Mite species (Acari) on blackberry cultivars in organic and conventional farms in Florida and Georgia, USA
Akyazı, Rana1 ; Welbourn, Cal2 and Liburd, Oscar E.3
1✉ Department of Plant Protection, Ordu University, Ordu, Turkey & Entomology and Nematology Department, University of Florida, Gainesville, USA.
2Division of Plant Industry, FSCA, Gainesville, USA.
3Entomology and Nematology Department, University of Florida, Gainesville, USA.
2021 - Volume: 61 Issue: 1 pages: 31-45
https://doi.org/10.24349/acarologia/20214414Original research
Keywords
Abstract
\subsubsubsection*
Part of this research was presented as a poster at the 19th International Conference on Entomology ICE 2017 (19-20 October 2017 Paris, France) and published as an abstract
Introduction
Blackberries (Rubus spp. (Rosaceae)) are a major small fruit crop that is grown throughout Europe and the United States (Strik 2007). Mexico is the leading producer of blackberries worldwide. In the US, the crop is valued at 50 million USD (USDA-NASS 2016) with 90% of its production in the northwest region (Oregon). Production is expanding in the southern United States (Arkansas to Florida) and several new cultivars are being developed that do not require the heat units for satisfactory production that is required of traditional cultivars.
Several mite species have been found in association with blackberry plants (Vincent et al. 2010) and most of these mites are agricultural pests that are known to cause economic damage. The broad mite, Polyphagotarsonemus latus (Banks) is one of two species in the family Tarsonemidae that cause extensive damage to crop plants (Liburd et al. 2020). Polyphagotarsonemus latus has been found in association with leaf-curling symptoms on primocane-fruiting blackberry (Rubus L. subgenus Rubus Watson) in Arkansas (Vincent et al. 2010), which negatively affect plant growth and development.
Davies et al. (2001) examined microhabitats and aggregation patterns of the phytophagous mite, Acalitus essigi (Hassan) (Trombidiformes: Eriophyidae), on Rubus fruticosus L. (Rosaceae). They found that A. essigi was a refugee inhabiting species that resided in the buds and leaf axils on primocanes and fructocanes. Scott et al. (2008) collected the same species of eriophyid mites from the fruits of three species of the weedy blackberry (R. anglocandicans A. Newton, R. laudatus A. Berger and R. ulmifolius Schott) in south-west Australia. This was the first record for this species in Western Australia and these plants appear to be new host records for A. essigi. Similarly, Cetin et al. (2010) recorded A. essigi as a new pest in blackberry plantings in the Marmara region of Turkey in 2009.
Marchetti and Ferla (2011) determined the diversity and population fluctuation of mites on blackberry (R. fruticosus) in Rio Grande do Sul, Brazil. A total of 26 mite species belonging to 12 families were found. Most of the mites were phytophagous species belonging to the families Diptilomiopidae (80.9%) and Tetranychidae (13.9%). The most common families of predaceous mites were Stigmaeidae (2.1%) and Phytoseiidae (0.4%). Recently, Trinidad et al. (2019) evaluated the occurrence of phytophagous and predatory mites in different blackberry genotypes in the municipality of Pelotas, RS, Brazil. They recorded a total of 12 mite species belonging to the families Tetranychidae, Diptilomiopidae, Eriophyidae, Tarsonemidae, Tenuipalpidae, Stigmaeidae, Tydeidae, Phytoseiidae. The families Tydeidae, Diptilomiopidae, Tetranychidae, and Eriophyidae showed a higher representability.
Ozsisli and Cobanoglu (2019) determined predatory and harmful mite species on R. fruticosus, and Rubus sanctus Schreber in Turkey (Adana, Hatay, Kahramanmaraş provinces). Phytoseius finitimus Ribaga (Phytoseiidae), Pronematus ubiquitus (McGregor), Tydeus californicus (Banks) (Tydeidae), and Zetzelia mali (Ewing) (Stigmaeidae) were predatory mite species obtained in the study. The phytophagous mites were Cenopalpus pulcher (Canestrini & Fanzago), C. spinosus (Donnadieu) (Tenuipalpidae) and Tetranychus urticae Koch (Tetranychidae).
In a recent study, Akyazi and Liburd (2019) assessed the effectiveness of a commercially available predator, Neoseiulus californicus (McGregor) to reduce T. urticae populations in a commercial blackberry planting and recommended that an assessment of local phytoseiids and other predators should be considered before further releases of N. californicus are considered.
To our knowledge a survey of mite fauna associated with blackberries have not been conducted in Florida and Georgia despite an expanding blackberry industry in the region. This study was carried out to determine phytophagous and predatory mite species on nine commercially available blackberry cultivars in Florida and Georgia, USA in 2016.
Material and Methods
Sampled Areas
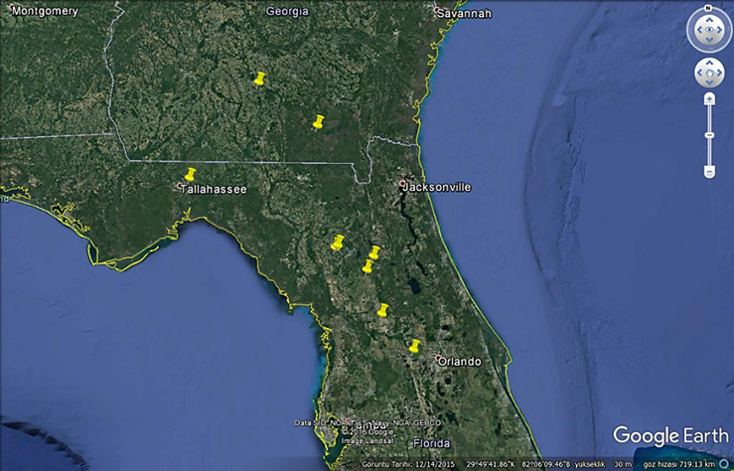
The survey was conducted in selected organic and conventional commercial blackberry farms in Florida and Georgia, USA (Figure 1). Geographical coordinates were recorded using a GPS mobile device.


Sampled Blackberry Cultivars
This study was carried out on nine blackberry cultivars including'Arapaho','Choctaw','Freedom','Kiowa','Natchez','Navaho','Osage','Ouachita' and'Von' (Table 1).



Sampling Method
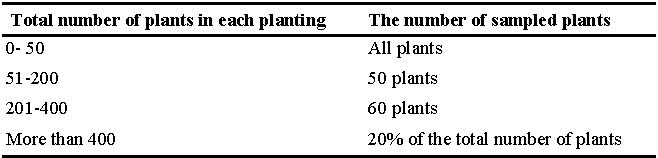
The survey was conducted from June to October 2016. Leaf samples were collected monthly. The number of sampled plants per site was determined according to the total number of the plants in each planting (Table 2). On each sampling date, leaves were taken from different parts of the bush canopy, i.e. lower, middle, and upper canopy. Approximately 20 leaves per blackberry plant were collected. The samples were placed in paper bags and then later in Ziplock plastic bags, labeled and transferred to the University of Florida, Small Fruit and Vegetable IPM (UF-SFVIPM) laboratory.


Extraction, preparation, and identification of mite specimens
The mites were collected with a 0 or 00 paint brush under a stereomicroscope LEICA M205 C (Leica Microsystems Inc., Buffalo Grove, Illinois, USA) directly from the leaves. In this way, all mites were separated into families before examination using 40 x to 160 x magnification. Specimens were preserved in vials containing 70% ethanol (according to cultivar and family). All mites were cleared in Lacto-phenol medium. Each mite was mounted in a drop of Hoyer's medium on microscope slides and dried in an oven at 50 °C for 5-7 days according to the method of Krantz and Walter (2009).
Species identifications were made in Division of Plant Industry, FSCA, Gainesville, USA by the second author. The mite specimens were deposited in the Mite Collection at the Division of Plant Industry, FSCA, Gainesville, USA.
Results and Discussion
During the surveys, a total of 20 mite species belonging to 7 families were identified (Table 3) as follows: nine species of Phytoseiidae, four Tetranychidae, three Tarsonemidae, one for each of Ascidae, Stigmaeidae, Cheyletidae and Erythraeidae.



Predatory mite species on nine different blackberry cultivars in selected organic and conventional commercial blackberry farms in Florida and Georgia, USA
Family Phytoseiidae
Galendromus (Galendromus) floridanus (Muma)
Typhlodromus floridanus (original designation) Muma
Galendromus floridanus Muma
Metaseiulus (Galendromus) floridanus Karg
Galendromus (Galendromus) gratus (Chant), synonymy according to Demite et al. (2020)
Galendromus (Galendromus) helveolus (Chant), synonymy according to Demite et al. (2020)
Material Examined (n = 8; 7 ♀♀, 1 ♂) — 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 2 August 2016, Osage, Organic Farm), 1♀ (30°26'18.19'' N, 84°11'55.83'' W, 39 m, 31 July 2016, Navaho, Organic Farm), 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 11 August 2016, Choctaw, Organic Farm), 1♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016, Choctaw, Conventional Farm), 1♂ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016, Choctaw, Conventional Farm), 1♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016, Ouachita, Conventional Farm), 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 25 August 2016, Osage, Organic Farm), 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 15 July 2016, Choctaw, Organic Farm).
Comments — G. (G.) floridanus was recorded in Costa Rica, Cuba, El Salvador, Jamaica, Mexico, Nicaragua, Colombia, Ecuador, Guadeloupe, Guatemala, Honduras, Martinique, Panama Venezuela, and USA- Florida. It was collected from Citrus sp. (Rutaceae) and Thespesia populnea L. (Malvaceae) (Demite et al. 2020). In the present study, G. floridanus was collected together with P. latus, Tarsonemus (Tarsonemus) bilobatus Suski, Tarsonemus (Tarsonemus) confusus Ewing (Trombidiformes: Tarsonemidae), Eotetranychus carpini (Qudemansi), Tetranychus schoenei McGregor and Tetranychus urticae Koch (Trombidiformes: Tetranychidae).
Phytoseius chanti Denmark
Phytoseius (Dubininellus) chanti Denmark
Phytoseius (Phytoseius) chanti Muma & Denmark
Phytoseius chantii Chant & McMurtry
Material Examined (n = 4; 4 ♀♀) — 1♀ (22 August 2016, Kiowa); 2♀♀ (15 July 2016, Arapaho); 1♀ (15 July 2016, Choctaw); 29°24'30.77'' N, 82°10'16.13'' W, 19 m, Organic Farm.
Comments — Phytoseius chanti was detected in USA- Florida (Denmark 1966; Denmark and Evans 2011; Muma and Denmark 1970). It was found on Quercus virginiana Mill (Fagaceae) (Denmark 1966), Calocarpum sapota (Jacq.) Merr (Sapotaceae), Cynadon dactylon (L.) (Poaceae), Diospyros sp. (Ebenaceae), Pisium sp. (Fabaceae), Quercus durandii Buckley, Quercus sp. (Fagaceae), Rhus copallinum L. var. leucantha (Jacq.) (Anacardiaceae), Styrax americana Lam (Styracaceae) and Vitis sp. (Vitaceae) (Muma and Denmark 1970). During the present study, P. chanti was collected together with P. latus, E. carpini, T. bilobatus, T. confusus, T. schoenei and T. urticae.
Proprioseius meridionalis Chant
Amblyseius (Proprioseius) meridionalis Pritchard & Baker
Phytoseiulus (Proprioseius) meridionalis Wainstein
Amblyseius (Amblyseius) meridionalis Tseng
Proprioseius (Proprioseius) meridionalis Karg
Typhlodromus psychotriae Hirschmann (unjustified replacement name for Proprioseius meridionalis)
Material Examined (n=4; 4 ♀♀) — 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 22 August 2016, Arapaho, Organic Farm), 1♀ (29°39'15.95'' N, 82°31'25.49'' W, 32 m, 27 July 2016, Arapaho, Organic Farm), 2♀♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016, Choctaw, Conventional Farm).
Comments — Proprioseius meridionalis was recorded previously on wide range of host plants including blackberry, Rubus sp. in USA, Florida (Chant 1957; De Leon 1959; Denmark and Evans 2011; Denmark and Muma 1966; Muma and Denmark 1970; Walter and Denmark 1991) and New Jersey (Dyer and Swift 1979). In the current study, this species was collected with all phytophagous mite species, which were collected during the present study.
Typhlodromips dentilis (De Leon)
Amblyseius dentilis Athias-Henriot
Amblyseius (Typhlodromopsis) dentilis Muma
Material Examined (n=5; 5 ♀♀) — 1♀(29°32'35.80'' N, 82°5'7.34'' W, 28 m, 11 August 2016, Natchez, Organic Farm), 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 25 August 2016, Von, Organic Farm), 1♀ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 20 September 2016, Ouachita, Organic Farm), 1♀ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 28 June 2016, Arapaho, Organic Farm), 1♀ (28°57'13.97'' N, 82°1'49.14'' W, 30 m, 12 July 2016, Natchez, Conventional Farm).
Comments — Typhlodromips dentilis has been found on many different types of plants worldwide (Demite et al. 2020). In Florida, it was recorded for the first time on Rhus copallina in Miami, Florida, USA and subsequently in various localities in Florida (Denmark and Evans 2011; Muma 1964a b; Muma and Denmark 1970). During the present study, T. dentilis was found together with populations of P. latus, T. schoenei, E. carpini and T. confusus.
Typhlodromalus peregrinus (Muma)
Typhlodromalus evansi (Chant)
Typhlodromalus primulae (Chant)
Typhlodromalus robiniae (Chant) Typhlodromalus sextus (Garman)
Typhlodromalus aripo (De Leon)
Material Examined (n=29; 29 ♀♀) — 2♀ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 11 August 2016, Natchez, Organic Farm), 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, Choctaw, Organic Farm), 2♀♀ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 18 August 2016, Natchez, Conventional Farm), 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 22 August 2016, Choctaw, Organic Farm), 2♀♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 25 August 2016, Von, Organic Farm), 1♀Natchez, 1♀Osage, 1♀ Ouachita (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 20 September 2016, Organic Farm), 1♀Natchez, 2♀♀Osage, 2♀♀ Ouachita (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 21 September 2016 Organic Farm), 1♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 22 September 2016, Ouachita, Conventional Farm), 8♀♀ Natchez, 1♀Osage, 3♀♀ Ouachita (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 22 September 2016, Conventional Farm).
Comments — According to Demite et al. (2020), to date, T. peregrinus was reported from 18 countries including the US. In the USA, it was detected in Florida, Georgia, Massachusetts, Missouri, New, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, and Virginia (Demite et al. 2020). It was generally found on citrus (Childers 1994; Fadamiro et al. 2008, 2009; Muma 1955a, 1967; Pena 1992; Villanueva and Childers 2004, 2005) and solanaceous plants (Fiaboe et al. 2007; McMurtry 1983; Silva et al. 2016). This species was also collected from ground cover vegetation (weeds) of Alabama (Fadamiro et al. 2008, 2009) and Florida (Childers and Denmark 2011) citrus orchards (Kreiter et al. 2018). Vieira de Souza et al. (2015) detected it on Cocos nucifera (L.) (Arecaceae), Theobroma cacao L. (Malvaceae), Psidium guajava L. (Myrtaceae), Carica papaya L. (Caricaceae). It was also found on Alchornea triplinervea (Spreng.) Müll.Arg. (Euphorbiaceae) by Zacarias and De Moreas (2001). Kreiter et al. (2018) collected this species from Neonotonia wightii (Wight and Arn.) Pueraria phaseoloides (Roxb.) Macroptilium atropurpureum (DC.) (Fabaceae) and Paspalum notatum Flügge cv. Pensacola (Poaceae). However, according to Demite et al. (2020), there is no known record on Rubus spp. This species was associated with many insect and mite species by different researchers. While Aleyrodidae, Coccidae and Tetranychidae were evaluated as optimal prey for this species by Muma (1971), Childers and Denmark (2011) indicated that this species is a predator of thrips. According to Muma (1955b, 1971), it is a facultative predator of Chrysomphalum aonidum (L) and Lepidosaphes beckii (Newn.) (Hemiptera: Diaspididae) (Pena et al.1989). It was also collected from miners of Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) and whitefly exuvia, empty scale armour, clump, dead scale insects, sooty mold (Childers 1994; Muma 1967; Villanueva and Childers 2011). It was found associated with Panonychus citri (McGregor), Eotetranychus sexmaculatus (Riley) (Trombidiformes: Tetranychidae), Parlatoria pergandii Comstock (Hemiptera: Diaspididae) (Muma 1969), Phyllocoptruta oleivora (Ashmead) (Trombidiformes: Eriophyidae) (Pena, 1992; Kretier et al., 2018). Silva et al. (2016) noted that this predator also was able to feed on all stages of P. latus. Immature stages of P. citri, all stages of T. urticae, and pollens of Malephora crocea (Jacq.) Schwant (Aizoaceae), Quercus virginiana Miller (Fagaceae), and Typha latifolia L. (Typhaceae) were evaluated as suitable diet in the laboratory by Fouly et al. (1995). In the present study, T. peregrinus was found together with populations of P. latus, E. carpini, T. bilobatus, T. confusus, T. schoenei and T. urticae.
Amblyseius sp.
Material Examined (n=3; 1 ♀, 2 ♂♂) — 1♂ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 25 August 2016, Ouachita, Organic Farm), 1♂ (29°40'37.56'' N, 82°29'32.73'' W, 32 m, 1 August 2016, Kiowa, Organic Farm), 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 21 September 2016, Natchez, Organic Farm).
Phytoseius sp. 1
Material Examined (n=5; 5 ♀♀) — 1♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016 Ouachita, Conventional Farm), 2♀♀ (22 August 2016, Arapaho, Organic Farm), 1♀ 22 August 2016, Kiowa, 1♀ 20 September 2016, Kiowa (29°24'30.77'' N, 82°10'16.13'' W, 19 m Organic Farm)
Phytoseius sp. 2
Material Examined (n=6; 6 ♀♀) — 1♀ (22 August 2016, Arapaho), 1♀ (22 August 2016, Choctaw), 1♀ (22 August 2016, Kiowa); 2♀♀ (20 September 2016, Kiowa); 1♀ (15 July 2016, Kiowa) (29°24'30.77'' N, 82°10'16.13'' W, 19 m Organic Farm)
Proprioseiopsis sp.
Material Examined (n=4; 3 ♀♀, 1 ♂) — 1♀ 11 August 2016, Osage, 1♀ 15 July 2016, Ouachita (29°32'35.80'' N, 82°5'7.34'' W, 28 m, Organic Farm), 1♂ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 22 August 2016, Choctaw, Organic Farm), 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 2 August 2016, Ouachita, Organic Farm).
Family Cheyletidae
Oudemanscheyla denmarki (Yunker)
Cheletomimus denmarki Yunker
Material Examined (n=2; 2 ♀♀) — 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 2 August 2016, Natchez, Organic Farm), 1♀ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 18 August 2016, Ouachita, Conventional Farm).
Comments — Yunker (1961) and Muma (1964c) recorded this species from Florida citrus. It was recorded from Lantana camara L. (Verbenaceae) leaves collected from native forests in southeastern Queensland, Australia by Walter (1999). Hagstrum (2013) detected this species on betel nut (Areca catechu L., Arecaceae) in Taiwan. It was observed feeding on tydeid mites in domatia on leaves of wild grapes by Walter and Denmark (1991). In the current study, it was collected together with P. latus, T. confusus, E. carpini and T. schoenei phytophagous mites.
Family Stigmaeidae
Agistemus sp.
Material examined (n=8; 8 nymph) — 1N (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 2 August 2016, Von, Organic Farm), 2N (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 11 August 2016, Osage, Organic Farm), 3N Natchez, 2N Osage (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 15 July 2016, Organic Farm).
Family Ascidae
Asca sp. (n=1; 1♂)
Material examined — 1♂ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 11 August 2016, Arapaho, Organic Farm)
Family Erythraeidae
Lasioerythraeus sp.
Material examined (n=1, 1 Larva) — 1L (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 15 July 2016, Arapaho, Organic Farm)
Phytophagous mite species on nine different blackberry cultivars in selected organic and conventional commercial blackberry farms in Florida and Georgia, USA
Family Tarsonemidae
Polyphagotarsonemus latus (Banks)
Acarus translucens Green
Polyphagotarsonemus latus (Banks)
Acarus translucens Green
Hemitarsonemus latus Banks
Tarsonemus translucens Green
Avrosita translucens Oudemans
Tarsonemus latus Banks
Tarsonemus phaseoli Bondar
Neotarsonemus latus Smiley
Material examined (n=10; 5 ♀♀, 5 ♂♂) — 1♀ Ouachita, 1♂ Osage (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 11 August 2016, Organic Farm); 1♂ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 28 June 2016, Ouachita, Organic Farm); 1♂ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016, Ouachita, Conventional Farm); 1♀ Osage, 1♀ Von (28°34'5.62'' N, 81°41'22.17'' W24 m, 25 August 2016, Organic Farm); 1♂ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 22 September 2016, Ouachita, Conventional Farm), 1♀ (28°57'13.97'' N, 82°1'49.14'' W, 30 m, 12 July 2016, Ouachita, Conventional Farm); 1♂ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 21 July 2016, Osage, Conventional Farm). 1♀ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 21 July 2016, Natchez, Conventional Farm).
Comments — Polyphagotarsonemus latus is distributed worldwide and has a wide host range (Fasulo 2019; Azzazy and Alhewairini 2018; Gerson 1992; Johnson et al. 2016; Pena and Campel 2005). It was first described by Banks (1904) as Tarsonemus latus from the terminal buds of mango in a greenhouse in Washington, D.C., USA (Denmark 1980; Fasulo 2019). This mite was recently found in multiple states in the US causing yield reductions in blackberries (Johnson et al. 2016; Lefors et al. 2017). Vincent et al. (2010) reported it on blackberry in organic production in Arkansas. Rebek (2017) detected it in Oklahoma Blackberry orchards. Demchak and Johnson (2017) noticed that it has been problematic for blackberry growers in Pennsylvania. Rebek (2017) also found it on blackberries in Oklahoma. Finally, in 2017, this mite has been detected on blackberries in Arkansas, Illinois, Indiana, Maryland, North Carolina, South Carolina, Pennsylvania, Virginia, California, and Oklahoma (Rebek 2017). Renkema et al. (2017) recorded it from commercial strawberries in Florida. It is also considered a serious pest of Pittosporum spp. (Pittosporaceae) in Florida (Johnson and Lyon 1991). Pena et al. (2000) observed P. latus on citrus.
Tarsonemus (Tarsonemus) bilobatus Suski
Tarsonemus (Tarsonemus) hungaricus Schaarschmidt
Lupotarsonemus bilobatus Suski & Schaarschmidt
Material examined (n=1; 1 ♀) — 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m 11 August 2016 Arapaho Organic Farm)
Comments — Tarsonemus bilobatus has been reported on many plant species in Central America (Costa Rica), Europe (Byelorussia, Hungary, Italy, Poland, Ukraine), Asia (China, India Japan, Korea,), Africa (Egypt) (Lin and Zhang 2002; Zhang 2003) and South America (Brazil) (Lofego et al. 2005; Rezende et al. 2012). It was also collected from bacterial and fungal cultures, stored food products, litter and soil. It is primarily a fungivorus species (Zhang 2003).
Tarsonemus (Tarsonemus) confusus Ewing
Tarsonemus assimilis Banks
Tarsonemus confusus Ewing; Beer; Smiley; Kaliszewski
Material Examined (n=5; 5 ♀♀) — 1♀ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 11 August 2016, Arapaho, Organic Farm); 1♀ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 18 August 2016, Ouachita, Conventional Farm); 2♀♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016 Kiowa Conventional Farm); 1♀ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 28 June 2016, Ouachita, Organic Farm).
Comments — Tarsonemus confusus occurs on many types of plants in Canada, Byelorussia, Egypt, China, Ireland, Italy, Japan, Germany, Korea, Netherlands, Ukraine, Poland, Russia, Turkey (Lin and Zhang 2002), Hungary (Ripka et al. 2005) and Brazil (Lofego et al. 2005). It was also previously recorded from multiple states in the US on various plants including Rubus sp. (Lin and Zhang 2002).
Family Tetranychidae
Eotetranychus carpini (Oudemans)
Material Examined (n=22; 4 ♀♀, 18 ♂♂) — 1♂ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 2 August 2016, Osage, Organic Farm); 11♂♂ 2♀♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 21 September 2016, Von, Organic Farm); 1♂ Von, 1♂2♀♀ Ouachita, 28°34'5.62'' N, 81°41'22.17'' W24 m, 25 August 2016, Organic Farm; 1♂ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 15july 2016, Kiowa, Organic Farm), 2♂♂ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 22 August 2016, Arapaho, Organic Farm), 1♂ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 11 August 2016, Osage, Organic Farm)
Comments — Eotetranychus carpini has been widely reported in Europe (Migeon et al. 2007; Malagnini et al., 2012)) . It is also found in Mexico (Beer and Lang 1958) and USA (McGregor 1917). To date, it was reported from 30 countries in two major regions of Nearctic and Palearctic (Migeon and Dorkeld 2020). This species also exploits a wide range of host plants and have been reported on 54 host plants including Rubus idaeus (Migeon and Dorkeld 2020) and Rubus sp. (Bolland et al. 1998).
Tetranychus schoenei McGregor
Septanychus schoenei McGregor
Tetranychus schoenei Pritchard & Baker
Tetranychus (Polynychus) schoenei Flechtmann & Hunter
Material Examined (n=23; 6 ♀♀, 17 ♂♂) — 1♀ Osage, 1♀ Ouachita (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 18 August 2016, Conventional Farm); 1♀ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 18 August 2016, Ouachita, Conventional Farm); 1♀ Kiowa, 1♂ Arapaho, 1♂ Choctaw (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 22 August 2016, Organic Farm), 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 25 August 2016, Osage, Organic Farm); 2♂♂ Natchez, 2♂♂ Osage (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 20 September 2016, Organic Farm); 1♂ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 28 June 2016, Ouachita, Organic Farm); 1♀ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 21 September 2016, Von, Organic Farm); 3♂♂ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 22 September 2016, Ouachita, Conventional Farm); 1♂ (29°32'35.80'' N, 82°5'7.34'' W, 28 m, 15 July 2016, Osage, Organic Farm); 1♂ Osage, 4 ♂♂ Ouachita (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 21 July 2016, Conventional Farm); 1♂ Osage (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 21 July 2016, Conventional Farm).
Comments — Tetranychus schoenei is widely distributed over the eastern and southwestern United States. Previous distribution records included Georgia (Flechtmann and Hunter 1971). It is also found in Iran (Beyzavi et al. 2013). According to Migeon and Dorkeld (2020), to date, it was reported from 2 countries and on 52 host plants including Rubus allegheniensis, R. idaeus, R. occidentalis (Reeves 1963), Rubus sp. (Reeves 1963; Seeman and Beard 2011).
Tetranychus urticae Koch
Fifty species names have been synonymized with T. urticae in spider Mites Web (Migeon and Dorkeld 2020).
Material Examined (n=5; 5 ♂♂) — 1♂ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 18 August 2016, Osage, Conventional Farm); 1♂ (29°24'30.77'' N, 82°10'16.13'' W, 19 m, 22 August 2016, Choctaw, Organic Farm); 2♂♂ (31°22'49.19'' N, 83°19'8.57'' W, 89 m, 21 July 2016, Osage, Conventional Farm); 1♂ (29°39'15.95'' N, 82°31'25.49'' W, 32 m, 27 July 2016, Navaho, Organic Farm).
Comments — Tetranychus urticae has an almost cosmopolitan distribution. It is also a highly polyphagous species. To date, it is reported on 1169 host plants including many Rubus spp. from 124 countries (Migeon and Dorkeld 2020).
Tetranychus sp.
Material Examined (n=6; 6 ♂) — 1♂ Osage 1♂ Von (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 2 August 2016, Organic Farm); 1♂ (28°34'5.62'' N, 81°41'22.17'' W, 24 m, 25 August 2016, Von, Organic Farm); 1♂ Navaho, 1♂ Ouachita (29°39'15.95'' N, 82°31'25.49'' W, 32 m, 27 July 2016, Organic Farm); 1♂ (30°54'18.31'' N, 82°38'51.06'' W, 46 m, 21 July 2016, Ouachita, Conventional Farm).
Conclusion
In conclusion, the total number of mites collected in organic farms (105) was over 2-fold higher than in conventional farms (47). Moreover, while all of the twenty mite species identified during the study could be collected from organic farms, just nine could be obtained from conventional farms. The total number of beneficial mites collected in the organic farm was 57. In contrast, 23 beneficial mites were collected from conventional farms (Table 3). These results may be related to pesticide use on the conventional farms and are similar to that previously reported for mites (Incekulak and Ecevit 2002; Yanar and Ecevit 2008; Akyazı et al. 2016; Fathipour and Maleknia 2016; Soysal and Akyazı 2018).



Among the nine sampled cultivars, Quachita had 35 mites and 12 mite species, Osage had 27 mites, 9 species, Natchez had 26 mites, 9 species, Von had 22 mites, 7 species, Arapaho had 15 mites, 11 species, Choctaw had 13 mites, 8 species and Kiowa had 12 mites, 7 species. In contrast, no mite was found on the leaf samples taken from the Freedom cultivar, and just one mite was collected from Navaho (Table 4). We hypothesize that differences in mite numbers and diversity may be caused by different phytochemical components, morphological and histological leaf structure of blackberry cultivars as stated by Camporese and Duso (1996), Krips et al. (1999), Kretier et al. (2002), Kabicek (2008), Khan et al. (2008) and Ali et al. (2015) for different plants. It should be noted that a single factor is not responsible for the abundance and diversity of mites but a combination of factors. Since the effect of cultivars on the mite population is time-consuming, such results can only be observed in the long term. As far as we know, no previous research has investigated the effect of blackberry cultivars on the mite population. And, future investigations are necessary to validate the kinds of conclusions that can be drawn from this study.
Acknowledgments
We thank growers in Florida and Georgia for allowing collection of samples from their farms.
References
Akyazi R., Liburd O.E. 2019. Biological control of the twospotted spider mite (Trombidiformes: Tetranychidae) with the predatory mite Neoseiulus californicus (Mesotigmata: Phytoseiidae) in blackberries. Fla. Entomol., 102: 373-381. doi:10.1653/024.102.0217
Akyazı R., Ueckermann E.A., Soysal M., Akyol D., 2016. Population dynamics of mites (Acari) on Diospyros kaki Thunb. and Diospyros lotus L. (Ebenaceae) trees in Ordu, Turkey. Syst. Appl. Acarol., 21 (10): 1334-1345. doi:10.11158/saa.21.10.4
Al-Azzazy M.M., Alhewairini S.S. 2018. First Record of Polyphagotarsonemus latus in Saudi Arabia. J. Agric. Sci., 10(12): 228-232. doi:10.5539/jas.v10n12p228
Alford D.V. 2016. Eotetranychus carpini. Pests of Fruit Crops: A Colour Handbook, Second Edition, Boca Raton, CRC Press, pp. 462. doi:10.1201/b17030
Ali F.S., Afifi A.M., El-Saiedy E.M.A., Ahmed M.M. 2015. Effect of phytochemical components, morphological and histological leaf structure of five tomato hybrids on Tetranychus urticae Koch Infestation. Acarines, 9: 23-30.
Banks N. 1904. Class III, Arachnida, Order 1, Acarina, four new species of injurious mites. J. N. Y. Entomol. Soc., 12: 53-56.
Beer R.E., Lang D.S. 1958. The Tetranychidae of Mexico. Univ. Kans. Sci. Bull., 38: 1231-1259. doi:10.5962/bhl.part.10974
Beyzavi G., Ueckerman E.A., Faraji F., Ostovan H. 2013. A catalog of Iranian prostigmatic mites of superfamilies Raphignathoidea & Tetranychoidea (Acari). Persian J. Acarol., 2(3): 389-474.
Bolland H.R., Gutierrez J., Flechtmann C.H.W. 1998. World catalogue of the spider mite family (Acari: Tetranychidae), Leiden: Brill Academic Publishers. pp. 392.
Cetin G., Denizhan E., Erenoğlu B. 2010. Türkiye faunası için yeni bir kayıt: Acalitus essigi (Hassan, 1928) (Böğürtlen akarı) (Acari: Prostigmata: Eriophyoidea). [A new blackberry pest for Turkish fauna, Acalitus essigi (Hassan, 1928) (Acari: Prostigmata: Eriophyoidea)]. Bitki Koruma Bülteni, 50(2):45-49.
Chant D.A. 1957. Descriptions of two new phytoseiid genera (Acarina: Phytoseiidae), with a note on Phytoseius Ribaga, 1902. Can. Entomol., 89: 357-363. doi:10.4039/Ent89357-8
Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. doi:10.4039/entm9112fv
Childers C.C. 1994. Biological control of phytophagous mites on Florida citrus utilizing predatory arthropods. In: Rosen D., Bennet F., Capinera J. (Eds). Pest management in the subtropics: biological control Florida perspective. Intercept, Andover, United Kingdom. p. 255-288.
Childers C.C., Denmark H.A. 2011. Phytoseiidae (Acari: Mesostigmata) within citrus orchards in Florida: species distribution, relative and seasonal abundance within trees, associated vines and ground cover plants. Exp. Appl. Acarol. 54: 331-371. doi:10.1007/s10493-011-9449-1
Camporese P., Duso C. 1996. Different colonization patterns of phytophagous and predatory mites (Acari,Tetranychidae, Phytoseiidae) on three grape varieties, A case study. Exp. Appl. Acarol., 20: 1-22. doi:10.1007/s10493-012-9519-z
Davies J.T., Allen G.R., Williams M.A. 2001. Intraplant distribution of Acalitus essigi (Acari: Eriophyoidea) on blackberries (Rubus fruticosus agg.). Exp. Appl. Acarol., 25:625-639. doi:10.1023/A:1016179817089
De Leon D. 1959. Two new genera of phytoseiid mites with a note on Proprioseius meridionalis Chant (Acarina: Phytoseiidae). Entomol. News., 70:257-262.
Demchak K., Johnson D. 2017. Small Fruit Mite - Broad Mites on Blackberries. College of Agricultural Sciences The Pennsylvania State University. https://extension.psu.edu/small-fruit-mite-broad-mites-on-blackberries
Demite P.R., Moraes G.J., de McMurtry J.A., Denmark H.A., Castilho R.C. 2020. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae.
Denmark H.A. 1966. Revision of the genus Phytoseius Ribaga, 1904 (Acarina: Phytoseiidae). Fla Dep. Agric. Bull. 6:1-105.
Denmark H.A. 1980. Broad mite, Polyphagotarsonemus latus (Banks). FDACS-DPI Bureau of Entomology Circular No. 213 pp. 2.
Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, pp. 451.
Denmark H.A., Evans G.A., Aguilar H., Vargas C., Ochoa R. 1999. Phytoseiidae of Central America. Indira Publishing House, West Bloomfield, Michigan, USA, pp. 125.
Denmark, H.A., Muma, M.H. 1966. Revision of the genus Proprioseius Chant (Acarina: Phytoseiidae). Flo. Entomol., 49(4):253-264. doi:10.2307/3493888
Dyer J.G., Swift F.C. 1979. Sex ratio in field populations of Phytoseiid mites (Acarina: Phytoseiidae). Ann. Entomol. Soc. Am., 72(1):149-154. doi:10.1093/aesa/72.1.149
Fadamiro H.Y., Xiao Y., Hargroder T., Nesbitt M., Childers C.C. 2009. Diversity and seasonal abundance of predacious mites in Alabama Satsuma citrus. Ann. Entomol. Soc. Am. 102(4): 617-628. doi:10.1603/008.102.0406
Fadamiro H.Y., Xiao Y., Hargroder T., Nesbitt M., Umeh V., Childers C.C. 2008. Seasonal occurrence of key arthropod pests and associated natural enemies in Alabama satsuma citrus. Environ. Entomol. 2: 555-567. doi:10.1093/ee/37.2.555
Fasulo T. 2019. Broad Mite, Polyphagotarsonemus latus (Banks) (Arachnida: Acari: Tarsonemidae). EENY-183. The Institute of Food and Agricultural Sciences, University of Florida, USA.
Fathipour Y., Maleknia B. 2016. Mite Predators. In: Omkar (Ed.), Ecofriendly pest management for food security. Elsevier, San Diego, USA, pp. 329-366. doi:10.1016/B978-0-12-803265-7.00011-7
Fernandez G., Garcia E., Lockwood D. 2015. Cultivars. Available from: https://content.ces.ncsu.edu/southeast-regional-caneberry-production-guide/cultivars

Fiaboe K.K.M., Gondim M.G.C. Jr., Moraes G.J. de, Ogol C.K.P.O., Knapp. M. 2007. Surveys for natural enemies of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) in the northeastern and southeastern Brazil. Zootaxa, 1395:33-58. doi:10.11646/zootaxa.1395.1.2
Flechtmann, C.H.W., Hunter, P.E. 1971. The spider mites (Prostigmata: Tetranychidae) of Georgia. J. Ga. Entomol. Soc., 6:16-30.
Fouly A.H., Abou-Setta M.M., Childers C.C. 1995. Effects of diets on the biology and life tables of Typhlodromalus peregrinus. Environ. Entomol., 24: 870-877. doi:10.1093/ee/24.4.870
Gerson U. 1992. Biology and control of the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Tarsonemidae). Exp Appl Acarol., 13:163-178. doi:10.1007/BF01194934
Hagstrum D.W., Klejdysz T., Subramanyam B., Nawrot J. 2013. Atlas of Stored-Product Insects and Mites. AACC International Press, St. Paul, Minnesota, USA, pp. 600. doi:10.1016/B978-1-891127-75-5.50009-5
Incekulak R., Ecevit O. 2002. A research on determination of harmful and beneficial mite species in apple orchards in Amasya and their population densities. Vth Turkish national congress of biological control (Erzurum, Turkey), pp. 297-314.
Johnson D.T., Garcia M.E., Rom C., Freeman L., Kim Soo-Hoon, Lewis B. 2016. Management of arthropods on blackberries and raspberries in Arkansas, USA. Acta Hortic. 1133:437-444. doi:10.17660/ActaHortic.2016.1133.67
Johnson W.T., Lyon H.H. 1991. Insects that Feed on Trees and Shrubs. 2nd ed., rev. Comstock Publishing Associates. pp. 560.
Kabicek J. 2008. Cohabitation and intraleaf distribution of phytoseiid mites (Acari, Phytoseiidae) on leaves of Corylus avellana. Plant. Prot. Sci., 44(1): 32-36. doi:10.17221/3/2008-PPS
Khan M.A., Khaliq A., Subhani M.N., Saleem M.W. 2008. Incidence and development of Thrips tabaci and Tetranychus urticae on field grown cotton. Int. J. Agric. Biol., 10: 232-234.
Krantz G.W., Walter D.E. 2009. A Manual of Acarology. 3rd Edition. Texas Tech University Press, Lubbock: pp. 807.
Kreiter S., Tixier M.S., Croft A., Auger P., Barret D. 2002. Plants and leaf characteristics influencing the predaceous mite Kampimodromus aberrans (Acari, Phytoseiidae) in habitats surrounding vineyards. Environ. Entomol., 31(4): 648-660. doi:10.1603/0046-225X-31.4.648
Kreiter S., Zriki G., Ryckewaert P., Pancarte C., Douin M., Tixier M.S. 2018. Phytoseiid mites of Martinique, with redescription of four species and new records (Acari: Mesostigmata). Acarologia, 58(2): 366-407.
Krips O.E., Kleijn P.W., Willems P.E.L., Gols G.J.Z., Dicke M. 1999. Leaf hairs influence searching efficiency and predation rate of the predatory mite Phytoseiulus persimilis (Phytoseiidae, Acarina). Exp. Appl. Acarol, 23(2): 119-131. doi:10.1023/A:1006098410165
LeFors J.A., Johnson D.T., Woodruff T. 2017. Acaricidal control of broad mites on blackberry, 2016. Arthropod Management Tests 42. Available from: https://doi. org/10.1093/amt/tsx113

Liburd O.E., Lopez L., Carrillo D., Revynthi A.M., Olaniyi O., Akyazi R. 2020. Integrated pest management of mites. In: Kogan M., Heinrichs E. (Eds). Integrated management of insect pests, current and future developments. Burleigh Dodds series in agricultural sciences; Burleigh Dodds Science Publishing. Cambridge UK. doi:10.19103/AS.2019.0047.26
Lin J.Z., Zhang Z.Q. 2002. Tarsonemidae of the World: Key to Genera, Geographical Distribution, Systematic Catalogue & Annotated Bibliography. Systematic & Applied Acarology Society, London, pp. 440.
Lofego A.C., Ochoa R., Moraes G.J. 2005. Some tarsonemid mites (Acari: Tarsonemidae) from the Brazilian $''$Cerrado$''$ vegetation, with descriptions of three new species. Zootaxa, 823:1-27. doi:10.11646/zootaxa.823.1.1
Madanlar N., Kısmalı S. 1991. İzmir ilinde turunçgillerde bulunan Acarina türleri ve popülasyon yoğunluklarının saptanması üzerinde araştırmalar. Doktora Tezi, Ege Üniversitesi Fen Bilimleri Enstitüsü, İzmir s. 258.
Malagnini V., Navajas M., Migeon A., Duso C. 2012. Differences between sympatric populations of Eotetranychus carpini collected from Vitis vinifera and Carpinus betulus: insights from host-switch experiments and molecular data. Exp. Appl. Acarol., 56:209-219. doi:10.1007/s10493-012-9511-7
Marchetti M.M., Ferla N.J. 2011. Diversity and population fluctuation of mites (Acari) in blackberry (Rubus fruticosus, Rosaceae) in the state of Rio Grande do Sul, Brazil. Iheringia Ser. Zool., 101:43-48. doi:10.1590/S0073-47212011000100005
McGregor E.A. 1917. Description of seven new species of red spiders. Proc. U. S. Natl. Mus., 51: 581-590. doi:10.5479/si.00963801.51-2167.581
McMurtry J.A. 1983. Phytoseiid mites from Guatemala, with descriptions of two new species and redefinitions of the genera Euseius, Typhloseiopsis, and the Typhlodromus occidentalis species group (Acari: Mesostigmata). Intern. J. Entomol. 25(4): 249-272.
Migeon A., Dorkeld F. 2020. SpmWeb: Spider Mites Web (version Jul 2011). In: Species 2000 & ITIS Catalogue of Life, [2020-01-10] Beta Roskov Y., Ower G., Orrell T., Nicolson D., Bailly N., Kirk P.M., Bourgoin T., DeWalt R.E., Decock W., Nieukerken E. van, Penev L., (Eds). Digital resource at www.catalogueoflife.org/col. Species 2000: Naturalis, Leiden, the Netherlands. ISSN 2405-8858.
Migeon A., Malagnini V., Navajas M., Duso C. 2007. Notes on the genus Eotetranychus (Acari: Tetranychidae) in Italy and France with a redescription of Eotetranychus fraxini Reck, new record for Italy and Western Europe. Zootaxa, 1509: 51-60. doi:10.11646/zootaxa.1509.1.5
Muma M.H. 1955a. Phytoseiidae (Acarina) associated with citrus in Florida. Ann. Entomol. Soc. Amer., 48: 262-272. doi:10.1093/aesa/48.4.262
Muma M.H. 1955b. Factors contributing to the natural control of citrus insects and mites in Florida. J. Econ. Entomol., 48: 432-438. doi:10.1093/jee/48.4.432
Muma M.H. 1964a. The population of Phytoseiidae on Florida citrus. Fla. Entomol., 47: 5-11. doi:10.2307/3493754
Muma, M.H. 1964b. Annotated list and keys to Phytoseiidae (Acarina: Mesostigmata) associated with Florida citrus. Bull. - Agric. Exp. Stn. (Fla.), 685:1-42.
Muma M.H. 1964c. Cheyletidae (Acarina: Trombidiformes) associated with citrus in Florida. Flo. Entomol., 47:239-253. doi:10.2307/3493742
Muma M.H. 1967. Typhlodromalus peregrinus (Muma) (Acari: Phytoseiidae) on Florida citrus. Proceedings, 2nd International Congress of Acarology. Sutton Bonington, 19-25 July 1967, England. Akade'miai Kiado', Budapest, Hungary: pp. 135-148.
Muma M.H. 1969. Biological control of various insects and mites on Florida citrus. Proceedings, 1st International Citrus Symposium, 16-26 March 1969, Riverside, CA. University of California, Riverside: pp. 863-870.
Muma M.H. 1971. Food habits of Phytoseiidae (Acarina: Mesostigmata) including common species on Florida citrus. Fla Entomol. 54(1): 21-34. doi:10.2307/3493786
Muma M.H., Denmark H.A. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighboring land areas, 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, USA, pp.150.
Ozsisli T., Cobanoglu S. 2019. Mite (Acari: Prostigmata, Mesostigmata) Species Determined on Blackberry Plant (Rubus fruticosus L., and Rubus sanctus Schreber, Rosaceae). pp. 1482-1488. International Symposium on Advanced Engineering Technologies (ISADET). May 02-04, 2019, Kahramanmaraş, Turkey.
Pena J.F. 1992. Predator-prey interactions between Typhlodromalus peregrinus and Polyphagotarsonemus latus: effects of alternative prey and other food resources. Fla. Entomol. 75: 241-248. doi:10.2307/3495626
Pena J.E., Baranowski R.M., Denmark H.A. 1989. Survey of Predators of the Broad Mite in Southern Florida. Flo. Entomol., 72(2): 373-377. doi:10.2307/3494921
Pena J.E., Campbell C.W. 2005. Broad mite. University of Florida, Institute of Food and Agricultural Sciences. Fact sheet ENY-618. The Institute of Food and Agricultural Sciences, University of Florida, USA.
Pena J.E., Ochoa R., Erbe E., 2000. Polyphagotarsonemus latus (Acari: Tarsonemidae) Research Status on Citrus. PROC. INTL. Soc. Citricult. IX Congr. pp. 754-759.
Rebek E.J. 2017 Broad mites on blackberries. Oklahoma State University Extension Entomology and Plant Pathology. Pest e-alerts 16(28):1-5.
Reeves R.M. 1963. Tetranychidae infesting woody plants in New York State, and a life history study of the elm spider mite Eotetranychus matthyssei n. sp. Memoir of the Cornell University Agricultural Experiment Station, New York State College of Agriculture, Ithaca, New York, 380:1-99.
Renkema J.M., LeFors J.A., Johnson D.T. 2017. First report of broad mite (Acari: Tarsonemidae) on commercial strawberry in Florida. Fla. Entomol. 100(4):804-806. doi:10.1653/024.100.0406
Rezende J.M., Lofego A.C., Navia D., Roggia S., 2012. Mites (Acari: Mesostigmata, Sarcoptiformes and Trombidiformes) Associated to Soybean in Brazil, Including New Records from the Cerrado Areas. Fla. Entomol., 95(3): 683-693. doi:10.1653/024.095.0319
Ripka G., Fain A., Kazmierski A., Kreiter S., Magowski W. 2005. New data to the knowledge of the mite fauna of Hungary (Acari: Mesostigmata, Prostigmata and Astigmata). Acta Phytopathol. Entomol. Hung., 40:159-176. doi:10.1556/APhyt.40.2005.1-2.13
Rodriguez N., Farinas M.E., Sibat R. 1983. Acaros depredadores del genero Galendromus (Acarina: Phytoseiidae) en citricos de Cuba. Ciencia y Tecnica de la Agricultura. Serie Citricos y Otros Frutales, 6(1): 7-14.
Scott J.K., Yeoh P.B., Knihinicki D.K. 2008. Redberry mite, Acalitus essigi (Hassan) (Acari: Eriophyidae), an additional biological control agent for Rubus species (blackberry) (Rosaceae) in Australia. Aust. J. Entomol. 47:261-264. doi:10.1111/j.1440-6055.2008.00654.x
Seeman O.D. Beard J.J. 2011. Identification of exotic pest and Australian native and naturalised species of Tetranychus (Acari: Tetranychidae). Zootaxa, 2961: 1-72. doi:10.11646/zootaxa.2961.1.1
Silva A.S., Tavares S.R., Lofego A.C., Almeida E.H., Silva E.S. 2016. Predatory mites (Acari: Mesostigmata) associated with Polyphagotarsonemus latus (Prostigmata: Tarsonemidae) on solanaceous plants. Syst. Appl. Acarol., 21(8):1133-1144. doi:10.11158/saa.21.8.13
Soysal M., Akyazı R. 2018. Mite species of the vegetable crops in Ordu province with first report of Amblyseius rademacheri Dosse, 1958 (Mesostigmata: Phytoseiidae) in Turkey. Turk. J. Entomol., 42(4), 265-286. doi:10.16970/entoted.447218
Strik B.C., Clark J.R., Finn C., Barnados M.P. 2007. Worldwide production of blackberries. Acta Hortic., 777:209-218. doi:10.21273/HORTTECH.17.2.205
Trinidad C.T.O., Fagundes J.P., Zorzo B., Nava D.E., da Cunha U.S. 2019. Diversity of mites in blackberry genotypes in Pelotas, Rio Grande do Sul, Brazil. Ciência Rural, 49(2):e20170734. doi:10.1590/0103-8478cr20170734
\biblio{USDA-NASS 2016. Noncitrus Fruits and Nuts 2015 Summary July 2016. Available from:
https://downloads.usda.library.cornell.edu/usda-esmis/files/zs25x846c/p2676z01hfq977x78g/ Vieira de Souza I., Argolo P.S., Gondim Júnior M.G.C., de Moraes G.J., Bittencourt M.A.L., Oliveira A.R. 2015. Phytoseiid mites from tropical fruit trees in Bahia State, Brazil (Acari, Phytoseiidae). ZooKeys, 533: 99-131. doi:10.3897/zookeys.533.5981
Villanueva R.T., Childers C.C. 2004. Phytoseiidae increase with pollen deposition on citrus leaves. Fla. Entomol. 4:609-611. doi:10.1653/0015-4040(2004)087[0609:PIWPDO]2.0.CO;2
Villanueva R.T., Childers C.C. 2005. Diurnal and spatial patterns of Phytoseiidae in the citrus canopy. Exp. Appl. Acarol., 35(4): 269-280. doi:10.1007/s10493-004-5728-4
Villanueva R.T. Childers C.C. 2011. Mine-damaged leaves by Phyllocnistis citrella Stainton provide refuge for phytoseiids on grapefruit in Florida and Texas. Zoosymposia, 6:118-123. doi:10.11646/zoosymposia.6.1.20
Vincent C.I., García M.E., Johnson D.T., Rom C.R. 2010. Broad mite on primocane fruiting blackberry in organic production in Arkansas. Hort. Technology, 20: 718-723. doi:10.21273/HORTTECH.20.4.718
Walter D.E. 1999. Cryptic inhabitants of a noxious weed: mites (Arachnida: Acari) on Lantana camara L. invading forests in Queensland. Aust. J. Entomol., 38: 197-200. doi:10.1046/j.1440-6055.1999.00101.x
Walter D.E., Denmark H.A. 1991. Use of leaf domatia on wild grape (Vitis munsoniana) by arthropods in central Florida. Fla. Entomol., 74:440-446. doi:10.2307/3494838
Yunker C.E. 1961. The genera Bak, new genus, and Cheletomimus Oudemans, with descriptions of three new species (Acarina: Cheyletidae), Can. Entomol., 93:1023-1035. doi:10.4039/Ent931023-11
Yanar D., Ecevit O. 2008. Species composition and seasonal occurrence of spider mites and their predators in sprayed and unsprayed apple orchards in Tokat, Turkey. Phytoparasitica, 36(5): 491-501. doi:10.1007/BF03020296
Zacarias M.S., De Moraes G.J. de 2001. Phytoseiid mites (Acari) associated with rubber trees and other euphorbiaceous plants in southeastern Brazil. Neotrop. Entomol., 30:579-586. doi:10.1590/S1519-566X2001000400011
Zhang Z.Q. 2003. Mites of greenhouses identification, biology and control. CABI Publishing, pp. 244. doi:10.1079/9780851995908.0000
NoncFruiNu-07-06-2016.pdf



2020-09-17
Date accepted:
2021-01-06
Date published:
2021-01-14
Edited by:
Migeon, Alain

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Akyazı, Rana; Welbourn, Cal and Liburd, Oscar E.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



