informative sites were calculated in MEGAX (Kumar et al. 2018). Mean intra- and interspecific genetic distances (p-distances; proportion (p) of nucleotide sites) were calculated by MEGAX. The maximum likelihood (ML) tree was constructed using the best-fit model (Kimura 2-Parameter model with gamma distribution) chosen by MEGAX. Branch robustness was tested by bootstrap analysis with 1,000 replications.
Results
Taxonomy
Neoseiulus longispinosus (Evans, 1952)
[Japanese name: Minami-kenaga-kaburidani]
(Figs. 1-4) (Table 2)
Description



Female (n=10)



Dorsum (Fig. 1A). Dorsal shield smooth, with few striae anterolaterally; with five pairs of solenostomes (gd2 , gd4 , gd6 , gd8 , gd9 ) and 13 pairs of poroids (id1 , idla , id2 , id4 , idm2 , idm3 , idm4 , idm5 , idm6 , isl, idl1 , idl3 , idl4 ); 328–347 long and 178–189 wide at s4 level; j1 17–21, j3 62–66, j4 63–65, j5 66–70, j6 74–77, J2 79–81, J5 7–9, z2 70–75, z4 69–76, z5 35–38, Z1 74–84, Z4 74–77, Z5 78–80, s4 69–83, S2 76–80, S4 56–59, S5 22–24, r3 64–69, R1 63–70. All setae very finely serrated, except setae j1, J5 and S5 short and smooth.
Venter (Fig. 1B). Sternal and genital shields striated, distance between setae st1–st3 59–64, st2–st2 58–62, st5–st5 49–53. Sternal shield with two pairs of poroids (iv1 , iv2 ). Ventrianal shield striated, 109–115 long, 90–99 wide at level ZV2 and 71–76 wide at the anus level; distance between setae JV2–JV2 46–58; one distinct pair of pores (gv3 ) posteromesad JV2, gv3–gv3 16–28; JV5 62–66.
Peritreme. Extending to the level between setae j1 and j3.
Chelicera (Fig. 1C). Movable cheliceral digit 27–30 long, with 2 teeth. Fixed cheliceral digit 25–28 long, with 4-5 teeth and a pilus dentilis.
Spermatheca (Fig. 1D). Calyx long, thin, flared distally, 19–25 long, 4–7 wide, constricted basally forming a short stalk at junction with a large semicircular atrium. Minor duct indistinct.
Legs. Leg IV with a long macroseta on basitarsus: StIV 78–83 (Fig. 1E). Chaetotactic formulae are shown in Table 3.



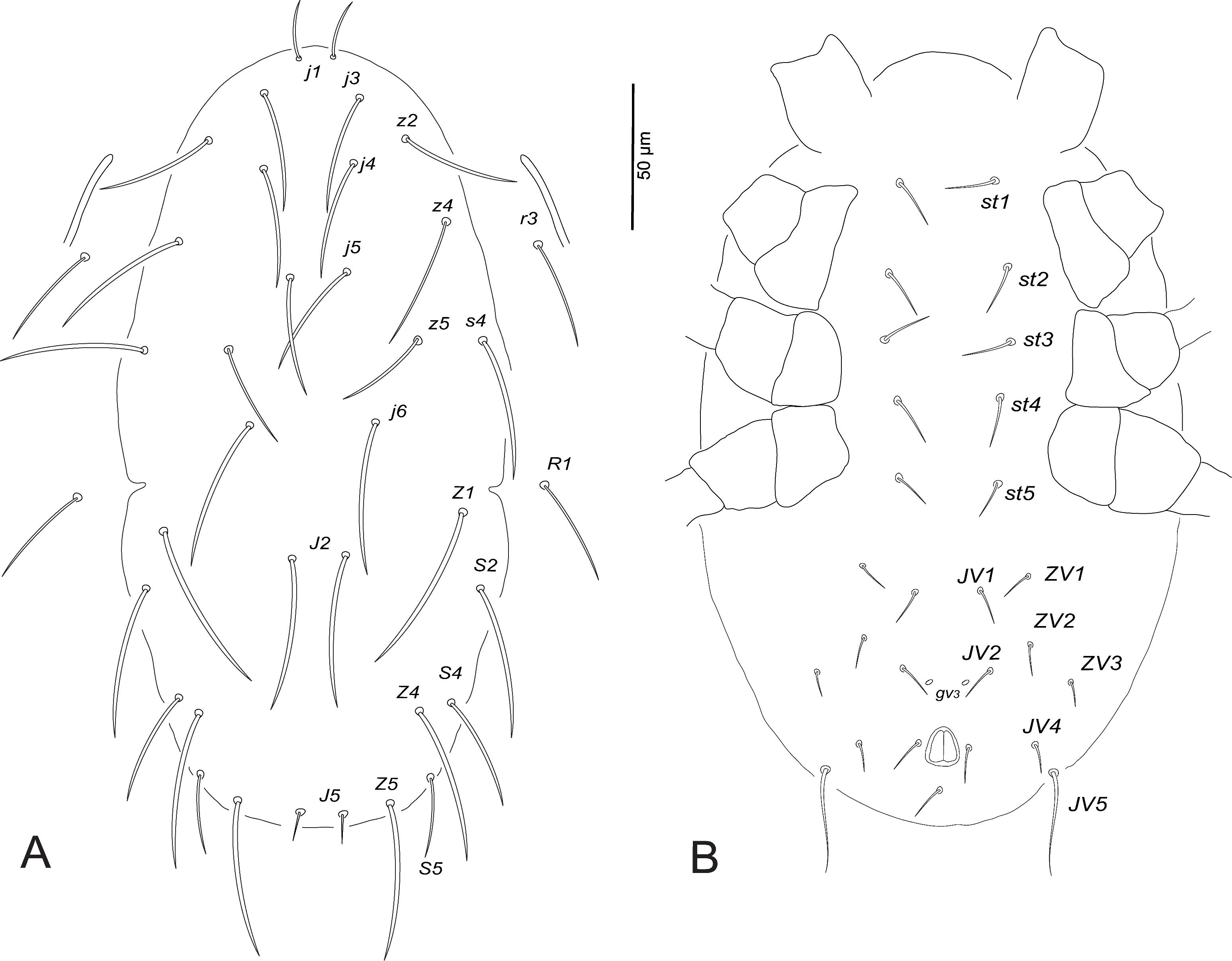
Male (n=7)
Dorsum. Dorsal shield smooth, with few striae anterolaterally; 253–261 long and 173–182 wide at s4 level; j1 14–16, j3 57–60, j4 49–55, j5 57–63, j6 63–65, J2 66–72, J5 5–6, z2 56–60, z4 60–64, z5 30–33, Z1 55–59, Z4 58–61, Z5 60–64, s4 64–70, S2 58–63, S4 36–40, S5 14–16, r3 44–57, R1 41–46.
Venter. Sternogenital shield striated, st1–st5 105–109, st2–st2 51–55. Ventrianal shield entirely striated, wider than long, 104–122 long and 143–149 wide at the anterior corners level; with three pairs of pre-anal setae, one distinct pair of pores (gv3 ) posteromesad JV2; JV5 50–59 (Fig. 1F).
Peritreme. Extending to level between setae z2 and z4.
Spermatodactyl (Fig. 1G). Almost T-shaped, shaft 20–22 (transverse part). Movable cheliceral digit 18–20 long, fixed digit 16–17 long.
Legs. Leg IV with long setaceous macroseta on basitarsus: StIV 67–70. Chaetotactic formulae are shown in Table 3.
Deutonymph (female) (n=7)
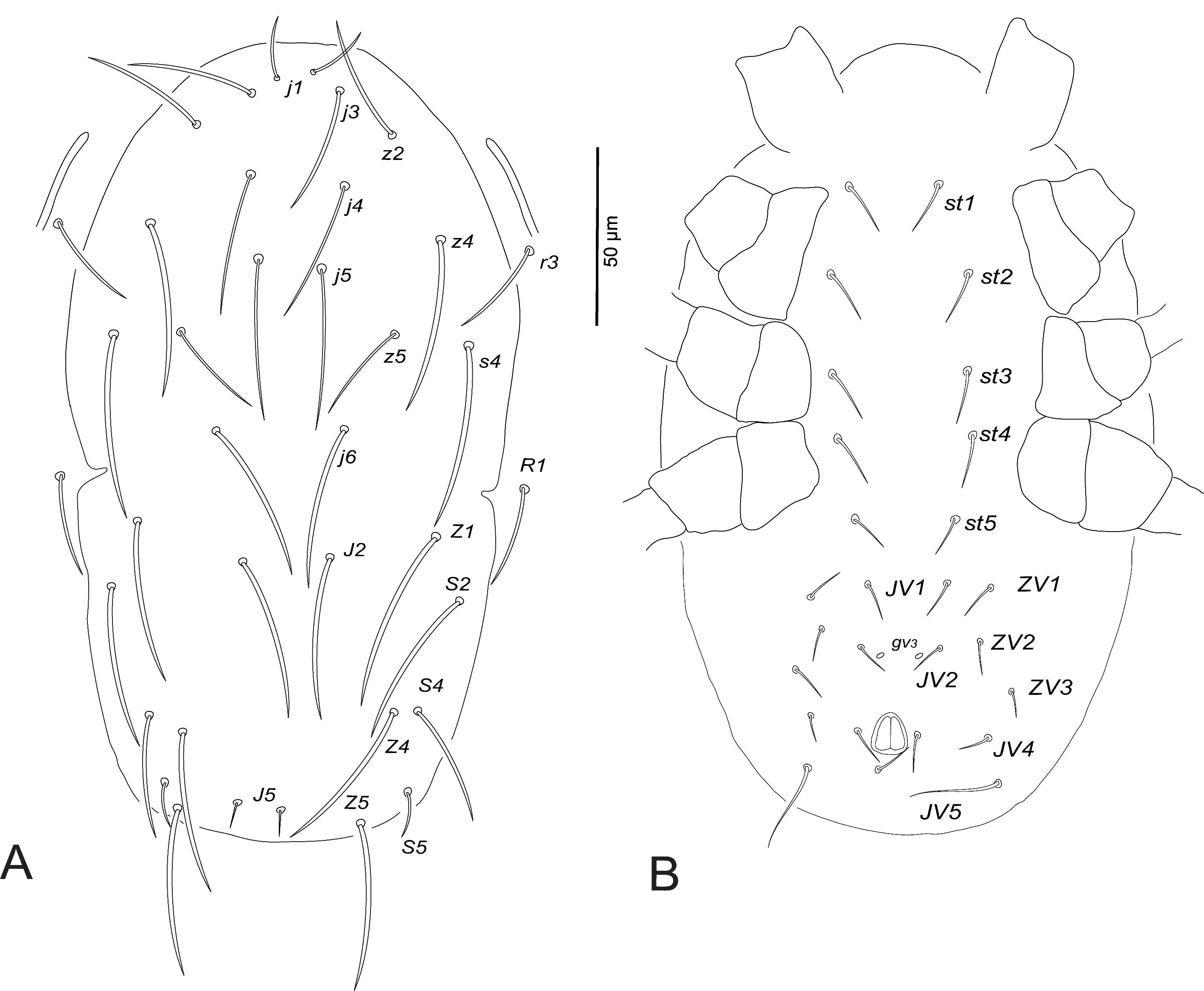


Dorsum (Fig. 2A). Dorsal shield faint and smooth; 260–270 long and 170–179 wide at s4 level; j1 15–17, j3 36–47, j4 40–51, j5 45–55, j6 57–63, J2 42–47, J5 7–8, z2 44–49, z4 49–61, z5 32–36, Z1 46–52, Z4 39–53, Z5 40–46, s4 55–65, S2 48–53, S4 34–43, S5 10–13, r3 30–36, R1 29–34.
Venter (Fig. 2B). Ventral shields indistinct, distance between setae st1–st3 68–71, st2–st2 50–56, st5–st5 35–37, st1–st5 120–126. Distances JV1–JV1 30–34, JV1–JV5 70–76; one distinct pair of pores (gv3 ) posteromesad JV2; JV5 24–27.
Peritreme. Extending to the level between setae z2 and z4, closer to z2.
Legs. Leg IV with one macroseta, StIV 65–70. Chaetotactic formulae are shown in Table 3.
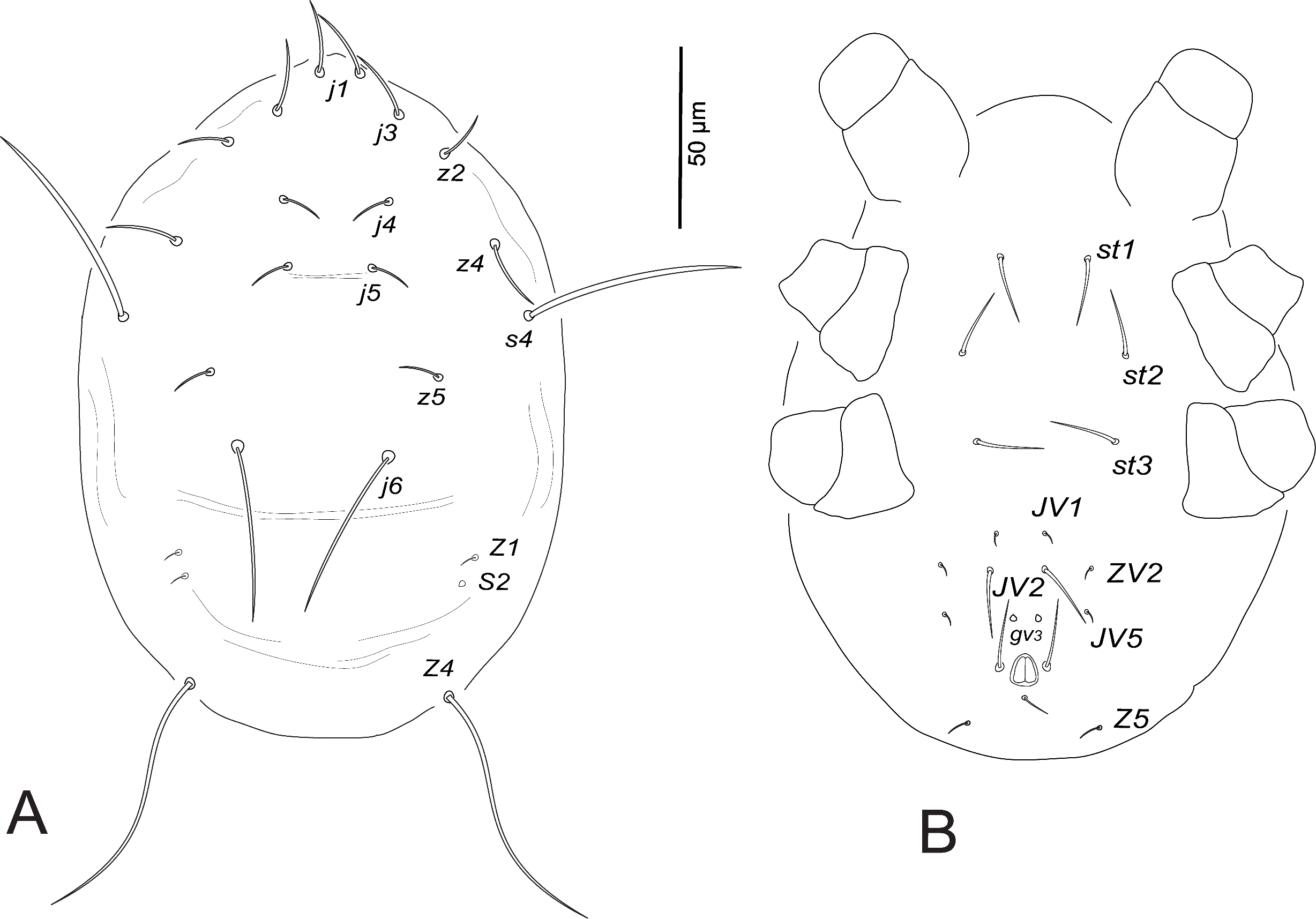
Protonymph (n=6)



Dorsum (Fig. 3A). Dorsal shield indistinct; 238–245 long and 162–171 wide at s4 level; j1 14–15, j3 33–43, j4 35–38, j5 38–40, j6 43–45, J2 31–37, J5 4, z2 40–42, z4 47–50, z5 25–28, Z1 27–34, Z4 33–37, Z5 33–40, s4 55–58, S2 33–36, S4 27–34, S5 8–10, r3 25–28, R1 23–29.
Venter (Fig. 3B). Sternal and genital shields indistinct, distance between setae st1–st3 65–67, st2–st2 51–55. Distances JV1–JV1 25–27, JV1–JV5 64–70; pores (gv3 ) posteromesad JV2; JV5 18–21.
Peritreme. Very short, extending to level between setae j6 and s4.
Legs. Leg IV with macroseta, StIV 61–67. Chaetotactic formulae are shown in Table 3.
Larva (n=7)


Dorsum (Fig. 4A). Dorsal shield indistinct; 186–191 long and 133–137 wide at s4 level; j1 18–23, j3 17–23, j4 10–13, j5 11–15, j6 53–57, z2 14–16, z4 17–23, z5 9–10, Z1 8–10, Z4 69–90 (whip-like), s4 54–61, S2 9–11.
Peritreme. Indistinct.
Venter (Fig. 4B). Distances between ventral setae st1–st3 62–66, st2–st2 54–57, JV1–JV1 19–22; pores (gv3 ) present and setae ZV1, ZV3, JV4 absent. Dorsal seta Z5 on posterior margin ventrally. Chaetotactic formulae of legs are shown in Table 3.
Material examined
10 females, 7 males, 7 deutonymphs, 6 protonymphs, 7 larvae (voucher specimen no. 900), Tarama, Okinawa, Japan (24˚40' N – 124˚41' E, T. Gotoh leg.), on Bidens sp. (Asteraceae); 10 females, 8 males, 10 deutonymphs, 8 protonymphs, 8 larvae (voucher specimen no. 906), Taitung, Taiwan (23˚02' N – 121˚09' E, T. Gotoh leg.), on Fragaria × ananassa Duchesne (Rosaceae); 10 females (voucher specimen no. 725), Bangkok, Thailand (13˚45' N – 100˚52' E, M. Kongchuensin leg.), on F. × ananassa (Table 1).
Neoseiulus womersleyi (Schicha)
[Japanese name: Kenaga-kaburidani]
(Figs. 5-8) (Table 4)
Description



Female (n=10)



Dorsum (Fig. 5A). Dorsal shield smooth, with few striae anterolaterally; with five pairs of solenostomes (gd2 , gd4 , gd6 , gd8 , gd9 ) and 13 pairs of poroids (id1 , idla , id2 , id4 , idm2 , idm3 , idm4 , idm5 , idm6 , isl, idl1 , idl3 , idl4 ); 331–359 long and 175–203 wide at s4 level; j1 16–19, j3 52–57, j4 47–57, j5 55–59, j6 67–70, J2 74–81, J5 8–10, z2 52–66, z4 64–73, z5 31–35, Z1 73–76, Z4 73–77, Z5 84–90, s4 73–76, S2 68–77, S4 62–66, S5 45–51, r3 47–56, R1 50–55. All setae very finely serrated, except setae j1 and J5 short and smooth.
Venter (Fig. 5B). Sternal and genital shields striated, distance between setae st1–st3 55–60, st2–st2 59–63, st5–st5 52–59. Sternal shield with two pairs of poroids (iv1 , iv2 ). Ventrianal shield striated, 99–121 long, 77–93 wide at level ZV2 and 71–75 wide at the anus level; distance between setae JV2–JV2 42–56; pores (gv3 ) posteromesad JV2, gv3–gv3 14–21; JV5 62–69.
Peritreme. Extending to bases of setae j3.
Chelicera (Fig. 5C). Movable cheliceral digit 24–29 long, with 2 teeth. Fixed cheliceral digit 22–27 long, with 4 teeth and a pilus dentilis.
Spermatheca (Fig. 5D). As in females of N. longispinosus. Calyx 12–21 long, 4–5 wide, slightly flared distally, constricted basally at junction with atrium. Atrium bulged and rounded, deeply forked at the junction with the major duct. Minor duct indistinct.
Legs. Leg IV with long, smooth, pointed macroseta on basitarsus: StIV 69–80 (Fig. 5E). Chaetotactic formulae are shown in Table 3.
Male (n=8)
Dorsum. Dorsal shield smooth, with few striae anterolaterally; 243–254 long and 162–170 wide at s4 level; j1 11–14, j3 33–36, j4 29–33, j5 31–33, j6 45–49, J2 47–51, J5 6–7, z2 37–40, z4 43–46, z5 22–26, Z1 45–48, Z4 49–54, Z5 56–63, s4 50–56, S2 45–48, S4 32–34, S5 22–26, r3 22–27, R1 24–29.
Venter. Sternogenital shield striated; st1–st5 98–101, st2–st2 50–54. Ventrianal shield entirely striated, wider than long, 102–131 long and 139–157 wide at the anterior corners level; with three pairs of pre-anal setae; pores (gv3 ) posteromesad JV2; JV5 41–51 (Fig. 5F).
Peritreme. Extending to level beyond setae z2.
Spermatodactyl (Fig. 5G). T-shaped, shaft 21–26 (transverse part). Movable cheliceral digit 16–19 long, fixed digit 17–19 long.
Legs. Leg IV with macroseta: StIV 57–63. Chaetotactic formulae are shown in Table 3.
Deutonymph (female) (n=7)


Dorsum (Fig. 6A). Dorsal shield smooth; 264–270 long and 145–151 wide at s4 level; j1 13–20, j3 42–46, j4 36–37, j5 42–45, j6 52, J2 47–50, J5 5, z2 41–44, z4 46–56, z5 28–30, Z1 47–59, Z4 48–54, Z5 54–58, s4 49–60, S2 50–54, S4 35–39, S5 22–27, r3 27–38, R1 31–38.
Venter (Fig. 6B). Ventral shields indistinct, distance between setae st1–st3 65–69, st2–st2 51–54, st5–st5 39–41, st1–st5 119–124. Distances JV1–JV1 32–34, JV1–JV5 75–80; pores (gv3 ) distinct, posteromesad JV2; JV5 35–38.
Peritreme. Extending to the same level of setae j4.
Legs. Leg IV with macroseta: StIV 62–68. Chaetotactic formulae are shown in Table 3.
Protonymph (n=7)
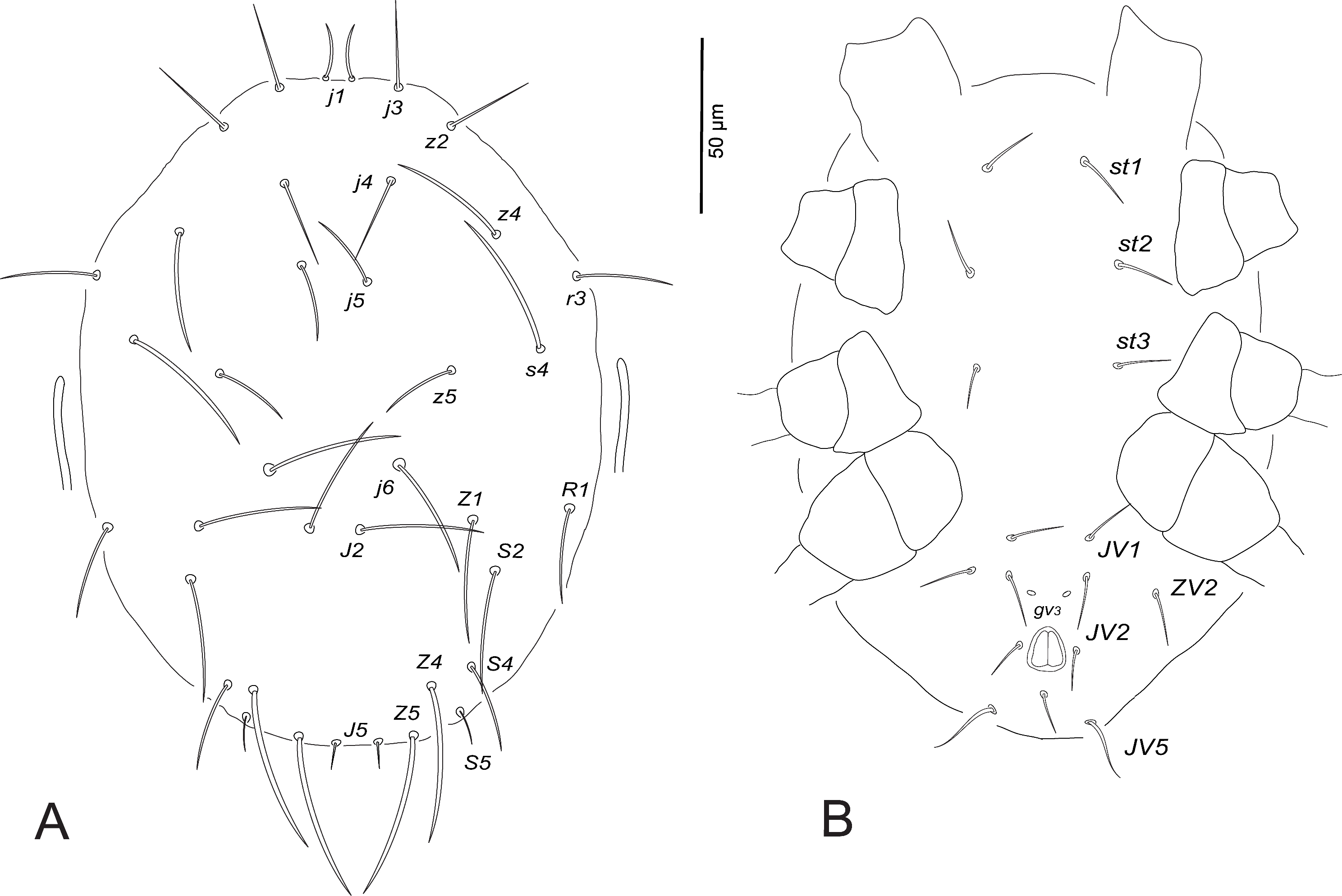


Dorsum (Fig. 7A). Dorsal shield indistinct; 189–210 long and 140–164 wide at s4 level; j1 14–15, j3 25–33, j4 25–31, j5 22–32, j6 32–39, J2 35–36, J5 5–6, z2 26–28, z4 35–37, z5 21–26, Z1 35–37, Z4 39–51, Z5 40–51, s4 43–53, S2 35–38, S4 22–26, S5 10–12, r3 18–28, R1 24–28.
Venter (Fig. 7B). Ventral shields indistinct, distance between setae st1–st3 60–64, st2–st2 48–52. Distances JV1–JV1 21–24, JV1–JV5 46–50; pores (gv3 ) distinct, posteromesad JV2; JV5 17–18.
Peritreme. Very short, extending to beyond the level of setae s4.
Legs. Leg IV with macroseta: StIV 60–65. Chaetotactic formulae are shown in Table 3.
Larva (n=10)
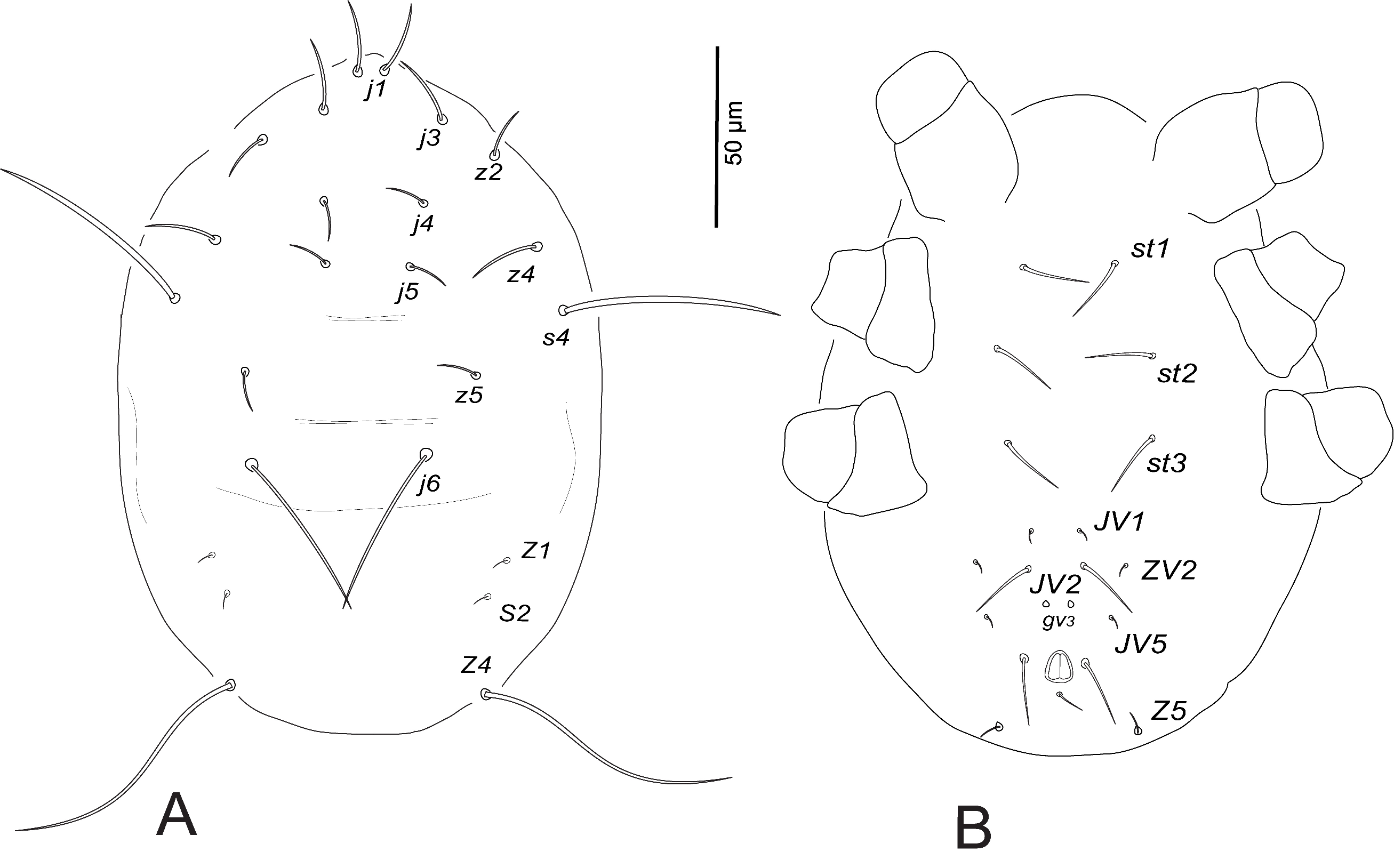


Dorsum (Fig. 8A). Dorsal shield indistinct; 170–177 long and 139–144 wide at s4 level; j1 17–19, j3 16–20, j4 10–14, j5 10–12, j6 48–54, z2 13–16, z4 16–18, z5 6–8, Z1 4–10, Z4 75–99 (whip-like), s4 57–60, S2 6–9.
Peritreme. Indistinct.
Venter (Fig. 8B). Distances between ventral setae st1–st3 63–67, st2–st2 56–58, JV1–JV1 20–23; pores (gv3 ) present and setae ZV1, ZV3, JV4 absent. Dorsal seta Z5 on posterior margin ventrally. Chaetotactic formulae of legs are shown in Table 3.
Material examined
10 females, 8 males, 7 deutonymphs, 7 protonymphs, 10 larvae (voucher specimen no. 907), Morioka, Iwate, Japan (39˚76' N – 141˚13' E, H. Kishimoto leg.), on Malus domestica Borkh (Rosaceae); 10 females, 10 males, 8 deutonymphs, 8 protonymphs, 8 larvae (voucher specimen no. 828), Taitung, Taiwan (23˚53' N – 121˚11' E, T. Gotoh leg.), on Ipomoea nil (L.) Roth (Convolvulaceae); 10 females (voucher specimen no. 908), Main Island, Okinawa, Japan (26˚10' N– 127˚43' E, T. Gotoh leg.), on Luffa sp. (Cucurbitaceae) (Table 1).
Molecular analyses
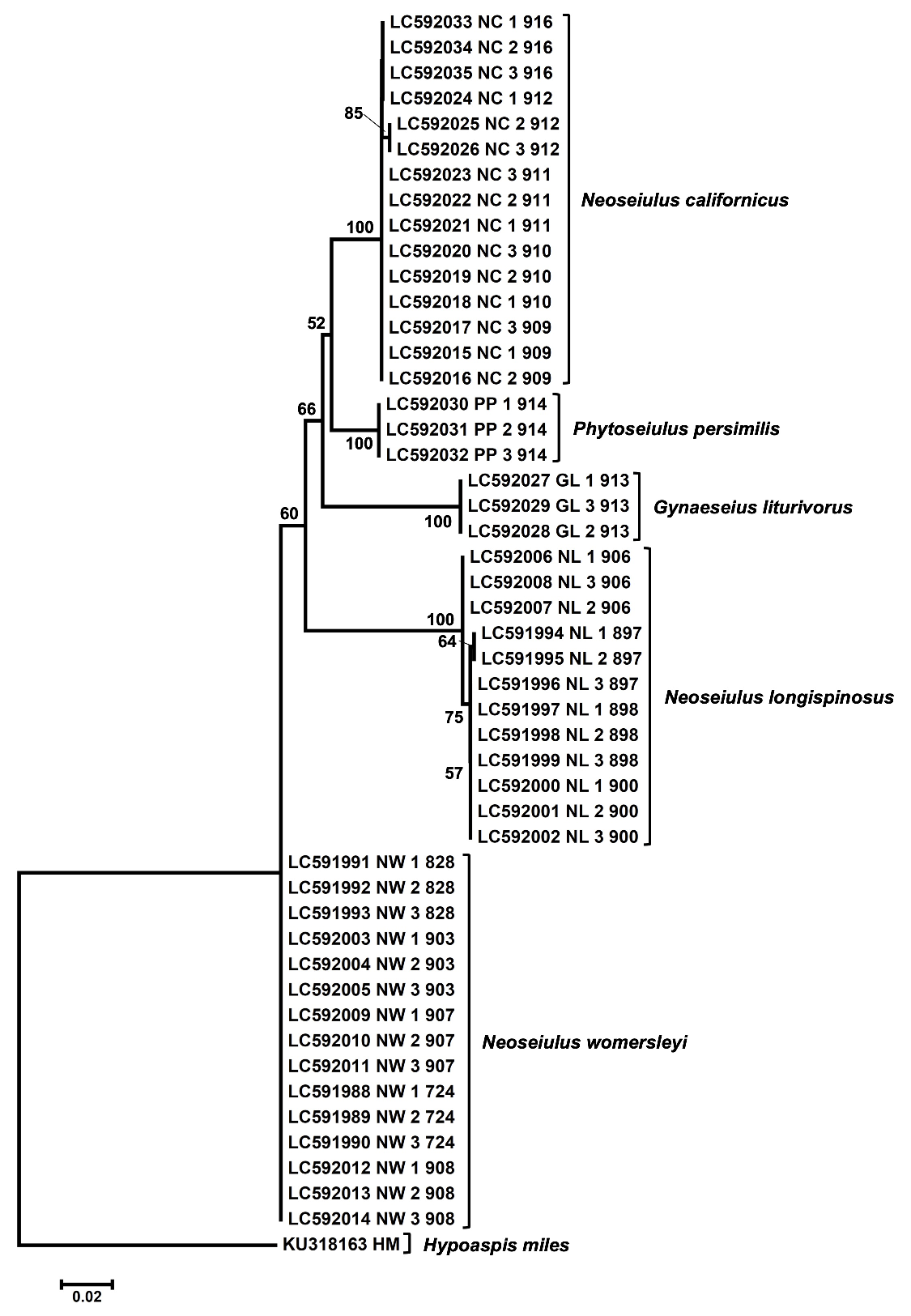


After alignment, the 28S fragment had 666 nucleotide sites, of which 93 were parsimony informative. Comparison of the mean genetic distances of the 28S region showed an interspecific divergence (3.32-9.33%) of phytoseiid mites (Table 5) and it was obviously higher than intraspecific divergence (0-0.17%). This result is consistent with our previous study of herbivorous spider mites (Tetranychidae, genus Oligonychus) that the genetic distances of the 28S region showed an intra- and interspecific divergence, 0-0.1% and 0.4-10.7%, respectively (Matsuda et al. 2012). A maximum likelihood tree of five species of phytoseiid mites based on the 28S nrDNA clearly separated the five species (Fig. 9). N. longispinosus and N. womersleyi can be distinguished from each other by the 28S region of nrDNA sequences as well as by morphological characters. In the ML tree, the genus Neoseiulus appeared to be polyphyletic.



Discussion
The present study provides morphological and molecular information to support the species identification of N. longispinosus and N. womersleyi. Because females of these species are morphologically close, there has been confusion in species delimitation (Xin et al. 1981; Collyer 1982; Mallik and ChannaBasavanna 1983). Xin et al. (1981) misidentified N. womersleyi specimens and described them as new species, namely N. pseudolongispinosus. Collyer (1982) noted that the length of seta S5 is highly variable and synonymized N. longispinosus with N. womersleyi. Some of Collyer's N. longispinosus specimens seemed to be N. womersleyi (Minor 2020). In the present study, the length of seta S5 and the ratio of setae Z5/S5 in adult females greatly helped to separate the examined strains. Setae S5 in N. longispinosus (20–26µm) are distinctly shorter than those of N. womersleyi (45–53µm); and setae Z5 are about three to four times longer than S5 in N. longispinosus (Z5/S5 = 3.03-4.25), but only around two times longer in N. womersleyi (Z5/S5 = 1.56-2.08; Tables 2 and 4). Furthermore, Schicha (1975) redescribed N. longispinosus from Australia and separated it from N. womersleyi based on the length of seta S5 and the distance between the two ventrianal pores (gv3). He also indicated that the distance gv3–gv3 was about twice as long in N. womersleyi as it was in N. longispinosus. However, we found this feature variable among individuals of the examined strains, considering it unreliable intra- and interspecific variation: gv3–gv3 was 16-28µm for N. longispinosus and 14-21µm for N. womersleyi.
The measurements of N. longispinosus adult females in the examined strains are relatively similar to those published in the previous works (Table 2); however, the length of seta S5 of the Korean specimens reported by Lee and Ryu (1989) (33.3-51.0µm) is questionable because it is noticeably longer than normal setal range. High similarity in S5 measurements was also observed between the three strains of N. longispinosus examined in the present study (20-26µm) and those previously described from Taiwan (17µm; Tseng 1983), India (18µm; Gupta 1986) and Thailand (18-25µm; Oliveira et al. 2012); however, the length of S5 is little shorter in the specimens described from Martinique (13-15µm; Kreiter et al. 2018), China (16.2µm; Xin et al. 1981) and the Dominican Republic (12, 15µm; Abo-Shnaf et al. 2016). Regarding N. womersleyi, the measurements of adult females collected in the present study were compared with those of previous studies (Table 4). To our knowledge, no descriptions have been done for N. womersleyi except for the main works of Womersley (1954), Schicha (1975), Ehara (1958), and most recently by Liao et al. (2020). Interestingly, Womersley (1954) redescribed N. longispinosus based on specimens collected from Australia, and although he noted that setae S5 and j1 are longer than that in the original description of N. longispinosus, he did not describe the Australian specimens as a new species but considered this variation as intraspecific. Conversely, Schicha (1975) emphasized the importance of this trait and described Womersley's specimens along with other collected materials as new species, namely N. womersleyi.
Recently, there has been more interest in studying the ontogeny of mite groups to address the morphological variations among immatures of different species (Zhang 2018). Several studies have also described the immature stages of phytoseiid mites (MacGill 1939, Evans 1953, Chant 1958, Prasad 1973, Xin et al. 1981, El-Banhawy and Abou-Awad 1985; Aponte and McMurtry 1987; Li et al. 2020; Ma et al. 2020a, b). As for the immature stages of the present specimens of N. longispinosus, the dorsal chaetotaxy of the larvae has 12 setae (Fig. 4A), whereas Mallik and ChannaBasavanna (1983) described the larva collected from India with only nine pairs of dorsal setae, apparently overlooking setal pairs z5, Z1 and S2. Also, the present specimens have eight pairs of ventral setae (excluding the para- and postanal setae) for the larva, but Mallik and ChannaBasavanna (1983) depicted only five pairs of ventral setae, missing the setae ZV2, JV5 and Z5. For the protonymphs, the dorsal setae appear likewise in the adults (with 19 pairs), while the ventral side still lacks some setae (ST4, ST5, ZV1, ZV3, JV4). Subsequently, the deutonymph gets complete dorsal and ventral setations just as in the adult stages. It is worth mentioning that the deutonymphal stages reflect the adult differences (lengths of setae S5 and Z5) in contrary to protonymphs, which have little variation in the length of setae Z5 (37-40µm for N. longispinosus and 40-45µm for N. womersleyi). Moreover, the chaetotaxy of legs is also similar in the developmental stages of N. longispinosus and N. womersleyi strains examined in the present study (Table 3), with little setal variations from the redescribed species, N. barkeri Hughes, N. californicus (McGregor) and N. kikuya Ma, Fan & Zhang (Li et al. 2020; Ma et al. 2020a, b).






Concerning the geographical distribution of N. longispinosus and N. womersleyi, Evans (1952) first described N. longispinosus from Indonesia, while Schicha (1975) described N. womersleyi from Australia. Since then, both predatory species have been found in several countries (Ho et al. 1995; Ehara 2002). It seems that N. longispinosus has broader distribution than N. womersleyi. N. longispinosus is distributed latitudinally around the globe between 30°N and 30°S. On the other hand, N. womersleyi is distributed longitudinally from Siberia, Russia, to Australia along the Pacific coast region of East and Southeast Asia and Oceania (Akimov and Kolodochka 1991; Ho et al. 1995, 2003; Lin 2000; Ehara 2002; Ehara and Amano 2004; Moraes et al. 2004; Ohno et al. 2012). However, some overlapping distributions exist between the two species (Fig. 10), which are correctly confirmed by literatures as well as specimens, considering that there may be some more misidentifications. For example, Akimov and Kolodochka (1991) mentioned that N. longispinosus is distributed in Siberia, but their drawing clearly shows that it is N. womersleyi due to having long S5 (Z5/S5 = 2.0). In Japan, N. longispinosus is limited to Tarama Isl. and Irabu Isl. (ca. 24°N) of Okinawa Prefecture (Ohno et al. 2012), whereas N. womersleyi has been reported from more than 35 out of 47 prefectures from Hokkaido (ca. 44°N) to Okinawa (ca. 24°N) (Toyoshima et al. 2013). Although Ehara (1958) at first mistakenly redescribed N. womersleyi as N. longispinosus based on specimens collected from Fukushima Prefecture (Honshu; 37-38°N), but he subsequently corrected the species identity to N. womersleyi (Ehara et al. 1994). In Thailand, Kongchuensin et al. (2005) showed how the distributions of N. longispinosus strains were related to their host plant species. The body color of adult females differs in the current examined strains (Fig. 11). For example, N. longispinosus is reddish in the Taiwanese and Thai strains regardless of food types, but whitish in the Okinawa strain. However, N. womersleyi is whitish in all studied strains.
Crossbreeding and reproductive interference experiments carried out by Ho et al. (1995) and Ullah et al. (2017), respectively, confirmed that N. longispinosus and N. womersleyi represent distinct species. A significant genetic divergence was also reported between N. longispinosus and N. womersleyi by using a random amplified polymorphic DNA (RAPD) analysis (Yeh et al. 2000). In the present study, the 28S nucleotide sequences showed that the two Neoseiulus species are different species as they formed separate clades. The ML tree also showed that the genus Neoseiulus appeared to be polyphyletic, which agrees with previous phylogenies based on the 12S rRNA genes of mtDNA (Tsolakis et al. 2012) and the ITS region of nrDNA (Inak et al. 2020). The current morphological and molecular analyses strengthen the taxonomic separation of the two species. Several case studies have used integrative taxonomy and employed the same methods to separate close species (Famah Sourassou et al. 2012; Arabuli et al. 2019). We conclude that a combination of different methods is required to distinguish and separate between closely related species.
Acknowledgements
We are very grateful to Drs. Hidenari Kishimoto (NARO, Morioka), Hiroshi Oida (Hosei University), M. S. Ullah (Bangladesh Agricultural University) and Toshiyuki Tezuka (Agrisect Inc.) for providing phytoseiid mites. We also thank Drs. Suguru Ohno (Okinawa Prefectural Agricultural Research Center) and Yasuki Kitashima (Ibaraki University) for helping in mite collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abo-Shnaf R.I.A., Sánchez L., Moraes G.J. de. 2016. Plant inhabiting Gamasina mites (Acari: Mesostigmata) from the Dominican Republic, with descriptions of four new species of Lasioseius (Blattisociidae) and complementary descriptions of other species. Syst. Appl. Acarol., 21: 607-646. doi:10.11158/saa.21.5.5
- Akimov I.A., Kolodochka L.A. 1991. Predatory Mites in Greenhouse. Kyiv: Naukova Dumka, 1-144. (in Russian)
- Aponte O., McMurtry J.A. 1987. Description of the immature and adult stages of Amblyseius colimensis n.sp. (Acari: Phytoseiidae) from Mexico. Acarologia, 28: 201-220.
- Arabuli T., Negm M.W., Matsuda T., Kitashima Y., Abramishvili T., Akimov I.A., et al. 2019. Morphological identification of Amphitetranychus species (Acari: Tetranychidae) with crossbreeding, esterase zymograms and DNA barcode data. PLoS ONE, 14(9):e0221951. doi:10.1371/journal.pone.0221951
- Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides Protoadeniques, Phytoseiidae). Acarologia, 27: 20-29.
- Chant D.A. 1958. Immature and adult stages of some British Phytoseiidae Berl., 1916 (Acarina). J. Linn. Soc. London, 43: 599-643. doi:10.1111/j.1096-3642.1958.tb01581.x
- Chant D.A., McMurtry J.A. 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae). Part I. Neoseiulini new tribe. Int. J. Acarol., 29: 3-46. doi:10.1080/01647950308684319
- Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, 219 pp.
- Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Int. J. Acarol., 17: 187-199. doi:10.1080/01647959108683906
- Collyer E. 1982. The Phytoseiidae of New Zealand (Acarina) 1. The genera Typhlodromus and Amblyseius - keys and new species. N. Z. J. Zool., 9: 185-206. doi:10.1080/03014223.1982.10423848
- Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2020. Phytoseiidae Database. Available from: http://www.lea.esalq.usp.br/phytoseiidae/index.php (accessed 31/08/2020)
- Dowling A.P.G., OConnor B.M. 2010. Phylogenetic relationships within the suborder Dermanyssina (Acari: Parasitiformes) and a test of dermanyssoid monophyly. Int. J. Acarol., 36: 299-312. doi:10.1080/01647951003604569
- Ehara S. 1958. Three predatory mites of the genus Typhlodromus from Japan (Phytoseiidae). Annot. Zool. Jap., 31: 53-57.
- Ehara S. 2002. Phytoseiid mites (Acari: Phytoseiidae) from Sumatra with description of a new species. Acta Arachnol., 51: 125-133. doi:10.2476/asjaa.51.125
- Ehara S., Amano H. 2004. Checklist and keys to Japanese Amblyseiinae (Acari: Gamasina: Phytoseiidae). J. Acarol. Soc. Jpn., 13: 1-30. doi:10.2300/acari.13.1
- Ehara S., Okada Y., Kato H. 1994. Contribution to the knowledge of the mite family Phytoseiidae in Japan (Acari: Gamasina). J. Fac. Educ. Tottori Univ., 42: 119-160.
- El-Banhawy E.M., Abou-Awad B.A. 1985. Comparative morphology of the immature stages of phytoseiid mites (Acari: Gamasina). Bull. Zool. Soc. Egypt, 35: 11-29.
- Evans G.O. 1952. A new typhlodromid mite predaceous on Tetranychus bimaculatus Harvey in Indonesia. Ann. Mag. Nat. Hist., 5: 413-416. doi:10.1080/00222935208654311
- Evans G.O. 1953. On some mites of the genus Typhlodromus Scheuten, 1857, from S. E. Asia. Ann. Mag. Nat. Hist., 6: 449-467. doi:10.1080/00222935308654444
- Famah Sourassou N., Hanna R., Zannou I., Breeuwer J.A.J., Moraes G. de, Sabelis M.W. 2012. Morphological, molecular and cross-breeding analysis of geographic populations of coconut-mite associated predatory mites identified as Neoseiulus baraki: evidence for cryptic species? Exp. Appl. Acarol., 57: 15-36. doi:10.1007/s10493-012-9534-0
- Gupta S.K. 1986. Fauna of India (Acari: Mesostigmata) Family Phytoseiidae. Zoological Survey of India, Calcutta, India, 350 pp.
- Ho C.C., Lo K.C., Chen W.H. 1995. Comparative biology, reproductive compatibility, and geographical distribution of Amblyseius longispinosus and A. womersleyi (Acari: Phytoseiidae). Environ. Entomol., 24: 601-607. doi:10.1093/ee/24.3.601
- Ho C.C., Shih H.T., Chen W.H. 2003. Eight phytoseiid mites from the Matsu Island. Plant. Prot. Bull., 45: 145-154. (in Chinese)
- Inak E., Cobanoglu S., Sade E., Tixier M.-S. 2020. Molecular characterization of phytoseiid mites in Turkey based on the internal trnscribed spacer (ITS) region, with a new record for the country. Exp. Appl. Acarol., 81: 201-213. doi:10.1007/s10493-020-00504-3
- Kongchuensin M., Charanasri V., Takafuji A. 2005. Geographic distribution of Neoseiulus longispinosus (Evans) and its habitat plants in Thailand. J. Acarol. Soc. Jpn., 14: 1-11. doi:10.2300/acari.14.1
- Kreiter S., Payet R.-M., Douin M., Fontaine O., Fillâtre J., Le Bellec F. 2020. Phytoseiidae of La Réunion Island (Acari: Mesostigmata): three new species and two males described, new synonymies, and new records. Acarologia, 60: 111-195. doi:10.24349/acarologia/20204361
- Kreiter S., Zriki Z., Ryckewaert P., Pancarte C., Douin M., Tixier M.-S. 2018. New phytoseiid mites of Martinique, with redescription of four species and new records. Acarologia, 58: 366-407. doi:10.24349/acarologia/20184248
- Kumar S., Stecher G., Li M., Knyaz C., Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol., 35:1547-1549. doi:10.1093/molbev/msy096
- Lee W.K., Ryu M.O. 1989. A taxonomic study on the phytoseiid mites (Acarina: Arachnida) in Korea. Korean J. Entomol., 19: 215-225.
- Li D.-D., Yi T.-C., Jin D.-C. 2020. Morphological changes in Neoseiulus californicus (Acari: Phytoseiidae). Zootaxa, 4857: 71-96. doi:10.11646/zootaxa.4857.1.5
- Liao, J.R., Ho, C.C., Lee, H.C. & Ko, C.C. 2020. Phytoseiidae of Taiwan. Acari: Mesostigmata. Taipei, Taiwan, National Taiwan University Press, 552 pp.
- Lin J.Z., Zhang Z.Q., Liu Q.Y., Ji J. 2000. Checklist of mites from moso bamboo in Fujian, China. Syst. Appl. Acarol., Special Publ., 4: 81-92. doi:10.11158/saasp.4.1.9
- Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can., 47: 1-64. doi:10.4039/entm9747fv
- Ma M., Fan Q.-H., Zhang Z.-Q. 2020a. Neoseiulus kikuyu sp. nov. (Mesostigmata: Phytoseiidae): descriptions of all life stages from New Zealand. Syst. Appl. Acarol., 25: 2098-2114. doi:10.11158/saa.25.11.13
- Ma M., Zhang B., Fan Q.-H., Zhang Z.-Q. 2020b. Ontogenetic changes in the morphology of Neoseiulus barkeri (Acari: Phytoseiidae). Zootaxa, 4900: 5-19. doi:10.11646/zootaxa.4900.1.4
- MacGill E. 1939. A gamasid mite (Typhlodromus thripsi n. sp.), a predator of Thrips tabaci Lind. Ann. Appl. Biol., 26: 309-317. doi:10.1111/j.1744-7348.1939.tb06973.x
- Mallik B., ChannaBasavanna G.P. 1983. Life history and life tables of Tetranychus ludeni and its predator Amblyseius longispinosus (Acari: Tetranychidae; Phytoseiidae). Indian J. Acarol., 8: 1-12.
- Matsuda T., Hinomoto N., Singh R.N., Gotoh T. 2012. Molecular-based identification and phylogeny of Oligonychus species (Acari: Tetranychidae). J. Econ. Entomol., 105: 1043-1050. doi:10.1603/EC11404
- McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Annu. Rev. Entomol., 42: 291-321. doi:10.1146/annurev.ento.42.1.291
- Minor M. 2020. Phytoseiidae of New Zealand (version 1.0, 2008). Available from: https://keys.lucidcentral.org/keys/v3/phytoseiidae/key/phytoseiidae/Media/Html/about.htm
 (accessed 10/10/2020)
(accessed 10/10/2020) - Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. doi:10.11646/zootaxa.434.1.1
- Nguyen V.H., Jonckheere W., Nguyen D.T., Moraes G.J. de, van Leeuwen T., Clercq P.D. 2019. Phytoseiid mites prey effectively on thrips eggs: evidence from predation trails and molecular analyses. Biol. Control, 137: 104012. doi:10.1016/j.biocontrol.2019.104012
- Ohno S., Gotoh T., Miyagi A., Ganaha-Kikumura T., Kurima M., Kijima K., Ooishi T. 2012. Geographic distribution of phytoseiid mite species (Acari: Phytoseiidae) on crops in Okinawa, a subtropical area of Japan. Entomol. Sci., 15: 115-120. doi:10.1111/j.1479-8298.2011.00469.x
- Okassa M., Tixier M.-S., Cheval B., Kreiter S. 2009. Molecular and morphological evidence for a new species status within the genus Euseius (Acari: Phytoseiidae). Can. J. Zool., 87: 689-698. doi:10.1139/Z09-057
- Okassa M.B.M., Ntabi D.M., Lenga A. 2020. Morphological and molecular identification of specimens in the genus Euseius (Acari: Phytoseiidae) from the Republic of Congo. Zootaxa, 4768: 479-498. doi:10.11646/zootaxa.4768.4.2
- Oliveira D.C., Charanasri V., Kongchuensin M., Konvipasruang P., Chandrapatya A., Moraes G.J. de. 2012. Phytoseiidae of Thailand (Acari: Mesostigmata), with a key for their identification. Zootaxa, 3453: 1-24. doi:10.11646/zootaxa.3453.1.1
- Prasad V. 1973. Description of life stages of the predatory mite Phytoseiulus macropilis (Banks) (Acarina: Phytoseiidae). Acarologia, 15: 391-399.
- Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. doi:10.4039/Ent110859-8
- Schicha E. 1975. A new predacious species of Amblyseius Berlese from strawberry in Australia, and A. longispinosus (Evans) redescribed (Acari: Phytoseiidae). J. Aust. Entomol. Soc., 14: 101-106. doi:10.1111/j.1440-6055.1975.tb02010.x
- Sonoda S., Kohara Y., Siqingerile, Toyoshima S., Kishimoto H., Hinomoto N. 2012. Phytoseiid mite species composition in Japanese peach orchards estimated using quantitative sequencing. Exp. Appl. Acarol. 56: 9-22. doi:10.1007/s10493-011-9485-x
- Toyoshima S., Kishimoto H., Amano H. 2013. Phytoseiid Mite Portal. Available from: http://phytoseiidae.acarologyjapan.org/
 (accessed 15/09/2020).
(accessed 15/09/2020). - Tseng Y.H. 1983. Further study on phytoseiid mites from Taiwan (Acarina: Mesostigmata). Chin. J. Entomol., 3: 33-74.
- Tsolakis H., Tixier M.-X., Kreiter S., Ragusa S. 2012. The concept of genus within the family Phytoseiidae (Acari: Parasitiformes): historical review and phylogenetic analyses of the genus Neoseiulus Hughes. Zool. J. Linn. Soc., 165: 253-273. doi:10.1111/j.1096-3642.2011.00809.x
- Ullah M.S., Sugimoto R., Matsuda T., Wang C.-H., Kongchuensin M., Konvipasruang P., Gotoh T. 2017. Interspecific interference in the closely related predatory mites Neoseiulus womersleyi and N. longispinosus (Acari: Phytoseiidae). Int. J. Acarol., 43: 296-301. doi:10.1080/01647954.2017.1293732
- Vicente dos Santos V., Tixier M.-S. 2017. Which molecular markers for assessing which taxonomic level? The case study of the mite family Phytoseiidae (Acari: Mesostigmata). Cladistics, 33: 251-267. doi:10.1111/cla.12166
- Vicente dos Santos V., Tixier M.-S. 2018. Integrative taxonomy approach for analysing evolutionary history of the tribe Euseiini Chant & McMurtry (Acari: Phytoseiidae). Syst. Biodiver., 16: 302-319. doi:10.1080/14772000.2017.1401562
- Womersley H. 1954. Species of the subfamily Phytoseiinae (Acarina: Laelaptidae) from Australia. Aust. J. Zool., 2: 169-191. doi:10.1071/ZO9540169
- Xin J.L., Liang L.R., Ke L.S. 1981. A new species of the genus Amblyseius from China (Acarina: Phytoseiidae). Int. J. Acarol., 7: 75-80.
- Yeh W.B., Ho C.L., Hui C.F., Ho C.C. 2000. Genetic diversity of Amblyseius longispinosus and A. womersleyi (Acari: Phytoseiidae) using RAPD analysis. Chin. J. Entomol., 20: 335-345. (in Chinese with English abstract)
- Zhang Z.-Q. 2018. Accelerating studies on the ontogeny and morphological diversity in immature mites. Zootaxa, 4540: 5-6. doi:10.11646/zootaxa.4540.1.3



2021-01-19
Date accepted:
2021-05-01
Date published:
2021-05-07
Edited by:
Tixier, Marie Stéphane

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Negm, Mohamed W.; Matsuda, Tomoko; Kayukawa, Takumi; Ho, Chyi-Chen; Hsu, Yu-Tzu; Kongchuensin, Manita; Konvipasruang, Ploychompoo and Gotoh, Tetsuo
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



