Seasonal dynamics of mites (Acari) in pastures and meadows in Poland, with species analysis of Oribatida
Pacek, Sylwiusz1 ; Seniczak, Stanislaw2 ; Graczyk, Radomir3 ; Chachaj, Bogusław4 and Seniczak, Anna5
1✉ Department of Animal Biology and Environment, Faculty of Animal Breeding and Biology, UTP University of Technology and Life Sciences, Bydgoszcz, Poland.
2Department of Evolutionary Biology, Faculty of Biological Sciences, Kazimierz Wielki University, Bydgoszcz, Poland.
3Department of Animal Biology and Environment, Faculty of Animal Breeding and Biology, UTP University of Technology and Life Sciences, Bydgoszcz, Poland.
4Department of Animal Biology and Environment, Faculty of Animal Breeding and Biology, UTP University of Technology and Life Sciences, Bydgoszcz, Poland.
5Department of Natural History, University Museum of Bergen, University of Bergen, Bergen, Norway.
2020 - Volume: 60 Issue: 4 pages: 668-683
https://doi.org/10.24349/acarologia/20204395Original research
Keywords
Abstract
Introduction
Mites (Acari), and particularly Oribatida, are considered one of the most abundant arthropod groups in the soil (Walter and Proctor 2013). Their densities in meadows and pastures are comparable and range from several thousand to several tens of thousands of individuals per 1 m2. For example, in north-central Poland, mean density of Oribatida in meadows in a growing season varied from 6.000 to 60.000 individuals/m2 and they constituted 32–75% of all mites. Density of Mesostigmata in the same meadows ranged between 3.000–10.000 individuals/m2 (Chachaj and Seniczak 2006; Szczukowska 2015; Seniczak et al. 2017; Pacek et al. 2020). Mite density in pastures depended on the grazing species (Chachaj and Seniczak 2006; Pacek et al. 2020). The highest density of Oribatida was observed in a goose pasture (57.000 individuals/m2),\up where they made up 83% of mites, and the lowest in a sheep pasture (3.500 individuals/m2), where Oribatida made up only 38% of mites. Density of Mesostigmata ranged from 2.000 individuals/m2 in a cattle pasture to 10.000 individuals/m2 in a goose pasture (Chachaj and Seniczak 2006; Pacek et al. 2020). High abundance of oribatid mites in soils translates into their ecological importance (Walter and Proctor 2013). These mites play important roles in decomposition of soil organic matter, formation and changes in the soil properties, and they help with supporting plants with nutrients (Wasilewski 2006; Walter and Proctor 2013). In Poland, the number of oribatid species in pastures ranged between 19 and 22, and in meadows between 24 and 31 (Chachaj and Seniczak 2005). In the Czech Republic the number of oribatid species in cow pastures and meadows ranged between 15 and 30 (Hubert 2000). However, some oribatid species negatively affect domestic and wild animals, as they are intermediary hosts of tapeworms (e.g. Zbikowska-Zdun and Koczara 2013; Václav and Kalúz 2014; Roczeń-Karczmarz and Tomczuk 2016; Tomczuk et al. 2017). Mesostigmata are mostly predators, and play a positive role in ecosystems by controlling and regulating the density of saprophages, including oribatid mites (Walter and Proctor 2013).
Ecological investigations of the mite fauna in grasslands have focused mainly on the effects of fertilizing (Bolger and Curry 1980; Bielska 1986; Bielska and Paszewska 1995; Domek-Chruścicka and Seniczak 2005; Parfitt 2005; Sokołowska and Seniczak 2005; Graczyk et al. 2008, 2010; Wasińska-Graczyk et al. 2009; Kruczyńska and Seniczak 2010; Seniczak et al. 2017), grazing (Hubert 2000; Clapperton et al. 2002; Seniczak et al. 2007a,b; Schon et al. 2008; Pacek et al. 2020), or ecotone zones (Bardgett and Cook 1998).
Grazing is a strong factor that can change seasonal dynamics of oribatid mites. It mainly depends on the weight of grazing animals, their behavior, hoof type (Greenwood et al. 1997; Parfitt et al. 2005) and the period of grazing, i.e. the longer the animals stay in the pasture, the more compact the soil becomes (Amiri et al. 2008). Since the compaction of soil worsens living conditions of mites, it can be expected that grazing affects mite communities (Chachaj and Seniczak 2006). In addition, grazing changes the composition of vegetation and root biomass between the seasons, and affects the content of organic matter in the soil, which together may alter microhabitat conditions for oribatid mites (Di et al. 2001; Altesor et al. 2006).
Farm animal feces affect chemical composition of soil and consequently seasonal dynamics of oribatid mites in different ways. For example, goose manure increases nitrogen and phosphorus content, while goat manure increases the level of potassium (Ligęza 2009; Uwah and Eyo 2014).
As majority of studies on acarofauna from grasslands have focused on one season only or presented averaged values from a few seasons (Clapperton 2002; Chachaj and Seniczak 2006; Schon et al. 2010), relatively little is known about seasonal changes in the mite communities in pastures and meadows. According to Norton (1994), the effect of seasons on oribatid communities in pastures is less pronounced because seasons are strongly associated with the availability of organic matter in the soil. This, in turn, is connected with life cycle of plants and circulation of matter in the environment, which can affect the abundance of Oribatida (Belnap and Lange 2001; Wasilewski 2006). Seasonal agrotechnical treatments may also influence Oribatida communities (Cookson et al. 1998; Vreeken-Buijs et al. 1998; Wickings et al. 2011; Wickings and Grandy 2013). Mowing of meadows or herbicide use can reduce abundance and species diversity of oribatid mites and change their age structure (Minor and Norton 2008), while moderate fertilizing can improve their living conditions (Beltman et al. 2003). However, significant differences in seasonal dynamics of oribatid species were more often observed in pastures than in meadows (Bedano et al. 2006; Chachaj and Seniczak 2006).
Studies on seasonal dynamics of Oribatida in pastures are particularly important in the regions seriously affected by tapeworm parasites with their resulting detrimental economic impact on farm animals (Denegri and Martinez 2007). There are at least 127 oribatid species known to be intermediary hosts of 27 species of anoplocephalid tapeworms (Denegri 1993). Denegri and Martinez (2007) demonstrated that increased number of oribatid mites in sheep pastures and rapid maturation of cysticercoids coincided with lambing season and lamb weaning, leading to high lamb mortality. Knowledge of the seasonality of intermediary hosts can be used to help control tapeworms through the rotation of pasture plots.
Our aim was to study seasonal differences in the abundance of mites and, more specifically, oribatid communities, in pastures grazed by geese, goats and fallow deer, and in meadows (control plots) situated close to these pastures. Based on the literature mentioned above, we hypothesized that seasonal changes in mites and particularly in Oribatida, were more pronounced in the pastures than in the meadows.
Materials and methods
Study area
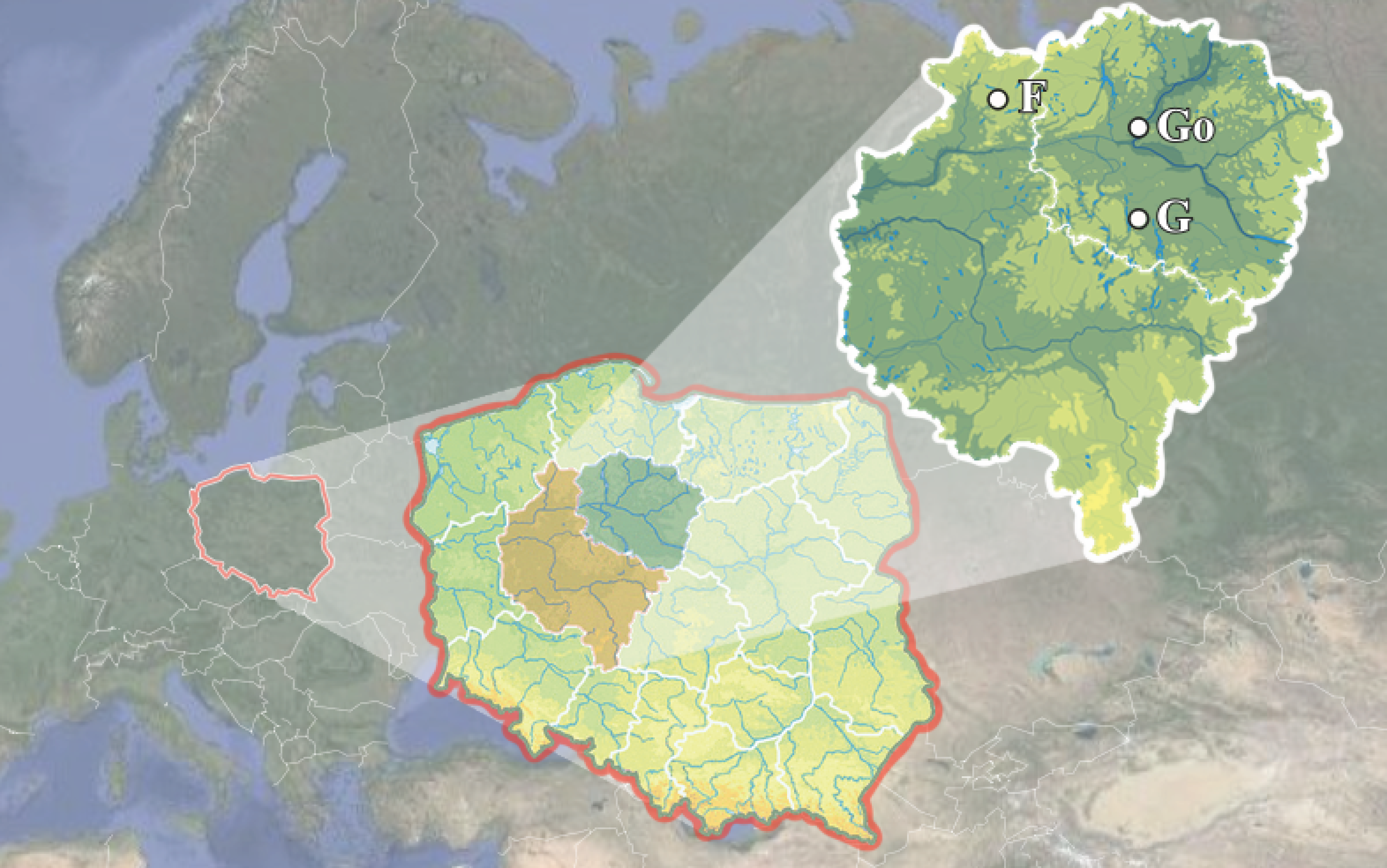
The study sites were three pastures and three meadows situated in north-central Poland (Figure 1). The pastures were grazed by geese (later referred to as GP, 52.4427° N, 18.0914° E), goats (GoP, 53.1046° N, 18.1048° E) and fallow deer (FP, 53.1743° N, 17.0146° E); the meadows were situated near each pasture (in the closest possible location) (control for geese, GM, 52.4655° N, 18.0804° E, goat – GoM, 53.1047° N, 18.1040° E and fallow deer – FM, 53.1746° N, 17.0133° E). All study plots were fenced. The pastures provided stable conditions and had a documented history of usage. The livestock density was established at the beginning of the study and did not change over time. It was expressed as LU/ha, (livestock unit per hectare), i.e. a unit of animal density on a pasture, based on the animal weight and food demand (Rozporządzenie Rady Ministrów 2010), and reached 31.27 LU/ha for geese, 21.12 LU/ha for goats, and 6.60 LU/ha for fallow deer. From early spring to mid-autumn the animals spent most of the day in the pastures. The meadows were extensively used and mowed twice a year.


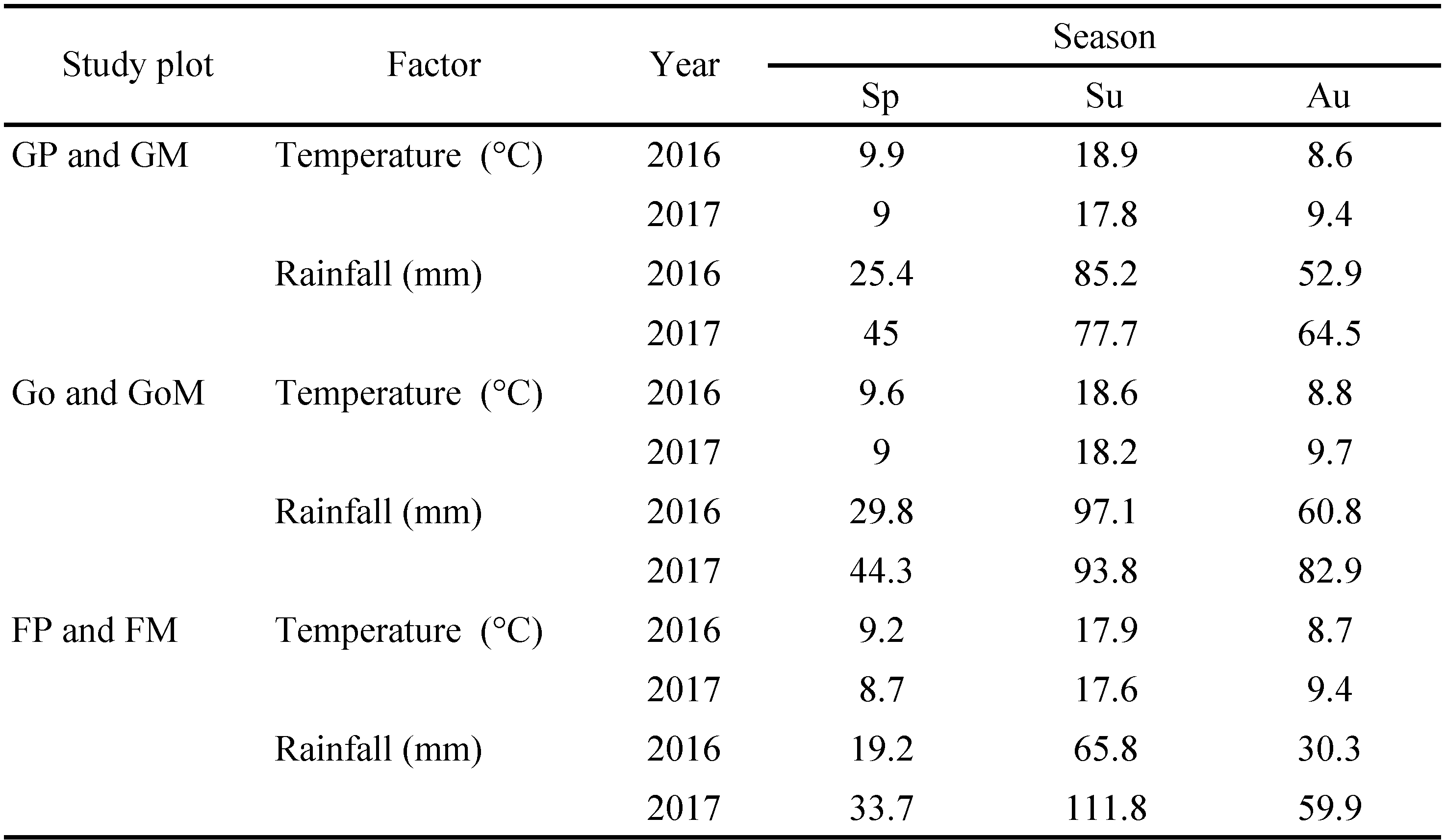
Temperature and rainfall in the study years (2016–2017) were similar (Table 1). In all study plots the highest temperature and rainfall were registered in summer, while in spring and autumn they were similar. Data on the soil and vegetation in the study plots were published before (Pacek et al. 2020). Soil in GP was the richest in phosphorus and organic carbon, in GoP in potassium, and in FP in nitrogen. In two pastures (GP and FP) vegetation belonged to Lolio-Plantaginetum association, and in GoP it belonged to Festuco-Brometea class. Two meadows (GM and GoM) harbored plant association Arrhenatheretum elatioris, while the remaining one (FM) was covered with Poa pratensis-Festuca rubra.


Sampling and identification
Samples of soil and vegetation were collected from all experimental plots in spring (April), summer (July) and autumn (November) 2016 and 2017 in the grazing season. In each plot and season, the samples of 150 cm3 each were taken with a soil corer (to a depth of 6 cm and with 3 cm of lower parts of plants), in 10 replicates. In total, 360 samples were collected. The mites were extracted using high-gradient Tullgren funnels for 14 days and preserved in 75% ethanol. Oribatida were identified to species, including juvenile forms, using the key by Weigmann (2006) and other keys (Seniczak 1977, 1978, 1980a, 1980b, 1988, 1990; Seniczak and Klimek 1990; Seniczak and Seniczak 2008; Ermilov 2010; Pfingstl and Krisper 2011). Mesostigmata were sorted out using Lindquist et al. (2009), whereas the remaining acarofauna was treated together as `other mites'. Full names of oribatid species are used in Tables 3 and 4; in other tables and figures abbreviations are used.
Statistical analyses
Since weather conditions in two subsequent years were similar, the data from seasons from these two years were analyzed together. The oribatid communities were characterized by their abundance index (A, specimens/m2), number of species (S), and Shannon species diversity index (H′) (Odum 1982). Basic statistical descriptors included mean values ¯x and standard deviation (±SD). For the remaining statistical analyses, the values were log-transformed log (x + 1) (Łomnicki 2010). Normality of the distribution was tested with Kolmogorov–Smirnov test, while equality of variance in different samples with Levene test. Multifactorial analysis of variance was used to find significant differences between the means, and in the case of significant differences Tukey's post-hoc test was employed (Stanisz 2006). The analysis of the most numerous oribatid species, seasons and habitats was performed using detrended correspondence analysis (DCA) (Piernik 2008). INCOMPLETE DCA, instead of PCA, was carried out because gradient length was 5.0 indicating that unimodal models were more appropriate than linear models (Leps and Smilauer 2003), as the structure of the data had unimodal character (i.e., each species occurs in particular range of a given habitat parameter) (Hill and Gauch 1980). The level of significance for all statistical tests was α=0.05. The statistical calculations mentioned above were carried out using MS Excel 2019 software (Microsoft 2019), STATISTICA 13.3 (Dell 2019) and MVSP 3.2 software (Kovach Computing Services 2019).
Results
Abundance and diversity of mite species
In total, 22.561 mites were extracted, in which Oribatida predominated (69% of all mites), followed by Mesostigmata (21%) and other mites (10%). Among oribatid mites, adult forms dominated (Figure 2), representing 51% of all mites. At most study plots, both in the pastures and meadows, total abundance of mites, of Oribatida as well as of Mesostigmata, did not differ significantly between the seasons. Only other mites were at all plots most abundant in spring and usually least abundant in autumn, and in all plots significant differences between some or all the seasons were found (Table 2).






Species diversity of Oribatida changed over seasons. In all pastures, it was the lowest in spring and the highest in summer, while in all meadows it was the highest in spring and decreased towards autumn (Table 2). In total, 21 oribatid species were found in the studied grasslands (Tables 2–4), but only about half of them [Platynothrus peltifer (C.L. Koch, 1839), Tectocepheus velatus (Michael, 1880), Eupelops occultus (C.L. Koch, 1835), Liebstadia similis (Michael, 1888), Sellnickochthonius immaculatus (Forsslund, 1942), Punctoribates punctum (C.L. Koch, 1839), Trichoribates novus (Sellnick, 1928), Achipteria coleoptrata (Linnaeus, 1758), Metabelba pulverosa (Strenzke, 1953) and Scheloribates laevigatus (C.L. Koch, 1835)] were relatively abundant, at least in some plots. The abundance of juvenile Oribatida in pastures changed significantly over the seasons, being the lowest in summer and the highest in autumn (Figure 2), while no significant changes were observed in the meadows.



Juveniles of some species (T. velatus, L. similis, E. occultus and P. peltifer) showed greater abundance in certain seasons. For example, at a majority of plots juveniles of T. velatus were most abundant in summer and those of P. peltifer, in autumn. In most species, juveniles were absent in some seasons (Table 5).



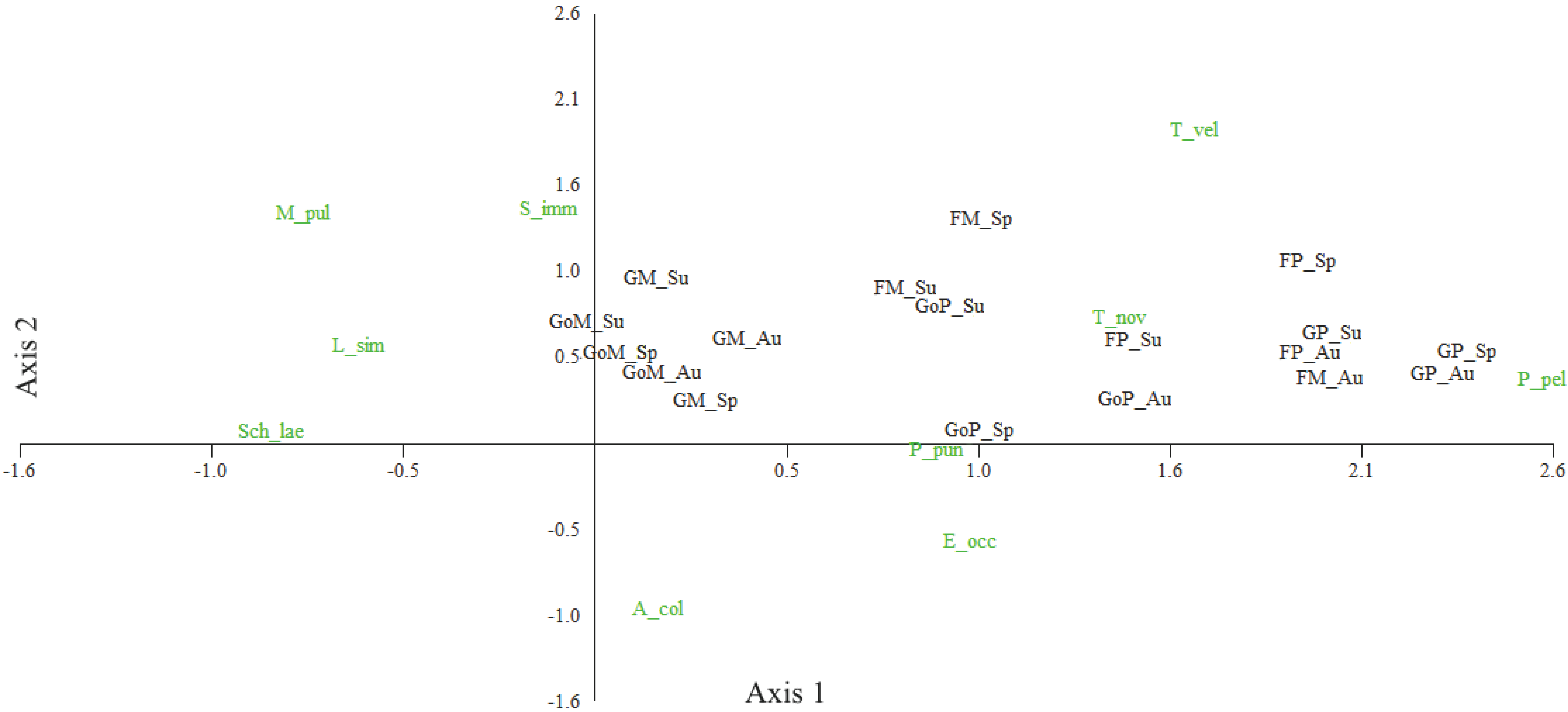
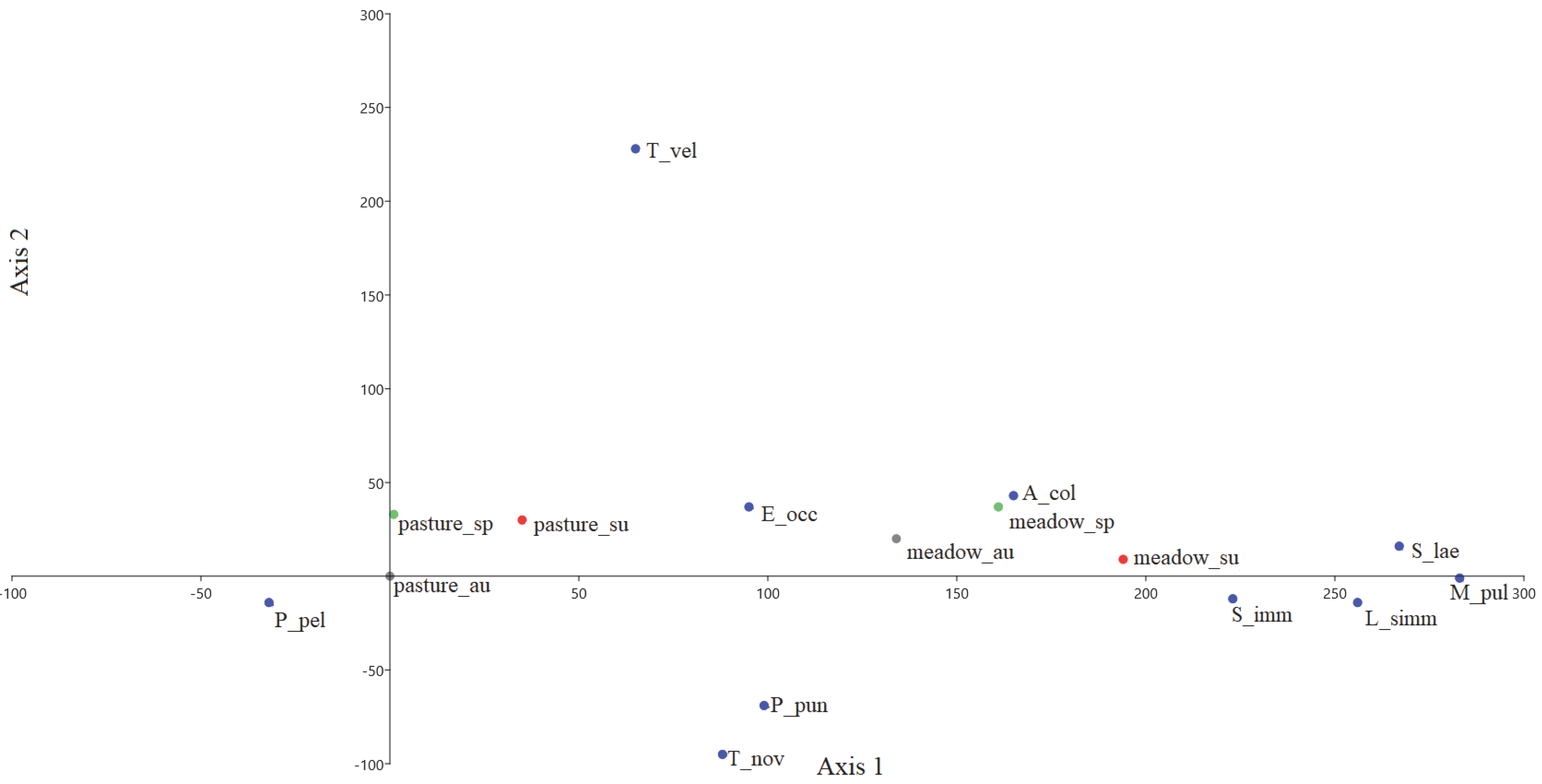
Oribatid mites were more influenced by the habitat than by season (Figure 3). Platynothrus peltifer was strongly associated with pastures, five species (A. coleoptrata, L. similis, M. pulverosa, S. laevigatus and S. immaculatus) showed stronger preferences for meadows, and two other (E. occultus and T. velatus) were abundant both in the pastures and the meadows. Albeit to a lesser degree, seasons had an impact, too (Figure 4). Eupelops occultus was most associated with spring and summer in the pastures, P. peltifer with autumn in the pastures, and L. similis and S. immaculatus with summer in the meadows.




Seasonal changes in the goose pasture/ meadow
Both at the goose pasture and meadow the abundance of Oribatida and Mesostigmata did not differ significantly between the seasons. Only the abundance of other mites was significantly higher in spring than in autumn (Table 2). In all studied seasons, total density of mites, as well as of Oribatida, was higher in the pasture than in the meadow with differences being insignificant.
Platynothrus peltifer was the most numerous species in the pasture but its abundance did not differ significantly between the seasons. Only in some species significant seasonal differences were observed. In the pasture, T. velatus was more abundant in summer than in other seasons and S. laevigatus was more abundant in summer than in spring. In the meadow, P. peltifer was more abundant in spring than in other seasons, T. novus was more abundant in summer than in other seasons, A. coleoptrata was more abundant in autumn then in summer, while M. pulverosa was more abundant in summer than in autumn (Table 3).



Seasonal changes in the goat pasture/ meadow
In the goat pasture, total density of mites and of their individual groups differed significantly between the seasons (Table 2). In this pasture, the density of Acari and of Mesostigmata was higher in autumn than in spring, density of Oribatida was higher in autumn than in the remaining seasons, while other mites were more abundant in spring than in summer and autumn. In the meadow, no seasonal differences were observed in total density of Acari and Oribatida but such differences were observed in Mesostigmata and other mites (Table 2). In many oribatid species significant seasonal differences were perceived. In the pasture, P. peltifer and L. similis were more abundant in autumn than in other seasons, T. novus and A. coleoptrata were more abundant in spring than in other seasons, and P. punctum was more abundant in summer and autumn than in spring. In the meadow, E. occultus was more abundant in spring and autumn than in summer, A. coleoptrata, in summer than in other seasons, and P. punctum, in autumn than in other seasons. Density of Mesostigmata in the goat pasture was significantly higher in autumn than in spring, and in the meadow, it was significantly higher in summer than in spring (Table 2).
Seasonal changes in the fallow deer pasture/ meadow
In the fallow deer pasture significant seasonal differences were observed only in other mites, which were more abundant in spring than in summer (Table 2). In the meadow, total density of Acari and of Oribatida was higher in spring than in summer, and density of other mites was higher in spring than in other seasons. Abundance of some oribatid species differed significantly between the seasons: in the pasture, P. peltifer was more abundant in autumn than in the remaining seasons, E. occultus, in summer than in spring; in the meadow, T. velatus, L. similis and T. novus were more abundant in spring than in other seasons, and E. occultus was more abundant in autumn than in other seasons.
Discussion
In this study, oribatid mites were more influenced by habitat than by season, which is consistent with the literature. Some authors (Clapperton 2002; Chachaj and Seniczak 2006; Schon et al. 2010) showed that in pastures grazed by cows and horses, the number of species and species diversity of Oribatida did not clearly change throughout the year. Studies on the impact of climate on Oribatida also showed that habitat factors can be more important than seasons (Corral-Hernandez 2006). Mowing of meadows or grazing and trampling of pastures by domestic animals have such strong effects on oribatid communities (Maraun and Scheu 2000) that the effects of other factors, e.g. seasonality, can be less visible (Eitminavičiūtė 2006; Eitminavičiūtė et al. 2008). In addition, if humidity and temperature remain within the tolerance range of oribatid species, they have little effect on the abundance and structure of the mite communities (Maraun and Scheu 2000). Oribatid species with greater tolerance of environmental factors respond only slightly to short-term changes in the environment (e.g. amount of precipitation, temperature) and availability of food (Clapperton et al. 2002). Additionally, pastures and meadows are inhabited by species more tolerant to environmental factors such as drying and low or high temperature (Siepel 1996). Oribatida were shown to be less responsive to seasonal changes in root structure and plant productivity in grazed areas (Bardgett et al. 1996; Bardgett et al. 1998; Clapperton et al. 2002). Our results suggest that this also partly applies to mowed meadows. In contrary to that, in natural ecosystems, such as bogs, Oribatida respond clearly to seasonal changes, both in terms of their abundance and species diversity (Seniczak et al. 2019).
Although in our study some effects of seasons on the abundance of all mite groups were observed in the goat pasture, it needs to be emphasized that total density of mites was very low there. Goats negatively affect mite communities due to intensive grazing and trampling (Pacek et al. 2020), and also goat manure has a limiting effect on the oribatid fauna (Seniczak et al. 2017). In the meadows, there were no clear trends in the abundance of mites and Oribatida, which supports our hypothesis that seasonal effects are more pronounced in the pastures than in the meadows, probably due to regular disturbances in meadows caused by agrotechnical treatments (Siepel 1996; Cookson et al. 1998; Vreeken-Buijs et al. 1998; Wickings et al. 2011; Wickings and Grandy 2013).
Clear seasonal changes were seen in species diversity and in the number of species, which increased during the year in the pastures and decreased in the meadows. In the pastures, these changes could be caused by increased influence of grazing, which promotes fungivorous and parthenogenetic species of Oribatida (Rajski, 1967; Niedbała, 1980; Parfitt et al., 2005; Amiri et al. 2008). Additionally, some oribatid species reacted clearly to seasonal changes, both in the pastures and the meadows (Table 3). Many oribatid species are sensitive to grazing, mowing and drying and therefore they may react more strongly to seasonal changes related to agrotechnical treatments and atmospheric factors (Rajski 1967; Olszanowski et al. 1996; Siepel 1996; Claperton 2002; Maraun and Scheu 2000; Chachaj and Seniczak 2006).
Such species include e.g. E. occultus, L. similis, T. novus, A. coleoptrata and M. pulverosa, that are sensitive to organic fertilizers and prefer natural meadows (Weigmann 2006; Szczukowska 2015). However, in our study, more tolerant species like P. peltifer and T. velatus reacted to seasonal changes. Platynothrus peltifer is a hygrophilous species (Travé 1963; Lebrun 1965) and prefers soils rich in organic matter (Rajski 1967; Niedbała 1980), and T. velatus shows a broad tolerance to many ecological factors (Rajski 1967; Siepel 1996). In these cases, the seasonal differences in abundance could be caused by the concentration of macroelements in the soil, the livestock species and plant cover (Pacek et al. 2020). In the goose pasture P. peltifer had favorable conditions in all investigated seasons, which could be the reason for lack of differences in its abundance between the seasons. Although the goose pasture had the highest livestock density, in autumn Oribatida achieved the highest abundance there. Geese have small body weight and are incapable of compacting the soil by trampling. Therefore, they did not affect the soil fauna in any negative way during the grazing season and the mites could reproduce and achieve their highest density. The goose pasture had also the most abundant plant cover as the animals did not eat some plant species, and the soil was well protected against drying and erosion. The goose pasture was also the richest in organic matter (Pacek et al. 2020) and water, thus providing very favorable conditions for P. peltifer.
Heavier animals, in particular ungulates like goats and fallow deer, strongly compact the soil to a depth of 5 cm and destroy its structure (Greenwood et al. 1997; Di et al. 2001; Parfitt 2005), thus decreasing the abundance and species richness of oribatid mites (Siepel 1996; Hubert 2000; Clapperton et al. 2002; Chachaj and Seniczak 2005; Seniczak 2007a; Schon et al. 2008). In the pastures of goats and fallow deer, mites, including Oribatida, were significantly less abundant than in the goose pasture (Pacek et al. 2020). However, also in the pastures grazed by goats and fallow deer, the mites and Oribatida were the most abundant in autumn, the difference especially noteworthy with regard to the goat pasture. In our study, livestock density did not clearly change the abundance of mites between seasons.
Platynothrus peltifer, L. similis, S. laevigatus and less abundant in pastures A. coleoptrata, Oppiella nova (Oudemans, 1902), Ceratozetes gracilis (Michael, 1884) and Scheloribates latipes (C.L. Koch, 1844) are intermediary hosts of Anoplocephalidae, which pose serious problems on horse pastures (Denegri 1993; Roczeń–Karczmarz and Tomczuk 2016; Tomczuk et al. 2017). These species are of epidemiological significance where the problem of tapeworms exists. Additionally, P. peltifer was highly abundant in pastures, which increased the likelihood of contracting tapeworms in farm animals.
In conclusion, we can state that oribatid communities in pastures and meadows were more affected by habitat than by season. Mowing of meadows, grazing or trampling of pastures by domestic animals had strong effects on Oribatida. However, a seasonal effect on the mites was also observed. Studies of this kind expand our knowledge on the ecology of oribatid mites in pastures and meadows, and can have practical implications for the pasture management and tapeworm prevention.
Acknowledgements
We are very grateful to Magdalena Pacek for the graphical work and editing.
References
Altesor A., Pińeiro G., Lezama F., Jackson R.-B., Sarasola M. et al. 2006. Ecosystem changes associated with grazing in subhumid South American grasslands. J. Veg. Sci., 17: 323-332. doi:10.1111/j.1654-1103.2006.tb02452.x
Amiri F., Ariapour A., Fadai S. 2008. Effects of Livestock Grazing on Vegetation Composition and Soil Moisture Properties in Grazed and Non-Grazed Range Site. J. Bio. Sci., 8: 1289 - 1297. doi:10.3923/jbs.2008.1289.1297
Bardgett R.-D., Cook R. 1998. Functional aspects of soil animal diversity in agricultural grasslands. Soil Ecol., 10: 263-276. doi:10.1016/S0929-1393(98)00125-5
Bardgett R.-D., Hobbs P.-J., Frostegård Å. 1996. Changes in soil fungal:bacterial biomass ratios following reductions in the intensity of management of an upland grassland. Biol. Fert. Soils, 22: 261-264. doi:10.1007/BF00382522
Barkman J.-J., Doing H., Segal S. 1964. Kritische bemerkungen und vorschläge zur quantitativen vegetationsanalyse. Acta Bot. Neerl., 13: 394-419. doi:10.1111/j.1438-8677.1964.tb00164.x
Bedano J.-C., Cantu M.-P., Doicet M.-E. 2006. Influence of three different land management practices on soil mite (Arachnida: Acarii) densities in relation to a natural soil. Soil Ecol., 32: 293-304. doi:10.1016/j.apsoil.2005.07.009
Belnap J., Lange O.-L., 2001. Biological Soil Crust: Structure, Function and Management. Springer-Verlag. doi:10.1007/978-3-642-56475-8_1
Beltman B., Broek T., Martin W., Cate M., Güsewell S. 2003. Impact of mowing regime on species richness and biomass of a limestone hay meadow in Ireland. Bull. of the Geobotanical Inst. ETH. 69: 17-30.
Bielska I. 1986. Communities of moss mites (Acari, Oribatei) of chosen grasslands periodically flooded with liquid manure. Pol. Ecol. Stud., 12: 163-178.
Bielska I., Paszewska H. 1995. The Oribatida (Acari, Oribatida) communities of meadows ferti¬lized and non-fertilized with liquid manure. Pol. Ecol. Stud., 21: 277-292.
Bolger T., Curry J.-P. 1980. Effects of cattle slurry on soil arthropods in grassland. Pedobiologia, 20: 246-253.
Chachaj B. and Seniczak S. 2005. The Influence of Sheep, Cattle and Horse Grazing on Soil Mites (Acari) of Lowland Meadows. Folia Biol., 53: 127-132. doi:10.3409/173491605775789362
Chachaj B., Seniczak S. 2006. Seasonal dynamics of density of Oribatida (Acari) in a lowland meadow and pastures. Biol. Lett., 43: 145-149.
Clapperton M.-J., Kanashiro D.-A., Behan-Pelletier V.-M. 2002. Changes in abundance and diversity of microarthropods associated with Fescue Prairie grazing regimes. Pedobiologia, 46: 496-511. doi:10.1078/0031-4056-00155
Cookson W.-R., Beare M.-H., Wilson P.-E. 1998. Effects of prior crop residue management on microbial properties and crop residue decomposition. Appl. Soil Ecol. 7: 179-188. doi:10.1016/S0929-1393(97)00032-2
Corral-Hernández E., Balanzategui I., Iturrondobeitia J. 2016. Ecosystemic, climatic and temporal differences in oribatid communities (Acari: Oribatida) from forest soils. Exp. Appl. Acarol., 69. doi:10.1007/s10493-016-0052-3
Dell 2019. STATISTICA 13.3 (computer software). Round Rock.
Denegri G.-M. 1993. Review of oribatid mites as intermediate hosts of tapeworms of the Anoplocephalidae. Exp. Appl. Acarol., 17: 567-580 doi:10.1007/BF00053486
Denegri G. and Martinez P. 2007. Population dynamics of oribatid mites in an endemic zone of sheep cestodosis in Argentina. Rev. Vet., 18: 92-94.
Di H.-J., Cameron K.-C., Milne J., Drewry J.-J., Smith N.-P. et al. 2001. Amechanical hoof for simulating animal treading under controlled conditions. New Zeal. J. Agr. Res., 44: 111-116. doi:10.1080/00288233.2001.9513465
Domek-Chruścicka K. and Seniczak S. 2005. The Effect of Pig Liquid Manure Fertilization on the Crop of Alternating Grassland and Some Groups of Soil Mesofauna. Folia Biol., 53. doi:10.3409/173491605775789470
Eitminaviciute I. 2006. Microarthropod communities in anthropogenic urban soils. 2. Seasonal dynamics of microarthropod number in crossing curb soils. Zool. Zh., 85: 1309-1320. doi:10.1134/S0013873806110030
Eitminavičiūtė I., Petrauskiene A., Augustaitis A. 2008. Dynamic and seasonal fluctuations of microarthropod complex in coniferous forest soil. Ekolo., 54: 201-215. doi:10.2478/v10055-008-0031-z. doi:10.2478/v10055-008-0031-z
Ermilov G. 2010. Morphology of juvenile instars of Metabelba Papillipes (Acari, Oribatida, Damaeidae). Acarina, 18: 273-279.
Graczyk R., Seniczak S., Wasińska B. 2008. The effect of cattle liquid manure fertilization on the soil mites (Acari) in permanent meadow in Poland. J. Cent. Eur. Agric., 9: 651-658.
Graczyk R., Seniczak S., Wasińska-Graczyk B. 2010. Effect of cattle liquid manure fertilization and disinfectant on seasonal dynamics of Oribatida (Acari) in a permanent lowland meadow in Poland. Biol. Lett., 47: 59-64. doi:10.2478/v10120-009-0021-1
Greenwood K.-L., MacLeod D.-A., Hutchinson K.-J. 1997. Long-term stocking rate effects on soil physical properties. Aust. J. Exp. Agric., 37: 413-419. doi:10.1071/EA96131
Hill M.-O., Gauch H.-G. 1980. Detrended correspondence analysis, an improved ordination. Vegetatio, 42: 47-58. doi:10.1007/978-94-009-9197-2_7
Hubert J. 2000. The oribatid community (Acari: Oribatida) on a dry cow pasture. Ekol. Bratislava, 19: 354-364.
Kovach Computing Services 2019. MVSP 3.2 (computer software).
Kruczyńska K., Seniczak S. 2010. Effect of cattle liquid manure fertilization on soil mites (Acari) of lowland meadow. Zesz. Nauk. Nr 255 - Zootechnika, 38: 13-17. doi:10.2478/v10120-011-0003-y
Lebrun P. 1965. Contribution a l'étude écologique des Oribates de la litiere dans une foret de Moyenne-Belgique. Mem. Inst. r. Sci. nat. Belg., 153: 1-96.
Leps J., Smilauer P. 2003. Multivariate analysis of ecological data using CANOCO. doi:10.1017/CBO9780511615146
Ligęza S. 2009. Zagrożenie gleb na fermach gęsi związkami azotu i fosforu. Zesz. Prob. Post. Nauk Rol., 535: 261-268.
Lindquist E.E., Krantz G.W., Walter D.E. 2009. Classification. In: Krantz G.W, Walter D.E. (Eds). A Manual of Acarology. TTU Press, pp. 97-103.
Łomnicki A. 2010. Wprowadzenie do statystyki dla przyrodników. PWN.
Maraun M., Scheu S. 2000. The structure of oribatid mite communities (Acari, Oribatida): Patterns, mechanisms and implications for future research. Ecography, 23: 374-382. doi:10.1111/j.1600-0587.2000.tb00294.x
Microsoft 2019. MS Excel 2019 (computer software).
Niedbała W. 1980. Mechowce - roztocze ekosystemów lądowych. PWN.
Minor M., Norton R. 2008. Effects of weed and erosion control on communities of soil mites (Oribatida and Gamasina) in short-rotation willow plantings in central New York. Can. J. For. Res., 38. doi:10.1139/X07-207
Norton R.A. 1994. Evolutionary aspects of oribatid mites life histories and consequences for the origin of the Astigmata. In: Houck, M.A. (Eds) Mites: Ecological and Evolutionary Analysis of Life-History Patterns. C&H, pp. 99-135. doi:10.1007/978-1-4615-2389-5_5
Odum E.-P. 1982. Podstawy ekologii. PWRiL.
Olszanowski Z., Rajski A., Niedbała W. 1996. Katalog Fauny Polski. Acari. Oribatida [Catalogus faunae Poloniae. Acari. Oribatida]. PAN, Muz. i Inst. Zool., SORUS, 34: 243.
Pacek S., Seniczak A., Graczyk R., Chachaj B., Waldon-Rudzionek B. 2020. The effect of grazing by geese, goats and fallow deer on soil mites (Acari). Turk. J. Zool., 44, 3, 254-265 doi:10.3906/zoo-1910-22
Parfitt R.-L., Yeates G.-W., Ross D.-J., Mackay A.-D., Budding P.-J. 2005. Relationship between soil biota, nitrogen and phosphorus availability, and pasture growth under organic and conventional management. Soil Ecol., 28: 1-13. doi:10.1016/j.apsoil.2004.07.001
Pfingstl T., Krisper G. 2011. No difference in the juveniles of two Tectocepheus species (Acari: Oribatida, Tectocepheidae). Acarologia, 51(2): 199-218. doi:10.1051/acarologia/20112005
Piernik A. 2008. Metody numeryczne w ekologii na przykładzie zastosowań pakietu MVSP. Wyd. Nauk. UMK.
Rajski A. 1967. Autecological-Zoogeographical Analysis of Moss Mites (Acari, Oribatei) on the basis of Fauna in the Poznan Environs. Part I. Pol. J Entomol., 37: 69-166. doi:10.3161/00159301FF1968.14.12.277
Roczeń-Karczmarz M., Tomaczuk K. 2016. Oribatid mites as vectors of invasive diseases, Acarologia, 56: 613-623. doi:10.1051/acarologia/20164143
Rozporządzenie Rady Ministrów 2010. Rozporządzenie Rady Ministrów z dnia 9 listopada 2010 r. w sprawie przedsięwzięć mogących znacząco oddziaływać na środowisko. Dz. Ust., 213.
Schon N.-L., Mackay A.-D., Minor M.-A., Yeates G.-W., Hedley M.-J. 2008. Soil fauna in grazed New Zealand hill country pastures at two management intensities. Soil ecol., 40: 218-228. doi:10.1016/j.apsoil.2008.04.007. doi:10.1016/j.apsoil.2008.04.007
Schon N.-L., Mackay A.-D., Yeates G.-W., Minor M.-A. 2010. Separating the effects of defoliation and dairy cow treading pressure on the abundance and diversity of soil invertebrates in pastures. Soil Ecol., 46: 209-221. doi:10.1016/j.apsoil.2010.08.011
Szczukowska H. 2015. Wpływ nawożenia obornikiem świńskim i kozim na akarofaunę łąki [Phd Thesis]. Wyd. Ucz. UTP.
Seniczak A., Seniczak S., Graczyk R., Waldon-Rudzionek B., Nowicka A., Pacek S. 2019. Seasonal dynamics of oribatid mites (Acari, Oribatida) in a bog in Poland. Wetlands 39: 853-864. doi:10.1007/s13157-019-01125-2
Seniczak A., Seniczak S., Szczukowska H., Graczyk R., Bukowski G. 2017. Preliminary study of the impact of pig or goat manure fertilization of a meadow on oribatid mites Biol. Lett., 53: 55-66. doi:10.1515/biolet-2017-0004
Seniczak S. 1977. The systematic position of moss mites of the genus Anachipteria Grandjean, 1953 (Acarina, Oribatei) in the light of ontogenetic studies. Acarologia, 18: 140-147.
Seniczak S. 1978. The morphology of juvenile stages of soil mites of the family Achipteriidae (Acari: Oribatei). Ann. Zool., 34: 89-99.
Seniczak S. 1980a. The morphology of the juvenile stages of moss mites of the family Scheloribatidae Grandjean, 1953 (Acari, Oribatei). Acta Zool. Cracov, 24: 487-500.
Seniczak S. 1980b. The morphology of juvenile stages of moss mites of the subfamily Trichoribatinae (Acari: Oribatei). Ann. Zool., 35: 83-92.
Seniczak S. 1988. The morphology of juvenile stages of moss mites of the family Pelopidae Ewing (Acarida, Oribatida). II. Ann. Zool., 41: 383-393.
Seniczak S. 1990. The morphology of juvenile stages of moss mites of the family Scheloribatidae (Acarida, Oribatida). II. Ann. Zool., 43: 299-308.
Seniczak S., Chachaj B. and Kaczmarek S. 2007a. Preliminary study on the influence of sheep, cattle and horse grazing on soil mites (Acari) of lowland meadow in Poland. Cont. to Soil Zool. in Cent. Europe II., 135 - 138.
Seniczak S., Gulvik M.-E., Seniczak A. 2007b. Effects of sheep treading on plant covering and soil Oribatida (Acari) in wooded hay meadow in Sogn (Norway). J. Cent. Eur. Agric., 8: 453-460.
Seniczak S., Klimek A. 1990. The morphology of juvenile stages of moss mites of the family Camisiidae (Acari, Oribatida). I. Zool. Anz., 225: 71-86.
Seniczak S., Seniczak A. 2008. Morphology of juvenile stages of three species of the genus Punctoribates Berlese, 1908 (Acari: Oibatida: Mycobatidae). Ann. Zool., 58: 473-485. doi:10.3161/000345408X364328
Siepel, H. 1996. The Importance of Unpredictable and Short-Term Environmental Extremes for Biodiversity in Oribatid Mites. Biodivers. Lett., 3: 26-34. doi:10.2307/2999707. doi:10.2307/2999707
Sokołowska L., Seniczak S. 2005. The effect of cattle liquid manure fertilization on alternating grassland and some groups of soil mesofauna. Folia Biol., 53: 133-137. doi:10.3409/173491605775789407
Stanisz A. 2006. Easy Course of Statistic Using Statistica PL and Medicine Examples, 1. Basic statistic. StatSoft Polska.
Szczukowska H. 2015. Wpływ nawożenia obornikiem świńskim i kozim na akarofaunę łąki [Phd Thesis]. UTP w Bydgoszczy.
Tomczuk K., Grzybek M., Szczepaniak K., Studzińska M., Demkowska-Kutrzepa M. and Roczeń-Karczmarz M., Zahrai A., Kostro K. and Junkuszew A. 2017. Factors affecting prevalence and abundance of A.perfoliata infections in horses from south-eastern Poland. Vet. Parasitol., 246. doi:10.1016/j.vetpar.2017.08.027
Travé J. 1963. Écologie et biologie des Oribates (Acariens) saxicoles et arboricoles. Vie et Milieu, 11: 1-267.
Uwah D. and Eyo V. 2014. Effects of Number and Rate of Goat Manure Application on Soil Properties, Growth and Yield of Sweet Maize (Zea mays, L. saccharata Strut). Sustain. Agric. Res., 3. doi:10.5539/sar.v3n4p75
Václav R. and Kalúz S. 2014. The effect of herbivore faeces on the edaphic mite community: Implications for tapeworm transmission. Exp. App. Acarol., 62: 377-390. doi:10.1007/s10493-013-9743-1
Walter D.E. and Proctor H.C. 2013. Mites: Ecology, Evolution & Behaviour. Springer. doi:10.1007/978-94-007-7164-2. doi:10.1007/978-94-007-7164-2
Wasilewski Z. 2006. Ocena jakości runi i darni spasanych użytków zielonych w różnych siedliskach. IMUZ, 6: 413-421.
Wasińska-Graczyk B., Seniczak S., Graczyk R. 2009. The effect of pig liquid manure fertilization on the density and species structure of Oribatida (Acari) in lowland meadow in Poland. Biol. Lett., 46: 57-63. doi:10.2478/v10120-009-0001-5
Weigmann G. 2006. Hornmilben (Oribatida) Die Tierwelt Deutschlands 76 Teil. G&E.
Wickings K., Grandy A.-S. 2011. The oribatid mite Scheloribates moestus (Acari: Oribatida) alters litter chemistry and nutrient cycling during decomposition. Soil Biol. Biochem., 43: 351-358. doi:10.1016/j.soilbio.2010.10.023
Wickings K., Grandy A.-S. 2013. Management intensity interacts with litter chemistry and climate to drive temporal patterns in arthropod communities during decomposition. Pedobiologia, 56: 105-112. doi:10.1016/j.pedobi.2013.01.001
Vreeken-Buijs M.-J., Hassink J., Brussaard L. 1998. Relationships of soil microarthropod biomass with organic matter and pore size distribution in soils under different land use. Soil Biol. Biochem., 30: 97-106. doi:10.1016/S0038-0717(97)00064-3
Zbikowska-Zdun K., Koczara M. 2013. Oribatid mites (Oribatida: Acari): the indirect host of Anoplocephalidae tapeworms in an area of small pastures in Krakow. Med. Wet., 69:565-567.



2020-04-22
Date accepted:
2020-09-23
Date published:
2020-09-28
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Pacek, Sylwiusz; Seniczak, Stanislaw; Graczyk, Radomir; Chachaj, Bogusław and Seniczak, Anna
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



