Review of Amblyseius Berlese (Acari: Phytoseiidae) in Western Siberia, Russia
Khaustov, Vladimir A.1
1✉ Tyumen State University, Tyumen, Russia.
2020 - Volume: 60 Issue: 4 pages: 769-805
https://doi.org/10.24349/acarologia/20204401Original research
Keywords
Abstract
Introduction
Phytoseiid mites are important natural enemies of phytophagous mites and other different small arthropods. Some of them, such as Phytoseiulus persimilis (Athias-Henriot), Amblyseius swirskii (Athias-Henriot), Transeius montdorensis (Schicha), Neoseiulus californicus (McGregor) are available commercially for use in greenhouses. Phytoseiid mites are widely distributed around the world and presently include more than 2,500 valid species in three subfamilies and 94 genera (Demite et al. 2020; Chant and McMurtry 2007). The genus Amblyseius is the largest in the subfamily Amblyseiinae, with 434 nominal species (Demite et al. 2020).
Twenty four Amblyseius species are reported from Russia (Wainstein 1960; Meshkov 1999; Wainstein and Beglyarov 1971; Wainstein 1978, 1979; Vysotskaya and Bregetova 1957; Wainstein 1975; Makarova 2009; Kolodochka 1990; Livshitz and Kuznetsov 1972; Kolodochka 1981, 2003, 2006).
The present paper aims to increase the knowledge of the mite fauna of Western Siberia, particularly the poorly studied fauna of phytoseiid mites (Tixier et al. 2008). As a result, three species are here reported for the first time from Russia and additional morphological information of apical sensorial setal cluster of tarsus I are provided to complete the description of all the species investigated. Redescription of the females of Amblyseius silvaticus Chant, 1959, A. ampullosus Wu and Lan, 1991, A. omaloensis Gomelauri, 1968a and A. myrtilli Papadoulis, Emmanouel and Kapaxidi, 2009 are provided and males of A. silvaticus and A. ampullosus are described for the first time. Other species such as A. rademacheri Dosse, 1958, A. krantzi Chant, 1959, A. meridionalis Berlese, 1914 and A. obtusus Koch, 1839 already redescribed in detail (Kolodochka and Gwiazdowicz [2016], Faraji et al. [2011], Döker et al. [2020]) and no need to provide full redescription in this study.
Materials and methods
The mites were collected directly from plant leaves using stereomicroscope Discovery V8 and placed in vials filled with 96% ethanol. Mites from bark and soil samples were extracted using Berlese-Tullgren funnels. Specimens were cleared in lactic acid solution and mounted in Hoyer's medium (Walter and Krantz 2009).
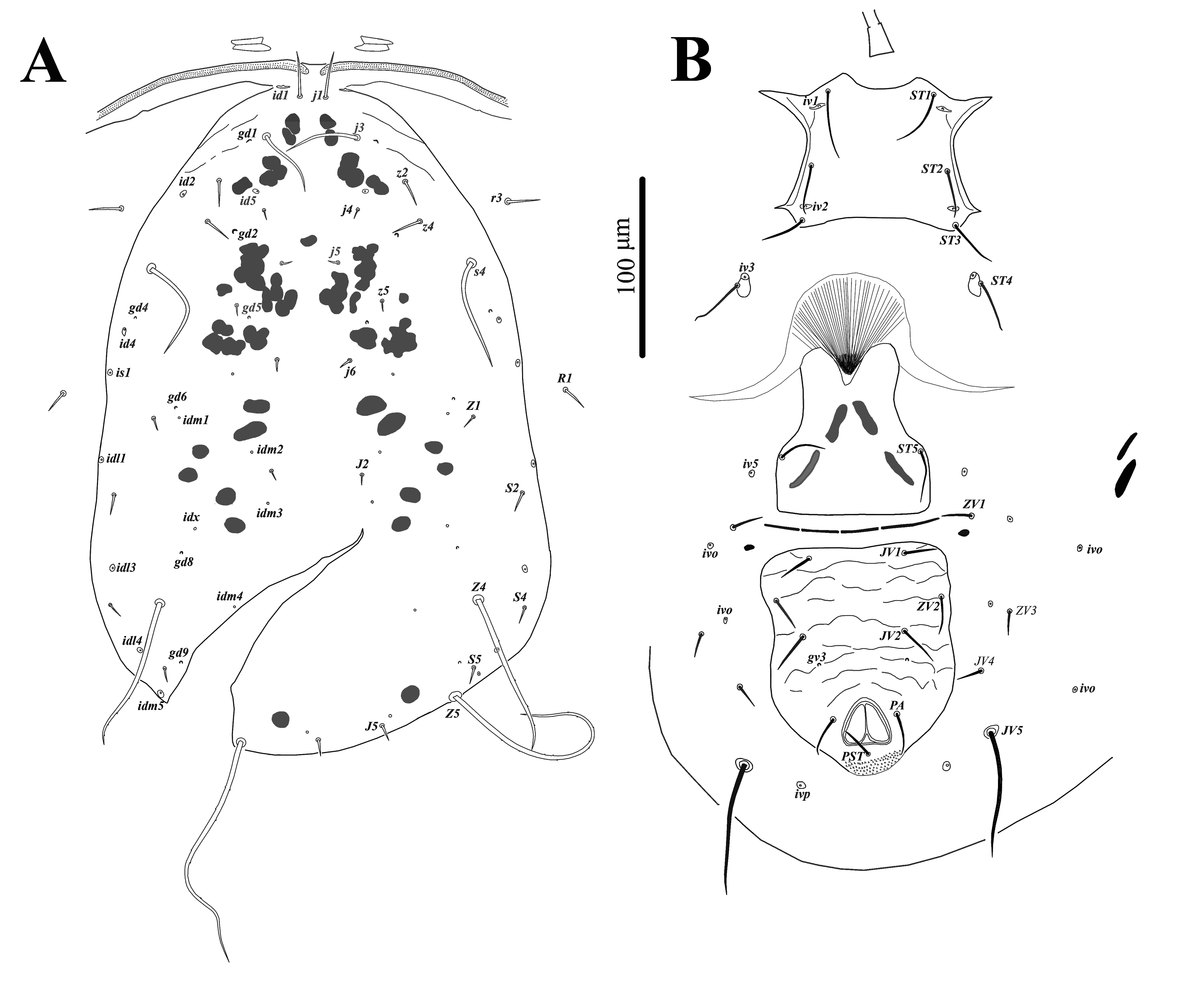
Systematics of Phytoseiidae follows that of Chant and McMurtry (2007). Setal nomenclature for the dorsal idiosoma follows that of Lindquist and Evans (1965) as adapted by Rowell et al. (1978), and Chant and Yoshida-Shaul (1991) for ventral idiosoma. The chaetotaxy of the palp tibia and tarsus and the distal part of tarsus I follows that of Jackson (1974). Chaetotaxy of other parts of legs and palps follows that of Evans (1963, 1964, 1969). For designation of dorsal solenostomes and poroids, nomenclature proposed by Athias-Henriot (1975) and Johnston and Moraza (1991) for ventral surface of idiosoma was used. Terminology of the morphological structures of spermatodactyl follows that of Beard (2001). World distribution of investigated species is based on Demite et al. (2020). Measurements of morphological structure are given in micrometers (µm) and presented as a mean followed by the range in parenthesis. Morphological observations, illustrations and measurements were made using compound microscope Axio Imager A2 (Carl Zeiss, Germany), equipped with differential interference contrast (DIC) and phase contrast optical system. Pictures were taken with Axiocam 506 color (Carl Zeiss, Germany). Dorsal shield length measured along the midline from j1 setae level to J5 setae level; dorsal shield width taken at R1 setae level. Length and width of sternal shield were measured as distance between bases of setae ST1-ST3 and ST2-ST2 for females and distance between bases of setae ST1-ST5, ST2-ST2 for males, respectively. Length of legs is from basis of the coxa to apex of the tarsus, excluding pre-tarsus.
The following abbreviations are used in this paper for morphological characters: Dsl – dorsal shield length; Dsw – dorsal shield width; Vsl – ventrianal shield length; Vsw ZV2 – ventrianal shield width at ZV2 setae level; Nbf – number of teeth on the fixed digit; Nbm – number of teeth on the movable digit.
All examined materials are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia.
Results
Family Phytoseiidae Berlese, 1916
Subfamily Amblyseiinae Muma, 1961
Tribe Amblyseiini Muma, 1961
Subtribe Amblyseiina Muma, 1961
Genus Amblyseius Berlese, 1914
Type species: Zercon obtusus Koch, 1839, by original designation.
Amblyseius silvaticus (Chant) (Figs. 1-8, 26A)
Typhlodromus (Amblyseius) silvaticus Chant, 1959: 94, Figs. 216, 217.
Amblyseius (Amblyseius) sylvaticus (sic): Muma 1961: 287.
Typhlodromus silvaticus: Hirschmann 1962: 24.
Amblyseius (Amblyseius) silvaticus: Denmark & Muma 1989: 73.
Amblyseius patrius Karg, 1970: 295 (synonymized by Denmark & Muma, 1989: 73).


















Material examined — two females, Russia, Tyumen province, vicinity of Tyunevo, 57°22' N, 65°41' E, 25 September 2018, A. Khaustov coll., on mushrooms of Armillaria genus; 8 females and 5 males, Russia, Tyumen province, Uspenka state zoological reserve, 57°04'N, 65°04'E, 28 June 2019, A. Khaustov coll., on bark of Picea obovata Ledeb., Pinus sylvestris L., (Pinaceae) and Betula pendula Roth., (Betulaceae).
World distribution — Cuba, England, Finland, Norway, Switzerland (Demite et al. 2020).
Redescription — Female (Figs. 1, 2B-2H, 3, 4, 6, 7A, 7B, 7D, 7E, 26A) (n = 10)
Idiosomal setal pattern – 10A:9B/JV–3:ZV.
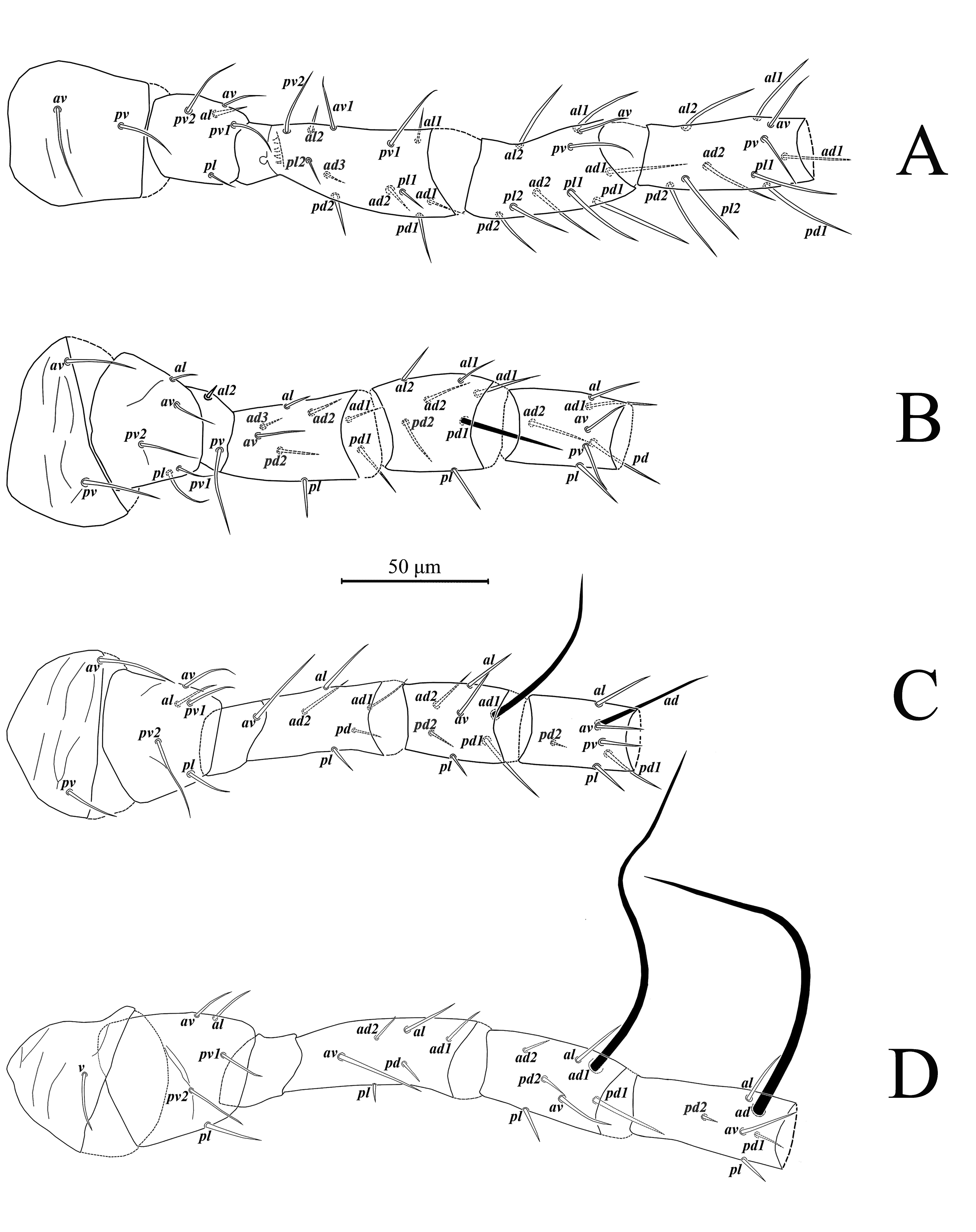
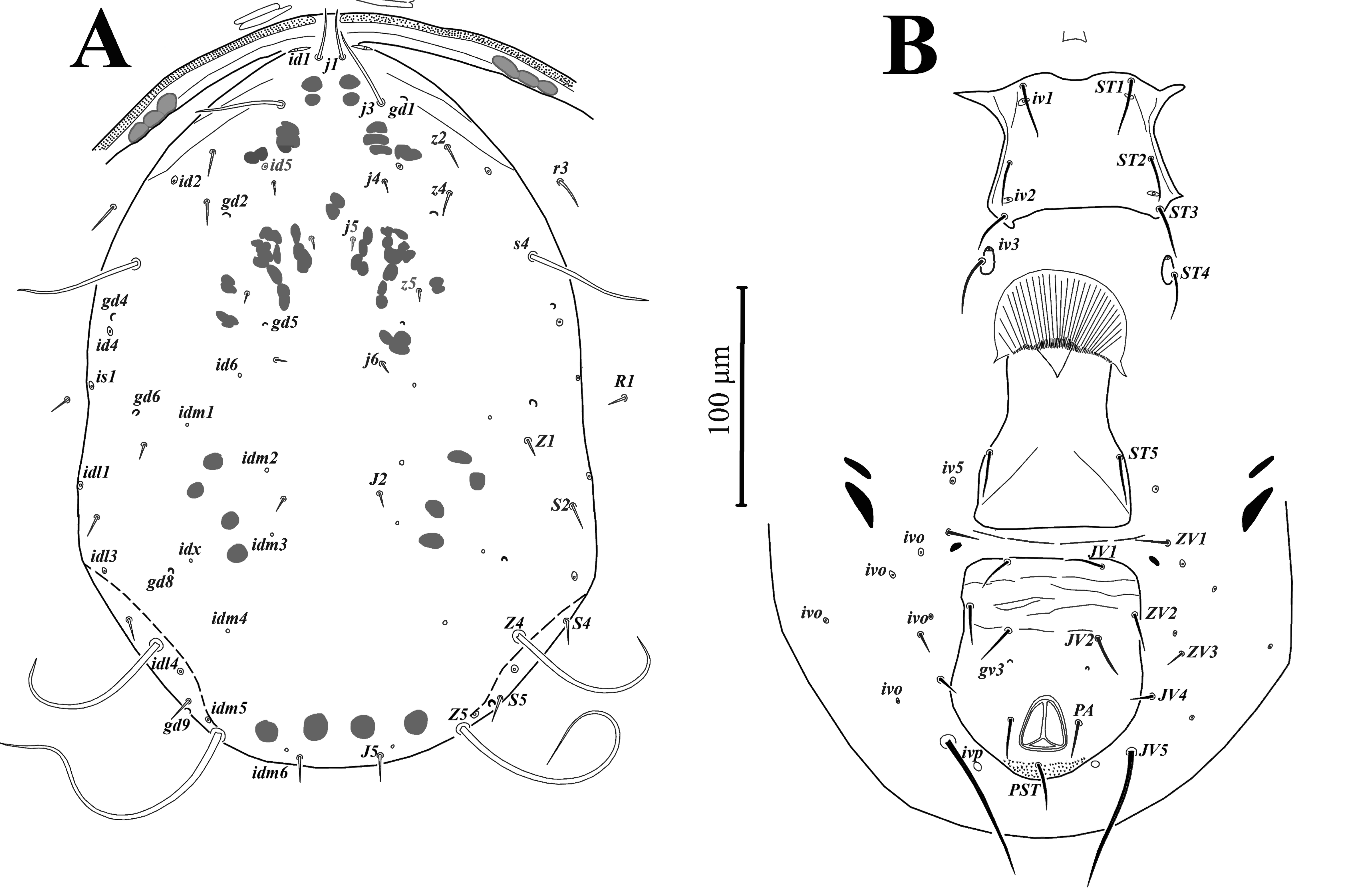
Dorsal idiosoma (Figs. 1A, 7A) – Dorsal shield oval, smooth, 372 (351-386) long and 250 (240-266) wide; with 19 pairs of setae (included r3 and R1), all setae smooth, except J5, Z4 and Z5, slightly barbed; length of setae: j1 34 (30-38), j3 61 (58-67), j4 6 (5-7), j5 5 (4-6), j6 6 (5-7), J2 6 (5-6), J5 9 (8-10), z2 20 (18-21), z4 37 (31-43), z5 5 (5-6), Z1 6 (5-7), Z4 146 (134-160), Z5 247 (233-258), s4 113 (107-116), S2 7 (7-9), S4 7 (6-7), S5 7 (6-8); setae r3 26 (24-28) and R1 9 (8-9) on lateral soft cuticle; dorsal shield with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, gd9) and 16 pairs of poroids.
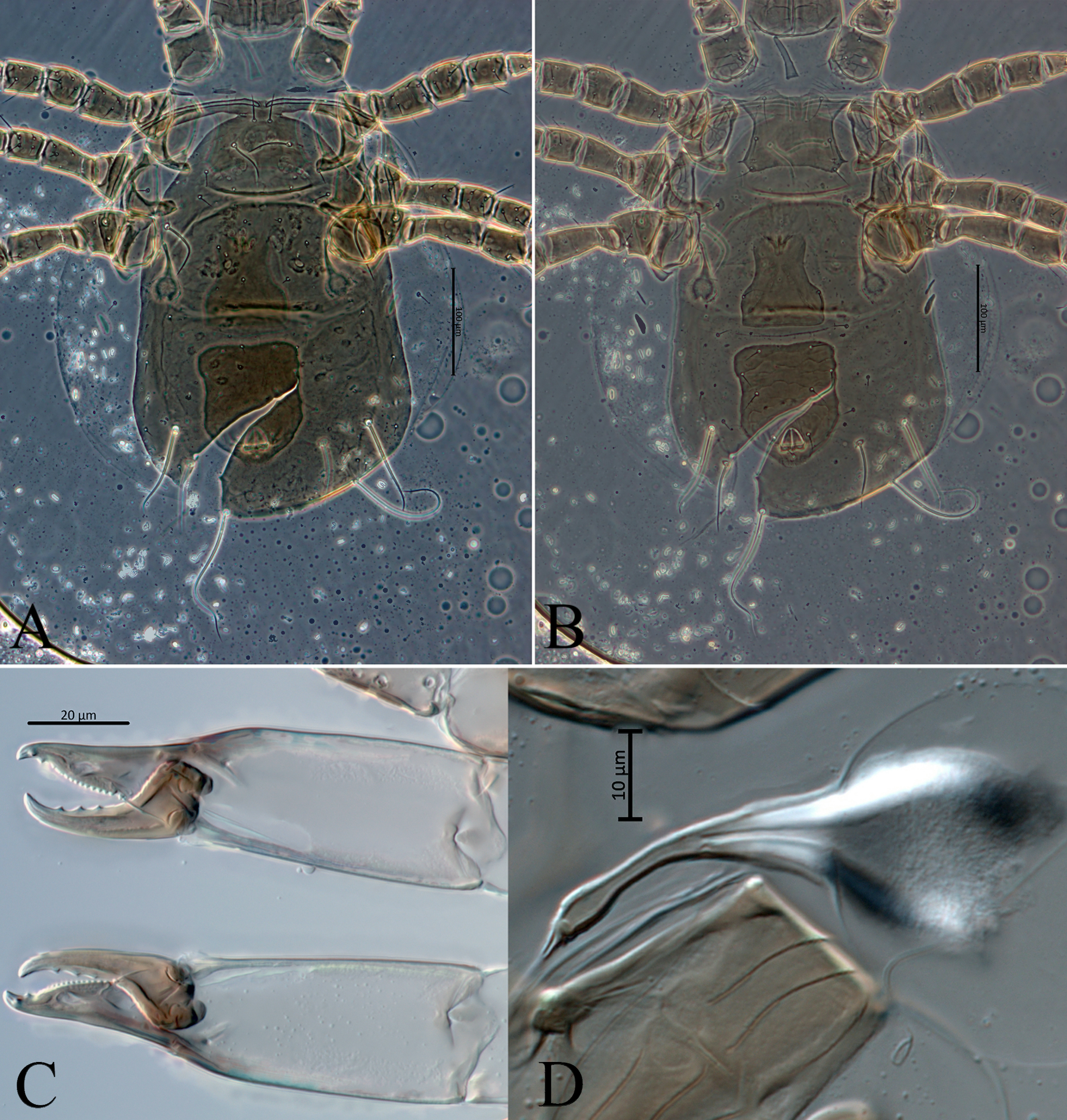
Gnathosoma (Figs. 2A – 2D, 2F, 2G) – Anterior margin of epistome bump-like and smooth. Hypostomal groove with seven transverse rows of denticles, each row with two teeth; subcapitular setae h1, h2, h3 subequal (28-30), slightly shorter than palp coxal setae (pc) 34-35. Chaetotaxy of palps: trochanter with two setae v1, v2; femur with five setae, thickened and apically spatulate antero-lateral al, three dorsal (d1, d2, d3) and one postero-lateral (pl); genu with six setae, antero-lateral setae (al1 and al2) thickened , three dorsal setae (d1, d2, d3) and one postero-lateral (pl); tibia with 14 setae, one antero-lateral (al), eight dorsal d1 – 8, two setae di-1, di-2, arise from the dorsal surface at the distal end, two ventral (v1, v2) and one postero-lateral (pl); tarsus with 15 setae (six simple d1, d2, d3, v1, v2, v3; nine stout setae with rounded tips di-1 to di-9) and two-tined apotele (Fig. 2C).
Chelicera (Figs. 2B, 7D) – fixed digit 34 (31-37) long, with 10-11 teeth and pilus dentilis; movable digit 34 (31-38) long, with three teeth.
Ventral idiosoma (Figs. 1B, 7B) – Tritosternum with paired pilose laciniae 96-97, fused basally 41-44, columnar base 16-17 × 12-13 wide. Sternal shield smooth, 71 (68-72) long and 80 (78-82) wide, with three pairs of setae ST1 38 (35-43), ST2 32 (30-35), ST3 32 (31-33) and two pairs of lyrifissures iv1, iv2. Setae ST4 34 (32-36) located on small separate metasternal platelets, each with one pore iv3.
Genital shield smooth, 80 (75-84) wide at level of base of setae ST5 35 (32-38), para-genital poroids iv5 on soft cuticle.
Opisthosomatic venter with two pairs of elongated metapodal platelets, primary 24 (22-27) and accessory 16 (12-19) long; four pairs of setae, ZV1 21 (19-24), ZV3 14 (12-16), JV4 16 (13-17), JV5 103 (92-109) long, all smooth, and four pairs of poroids.
Ventrianal shield pentagonal in shape, smooth, 120 (115-125) long and 96 (92-100) wide at level of setae ZV2, with three pairs of pre-anal setae JV1 23 (22-25), ZV2 21 (20-25), JV2 25 (23-26), with small rounded pre-anal pores gv3 (distance between pores 45 (43-47)); para-anal setae 22 (21-23) and post-anal seta 26 (24-28).
Peritreme (Figs. 1A, 2H) – extends anteriorly to setae j1.
Spermatheca (Figs. 2E, 7E) – Calyx bell-shaped, 10 (8-13) long and at the opening 8 (7-11) wide, sides of calyx curved; atrium C-shaped, connected without neck with calyx; major duct thick, minor duct not visible.
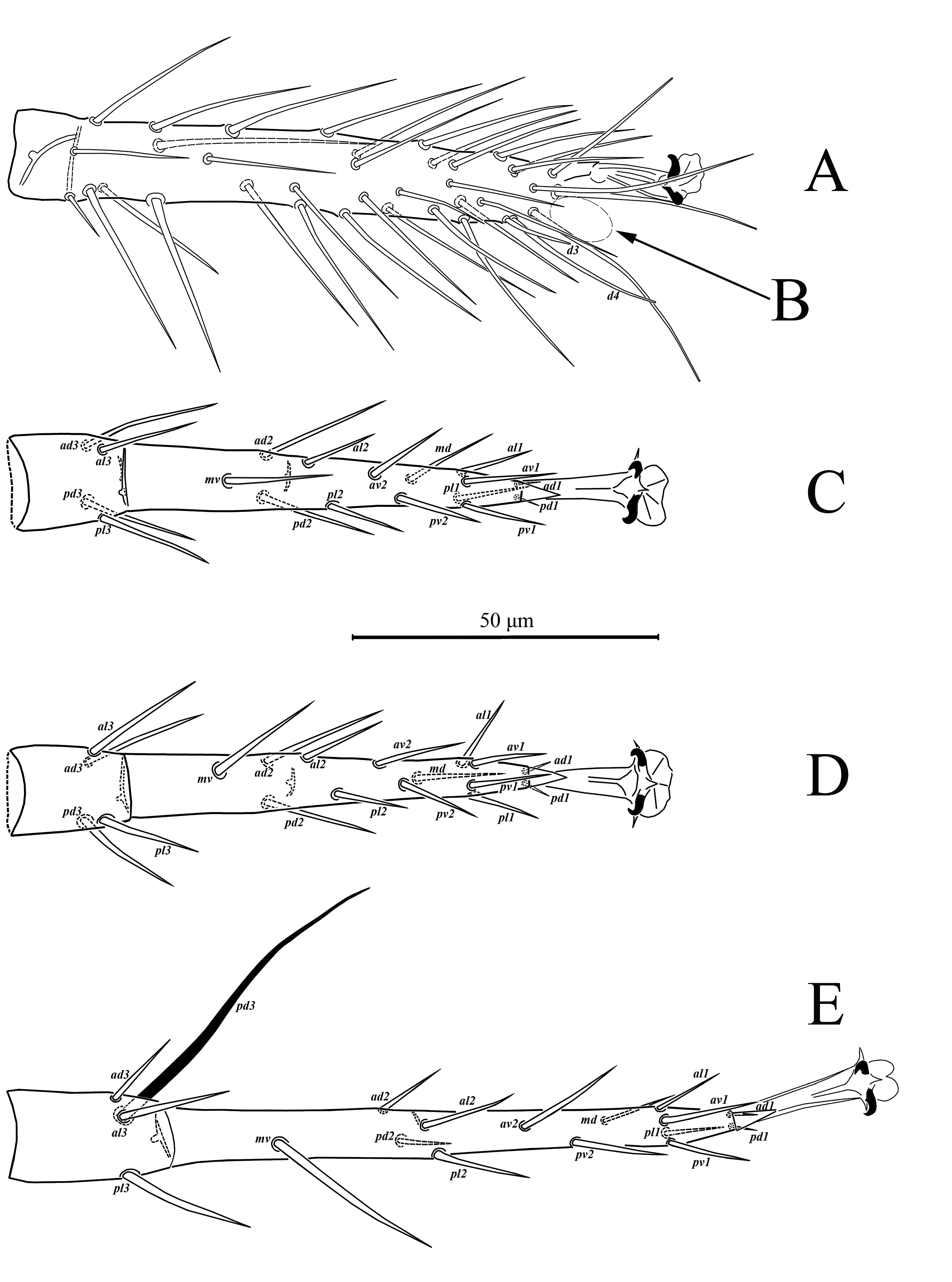
Legs (Figs. 3, 4) – Legs I 393 (386-405) and IV 425 (412-429) longer than legs II 320 (313-329) and III 320 (317-329). Chaetotaxy normal for phytoseiid mites: Leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/0 1, tibia 1 2/1 1/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1. Chaetotaxy of tarsi II-IV typical for Phytoseiidae and bears 18 setae 3 3/2 3/2 3 + mv, md. Tarsus I with 37 setae, excluding apical sensorial setal cluster. Setae d3 13-15 with rounded tip, d4 30-34. Apical sensorial setal cluster (Fig. 26A) includes 10 short setae of different shape. Setae df-1(12-14), df-3 (7-8) and df-7 (6-7) finger-shaped with blunt tips. Setae df-2 (5-6), df-5 (6-8), df-6 (12-13) and df-8 (11-12) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (8-9), df-9 (8-9) spur-like with lobe-like tips, setae df-10 (5-6) located between df-4 and df-9 also spur-like with acuminate tip. Measurements of macrosetae as follows: SgeI 36 (35-39), SgeII 40 (38-41), SgeIII 64 (58-73), SgeIV 143 (132-153), StiIII 43 (40-46), StiIV 108 (102-117), StIV 84 (78-93). All macrosetae are acuminate.
Male (Figs. 2A, 5, 7C, 8) (n = 5)



Idiosomal setal pattern – 10A:9B/JV-3,4:ZV-1,3.
Dorsal idiosoma (Figs. 5A, 8A) – Dorsal shield oval, smooth, 312 (306-316) long and 226 wide. As in female, dorsal shield with 19 pairs of setae, most of which smooth, except Z4 and Z5, slightly barbed. Setae r3 located on dorsal shield, setae R1 on lateral soft cuticle or on dorsal shield. Length of dorsal setae as follows: j1 29 (27-30), j3 49 (46-52), j4 5 (4-7), j5 5 (4-5), j6 5 (5-6), J2 5 (5-6), J5 8 (7-8), z2 18 (17-18), z4 29 (28-32), z5 5 (4-5), Z1 5, Z4 106 (104-118), Z5 168 (163-174), s4 84 (81-91), S2 7, S4 6 (5-6), S5 5 (5-6), r3 18 (17-20), R1 6 (5-6). Number and location of solenostomes and pores as in female, except gd3, which situated on dorsal shield posteriad base of setae s4.
Ventral idiosoma (Figs. 5B, 8B) – Sternogenital shield smooth with few sclerotized lines laterally, 125 (123-127) long (ST1-ST5) and 70 (69-72) wide (ST2-ST2); five pairs of setae ST1 30 (25-31), ST2 23 (21-26), ST3 24 (22-26), ST4 22 (20-25), ST5 22 (20-23) and three pairs of lyrifissures iv1, iv2, iv3.
Ventrianal shield reticulated only in anterior part, with three pairs of pre-anal setae JV1 17 (16-18), JV2 20 (17-22), ZV2 17 (16-20), one pair of anal setae PA 17 (16-17) and post-anal setae PST 20 (20-21), a pair of pre-anal pores gv3, posteriad base of setae JV2 and four pairs of poroids iv5, iv0, iv0, iv0. Ventrianal shield 139 (136-146) long, measured along midline; 185 (178-196) wide at level of anterolateral corners. Opisthogastric cuticle with one pair of setae JV5 53 (50-57) and one pair of poroids ivp.
Chelicera (Figs. 2A, 7C) – Fixed digit 25 (23-26) long, with 9-10 teeth and pilus dentilis; movable digit 25 long with 1 tooth. Spermatodactyl as in Figs. 2A, 7C; shaft of spermatodactyl 14 (13-14) long.
Legs – Legs I 344 (335-346) and IV 371 (367-374) longer than legs II 283 (278-289) and III 288 (279-289). Legs chaetotaxy as in female. Measurements of macrosetae as follow: SgeII 33 (31-36), SgeIII 45 (41-47), SgeIV 97 (91-102), StiIII 36 (33-38), StiIV 72 (66-73), StIV 71 (67-75).
Remarks — In Denmark and Muma (1989), Amblyseius silvaticus is placed in the silvaticus species group. Also, Denmark and Muma (1989) synonymized A. silvaticus and A. patrius Karg. Karg and Huhta (2009) then removed this synonymy and updated the identification key of the species group. According to the key of Karg and Huhta (2009), the present specimens from Western Siberia are intermediate between A. silvaticus and A. tavasticus in having ratio lengths of setae z2/z3 as in A. tavasticus and shape of spermatheca and length of setae Z5 as in A. silvaticus. Also, A. tavasticus in original description differs in number of teeth on fixed digit (14-16 versus 10-11 in the present Siberian specimens) and presence of delicate transverse lines on the dorsal shield. At my request, Drs Veikko Huhta (University of Jyväskylä, Finland) and Axel Christian (Senckenberg National Museum, Görlitz, Germany) examined paratypes of A. tavasticus deposited in Museum of Zoology, Helsinki, Finland and Senckenberg National Museum, Görlitz, Germany, respectively. They confirmed the absence of transverse lines on the dorsal shield. In specimens from Senckenberg National Museum, the number of teeth on fixed digit was about 10-11. The only relevant difference between A. silvaticus and A. tavasticus is thus the shape of calyx of spermatheca. In A. tavasticus it is wider than long, while in A. silvaticus the length and width of calyx is subequal (figure 2E). Siberian specimens show some variability in length/width ratio of calyx from equal length and width to length slightly longer than width. Both species have the same habitats and live on bark of trees, mainly in spruce forests. In my opinion, A. tavasticus is likely a potential junior synonym of A. silvaticus.



Amblyseius ampullosus Wu and Lan
Amblyseius ampullosus Wu & Lan, 1991: 316.
Amblyseius (Amblyseius) ampullosus: Wu et al. 2009: 199.

















Material examined — seven females, one male, Russia, Kurgan province, vicinity of Zverinogolovskoe, 54°27' N, 64°51' E, 28 September 2019, A. Khaustov coll., from sod.
World distribution — China, Iran (Demite et al. 2020).
Redescription — Female (Figs. 9, 10A, 10C – 10H, 11, 12, 14A – 14C, 14E, 26B) (n = 5)
Idiosomal setal pattern – 10A:9B/JV–3:ZV.
Dorsal idiosoma (Figs. 9A, 14A) – Dorsal shield oval, smooth, 333 (327-344) long, 230 (227-236) wide, with 19 pairs of setae (included r3 and R1), all setae smooth. Length of setae: j1 22 (21-24), j3 40 (39-43), j4 6, j5 5 (5-6), j6 5 (5-6), J2 5 (5-6), J5 11 (11-12), z2 12 (11-14), z4 12 (10-14), z5 5, Z1 6 (6-7), Z4 86 (82-91), Z5 139 (132-148), s4 58 (56-64), S2 9 (9-10), S4 10 (9-10), S5 11; setae r3 15 and R1 9 (8-9) situated on lateral soft cuticle; dorsal shield with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, gd9), and 16 pairs of poroids.
Gnathosoma (Figs. 10A-10D, 10F, 10G) – Anterior margin of epistom smoothly mucronate. Hypostomal groove with seven transverse rows of denticles, each row with two teeth; subcapitular setae h1, h2, h3 and palp coxal setae pc are subequal in length 21-24. Chaetotaxy of palps as in A. silvaticus.
Chelicera (Figs. 10A, 14C) – fixed digit 27 (26-27) long, with four teeth and pilus dentilis; movable digit 27 (26-28) long, with two teeth.
Ventral idiosoma (Figs. 9B, 14B) – Tritosternum with paired pilose laciniae 74-77, fused basally 28-31, columnar base 14-16 × 9-10 wide. Sternal shield smooth, 60 (58-62) long and 65 (64-65) wide, with 3 pairs of setae ST1 27 (26-28), ST2 23 (22-24), ST3 26 (24-27) and two pairs of lyrifissures iv1, iv2. Setae ST4 26 (24-26) situated on small separate metasternal platelets, each with one pore iv3.
Genital shield smooth, 59 (58-61) wide at level of base setae ST5 25 (24-26), para-genital poroids iv5 on soft cuticle.
Opisthosomatic venter with two pairs of elongate metapodal platelets, primary 22 (20-26) and accessory 14 (13-16) long; soft cuticle with four pairs of setae, ZV1 16, ZV3 10 (9-10), JV4 10 (8-11), JV5 68 (67-69) long, all smooth, and six pairs of poroids.
Ventrianal shield pentagonal, with reticulation in the anterior area, 104 (103-113) long and 86 (81-89) wide, with three pairs of pre-anal setae JV1 17 (16-17), ZV2 17 (16-18), JV2 20 (20-21) long with small rounded pre-anal pores gv3 (distance between pores 41 (35-43)); para-anal setae PA 18 (17-19) and post-anal seta PST 22 (21-22) long.
Peritreme (Figs. 9A, 10H) – extends anteriorly to setae j1.
Spermatheca (Figs. 10E, 14E) – Calyx funnel-shaped, 17 (16-20) long and at the opening 14 (13-15) wide; atrium nodular-like, connected without neck with calyx; major duct thick, minor duct visible.
Legs (Figs. 11, 12, 26B) – Legs I 351 (347-357) and IV 339 (335-342) longer than legs II 259 (255-264) and III 256 (250-258). Chaetotaxy normal for phytoseiid mites: Leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/1 1, tibia 1 2/1 1/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1. Chaetotaxy of tarsi II-IV typical for Phytoseiidae, with 18 setae 3 3/2 3/2 3 + mv, md. Tarsus I bears 37 setae, excluding apical sensorial setal cluster. Setae d3 26-28 with rounded tip, d4 33-35. Apical sensorial setal cluster (Fig. 26B) includes 10 short setae of different shape. Setae df-1(12), df-2 (6-7), df-3 (8) and df-7 (8) finger-shaped with blunt tips. Setae df-5 (6), df-6 (17) and df-8 (10-11) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (10-11), df-9 (8-10) spur-like with lobe-like tips, setae df-10 (5-6) located between df-4 and df-9 also spur-like with acuminate tip.
Measurements of macrosetae as follows: SgeII 30 (27-32), SgeIII 31 (29-34), SgeIV 69 (65-75), StiIV 53 (47-59), StIV 55 (51-59). All macrosetae are spine-like and acuminate.



Male (Figs. 10B, 13, 14D, 15) (n = 1)



Idiosomal setal pattern – 10A:9B/JV-3,4:ZV-1,3
Dorsal idiosoma (Figs. 13A, 15A) – Dorsal shield oval, smooth, 291 long and 219 wide. As in the female, dorsal shield with 19 pairs of setae, all smooth. Setae r3 and R1 situated on dorsal shield. Length of setae: j1 19, j3 33, j4 5, j5 5, j6 5, J2 5, J5 8, z2 9, z4 8, z5 5, Z1 5, Z4 70, Z5 107, s4 47, S2 9, S4 8, S5 8, r3 13, R1 7. Number and location of solenostomes and pores as in female, except gd3, which situated on dorsal shield posteriad base of setae s4.
Ventral idiosoma (Figs. 13B, 15B) —- Sternogenital shield smooth, 106 long (ST1-ST5) and 58 wide (ST2-ST2); 5 pairs of setae ST1 17, ST2 16, ST3 19, ST4 16, ST5 14 and three pairs of lyrifissures iv1, iv2, iv3.
Ventrianal shield reticulated on anterior part, with three pairs of pre-anal setae JV1 15, JV2 17, ZV2 14, pair of anal setae PA 15 and post-anal setae PST 21, a pair of pre-anal pores gv3, posteriad base of setae JV2 and four pairs of poroids iv5, ivo, ivo, ivo. Ventrianal shield 131 long, measured along midline; 152 wide at level of anterolateral corners. Opisthogastric soft cuticle with one pair of setae JV5 29 and three pair of poroids ivp, ivo, ivo.
Chelicera (Figs. 10B, 14D) – Fixed digit 23 long, with three teeth and pilus dentilis; movable digit 24 long with one tooth. Spermatodactyl as in Fig. 10B; spermatodactyl shaft 16 long.
Legs chaetotaxy as in female. Measurements of macrosetae as follow: SgeII 23, SgeIII 25, SgeIV 50, StiIV 39, StIV 51.
Remarks — Amblyseius ampullosus is very similar to A. verginensis Papadoulis, 1995, but differs in atrium shape of spermatheca, slightly bulbous and C-shaped in A. verginensis (vs. well sclerotized and nodular-like in A. ampullosus) (Figure 10E). This species was originally described from China, mountain He-Lan-Shan, on Artemisia sp. (Wu and Lan, 1991). It was also recorded and redescribed from Iran, from soil (Shirdel et al., 2009), and this is the first record from Russia.
My newly collected material agree very well with the original description given by Wu and Lan (1991).
The male of A. ampullosus is described for the first time.
Amblyseius krantzi (Chant)
Typhlodromus (Amblyseius) krantzi Chant, 1959: 83.
Amblyseius (Amblyseius) krantzi: Muma 1961: 287.
Typhlodromus krantzi: Hirschmann 1962: 23 Fig 232.
Amblyseius krantzi: Wainstein 1975: 920.
Typhlodromips krantzi: Moraes et al. 1986: 142
Typhlodromips krantzi: Moraes et al. 2004: 215.
World distribution — Alaska, Canada, Kazakhstan, Poland, Russia, USA (Demite et al. 2020).
Material examined — 12 females, Russia, Tyumen province, Uspenka state zoological reserve, 57°04'N, 65°04'E, 07 August 2018 and 28 June 2019, V.A. Khaustov coll., on leaves of Urtica dioica L. (Urticaceae) and Geranium sylvaticum L. (Geraniaceae); 32 females and 2 males, Russia, Tyumen province, vicinity of Tyunevo, 57°22' N, 65°41' E, 12 October 2017, 25 September 2018, V.A. Khaustov coll., on leaves of Geum rivale L. (Rosaceae), Aegopodium podagraria L. (Apiaceae) and Filipendula ulmaria (L.) Maxim., (Rosaceae); 1 female, Russia, Tyumen province, vicinity of lake Kuchak, 57°21'N, 66°03'E, 31 July 2018, V.A. Khaustov coll., on leaves of Rubus saxatilis L. (Rosaceae).
Supplementary description — Female Tarsus I bears 37 setae, excluding apical sensorial setal cluster. Setae d3 13-15 with rounded tip, d4 25. Apical sensorial setal cluster (Fig. 26C) includes 10 short setae of different shape. Setae df-1(13), df-2 (4), df-3 (9) and df-7 (8) finger-shaped with blunt tips. Setae df-5 (8-9), df-6 (12-13) and df-8 (11) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (10-11), df-9 (10) spur-like with lobe-like tips, setae df-10 (5-6) located between df-4 and df-9 also spur-like with acuminate tip.
Remarks — This species was originally described from Nakusp, British Columbia, Canada, on Ranunculus sp. as T. (Amblyseius) berlesei (Chant 1957). In 1959, this species was renamed to A. krantzi (Chant 1959). In Russia, this species was previously recorded from the Moscow (Meshkov 1999) and Yaroslavl provinces (Wainstein 1975). This species is recorded for the first time in Asian Russia. The characteristics of the specimens herein considered agree well with those of the descriptions given by Chant and Hunsell (1971), Congdon (2002) and Kolodochka and Gwiazdowicz (2016). However, the number of teeth on the movable digit in the material here observed is a variable character as some females had two teeth instead of three in the original description and further redescriptions. This species is common on grassy plants in dark spruce forests or in mixed forests with a predominance of spruce. It could be often found together with Amblyseius rademacheri Dosse (personal observation).
Amblyseius meridionalis Berlese
Amblyseius obtusus var. meridionalis Berlese, 1914: 144.
Typhlodromus obtusus var. meridionalis: Chant 1957: 306.
Amblyseius meridionalis: Athias-Henriot 1958: 32
Typhlodromus (Amblyseius) meridionalis: Chant 1959: 85.
Amblyseius (Amblyseius) meridionalis: Muma 1961: 287.
Typhlodromus meridionalis: Hirschmann 1962: 23.
Typhlodromus (Typhlodromus) meridionalis: Westerboer & Bernhard 1963: 690.
Amblyseius (Pauciseius) meridionalis: Denmark & Muma 1989: 131.
Amblyseius calicis Karg, 1960: 444 (synonymized by Karg, 1971: 214).
Amblyseius spiramentatus Athias-Henriot, 1961: 429 (synonymized by Ueckermann & Loots, 1988: 79).
World distribution — Algeria, Azerbaijan, Canada, France, Germany, Greece, Hungary, Iceland, Iran, Italy, Latvia, Moldova, Morocco, Poland, Spain, Russia (Livshitz and Kuznetsov 1972), Switzerland, Tunisia, Turkey, Ukraine, USA (Demite et al. 2020).
Material examined — three females, Russia, Tyumen Province, vicinity of Malinovka, 55°06' N, 65°04 E, 08 May 2019, A. Khaustov coll., in the soil.
Supplementary description — Female Tarsus I with 37 setae, excluding apical sensorial setal cluster. Setae d3 25 with rounded tip, d4 34. Apical sensorial setal cluster (26D) includes 10 short setae of different shape. Setae df-1(13), df-3 (8) and df-7 (8) finger-shaped with blunt tips. Setae df-2 (7), df-5 (5), df-6 (16) and df-8 (10) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (11), df-9 (8) spur-like with lobe-like tips, setae df-10 (6) situated between df-4 and df-9 also spur-like with acuminate tip.
Remarks — This species was originally described from Potenza, Basilicata, Italy, in humus (Berlese 1914). It is known from 21 countries in the Palearctic and Nearctic regions (Moraes et al. 2004; Demite et al. 2020). It was previously recorded from leaves of Poterium polygamum (Rosaceae) in Crimea, Russia by Livshitz and Kuznetsov (1972). Siberian specimens well agree with redescription given by Faraji et al. (2011). This species is recorded for the first time in Asian Russia.
Amblyseius rademacheri Dosse
Amblyseius rademacheri Dosse, 1958a: 44.
Typhlodromus (Amblyseius) rademacheri: Chant 1959: 89.
Amblyseius (Typhlodromopsis) rademacheri: Muma 1961: 287.
Typhlodromus rademacheri: Hirschmann 1962: 25 Fig 222.
Typhlodromus (Typhlodromus) rademacheri: Westerboer & Bernhard 1963: 658.
Amblyseius (Amblyseius) rademacheri: Ehara 1966: 23.
Amblyseius (Typhlodromips) rademacheri: Karg 1971:185.
Typhlodromips rademacheri: Moraes et al. 1986: 145.
Amblyseius (Neoseiulus) rademacheri: Ehara & Amano 1998: 31.
Typhlodromips khnzoriani Wainstein & Arutunjan, 1970: 1498 (synonymized by Wainstein 1975: 658).
World distribution — Armenia, Austria, Azerbaijan, China, Czechoslovakia, Denmark, Georgia, Germany, Hungary, Iran, Italy, Japan, Latvia, Moldova, Netherlands, Poland, Russia, Slovakia, Slovenia, South Korea, Spain, Switzerland, Ukraine (Demite et al. 2020).
Material examined — 22 females, 1 male, Russia, Tyumen Province, vicinity of lake Kuchak, 57°21'N, 66°03'E, 31 June 2018, V. Khaustov coll., on leaves of Geum rivale L. (Rosaceae); 28 females, 2 males, Russia, Tyumen Province, Uspenka state zoological reserve, 57°04'N, 65°04'E, 26 July 2018, 07 August 2018, 12 July 2019, 15 August 2019, V. Khaustov coll., on leaves of Arctium lappa L. (Asteraceae), Salix caprea L. (Salicaceae), Rubus idaeus L. (Rosaceae), Urtica dioica L. (Urticaceae); 29 females, 8 males, Tyumen Province, vicinity of Verkhniy Bor, 57°13'N, 65°28'E, 16 September 2017, 08 July 2018, 12 July 2018, V. Khaustov coll., on leaves of Lilium martagon L. (Liliaceae), Filipendula ulmaria (L.) Maxim., (Rosaceae), Valeriana wolgensis Kazak. (Valerianaceae), Geranium sylvaticum L. (Geraniaceae), Agrimonia pilosa Ledeb. (Rosaceae), Aegopodium podagraria L. (Apiaceae), Humulus lupulus L. (Cannabaceae); 14 females, 4 males, Russia, Tyumen Province, forest park Zatyumensky, 57°09' N, 65°27' E, 24 August 2019, 13 September 2019, V. Khaustov coll., on leaves of Geranium sylvaticum L. (Geraniaceae) and Aegopodium podagraria L. (Apiaceae).
Supplementary description — Female Tarsus I with 37 setae, excluding apical sensorial setal cluster. Setae d3 15 with rounded tip, d4 30-32. Apical sensorial setal cluster (27A) includes 10 short setae of different shape. Setae df-1(14), df-3 (9) and df-7 (7-8) finger-shaped with blunt tips. Setae df-2 (5-6), df-5 (8), df-6 (12) and df-8 (10-12) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (9), df-9 (9) spur-like with lobe-like tips, setae df-10 (6) situated between df-4 and df-9 also spur-like with acuminate tip.
Remarks — All measurements and morphological characters of Siberian specimens are very close to those of the redescription of Kolodochka and Gwiazdowicz (2016). This species is the most abundant in the investigated area. It is common on various grassy plants and often found with another species of Phytoseiidae mites in the present survey.
Amblyseius obtusus (Koch)
Zercon obtusus Koch, 1839: 13.
Seius obtusus: Berlese 1889: 7.
Typhlodromus obtusus: Chant 1957: 306.
Typhlodromus (Amblyseius) obtusus: Chant 1959: 90.
Amblyseius (Amblyseius) obtusus: Muma 1961: 287.
Typhlodromus affatisetus Wainstein, 1960: 683 (synonymized by Wainstein 1975: 916).
Amblyseius microsetae Muma, 1961: 289 (synonymized by Denmark & Muma 1989: 7).
Amblyseius hamizensis Athias-Henriot, 1961: 421 (synonymized by Wainstein 1975: 916).
Amblyseius rabdus Denmark, 1965: 95 (synonymized by Denmark & Muma, 1989: 7).
Amblyseius kurashvilii Gomelauri, 1968b: 515 (synonymized by Denmark & Muma 1989: 7).
Amblyseius isuki Chant & Hansell, 1971: 714 (synonymized by Wainstein 1975: 916).
Amblyseius bajulus Chaudhri, Akbar & Rasool, 1979: 70 (synonymized by Denmark & Muma 1989: 7).
World distribution — Algeria, Argentina, Armenia, Azerbaijan, Azores, Canada, Chile, Costa Rica, Croatia, Cuba, Czech Republic, England, France, Germany, Georgia, Greece, Haiti, Hawaii, Hungary, Iran, Ireland, Italy, Kazakhstan, Latvia, Moldova, Morocco, New Zealand, Norway, Pakistan, Poland, Portugal, Romania, Russia, Slovakia, Spain, Sweden, Tunisia, Turkey, Ukraine, USA, Venezuela (Demite et al. 2020).
Material examined — two females, Russia, Tyumen Province, vicinity of lake Kuchak, 57°21'N, 66°03'E, 27 April 2018, 11 August 2018, A. Khaustov coll., from humus; one female, Russia, Tyumen Province, forest park Zatyumensky, 57°09' N, 65°27' E, 19 April 2019, A. Khaustov coll., from moss.
Supplementary description — Female Tarsus I with 37 setae, excluding apical sensorial setal cluster. Setae d3 18-19 with rounded tip, d4 33-35. Apical sensorial setal cluster (27B) includes 10 short setae of different shape. Setae df-1(14), df-3 (8) and df-7 (8-9) finger-shaped with blunt tips. Setae df-2 (7), df-5 (6), df-6 (16-17) and df-8 (11-12) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (10), df-9 (9-10) spur-like with lobe-like tips, setae df-10 (5) situated between df-4 and df-9 also spur-like with acuminate tip.
Remarks — Amblyseius obtusus is a cosmopolitan species, reported in more than 30 countries (Demite et al., 2020). All measurements and morphological characters of Siberian specimens are very close to redescription of Döker et al. (2020). It is a first record of A. obtusus in Asian Russia.
Amblyseius omaloensis Gomelauri
Amblyseius omaloensis Gomelauri, 1968a: 702
Amblyseius benefactor Meshkov, 1991: 139 (synonymized by Meshkov 1999: 430).













World distribution — Georgia, Russia, Lithuania, Moldova (Kolodochka 2006; Demite et al. 2020).
Material examined — seven females, Russia, Tyumen Province, Uspenka state zoological reserve, 57°04'N, 65°04'E, 02 October 2017, 06 September 2019, A. Khaustov coll., from moss.
Redescription — Female (Figs. 16, 17, 18, 19, 20, 27C) (n = 4)
Idiosomal setal pattern – 10A:9B/JV–3:ZV.
Dorsal idiosoma (Figs. 16A, 20A) – Dorsal shield oval, smooth, 353 (340-371) long and 232 (229-236) wide; with 19 pairs of setae (included r3 and R1), all setae smooth, except Z4 and Z5, slightly barbed; length of setae: j1 26 (25-27), j3 42 (40-44), j4 6 (5-6), j5 5 (5-6), j6 5 (5-6), J2 5 (5-6), J5 9 (8-10), z2 15 (15-16), z4 15 (13-16), z5 5 (5-6), Z1 6 (5-6), Z4 91 (90-92), Z5 140 (132-147), s4 63 (62-67), S2 9 (8-10), S4 8 (7-9), S5 8 (7-8); setae r3 18 (17-18) and R1 14 (13-14) on lateral soft cuticle; dorsal shield with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, gd9) and 16 pairs of poroids.
Gnathosoma (Figs. 17A, 17C, 17E, 17F) – Anterior margin of epistome bump-like and smooth. Hypostomal groove with seven transverse rows of denticles, each row with two teeth; subcapitular setae h1 31 (30-32), h2 25 (24-25), h3 25 (24-25), palp coxal setae (pc) 31 (30-31). Chaetotaxy of palps: trochanter with two setae v1, v2; femur with five setae, thickened and apically spatulate antero-lateral al, three dorsal (d1, d2, d3) and one postero-lateral (pl); genu with six setae, antero-lateral setae (al1 and al2) thickened , three dorsal setae (d1, d2, d3) and one postero-lateral (pl); tibia with 14 setae, one antero-lateral (al), eight dorsal d1 – 8, two setae di-1, di-2, arise from the dorsal surface at the distal end, two ventral (v1, v2) and one postero-lateral (pl); tarsus with 15 setae (six simple d1, d2, d3, v1, v2, v3; nine stout setae with rounded tips di-1 to di-9) and two-tined apotele (Fig. 17F).
Chelicera (Figs. 17B, 20C) – fixed digit 30 (28-34) long, with 15-16 teeth and pilus dentilis; movable digit 34 (34-36) long, with three teeth.
Ventral idiosoma (Figs. 16B, 20B) – Tritosternum with paired pilose laciniae 81-84, fused basally 34-35, columnar base 13-15 × 10-12 wide. Sternal shield smooth, 65 (64-66) long and 68 (67-69) wide, with three pairs of setae ST1 32 (27-35), ST2 26 (24-30), ST3 26 (23-27) and two pairs of lyrifissures iv1, iv2. Setae ST4 27 (25-32) situated on small separate metasternal platelets, each with one pore iv3.
Genital shield smooth, 71 (70-71) wide at level of base of setae ST5 26 (24-28), para-genital poroids iv5 on soft cuticle.
Opisthosomatic venter with two pairs of elongated metapodal platelets, primary 20 (19-21) and accessory 14 (14-15) long; four pairs of setae, ZV1 16 (16-17), ZV3 12 (10-13), JV4 14 (13-14), JV5 66 (63-70) long, all smooth, and five pairs of poroids.
Ventrianal shield pentagonal in shape, reticulate, 115 (113-117) long and 93 (91-94) wide at level of setae ZV2, with three pairs of pre-anal setae JV1 18 (17-19), ZV2 18 (17-19), JV2 21 (20-21), with small rounded pre-anal pores gv3 (distance between pores 43 (42-45)); para-anal setae 19 (18-20) and post-anal setae 20 (19-21).
Peritreme (Figs. 16A, 17G, 20A) – extends anteriorly to setae j1.
Spermatheca (Figs. 17D, 20D) – Calyx tube-like, 25 (24-25) long and at the opening 6 (6-7) wide; atrium C-shaped, connected without neck with calyx; major duct thick, minor duct not visible.
Legs (Figs. 18, 19) – Legs I 344 (340-349) and IV 350 (345-353) longer than legs II 278 (276-279) and III 271 (267-273). Chaetotaxy normal for phytoseiid mites: Leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/1 1, tibia 1 2/1 1/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1. Chaetotaxy of tarsi II-IV typical for Phytoseiidae and bears 18 setae 3 3/2 3/2 3 + mv, md. Tarsus I with 37 setae, excluding apical sensorial setal cluster. Setae d3 13 with rounded tip, d4 28-32. Apical sensorial setal cluster (27C) includes 10 short setae of different shape. Setae df-1(13), df-3 (7) and df-7 (8) finger-shaped with blunt tips. Setae df-2 (4), df-5 (6), df-6 (16) and df-8 (9-10) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (9), df-9 (8) spur-like with lobe-like tips, setae df-10 (5) situated between df-4 and df-9 also spur-like with acuminate tip. Measurements of macrosetae as follows: SgeII 30 (28-31), SgeIII 34 (32-35), SgeIV 75 (70-80), StiIV 62 (60-64), StIV 59 (58-59). All macrosetae are acuminate.



Remarks — Amblyseius omaloensis is known only from Europe (Demite et al., 2020). All measurements and morphological characters of Siberian specimens are very close to description of Meshkov (1991). It is the first record of A. omaloensis in Asian Russia.
Amblyseius myrtilli Papadoulis, Emmanouel and Kapaxidi
Amblyseius myrtilli Papadoulis, Emmanouel & Kapaxidi, 2009: 57, Fig. 33.















World distribution — Greece (Demite et al. 2020).
Material examined — six females, Russia, Tyumen Province, forest park Zatyumensky, 57°09' N, 65°27' E, 26 April 2019, A, Khaustov coll., from soil; 2 females, Russia, Kurgan Province, vicinity of Zverinogolovskoe, 54°27' N, 64°51' E, 28 September 2019, A, Khaustov coll., from soil; 2 females, Russia, Tyumen Province, vicinity of lake Kuchak, 57°21'N, 66°03'E, 27 April 2018, A, Khaustov coll., from soil.
Redescription — Female (Figs. 21, 22, 23, 24, 25, 27D) (n = 4)
Idiosomal setal pattern – 10A:9B/JV–3:ZV.
Dorsal idiosoma (Figs. 21A, 25A) – Dorsal shield broadly oval, smooth, 362 (360-363) long and 262 (246-279) wide; with 19 pairs of setae (included r3 and R1), all setae smooth; length of setae: j1 24 (22-25), j3 43 (41-46), j4 6 (6), j5 6 (5-6), j6 6 (5-6), J2 7 (6-7), J5 11 (10-12), z2 13 (11-14), z4 9 (8-10), z5 6 (6), Z1 7 (6-7), Z4 89 (86-92), Z5 164 (156-173), s4 60 (57-62), S2 11 (10-12), S4 10 (9-10), S5 11 (10-11); setae r3 15 (14-15) and R1 10 (9-11) on lateral soft cuticle; dorsal shield with seven pairs of solenostomes (gd1, gd2, gd4, gd5, gd6, gd8, gd9) and 16 pairs of poroids.
Gnathosoma (Figs. 22B, 22C, 22D, 22E) – Anterior margin of epistome bump-like and smooth. Hypostomal groove with seven transverse rows of denticles, each row with two or three teeth; subcapitular setae h1 25 (24-26), h2 24 (23-24), h3 24 (23-24), palp coxal setae (pc) 28 (27-28). Chaetotaxy of palps: trochanter with two setae v1, v2; femur with five setae, thickened and apically spatulate antero-lateral al, three dorsal (d1, d2, d3) and one postero-lateral (pl); genu with six setae, antero-lateral setae (al1 and al2) thickened , three dorsal setae (d1, d2, d3) and one postero-lateral (pl); tibia with 14 setae, one antero-lateral (al), eight dorsal d1 – 8, two setae di-1, di-2, arise from the dorsal surface at the distal end, two ventral (v1, v2) and one postero-lateral (pl); tarsus with 15 setae (six simple d1, d2, d3, v1, v2, v3; nine stout setae with rounded tips di-1 to di-9) and two-tined apotele (Fig. 22C).
Chelicera (Figs. 22A, 25C) – fixed digit 28 (27-30) long, with 3-4 teeth and pilus dentilis; movable digit 28 long, with two small teeth.
Ventral idiosoma (Figs. 21B, 25B) – Tritosternum with paired pilose laciniae 78, fused basally 25, columnar base 15-16 × 12 wide. Sternal shield smooth, 62 (59-63) long and 66 (63-67) wide, with three pairs of setae ST1 31, ST2 25 (24-25), ST3 26 (25-28) and two pairs of lyrifissures iv1, iv2. Setae ST4 23 (22-25) situated on small separate metasternal platelets, each with one pore iv3.
Genital shield smooth, 67 (66-71) wide at level of base of setae ST5 31 (29-32), para-genital poroids iv5 on soft cuticle.
Opisthosomatic venter with two pairs of elongated metapodal platelets, primary 24 (22-26) and accessory 18 (17-19) long; four pairs of setae, ZV1 16 (16-17), ZV3 8, JV4 12 (12-13), JV5 68 (67-68) long, all smooth, and five pairs of poroids.
Ventrianal shield pentagonal in shape, noticeably wider than genital shield, with reticulation in anterior part, 120 (116-123) long and 111 (106-116) wide at level of setae ZV2, with three pairs of pre-anal setae JV1 17 (16-18), ZV2 18 (16-19), JV2 24 (23-25), with small rounded pre-anal pores gv3 (distance between pores 46 (44-48)); para-anal setae 20 (19-21) and post-anal setae 20 (20-21).
Peritreme (Figs. 21A, 22F, 25A) – extends anteriorly to setae j1.
Spermatheca (Figs. 22G, 25D) – Calyx sacculus-like, 25 (23-26) long and at the opening 15 (12-16) wide; atrium C-shaped, connected without neck with calyx; major duct thick, minor duct visible in some specimens.
Legs (Figs. 23, 24) – Legs I 351 (349-353) and IV 327 (318-336) longer than legs II 270 (264-279) and III 257 (248-264). Chaetotaxy normal for phytoseiid mites: Leg I: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/2 2, genu 2 2/1 2/1 2, tibia 2 2/1 2/1 2. Leg II: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 2 3/1 2/1 1, genu 2 2/0 2/0 1, tibia 1 2/1 1/1 1. Leg III: coxa 0 0/1 0/1 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/1 1. Leg IV: coxa 0 0/1 0/0 0, trochanter 1 0/1 0/2 1, femur 1 2/1 1/0 1, genu 1 2/1 2/0 1, tibia 1 1/1 2/0 1.
Chaetotaxy of tarsi II-IV typical for Phytoseiidae and bears 18 setae 3 3/2 3/2 3 + mv, md. Tarsus I with 37 setae, excluding apical sensorial setal cluster. Setae d3 22-24 with rounded tip, d4 30. Apical sensorial setal cluster (27D) includes 10 short setae of different shape. Setae df-1(12), df-3 (7-8) and df-7 (8-9) finger-shaped with blunt tips. Setae df-2 (7), df-5 (6), df-6 (16) and df-8 (9-10) baculiform with rounded tips, setae df-8 usually curved. Setae df-4 (12-14), df-9 (9) spur-like with lobe-like tips, setae df-10 (6) situated between df-4 and df-9 also spur-like with acuminate tip. Measurements of macrosetae as follows: SgeII 31 (30-32), SgeIII 32 (31-33), StiIII 25 (24-27), SgeIV 75 (72-80), StiIV 60 (59-60), StIV 55 (52-58). All macrosetae are acuminate.



Remarks — Amblyseius myrtilli is known only from Greece, on leaves of Vaccinium myrtillis (Ericaceae) and from litter under Juniperus sp. Morphological characteristics of Siberian specimens are consistent with those provided in the original description (Papadoulis et al., 2009). It is the second record of A. myrtilli in the world and the first report in Russia. The record of A. myrtilli in Russia is not accidental, since in Greece it was found in the mountains at an altitude of 1800 m. At this altitude, the climate is moderate and more or less similar to the southwestern Siberia.
Discussion
Usually, Phytoseiidae taxonomists, pay little attention to leg chaetotaxy and only report the length of macrosetae and chaetotaxy formula of genua II and III. The chaetotaxy of tarsus I is not considered, and the utility of some characters in Phytoseiidae species identifications is discussed below.
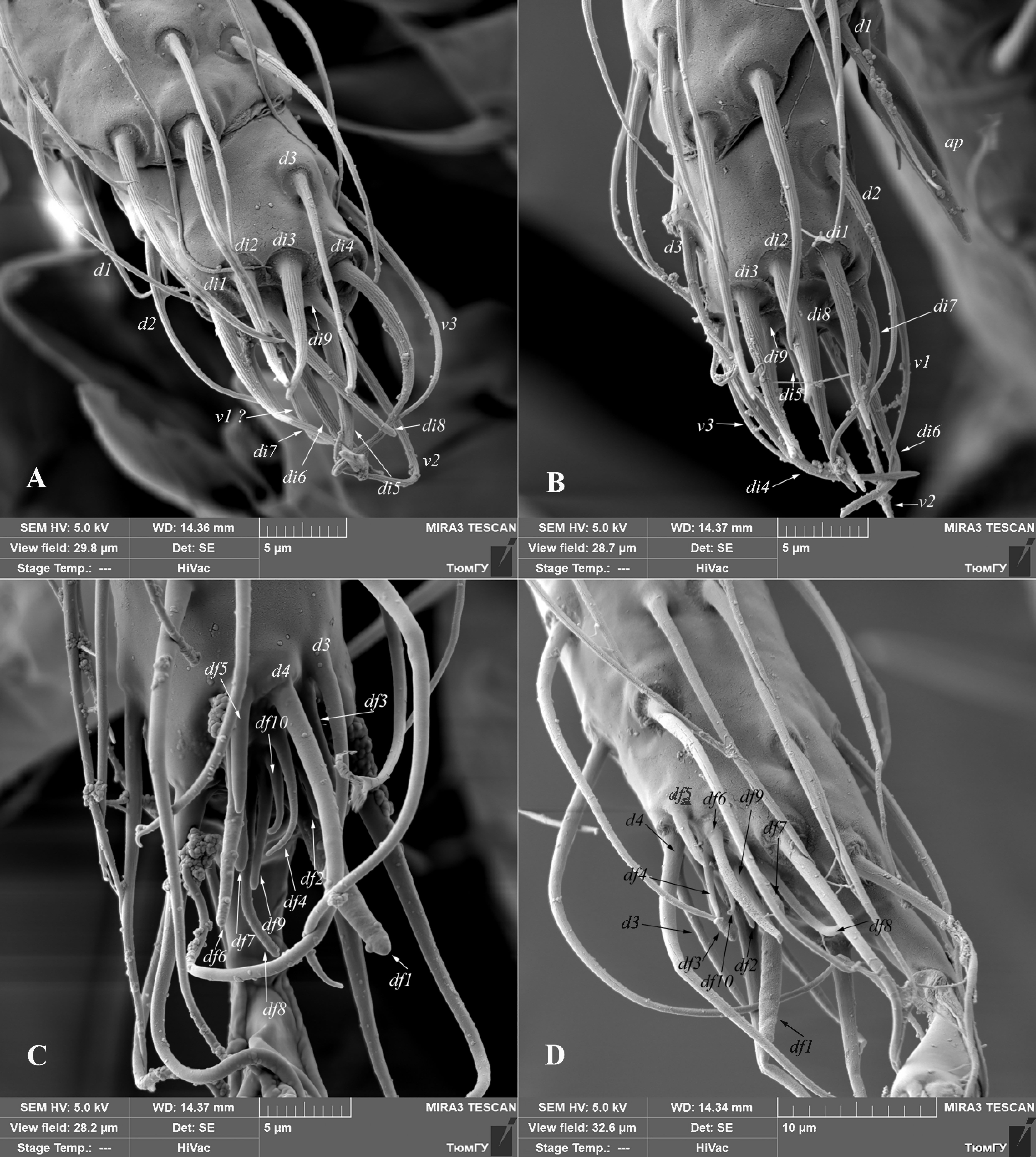
Jackson (1974) studied chaetotaxy of distal part of tarsus I, tarsus and tibia of palp in phytoseiid mites. He reviewed chaetotaxy of palp and apical sensorial setal cluster of tarsus I in detail, based on specimens of Phytoseiulus persimilis. On the distal part of tarsus I, he recognized nine group of short, peg- or spur-like setae and designated them by the prefix as df (’’dorsal field’’).



In all the Siberian Amblyseius specimens here considered, I identified on the distal part of tarsus I the group of ten modified, different in shape setae df1-df10 (Figs. 6C, 6D, 26, 27). Nine of them (df1-df9) are similar in shape and situated on the same positions as described for P. persimilis (Jackson 1974). However, I examined an additional seta in this complex, designated here as df10. This seta is similar in shape to setae df4 and df9 (spur-like), but shorter in length and always situated between them. Moreover, I discovered some differences in apical sensorial setal cluster between the Amblyseius species here considered. The most noticeable differences in the length of setae df6. For example, in A. silvaticus, A. rademacheri and A. krantzi this setae 12-13 in length vs 16-17 in other species (Figs. 26, 27). Also, the shape of setae df1 usually thickened finger-shaped in most Siberian Amblyseius species, except narrow baculiform in A. krantzi and A. rademacheri (Figs. 26C, 27A).



Relative length of setae d3 and d4 might also be useful as an additional diagnostic character. These two setae are situated posteriad df5 (Figs. 26, 27). According to relative length of setae d3 and d4, all the studied Siberian Amblyseius specimens can be divided into two groups: with relative length 1:2 (A. silvaticus, A. omaloensis, A. rademacheri, A. krantzi) and 1:1.2 (A. ampullosus, A. myrtilli, A. meridionalis) or 1:1.7 in case of A. obtusus.
All characters discussed above, are stable and were measured on several specimens of each Amblyseius species. In my opinion, tarsus I of phytoseiid mites is an important segment for identification on generic (at least Phytoseiulus and Amblyseius) and species levels and should be carefully studied in different Phytoseiidae genera. The use of sensory setae on tarsus I as diagnostic character is very limited in other groups of Mesostigmata. The best example is description of tarsal sensory cluster in some genera of Blattisocidae (Phytoseioidea) (Lindquist, Moraza 2010, 2012; Moraza, and Lindquist 2011). Leonovich (1989) provided the detailed morphological structure of setal structures in tarsal sensory complex and revealed the difference in its structure in different families of Gamasina. The correct study of all setae in tarsal sensory cluster requires high quality microscopes with DIC illumination as well as SEM microscopes; however, the most obvious diagnostic character (ratio of length of setae d3 and d4) is clearly discernable in phase contrast and even in bright field.
The chaetotaxy of palps and tarsus I are similar in the male and female for all studied species.
I studied tarsal sensory cluster only for abovementioned species of the genus Amblyseius. In the next papers on the systematics of Siberian Phytoseiidae, I am going to described tarsal sensory clusters in other specious genera, such as Transeius, Neoseiulus and Typhlodromus.
Acknowledgements
The author thanks to Drs Omid Joharchi and Alexander Khaustov (Tyumen State University) for valuable suggestions and materials of phytoseiid mites. Special thanks to Drs Veikko Huhta (University of Jyväskylä, Finland) and Axel Christian (Senckenberg National Museum, Görlitz, Germany) for the information about paratypes of Amblyseius tavasticus. I also thank to Alexey Gubin (Tyumen State University) for SEM photos. The present research was supported by the grant from the Russian Science Foundation, project No. 20-64-47015 to Dr. A. A. Khaustov.
References
Athias-Henriot C. 1958. Phytoseiidae et Aceosejidae d'Algérie. II. Phytoseiidae. Clé des genres Amblyseius Berlese (Suite) et Seiulus Berlese. Bull. Soc. Hist. Nat. Afr. Nord., 49: 23-43.
Athias-Henriot C. 1961. Mésostigmates (Urop. excl.) édaphiques Méditerranéens (Acaromorpha, Anactinotrichida). Acarologia, 3(4): 381-509.
Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides, protoadéniques, Phytoseiidae). Acarologia, 17(1): 20-29.
Beglyarov G.A. 1981. Keys to the determination of phytoseiid mites of the USSR. Information Bulletin International Organization for Biological Control of Noxious Animals and Plants, East Palearctic Section, Leningrad, Russia, 2: 97 pp. [In Russian].
Beard J.J. 2001. A review of Australian Neoseiulus Hughes and Typhlodromips De Leon (Acari: Phytoseiidae: Amblyseiinae). Inverterbr. Taxon., 15: 73-158. doi:10.1071/IT99017
Berlese A. 1889. Acari, Myriopoda et Scorpiones hucusque in Italia reperta. Tipografia del Seminario, Padova, 6(54): 7-9.
Berlese A. 1914. Acari nuovi. Manipulus IX. Redia, 10: 113-150.
Berlese A. 1916. Centuria terza di Acari nuovi. Redia 12: 289-338.
Congdon B.D. 2002. The family Phytoseiidae (Acari) in western Washington State with descriptions of three new species. Intern. J. Acarol., 28(1): 3-27. doi:10.1080/01647950208684275
Chant D.A. 1957. Descriptions of some phytoseiid mites (Acarina, Phytoseiidae). Part I. Nine new species from British Columbia with keys to the species of British Columbia. Part II. Redescriptions of eight species described by Berlese. Can. Entomol., 89(7): 289-308. doi:10.4039/Ent89289-7
Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. doi:10.4039/entm9112fv
Chant D.A., Hansell R.I.C. 1971. The genus Amblyseius (Acarina: Phytoseiidae) in Canada and Alaska. Can. J. Zool., 49(5): 703-758. doi:10.1139/z71-110
Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and subgenera of the Phytoseiidae of the world. Indira Publishing House, West Bloomfield, USA, 219 pp.
Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol., 17(3): 187-199. doi:10.1080/01647959108683906
Chaudhri W.M., Akbar S., Rasool A. 1979. Studies on the predatory leaf inhabiting mites of Pakistan. University of Agriculture, Faisalabad, Pakistan, 243 pp.
Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2020. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae

Denmark H.A. 1965. Four new Phytoseiidae (Acari: Mesostigmata) from Florida. Fla. Entomol., 48(2): 89-95. doi:10.2307/3493097
Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occasional Papers of the Florida State Collection of Arthropods, 4, 149 pp.
Döker I., Kazak C., Karut K. 2020. The genus Amblyseius Berlese (Acari: Phytoseiidae) in Turkey with discussion on the identity of Amblyseius meridionalis. Syst. Appl.Acarol, 25(8): 1395-1420. doi:10.11158/saa.25.8.4
Dosse G. 1958. Uber einige neue Raubmilbenarten (Acari: Phytoseiidae). Pflanz. Berichte, 21: 44-61.
Ehara S. 1966. A tentative catalogue of predatory mites of Phytoseiidae known from Asia, with descriptions of five new species from Japan. Mushi, 39: 9-30.
Ehara S., Amano H. 1998. A revision of the mite family Phytoseiidae in Japan (Acari: Gamasina), with remarks on its biology. Species Div., 3(1): 25-73. doi:10.12782/specdiv.3.25
Evans G.O. 1963. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari, Mesostigmata). Bull. Br. Mus. Nat. Hist., Zool., 10(5): 275-303. doi:10.5962/bhl.part.20528
Evans G.O. 1964. Some observations on the chaetotaxy of the pedipalps in the Mesostigmata (Acari). Ann. Mag. Nat. Hist., 13(6): 513-527. doi:10.1080/00222936308651393
Evans G.O. 1969. Observations on the ontogenetic development of the chaetotaxy of the tarsi of legs II-IV in the Mesostigmata (Acari). In: Evans, G.O. (ed.) Proceedings of the 2nd Intern. Cong, Acarol. 1967. Akadémiai Kiadó, Budapest, 195-200.
Faraji F., Roig J., Bakker F. 2011. Some new records of Phytoseiidae from southwest Europe with description of a new species from Spain (Acari: Mesostigmata). Intern. J. Acarol., 37(4): 331-346. doi:10.1080/01647954.2010.519722
Gomelauri L.A. 1968a. New species of the family Phytoseiidae (Berlese) from East Georgia (Acarina: Gamasoidea). Bull. Acad. Sci. Georg. SSR, Zool., 49(3): 701-706 [In Russian].
Gomelauri L.A. 1968b. Three new species of mites of the family Phytoseiidae in southern Georgia. Bull. Acad. Sci. Georg. SSR, Zool. Parasitol., 52(2): 515-520 [In Russian].
Hirschmann, W. 1962. Gangystematik der Parasitiformes. Acarologie Schriftenreihe fur Vergleichende Milbenkunde, Hirschmann-Verlag, Furth/Bay, 5(5-6): 80 pp. + 32 plates.
Jackson G.J. 1974. Chaetotaxy and setal morphology of the palps and first tarsi of Phytoseiulus persimilis A. - H. (Acarina, Phytoseiidae). Acarologia, 16(4) 583-594.
Johnston D.E., Moraza M.L. 1991. The idiosomal adenotaxy and poroidotaxy of Zerconidae. Modern Acarology, Academia, Prague and SPB Academic Publishing bv, The Hague, 2: 349-356.
Karg W. 1960. Zur Kenntnis der Typhlodromiden aus Acker-und Grunlandboden. Zeitsc. fur Angew. Entomol., 47: 440-452. doi:10.1111/j.1439-0418.1960.tb02848.x
Karg W. 1970. Neue Arten der Raubmilbenfamilie Phytoseiidae Berlese, 1916 (Acarina: Parasitiformes). Deut. Entomol. Z., 17: 289-301. doi:10.1002/mmnd.4810170402
Karg W. 1971. Acari (Acarina), Milben, Unterordnung Anactinochaeta (Parasitiformes): Die freilebenden Gamasina (Gamasides), Raubmilben. Die Tierwelt Deutschlands und der angrenzenden Meeresteile, 59. Teil, VEB Gustav Fischer Verlag, 475 pp.
Karg W., Huhta V. 2009. Taxonomic remarks on Phytoseiidae Berlese (Acari: Mesostigmata) with descriptions of three new species from Finland. Intern. J. Acarol., 35(6): 511-520. doi:10.1080/01647950903468257
Koch C.L. 1839. Deutschlands Crustaceen, Myriapoden und Arachniden. Ein Beitrag zur Deutschen Fauna. F. Pustet, Regensburg, 270 pp.
Kolodochka L.A. 1981. New phytoseiid mites from Crimea. I. Vestn. Zool., 15 (1): 18-22 [In Russian].
Kolodochka L.A. 1990. Three new phytoseiid mite species (Parasitiformes). Novosti Faunistiki Sistematiki, Naukova Dumka, Kiev, Ukraine, pp. 158-163 [In Russian].
Kolodochka L.A. 2003 A new species of Phytoseiid mites on the genus Amblyseius (Parasitiformes, Phytoseiidae) from Crimea. Vestn. Zool., 37(5): 73-76 [In Russian with English abstract].
Kolodochka L.A. 2006. Phytoseiid mites of the Palearctic region (Parasitiformes, Phytoseiidae): faunistics, taxonomy, ecomorphology, evolution. Vestn. Zool., Suppl. 21: 1-250.
Kolodochka, L.A., Gwiazdowicz D.J. 2016. Redescription of three species of phytoseiid mites (Acari, Mesistigmata) from Poland. Acarologia, 56(4): 625-632. doi:10.1051/acarologia/20164144
Krantz G.W., Walter D.E. 2009. A manual of acarology, 3<sup>rd</sup> ed. Lubbock, Texas Tech. University Press.
Leonovich S.A. 1989. Tarsal receptory complex and systematics of gamasid mites (Parasififormes, Mesostigmata, Gamasina). Parasitologia, 23(6): 469-479 [in Russian].
Lindquist, E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina Acarina: Mesostigmata. Mem. Entomol. Soc. Canada, 47: 1-64. doi:10.4039/entm9747fv
Lindquist E.E., Moraza M.L. 2010. Revised diagnosis of the family Blattisociidae (Acari: Mesostigmata: Phytoseioidea), with a key to its genera and description of a new fungus- inhabiting genus from Costa Rica. Zootaxa, 2479: 1-21. doi:10.11646/zootaxa.2479.1.1
Lindquist E.E., Moraza M.L. 2012. A new genus of fungus-inhabiting mites of the family Blattisocidae (Acari, Mesostigmata: Phytoseioidea) from Costa Rica, with an updated key to genera of the subfamily Blattisocinae. Redia, 95, 9-19.
Livshitz I.Z., Kuznetsov N.N. 1972. Phytoseiid mites from Crimea (Parasitiformes: Phytoseiidae). In: Pests and diseases of fruit and ornamental plants. The all-union V. I. Lenin Acad. Agric. Sci., The State Nikita Botanical Gardens, Proc., 61: 13-64 [In Russian].
Makarova O.L. 2009. The fauna of free-living gamasid mites (Parasitiformes, Mesostigmata) in the Northern Taiga: an analysis of the zonal specificity. Entomol. Rev., 8(9): 1177-1193. doi:10.1134/S0013873809090176
Meshkov Yu.I. 1991. A new and little known species of mites of the genus Amblyseius (Parasitiformes, Phytoseiidae) from Lithuania. Zool. Zh., 70: 139-142 [In Russian].
Meshkov Yu.I. 1999. Contribution to phytoseiid fauna (Parasitiformes, Phytoseiidae) of Moscow District. Zool. Zhurn., 78(4): 426-431 [In Russian].
Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A catalog of the mite family Phytoseiidae. References to taxonomy, synonymy, distribution and habitat. EMBRAPA - DDT, Brasilia, Brazil, 353 pp.
Moraes G.J. de, McMurtry J.A., Denmark H.A. Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. doi:10.11646/zootaxa.434.1.1
Moraza M.L., Lindquist E.E. 2011. Phytoseioidea from Middle America, with a key to genera and subgenera of the subfamily Blattisociinae. Zootaxa, 2758: 1-25. doi:10.11646/zootaxa.2758.1.1
Muma M.H. 1961. Subfamilies, genera, and species of Phytoseiidae. Fla St. Mus. Bull., 5(7): 267-302.
Papadoulis G.Th. 1995. A new species of Amblyseius Berlese (Acari: Phytoseiidae) from Greece. Intern. J. Acarol., 21(2): 93-97. doi:10.1080/01647959508684049
Papadoulis G.Th., Emmanouel N.G., Kapaxidi E.V. 2009. Phytoseiidae of Greece and Cyprus (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, 200 pp.
Rowell H.J., Chant D.A., Hansell R.J.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. doi:10.4039/Ent110859-8
Shirdel D., Arbabi M., Faraji F. 2009. Amblyseius ampullosus Wu & Lan, a new species record for the Iranian fauna. Syst. Appl. Acarol., 14(2): 136-139. doi:10.11158/saa.14.2.5
Tixier M.-S., Kreiter S., De Moraes G.J. 2008. Biogeographic distribution of the Phytoseiidae (Acari: Mesostigmata). Biol. J. Linn. Soc., 93: 845-856. doi:10.1111/j.1095-8312.2007.00937.x
Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomology Memoir, Department of Agriculture and Water Supply, Republic of South Africa, 73: 168 pp.
Vysotskaya C.O., Bregetova N.G. 1957. Gamasid mites - parasitic on mouse and living in nest in Priozersk region, Leningrad Province. Parazitologicheskiy sbornik Zoologicheskogo instituta Akademii nauk SSSR, 17: 5-37 [In Russian].
Wainstein B.A. 1960. New species and subspecies of the genus Typhlodromus Scheuten (Parasitiformes, Phytoseiidae) of the USSR fauna [in Russian]. Zool. Zh., 683-690.
Wainstein B.A. 1975. Predatory mites of the family Phytoseiidae (Parasitiformes) of Yaroslavl province. Entomol. Obozr., 54(4): 914-922 [In Russian].
Wainstein B.A. 1978. New species of mites of the family Phytoseiidae (Parasitiformes) in the Primorsky Territory. Zool. Zh.., 57: 1641-1650 [In Russian].
Wainstein B.A. 1979. Predatory mites of the family Phytoseiidae of the Primorsky Territory. Nazemnye Chlenistonogie Dal'nego Vostoka, Vladivistok, Russia, pp. 137-144 [In Russian].
Wainstein B.A., Arutunjan E.S. 1970. New species of predatory mites of the genera Amblyseius and Phytoseius (Parasitiformes: Phytoseiidae). Zool. Zh., 49: 1497-1504 [In Russian].
Wainstein B.A., Beglyarov G.A. 1971. New species of the genus Amblyseius (Parasitiformes: Phytoseiidae) from the Primorsky Territory. Zool. Zh., 50: 1803-1812 [In Russian].
Westerboer I., Bernhard F. 1963. Die Familie Phytoseiidae Berlese 1916. In: Stammer, H. (Ed.), Beitr. System. Ökol. Mitteleurop. Acarina. Bd. II, Mesostigmata I, pp. 451-791.
Wu W.N., Lan W.M. 1991. Five new species and one new record of Amblyseius from northwest China (Acari: Mesostigmata: Phytoseiidae). Acta Zootax. Sin., 16(3): 313-319 [In Chinese].
Wu W.N., Ou J.F., Huang J.L. 2009. Fauna Sinica, Invertebrata vol. 47. Arachnida Acari: Phytoseiidae. Science Press, Beijing, China. 511 pp. [In Chinese with English abstract].



2020-06-05
Date accepted:
2020-10-23
Date published:
2020-11-03
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Khaustov, Vladimir A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



