Immature development and survival of Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae) on eggs of Tyrophagus curvipenis (Fain & Fauvel) (Acari: Acaridae)
Li, Guang-Yun  1
; Pattison, Nick2
and Zhang, Zhi-Qiang
1
; Pattison, Nick2
and Zhang, Zhi-Qiang  3
3
1Key Laboratory of Entomology and Pest Control Engineering, College of Plant Protection, Southwest University, Chongqing 400716, China & Manaaki Whenua – Landcare Research, 231 Morrin Road, St John, Auckland, New Zealand.
2Ormiston Junior College, 285 Ormiston Rd, Flat Bush, Auckland, New Zealand.
3✉ Centre for Biodiversity and Biosecurity, School of Biological Sciences, University of Auckland, Auckland, New Zealand & Manaaki Whenua – Landcare Research, 231 Morrin Road, St John, Auckland, New Zealand.
2021 - Volume: 61 Issue: 1 pages: 84-93
https://doi.org/10.24349/acarologia/20214415Original research
Keywords
Abstract
Introduction
Phytoseiid mites (Acari: Phytoseiidae) are among the most important predatory species employed as biocontrol agents against agricultural pests such as phytophagous mites, thrips, and other small arthropods (van Lenteren 2012; Knapp et al. 2018; Rao et al. 2018). Many species in this family can be mass-reared with astigmatid mites (Acari: Astigmata) as factitious prey and are now commercially available (Gerson et al. 2003; Barbosa and Moraes 2015). Neoseiulus cucumeris (Oudemans) (Acari: Phytoseiidae), as one of the generalist predatory mites, is widely used (Jacobson et al. 2001; Zhang 2003; van Houten et al. 2005; McMurtry et al. 2013) ever since it was first used for biocontrol of thrips in 1985 (Knapp et al. 2018). It is well known worldwide for its biocontrol potential against a wide variety of pests such as mites, whiteflies, thrips, and psyllids of great agricultural and horticultural importance (Li & Zhang 2016, 2020; Kakkar et al. 2016; Li et al. 2017; Patel & Zhang 2017; Reitz et al. 2020).
The efficacy of biological control depends on many factors, including the characteristics of host plants and the availability of supplementary food. Biocontrol of thrips with N. cucumeris proved to be successful in sweet peppers: high densities of predators can be reached even in the absence of thrips because of pollen provided by the host plants (Ramakers 1988; 1990). However, by releasing predatory mites, some biological control programs were not successful when the pest population was small, or the plant could not offer the predators pollen as alternative food (Messelink et al. 2006; Skirvin et al. 2006). Therefore, in prophylactic control programs the key to successful biological control for the plants mentioned above is to provide the predators with supplementary food to maintain and enhance their population.
Some researchers suggested that dietary supplementary such as pollen, fungi (Skirvin et al. 2007), and the eggs of the Mediterranean flour moth Ephestia kuehniella Zeller (Lepidoptera: Phycitidae) (Delisle et al. 2015) should be provided to enhance the establishment and survival of predatory mites and to avoid cannibalism during the period of food scarcity (van Rijn et al. 2002; Nichols and Altieri 2004; Hoogerbrugge et al. 2008). Pollen is extensively studied as a dietary supplement for the natural enemy (e.g. Delisle et al. 2014; Liu & Zhang 2017; Pascua et al. 2020). However, many kinds of pollen from plants such as birch, hazel, and common cattail have been reported to have positive effects on the development and fecundity of secondary pests such as thrips (Van Rijn & Sabelis 1993; Hulshof et al. 2003; Skirvin et al. 2006). Although pollen can potentially reduce the efficiency of biological control, some studies, for example, Van Rijn (2002), have reported that Typha latifolia L. (Poales: Typhaceae) pollen is of benefit to the control of thrips even though it is edible for this pest, which contrasts with other kinds of pollens, which benefit thrips more than predators (Messelink et al. 2014). Therefore, the ideal alternative food for predatory mite should not only enhance the performance and boost the population of the predator but also not benefit the pest population. Although pollen, artificial diet, and moth eggs were feasible as a supplementary food source, exploring cost-effective alternative foods that can be commercially applied on a large scale in the field and greenhouses is a great challenge for biological control.
In this study, we explored a potential new alternative food, Tyrophagus curvipenis Fain & Fauvel (Sarcoptiformes: Acaridae), which is a common mite found on several plants in Australia, France, Portugal, and New Zealand (Fan and Zhang 2007). This species closely resembles Tyrophagus putriscentiae (Schrank) in morphology but differs in biology. It is more commonly found on living plants rather than in stored products and no obvious damage caused by this species to plants has been observed up to now (Ye and Zhang 2014). It was found by Zhang (2003) that the population of N. cucumeris increased with the presence of T. curvipenis. To evaluate the potential of T. curvipenis as a food source for N. cucumeris, an experiment was conducted to investigate whether the predatory mites can complete their development when fed on eggs of T. curvipenis at four prey densities. The following parameters in the immature stage were checked: survival rates, developmental time, predation rate and size at maturity. As it is well known that cannibalism is prevalent in generalist predatory mites when the food level is low, we further compared these responses with those of mites reared with conspecifics to understand how food levels and the presence of conspecifics influence the biology of predatory mites.
Material and methods
Mite culture
Neoseiulus cucumeris was obtained from Bioforce Ltd, New Zealand. The mite population was established in a petri dish with bran and T. putrescentiae. Dry yeast Saccharomyces cerevisiae was regularly added to this petri dish to provide enough food for T. putrescentiae.
Tyrophagus curvipenis was collected from pepper leaves in a greenhouse in St Johns, Auckland, New Zealand. A colony of T. curvipenis was established on a black plastic sheet (about 12 cm in diameter) with dry yeast, Saccharomyces cerevisiae (a common product used in the bakery, produced by Goodman Fielder Limited, New Zealand) sprinkled on it. This sheet was placed on water-saturated paper tissue in a Petri dish (15 cm in diameter) to avoid mites escaping.
Both these populations were put into boxes with a statured NaCl solution (common cook salt) to prevent the mites from escaping and maintaining a relatively suitable humidity (70 ± 10 % RH) for them.
Rearing units
The modified Munger cell (Ye and Zhang 2014) consisted of three plexiglass slides (3.8×2×0.3 cm) and a piece of black cloth. The middle slide, which was cone shaped (top diameter 10 mm and bottom diameter 7 mm), served as the cell, and the bottom slide was covered with a black cloth (to facilitate observation) and another plexiglass cone shape (to ensure the cell was well ventilated). The top slide was covered with a transparent glass fixed with two metal clips (Ye and Zhang 2014).
Collection of predator and prey eggs
The eggs of N. cucumeris were collected from the stock colony by providing to mites clean black cotton strings (about 1.5 cm in length) to facilitate oviposition. Next day, the black strings were checked, and the eggs produced within 24 h were collected using a small, fine hairbrush (size 00) and placed in a cell, individually or by pair.
The eggs of T. curvipenis were collected from the lab population regardless of their ages. These eggs were transferred to each cell with a series of densities of 20, 40, 60, 80 eggs per cell. Before being used to feed N. cucumeris, they were frozen at -18 °C for 24 hours to ensure they would not hatch during the experiments.
Effects of prey density and conspecific
To clarify the effects of food level (i.e. density of prey eggs) and the presence of conspecific (i.e. N. cucumeris of the same age) on the development and survival of N. cucumeris, a full factorial design was employed where we crossed prey density (20, 40, 60, 80 eggs per cell) with the presence of a conspecific (Yes/No), generating eight treatments. Fifteen rearing units (replicates) were set up for each treatment. The cells were checked at 24-hour intervals to monitor the survival and development of N. cucumeris. The exuvium was used as an indicator to confirm that N. cucumeris developed to the next stage. The number of eggs consumed was determined every day, with shrivelled and dried eggs recorded as already being consumed. During the experiment, the dead conspecific was not replaced. The experiment was terminated when the remaining predator became adult or eventually died.
Effect of prey density and conspecific on the body size
Mites that successfully developed into adults were mounted on microscope slides using Hoyer's medium (Krantz and Walter, 2009). After one week in the oven (50 °C), they were observed under a compound microscope at 1000× magnification and adult sex was determined. The length of the dorsal shield of predator mites was also measured as an indicator of body size. Mite specimens were vouchered in NZAC (Zhang 2018). All the experiments were conducted at a temperature of 25 ± 1 °C, a relative humidity of 80 ± 5 % RH, and a day/night cycle of 12:12 hours.
Statistical analyses
For statistical analyses, the parameters including developmental period and body size, which did not meet the assumption of normality, were subjected to Scheirer–Ray–Hare (SRH) test with prey density and the presence/absence of conspecific as factors. Non-parametric Mann-Whitney test was also conducted to explore the effects of sex on body size without considering prey density and the presence of conspecifics. The number of prey consumed per predator was analysed by a two-way ANOVA with prey density and the presence/absence of conspecific as factors. The percentage data, namely the hatching rate and the survival rate of the predator mite, were analysed with a Chi-square test. The correlation between prey density and the daily consumption of eggs was performed with the R package ''psych'' version 1.7.5 (Revelle 2017). These analyses were conducted at the significance level of 0.05. R 3.4.1 (R Core Team 2017) was employed to conduct the data analysis.
Results
Survival rates of the immature stages
The hatching rate of N. cucumeris was at least 93% (Table 1). Neither the prey density (χ2 =2.024, df =3, P=0.567) nor the presence of conspecifics (χ2 =0.451, df =1, P=0.501) significantly affected the hatching rates of N. cucumeris. While survivorship of protonymphs was not significantly affected by prey density (χ2 =1.703, df =3, P=0.636) and the presence of conspecifics (χ2 =0.000, df =1, P=1), a significant difference was shown in their interaction (χ2 =4.42, df =1, P=0.035). The survival rates of deutonymphs and adults dropped sharply because of food scarcity at the prey density of 20 and 40 per cell when compared with that of 60 and 80 prey per cell (χ2 =27.81, df =1, P\textless0.001 for deutonymphs; χ2 =36.67, df =1, P\textless0.001 for adults). Moreover, significantly lower survival rates were observed in deutonymphs and adults when predators were reared with conspecifics whatever the prey density (χ2 =5.31, df =1, P=0.021 for deutonymphs; χ2 =6.70, df =1, P=0.01 for adults).



Developmental periods of the immature stages
Neoseiulus cucumeris was able to complete its life cycle when it was offered enough T. curvipenis eggs. Approximately 6 days were needed to develop from egg to adult for the densities 60 and 80, for which it was possible to calculate this data (Table 2). At lower density, a too lower of deutonymphs survived. Prey density and conspecific presence (both P \textgreater0.05) did not significantly affect the developmental duration of larvae and protonymphs (for all prey densities). The duration of N. cucumeris deutonymphal stage was only compared at prey densities 60 and 80. The prey density significantly affected the deutonymphal stage, with the predatory mites reared at low prey density showing longer deutonymphal duration, with higher effects in presence of conspecifics (H=11.445, P=0.010; Table 2).



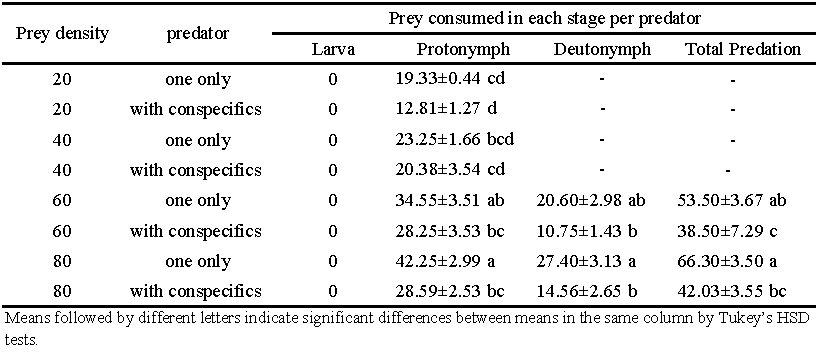
Predation rates of the immature stages
The consumption of N. cucumeris varied significantly according to the different life stages (Table 3). No predation was observed at larval stage. The number of T. curvipenis eggs consummed by predator during the protonymphal stage ranged from 12.81 at prey density of 20 with two predators to 42.25 at 80 with only one predator. At the protonymphal stage, both the prey density and the presence of conspecific affected the predation of N. cucumeris. At a same prey density, the predator with conspecifics ate significantly fewer eggs than the lone predator (F1, 68=13.46; P< 0.001). Significantly more eggs were consumed by the predator with increasing prey initial density (F3, 68=10.33; P\textless0.001). Deutonymphal predation was significantly affected by the presence of conspecifics (F1, 28=13.37; P=0.001), whereas, no influence was found among different prey densities (F1, 28=1.52; P=0.229). Significantly fewer prey were consumed by deutonymphs reared with conspecifics (F1, 28=25.02; P\textless0.001).


The total number of eggs consumed by predator generally increased with prey density, but showed an interaction effect with the presence of conspecific for males (Fig. 1). For both males and females, the predation rates increased significantly with prey density (R2 = 0.781, P = 0.02 for male, R2 = 0.771, P = 0.01 for female). In the presence of conspecifics, female predation rates were lower than without and no significant correlation between prey density and predation rates was observed (R2 = 0.40, P = 0.29 for female). On the contrary, the male predation rates were positively correlated with prey density (R2 = 0.98, P\textless0.001). When the male and female were pooled together regardless of the presence of a conspecific, there is a marginal effect of prey density on the number of prey consumed by the male (R2 =0.472, P=0.06 for male) but a significant effect on the number of prey consumed by the female (R2 =0.484, P=0.04).



Body size of N. cucumeris at maturity
Sexual size dimorphism was demonstrated in N. cucumeris: the body lengths of females were bigger than that of males regardless of prey densities and presence of conspecific (W=221.701, P\textless0.001; Fig. 2). No significant influence of the prey density (H=1.131, P=0.288) and the presence of conspecific on dorsal shield length was observed (H=0.084, P= 0.771) without considering sex.



Discussion
In mass production of natural enemies, selecting cost-effective food sources is of critical importance in reducing cost in production. More recently, it was also suggested that provisioning natural enemies with supplementary food improved the outcome of biocontrol through enhancing the survival and reproduction of predatory mites when prey is scarce or unavailable (Vacacela Ajila et al. 2019). In this study, we determined the immature biology of N. cucumeris when they were offered a potential food source, T. curvipenis, a mite species from the same family of the factitious prey T. putrescentiae. The results confirmed the usefulness of T. curvipenis as a food source for N. cucumeris.
Our results showed that N. cucumeris was able to complete its life cycle when fed on eggs of T. curvipenis, which was consistent with the previous report by Zhang et al. (2000) who showed that N. cucumeris can be reared on T. putrescentiae. By comparison, the N. cucumeris in our experiment developed almost as fast as those reared on cattail (Typha latifolia) pollen (Delisle et al. 2015; Nguyen et al. 2015), apple pollen (Delisle et al. 2014), and eggs of Tetranychus urticae Koch (Acari: Tetranychidae) (Li and Zhang, 2016), and much faster than those fed on the eggs of E. küehniella (Delisle et al. 2015) and artificial diet (Nguyen et al. 2015), suggesting that T. curvipenis as a suitable food source for N. cucumeris.
According to this study, higher mortality in N. cucumeris was observed when the predatory mites were reared together with their conspecifics. One reason is that when the predators encountered food scarcity, some of them died of starvation. Higher mortality can also be attributed to cannibalism, a common phenomenon in the animal kingdom where a species attacks, kills, and even eats its conspecifics. During this experiment, cannibalism occurred when deutonymph and protonymph attacked the protonymph and larvae, respectively. Cannibalism was reported in previous studies (Schausberger & Croft 2000; Schausberger 2003), that indicated that smaller N. cucumeris individuals such eggs, larvae, and protonymphs were often cannibalized by larger ones such as protonymphs, deutonymphs, and females. This only occurred when prey was scarce or absent, indicating that N. cucumeris could discriminate the eggs of T. curvipenis and immature of N. cucumeris, preferring the former.
Neoseiulus cucumeris larvae, as already documented, developped and survived without feeding (Zhang and Croft 1994). Reduced predation rates of N. cucumeris was witnessed when they were reared with conspecifics. A plausible explanation is that the competition between them reduced their consumption rates. In our previous study, immature N. cucumeris consumed about 107 to 150 T. urticae eggs in more than six days to develop into adults (Li and Zhang, 2016). In comparison, N. cucumeris intook only 39 to 65 T. curvipenis eggs per predator, about half of the spider mite eggs in the immature stage and developed a little bit faster. This indicates that the latter is a more optimal food source for this predatory mite.
Food availability is an important factor affecting both life-history characters (e.g. Mikolajewski et al. 2005; Carabio et al. 2017). Previous studies with other predators showed that food limitation affected body size at maturity and development of many species. Nevertheless, the response of these life-history traits varied greatly with species. For example, it was reported that prey (T. urticae egg) limitation did not influence the development of Amblyseius andersoni Chant (Acarina: Phytoseiidae) but accelerated that of Phytoseiulus persimilis Athias-Henriot (Acari: Phytoseiidae) and Neoseiulus californicus McGregor (Acari: Phytoseiidae) (Walzer and Schausberger 2011). For all these mite species, female body size at maturity increased significantly with the increasing density of prey (Walzer et al. 2004). However, in this study, we found that both development and body size were not significantly affected by prey density. This distinct response to food limitation may result from their difference in larval feeding plasticity. For N. cucumeris, no food is required for the development of larvae to protonymphs and the larvae do not feed even when food is provided (Zhang and Croft 1994; also in this study), the lower food requirement and high plasticity making this species more resilient to food stress than other species. Moreover, the predatory mites showed very short development time without any other cost such as body size, indicating the eggs of T. curvipenis are highly nutritious for them. This might mask the adverse effects of food shortage. Some limitations of this study such as the limited range of prey egg density and the long interval of observation might also contribute to the non-significant difference in development duration and body size of N. cucumeris.
In conclusion, the predatory mite N. cucumeris performed very well with T. curvipenis eggs as food, developing fast and resilient to food limitation. Compared with other suggested supplementary foods such as pollen, artificial diets, and Mediterranean flour moth eggs, T. curvipenis has some advantages over other alternative diets for N. cucumeris and other predators. N. cucumeris population could possibly establish readily as long as it was provided with T. curvipenis when the pest density was low at the very beginning of biological control or when pollen is rare on the host plants. Furthermore, T. curvipenis has not been observed to cause any damage to the plants (Ye & Zhang 2014). Thus, releasing T. curvipenis is cost-effective in terms of investment (Xie et al. 2018). However, this is the first study on this topic. Taking the complex factors in the field and greenhouses into account, further studies on other aspects of the interaction of this predator-prey interaction in field or semi-field experiments are needed to explore further whether T. curvipenis can be used optimally as an alternative food for biological agent N. cucumeris.
Acknowledgements
We thank Anne Austin (Manaaki Whenua – Landcare Research) and Kajal Patel (University of Auckland) for reviewing an early draft of this manuscript. We also appreciate the comments of two anonymous reviewers and the editor of Acarologia Dr Marie-Stéphane Tixier. Guang-Yun Li was funded by the joint PhD programme of the University of Auckland and China Scholarship Council. Nick Pattison was supported by the Royal Society Teacher fellowship. Zhi-Qiang Zhang was supported in part by New Zealand Government core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment's Science and Innovation Group.
Declaration of interest statement
No potential conflict of interest was reported by the authors.
References
Barbosa M.F.C., de Moraes G.J. 2015. Evaluation of astigmatid mites as factitious food for rearing four predaceous phytoseiid mites (Acari: Astigmatina; Phytoseiidae). Biol. Control., 91: 22–26. doi:10.1016/j.biocontrol.2015.06.010
Carabio M., Perazza G., Larrañaga F., Naya D.E. 2017. The effect of food availability on phenotypic plasticity and phenotypic integration in the hylid frog Hypsiboas pulchellus. Evol. Ecol. Res., 18(3): 281–291.
Delisle J.F., Brodeur J., Shipp L. 2015. Evaluation of various types of supplemental food for two species of predatory mites, Amblyseius swirskii and Neoseiulus cucumeris (Acari: Phytoseiidae). Exp. Appl. Acarol., 65(4): 483–494. doi:10.1007/s10493-014-9862-3
Delisle J.F., Shipp L., Brodeur J. 2014. Apple pollen as a supplemental food source for the control of western flower thrips by two predatory mites, Amblyseius swirskii and Neoseiulus cucumeris (Acari: Phytoseiidae), on potted chrysanthemum. Exp. Appl. Acarol., 65(4): 495–509. doi:10.1007/s10493-014-9863-2
Fan Q.H., Zhang Z.Q. 2007. Tyrophagus (Acari: Astigmata: Acaridae); Fauna of New Zealand No. 56, Manaaki Whenua Press, Lincoln, Christchurch, New Zealand, pp. 291
Gerson U., Smiley R.L. & Ochoa R. 2003. Mites (acari) for pest control. 2nd edition. Blackwell Publishing Ltd., Oxford, UK, pp. 539. doi:10.1002/9780470750995
Hoogerbrugge H., van Houten Y., van Baal E., Bolckmans K. 2008. Alternative food sources to enable establishment of Amblyseius swirskii (Athias-Henriot) on chrysanthemum without pest presence. IOBC WPRS Bulletin 32: 79–82.
Hulshof J., Ketoja E., Vänninen I. 2003. Life history characteristics of Frankliniella occidentalis on cucumber leaves with and without supplemental food. Entomol. Exp. Appl., 108(1): 19–32. doi:10.1046/j.1570-7458.2003.00061.x
Jacobson R.J., Croft P., Fenlon J. 2001. Suppressing establishment of Frankliniella occidentalis Pergande (Thysanoptera: Thripidae) in cucumber crops by prophylactic release of Amblyseius cucumeris Oudemans (Acarina: Phytoseiidae). Biocontrol. Sci. Technol., 11(1): 27–34. doi:10.1080/09583150020029718
Kakkar G., Kumar V., Seal D.R., Liburd O.E., Stansly P.A. 2016. Predation by Neoseiulus cucumeris and Amblyseius swirskii on Thrips palmi and Frankliniella schultzei on cucumber. Biol. Control., 92: 85–91. doi:10.1016/j.biocontrol.2015.10.004
Knapp M., Van Houten Y., Van Baal E., Groot T. 2018. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia 58(Suppl), 72–82.
Krantz G.W., Walter D.E. 2009. A manual of acarology. Texas Tech University Press, Texas, 3rd ed. pp. 807.
Li G.Y., Zhang Z.Q. 2016. Some factors affecting the development, survival and prey consumption of Neoseiulus cucumeris (Acari: Phytoseiidae) feeding on Tetranychus urticae eggs (Acari: Tetranychidae). Syst. Appl. Acarol., 21(5): 555–566. doi:10.11158/saa.21.5.1
Li G.Y., Zhang Z.Q. 2020. Can supplementary food (pollen) modulate the functional response of a generalist predatory mite (Neoseiulus cucumeris) to its prey (Tetranychus urticae)? BioControl, 65:165–174. doi:10.1007/s10526-019-09993-7
Li M., Yang N., Wan F., Liu L., Chen Y., Li J., Fu J. 2017. Functional response of Neoseiulus cucumeris (Oudemans)(Acari: Phytoseiidae) to Bemisia tabaci (Gennadius) on tomato leaves. Biocontrol. Sci. Techn., 27(5): 677–685. doi:10.1080/09583157.2017.1328484
Liu J.F., Zhang Z.Q. 2017. Development, survival and reproduction of a New Zealand strain of Amblydromalus limonicus (Acari: Phytoseiidae) on Typha orientalis pollen, Ephestia kuehniella eggs, and an artificial diet. Int. J. Acarology., 43(2):153–159. doi:10.1080/01647954.2016.1273972
McMurtry J.A., De Moraes G.J., Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297–320. doi:10.11158/saa.18.4.1
Messelink G.J., Bennison J., Alomar O., Ingegno B.L., Tavella L., Shipp L., Palevsky E., Wäckers F.L. 2014. Approaches to conserving natural enemy populations in greenhouse crops: current methods and future prospects. Biol. Control., 59(4): 377–393. doi:10.1007/s10526-014-9579-6
Messelink G.J., Van Steenpaal S.E., Ramakers P.M. 2006. Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. Biol. Control., 51(6): 753–768. doi:10.1007/s10526-006-9013-9
Mikolajewski D.J., Brodin T., Johansson F., Joop G. 2005. Phenotypic plasticity in gender specific life-history: effects of food availability and predation. Oikos, 110(1): 91–100. doi:10.1111/j.0030-1299.2005.13766.x
Nguyen D.T., Vangansbeke D., De Clercq P. 2015. Performance of four species of phytoseiid mites on artificial and natural diets. Biol. Control., 80: 56–62. doi:10.1016/j.biocontrol.2014.09.016
Nichols C.I., Altieri M.A. 2004. The agroecological engineering: for pest management. In: Gurr G, Wratten S, Tieri M (editors) Ecological engineering for pest management. CSIRO Publishing, Collingwood. pp. 33–54. doi:10.1079/9780851999036.0033
Pascua M.S., Rocca M., Greco N., De Clercq P. 2020. Typha angustifolia L. pollen as an alternative food for the predatory mite Neoseiulus californicus (McGregor)(Acari: Phytoseiidae). Syst. Appl. Acarol., 25(1): 51–62. doi:10.11158/saa.25.1.4
Patel K., Zhang Z.Q. 2017. Functional and numerical responses of Amblydromalus limonicus and Neoseiulus cucumeris to eggs and first instar nymph of tomato/potato psyllid (Bactericera cockerrelli). Syst. Appl. Acarol., 22: 1476–88. doi:10.11158/saa.22.9.12
Ramakers P.M.J. 1990. Manipulation of phytoseiid thrips predators in the absence of thrips. IOBC/WPRS Bulletin, 13(5): 169–172.
Ramakers P.M.J. 1988. Population dynamics of the thrips predators Amblyseius mckenziei and Amblyseius cucumeris (Acarina: Phytoseiidae) on sweet pepper. Neth. J. Agri. Sci., 36: 247–252. doi:10.18174/njas.v36i3.16676
Rao K.S., Padmanabhan A., Vishnupriya R. 2018. Recent update on the role of predatory mites in biological control programmes. Res. J. Agric. Sci., 9(3): 473–479.
R Core Team 2017. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
 .
.Reitz S.R., Gao Y., Kirk W.D., Hoddl M.S., Leiss K.A., Funderburk J.E. 2020. Invasion biology, ecology, and management of western flower thrips. Annu. Rev. Entomol., 65: 17–37. doi:10.1146/annurev-ento-011019-024947
Revelle W.R. 2016. psych: Procedures for personality and psychological research. R package v1.6.4. URL: http://CRAN.R-project.org/package=psych
 (accessed 2 May 2018)
(accessed 2 May 2018)Schausberger P. 2003. Cannibalism among phytoseiid mites: a review. Exp. Appl. Acarol., 29(3–4): 173–191. doi:10.1023/A:1025839206394
Schausberger P., Croft B.A. 2000. Cannibalism and intraguild predation among phytoseiid mites: Are aggressiveness and prey preference related to diet specialization? Exp. Appl. Acarol., 24(9): 709–725.
Skirvin D., Kravar-Garde L., Reynolds K., Jones J., De Courcy Williams M. 2006. The influence of pollen on combining predators to control Frankliniella occidentalis in ornamental chrysanthemum crops. Biocontrol. Sci. Techn., 16(1): 99–105. doi:10.1080/09583150500258636
Skirvin D.J., Kravar-Garde L., Reynolds K., Jones J., Mead A., Fenlon J. 2007. Supplemental food affects thrips predation and movement of Orius laevigatus (Hemiptera: Anthocoridae) and Neoseiulus cucumeris (Acari: Phytoseiidae). Bull. Entomol. Res., 97(03): 309–315. doi:10.1017/S0007485307005007
Vacacela Ajila H.E., Colares F., Lemos F., Marques P.H., Franklin E.C., Santos do Vale W., Oliveira E.E., Venzon M., Pallini A. 2019. Supplementary food for Neoseiulus californicus boosts biological control of Tetranychus urticae on strawberry. Pest. Manag. Sci., 75(7): 1986–92. doi:10.1002/ps.5312
van Houten Y.M., Ostilie, M.L., Hoogerbrugge H., Blockmans K. 2005. Biological control of western flower thrips on sweet pepper using the predatory mites Amblyseius cucumeris, Iphiseius degenerans, A. andersoni and A. swirskii. IOBC/WPRS Bulletin, 28: 283–286
van Lenteren J.C. 2012. The state of commercial augmentative biological control: plenty of natural enemies, but a frustrating lack of uptake. Biol. Control., 57(1): 1–20. doi:10.1007/s10526-011-9395-1
van Rijn P.C., van Houten Y.M., Sabelis M.W. 2002. How plants benefit from providing food to predators even when it is also edible to herbivores. Ecology, 83(10): 2664–2679. doi:10.1890/0012-9658(2002)083[2664:HPBFPF]2.0.CO;2
van Rijn, P.C.J., Sabelis M.W. 1993. Does alternative food always enhance biological control? The effect of pollen on the interaction between western flower thrips and its predators Amblyseius cucumeris. Bulletin OILB SROP (France), 16: 123–125
Walzer A., Paulus H., Schausberger P. 2004. Ontogenetic shifts in intraguild predation on thrips by phytoseiid mites: the relevance of body size and diet specialization. Bull. Entomol. Res., 94(6): 577–584. doi:10.1079/BER2004329
Walzer A., Schausberger P. 2011. Sex-specific developmental plasticity of generalist and specialist predatory mites (Acari: Phytoseiidae) in response to food stress. Biol. J. Linn. Soc., 102(3): 650-660. doi:10.1111/j.1095-8312.2010.01593.x
Xie L., Yan Y., Zhang Z.Q. 2018. Development, survival and reproduction of Stratiolaelaps scimitus (Acari: Laelapidae) on four diets. Syst. Appl. Acarol., 23(4): 779–794. doi:10.11158/saa.23.4.16
Ye S.S., Zhang Z.Q. 2014. Age and size at maturity in Tyrophagus curvipenis (Acari: Acaridae) when fed on three different diets. Syst. Appl. Acarol., 19(4): 506–512. doi:10.11158/saa.19.4.14
Zhang Y., Zhang Z.Q., Lin J., Ji J. 2000. Potential of Amblyseius cucumeris (Acari: Phytoseiidae) as a biocontrol agent against Schizotetranychus nanjingensis (Acari: Tetranychidae) in Fujian, China. Syst. Appl. Acarol. Special Publ., 4: 109–124. doi:10.11158/saasp.4.1.11
Zhang Z.Q., Croft B.A. 1994. A comparative life history study of immature Amblyseius fallacis, Amblyseius andersoni, Typhlodromus occidentalis and Typhlodromus pyri (Acari: Phytoseiidae) with a review of larval feeding patterns in the family. Exp. Appl. Acarol., 18(11–12): 631–657.
Zhang Z.Q. 2003. Mites of greenhouses: identification, biology and control. CABI Publishing, Wallingford, UK. pp.187. doi:10.1079/9780851995908.0000
Zhang Z.Q. 2018. Repositories for mite and tick specimens: acronyms and their nomenclature. Syst. Appl. Acarol., 23(12): 2432–2446. doi:10.11158/saa.23.12.12



2020-10-08
Date accepted:
2021-01-07
Date published:
2021-01-22
Edited by:
Tixier, Marie Stephane

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Li, Guang-Yun; Pattison, Nick and Zhang, Zhi-Qiang
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



