New Perscheloribates species (Acari, Oribatida, Scheloribatidae) phoretic on beetles (Insecta, Coleoptera)
Ermilov, Sergey G.1 and OConnor, Barry M.2
1✉ Institute of Environmental and Agricultural Biology (X-BIO), Tyumen State University, Tyumen, Russia.
2Department of Ecology and Evolutionary Biology (Museum of Zoology), University of Michigan, Ann Arbor, MI, USA.
2020 - Volume: 60 Issue: 2 pages: 289-300
https://doi.org/10.24349/acarologia/20204368ZooBank LSID: 4071E221-5201-4E6D-9459-EEE5430D2046
Original research
Keywords
Abstract
Introduction
The phoretic association of oribatid mites (Acari, Oribatida) with beetles (Coleoptera) is poorly known. Mainly, the available data refer to the phoresy on beetles of the family Passalidae, e.g.: unidentified Mesoplophora (later, it was identified by Niedbała (1985, 2000) as M. (M.) permodica Niedbała, 1985, Mesoplophora (Parplophora) flavida Niedbała, 1985; M. (P.) polita Niedbała, 1985, and M. (P.) subtilis Niedbała, 1981), Malaconothrus, Oppia, Protoribates, Scheloribates, and Metaleius spp. from the different regions of the world (Norton 1980); Neoamerioppia phoretica (Franklin & Woas, 1992) from Brazil (Franklin & Woas 1992); M. (P.) flavida, M. (P.) polita, Graptoppia (Stenoppia) luisi Ermilov & Frolov, 2009, Ramusella (Sabahoppia) blattarum (Oudemans, 1911), and Perscheloribates kontumensis Ermilov & Frolov, 2009) from Vietnam (Ermilov & Frolov 2019a); Graptoppia (Stenoppia) royi Ermilov, 2019 from Indonesia (Ermilov 2019). In addition, there are also some data on phoresy of oribatid mites on the beetles of the other families, e.g.: Euscheloribates samsinaki Kunst, 1958 and Mesoplophora sp. on Scarabaeidae from Slovakia and U.S.A. (Kunst 1958; Norton 1973); Eremella reticulatus (Woolley, 1969) on Elateridae from U.S.A. (Woolley 1969); Scheloribates sp. on Cerambycidae from Mexico (Norton 1980); Ramusella (Ramusella) clavipectinata (Michael, 1885) on Curculionidae from Russia (Ermilov et al. 2008); Dometorina sp., Metaleius sp., Siculobata (Paraleius) leontonycha (Berlese, 1910), S. (P.) leahae (Knee, 2017), and Tectocepheus velatus sarekensis Trägårdh, 1910 on Curculionidae from different countries (Norton 1980; Knee et al. 2013; Ahadiyat & Akrami 2015; Knee 2017); Ramusella (Dosangoppia) bochkovi Ermilov & Frolov, 2019 on Geotrupidae from Russia (Ermilov & Frolov 2019b).
During taxonomic identification of mites phoretic on passalid and zopherid (Zopheridae) beetles from the Philippines and the U.S.A., we found two new species of Perscheloribates (s.s.). The goal of this paper is to describe and illustrate these new species under the names Perscheloribates paracuriosus n. sp. and P. parakontumensis n. sp.
Perscheloribates comprises four subgenera and more than 50 species (Ermilov & Starý 2018), which are distributed in tropical and subtropical regions. The nominotypical subgenus comprises about 50 species, which have the same geographical distribution as the genus. The main generic characters of Perscheloribates (Perscheloribates) were summarized by Hammer (1973), Corpuz-Raros (1980), and Balogh & Balogh (1992). Identification keys for many species from different regions have been presented earlier by Balogh & Balogh (1990, 2002), Ermilov et al. (2011), and Ermilov & Martens (2014).
Methods
Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster. Notogastral width refers to the maximum width of the ventral plate in ventral view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometers. Some paratypes were dissected and mounted in lactic acid on temporary flat slides. Formulas for leg setation are given in parentheses according to the sequence trochanter-femur-genu-tibia-tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu-tibia-tarsus.
Drawings were made with a camera lucida using a Leica transmission light microscope ''Leica DM 2500''.
Morphological terminology used in this paper follows that of F. Grandjean: see Travé & Vachon (1975) for references, Norton (1977) for leg setal nomenclature, and Norton & Behan-Pelletier (2009) for overview.
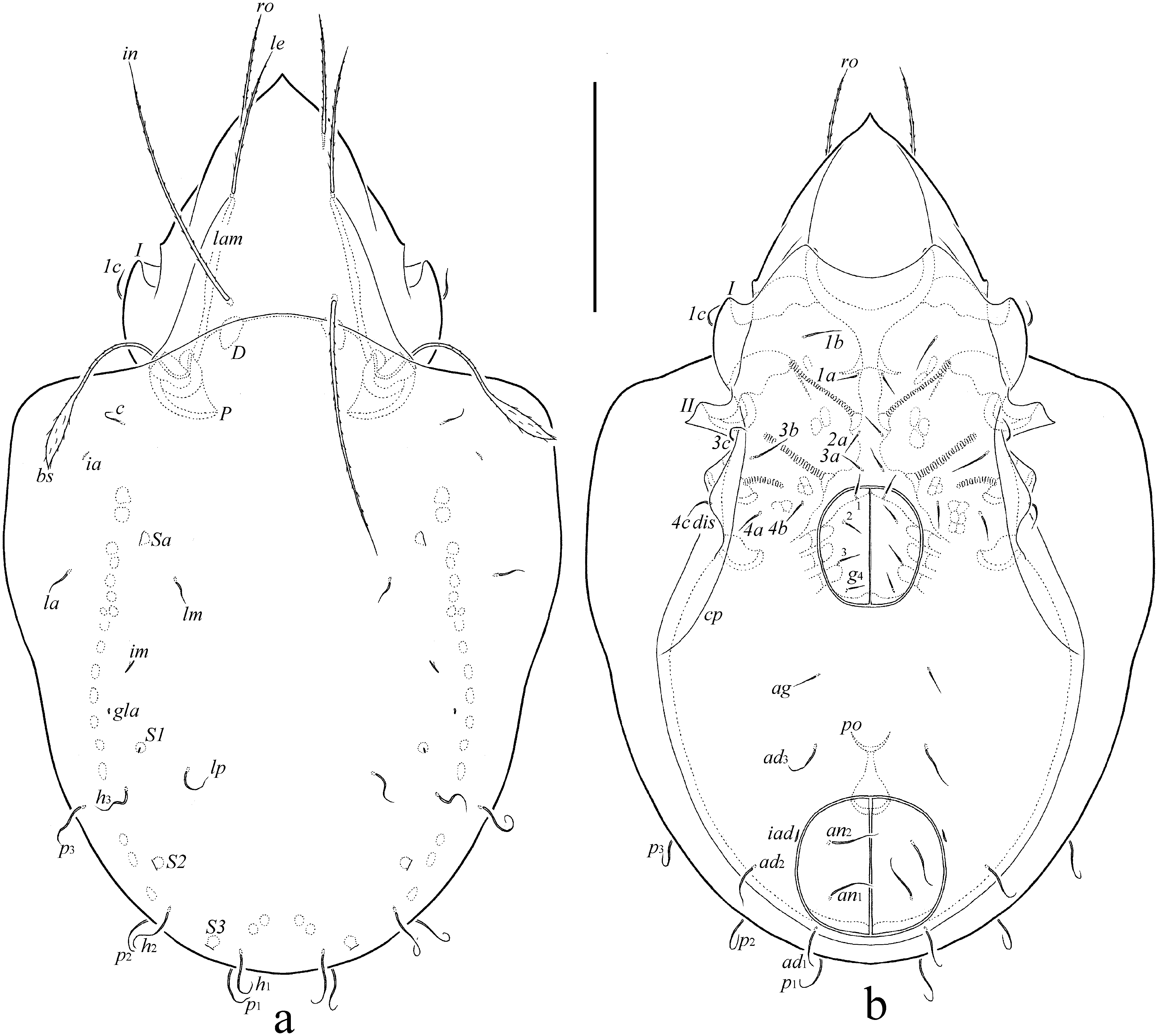
The following abbreviations are used (including text, figures and table): lam = lamella; plam = prolamella; slam = sublamella; Al = sublamellar porose area; kf = lateral keel-shaped ridge; ro, le, in, bs, ex = rostral, lamellar, interlamellar, bothridial and exobothridial setae, respectively; exv = alveolar vestige of second exobothridial seta; D = dorsophragma; P = pleurophragma; c, la, lm, dp, lp, h, p = notogastral setae; Sa, S1, S2, S3 = notogastral sacculi; ia, im, ip, ih, ips = notogastral lyrifissures; gla = opisthonotal gland opening; a, m, h = subcapitular setae; or = adoral seta; v, l, d, cm, acm, ul, sul, vt, lt = palp setae; ω = palp and leg solenidion; cha, chb = cheliceral setae; Tg = Trägårdh's organ; I, II = pedotecta I, II, respectively; 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c = epimeral setae; dis = discidium; cp = circumpedal carina; g, ag, an, ad = genital, aggenital, anal and adanal setae, respectively; iad = adanal lyrifissure; Amar = marginal porose area; po = preanal organ; Tr, Fe, Ge, Ti, Ta = leg trochanter, femur, genu, tibia, tarsus, respectively; pa = leg porose area; σ, φ = leg solenidia; ɛ = leg famulus; v, ev, bv, l, d, ft, tc, it, p, u, a, s, pv, pl = leg setae.
Systematics
Family Scheloribatidae
Genus Perscheloribates Hammer, 1973
Subgenus Perscheloribates (Perscheloribates) Hammer, 1973
Type species Perscheloribates clavatus Hammer, 1973
Perscheloribates (Perscheloribates) paracuriosus n. sp.
ZOOBANK: 4637B85A-2B7A-46E5-BCEB-1BD63BD07E7D ![]()
(Figures 1–3)





Diagnosis — Body size: 348–381 × 166–199. Rostrum pointed. Prolamella and translamella absent. Rostral, lamellar and interlamellar setae long, setiform, barbed; ro shortest, in longest. Bothridial seta long, lanceolate, barbed. Lateral keel-shaped ridge present. Notogastral seta short, setiform, roughened, c, la, lm short, simple, others longer, flexible. Epimeral and genital setae setiform, roughened. Anal and adanal setae flexible, roughened. Leg seta it absent on all tarsi.
Description — Measurements – a relatively small species. Body length: 365 (holotype, male), 348, 381 (two paratypes, two females); notogaster width: 182 (holotype), 166, 199 (two paratypes).
Integument – Body color light brown. Body surface punctate (visible under high magnification in dissected specimens). Lateral part of prodorsum slightly microgranulate.
Prodorsum (Figs 1a, 2a) – Rostrum pointed. Lamella located dorsolaterally, about half of prodorsum (measured in lateral view). Sublamella thin, slightly shorter than lamella. Sublamellar porose area (4) rounded. Prolamella and translamella absent. Rostral (53–57), lamellar (65–73) and interlamellar (102–110) setae setiform, barbed. Bothridial seta (82–86) lanceolate, barbed, with long stalk and shorter pointed apically head. Exobothridial seta (12–16) setiform, roughened. Alveolar vestige of second exobothridial seta and lateral keel-shaped ridge present. Sejugal porose area not observed. Dorsophragma slightly elongated.
Notogaster (Figs 1a, 2a-c) – Anterior notogastral margin convex medially. Pteromorph triangular, broadly rounded distally. Ten pairs of notogastral setae setiform, roughened, with different length and morphology; c, la, lm shorter (12–16), simple, others distinctly longer (lp, 24–36; others 32–41), with flexible tip. Four pairs of sacculi with small opening and drop-like chamber. Distance S1–S1 equal S2–S2. Opisthonotal gland opening and all lyrifissures distinct. Circumgastric scissure not observed. Circumgastric sigillar band visible.
Gnathosoma (Figs 2d-g) – Subcapitulum longer than wide (82–86 × 65–73). Three pairs of subcapitular setae setiform, barbed; h (28–32) longer than a (20–24) and m (20–24). Two pairs of adoral setae (10–12) setiform, barbed. Palp (49–53) with setation 0-2-1-3-9(+ω). Postpalpal seta (4) spiniform. Chelicera (90–94) with two setiform, barbed setae, cha (41–32) longer than chb (16–20). Trägårdh's organ narrowly triangular.
Epimeral and lateral podosomal regions (Figs 1b, 2a) – Epimeral setal formula 3-1-3-3. Setae setiform, roughened; 1b and 3b (24–28) longer than 1c, 3c, 4a, 4b, 4c (16–20) and 1a, 2a, 3a (10–12). Setae 1c inserted laterally on pedotectum I. Pedotectum II quadrangular in ventral view, with one protruding tip. Discidium rounded distally. Circumpedal carina long, reaching pedotectum II.
Anogenital region (Figs 1b, 2a-c) – Four pairs of genital (10–12) and one pair of aggenital (12–14) setae setiform, roughened. Two pairs of anal (32–41) and three pairs of adanal (32–41) setae flexible, roughened. Adanal lyrifissure located close and parallel to anal plates. Marginal porose area not observed. Preanal organ of typical, goblet-like form. Ovipositor elongated (138 × 20), blade (65) shorter than length of distal section (beyond middle fold; 73). Each of the three blades with four smooth setae, ψ1 ≈ τ1 (24) setiform, ψ2 ≈ τa ≈ τb ≈ τc (12) thorn-like. Six coronal setae (2) spiniform.



Legs (Figs 3a-d) – Claw of leg pretarsus sparsely barbed on dorsal side. Porose area on femora I–IV and on trochanters III, IV slightly visible; ventral porose area in basal part of tarsus and distal part of tibia not observed. Formulas of leg setation and solenidia: I (1-5-2-4-17) [1-2-2], II (1-5-2-4-13) [1-1-2], III (2-3-1-3-13) [1-1-0], IV (1-2-2-3-11) [0-1-0]; homology of setae and solenidia indicated in Table 1. Seta it absent on all tarsi, seta a' absent on tarsus IV. Famulus of tarsus I short, erect, slightly swollen distally, inserted between solenidion ω1 and seta ft''. Solenidion ω1 on tarsus I, ω1 and ω2 on tarsus II and σ on genu III bacilliform, other solenidia setiform.



Material examined — Holotype (male) and two paratypes (two females): Philippines, Negros Oriental, Sibulan Municipality, Lake Balinsasayao, 09°21'N, 123°10'E, 835 m a.s.l., phoretic on a specimen of Aceraius lamellatus Gravley, 1918 (Coleoptera, Passalidae), 9 August 1982 (P.D. Heideman). Data on localization of mites on A. lamellatus was not reported. Host specimen in the University of Michigan Museum of Zoology (UMMZ) bearing the voucher label, ''Mites removed, B.M. OConnor \#83-1800-024.'' The host specimen was collected in a decaying log in primary dipterocarp forest.
Type deposition — The holotype and one paratype are deposited in the collection of the University of Michigan Museum of Zoology, Ann Arbor, MI, USA; one paratype is deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia. All specimens are stored in ethanol with a drop of glycerol.
Etymology — The species name paracuriosus refers to the similarity between the new species and Perscheloribates curiosus Ermilov, 2016.
Remarks — In the presence of a pointed rostrum, lanceolate bothridial head and developed notogastral setae, and in the absence of prolamella, the new species is similar to Perscheloribates curiosus Ermilov, 2016 from Cuba (see Ermilov & Tolstikov 2016), but differs from the latter by the morphological differentiation of the notogastral setae (c, la, lm short, simple; others distinctly longer, flexible) (versus all setae short, similar in length, simple in P. curiosus), the presence of flexible anal and adanal setae (versus simple in P. curiosus) and a keel-shaped ridge on the lateral side of the prodorsum (versus absent in P. curiosus), and the absence of custodium (versus present in P. curiosus).
Perscheloribates (Perscheloribates) parakontumensis n. sp.
ZOOBANK: 0E24D8AB-6CBD-4AF0-BFDD-0A7CE11E7EE3 ![]()
(Figures 4–6)






Diagnosis — Body size: 348–365 × 182–199. Rostrum pointed. Prolamella and translamella absent. Rostral, lamellar and interlamellar setae long, setiform, barbed; ro shortest, in longest. Bothridial seta long, lanceolate, with long, setiform apex, barbed. Lateral keel-shaped ridge present. Notogastral setae short, setiform, roughened. Epimeral and anogenital setae setiform, roughened.
Description — Measurements – Small species. Body length: 348 (holotype, female), 348–365 (three paratypes, one male and two females); notogaster width: 182 (holotype), 182–199 (three paratypes).
Integument – Body color light brown. Body surface punctate (visible under high magnification in dissected specimens). Lateral part of prodorsum slightly microgranulate.
Prodorsum (Figs 4a, 5a) – Rostrum pointed. Lamella located dorsolaterally, about half of prodorsum (measured in lateral view). Sublamella thin, similar to lamella in length. Sublamellar porose area (4) rounded. Prolamella absent, but its basal part sometimes developed. Translamella absent. Rostral (53–61), lamellar (69–73) and interlamellar (77–82) setae setiform, barbed. Bothridial seta (65–73) lanceolate, barbed, with long stalk and shorter head having long setiform apex. Exobothridial seta (20–24) setiform, slightly barbed. Alveolar vestige of second exobothridial seta and lateral keel-shaped ridge present. Sejugal porose area not observed. Dorsophragma slightly elongated.
Notogaster (Figs 4a, 5a-c) – Anterior notogastral margin convex medially. Pteromorph triangular, broadly rounded distally. Ten pairs of notogastral setae (10–12) setiform, roughened. Four pairs of sacculi with small opening and drop-like chamber. Distance S1–S1 shorter than S2–S2. Opisthonotal gland opening and all lyrifissures distinct. Circumgastric scissure often not observed. Circumgastric sigillar band visible.
Gnathosoma (Fig. 4b) – Similar to Perscheloribates paracuriosus n. sp. Subcapitulum longer than wide (82–90 × 65–73). Three pairs of subcapitular setae setiform, barbed; h (24–28) longer than a (18–20) and m (10–12), m thinnest. Two pairs of adoral setae (10) setiform, barbed. Palp (49–53) with setation 0-2-1-3-9(+ω). Postpalpal seta (4) spiniform. Chelicera (90–94) with two setiform, barbed setae, cha (32–36) longer than chb (16–20). Trägårdh's organ narrowly triangular.
Epimeral and lateral podosomal regions (Figs 4b, 5a) – Epimeral setal formula 3-1-3-3. Setae setiform, roughened; 1b, 1c, 3b, 3c, 4c (16–24) longer than 1a, 2a, 3a, 4a, 4b (10–12). Setae 1c inserted laterally on pedotectum I. Pedotectum II quadrangular in ventral view, with one protruding tip. Discidium rounded distally. Circumpedal carina long, reaching pedotectum II.
Anogenital region (Figs 4b, 5a-c) – Four pairs of genital (8–10), one pair of aggenital (10–12), two pairs of anal (10–41) and three pairs of adanal (10–12) setae setiform, roughened. Adanal lyrifissure located close and parallel to anal plates. Marginal porose area complete, band-like. Preanal organ of typical, goblet-like form. Ovipositor elongated (134 × 28), blade (61) shorter than length of distal section (beyond middle fold; 73). Each of the three blades with four smooth setae, ψ1 ≈ τ1 (24) setiform, ψ2 ≈ τa ≈ τb ≈ τc (12) thorn-like. Six coronal setae (2) spiniform.
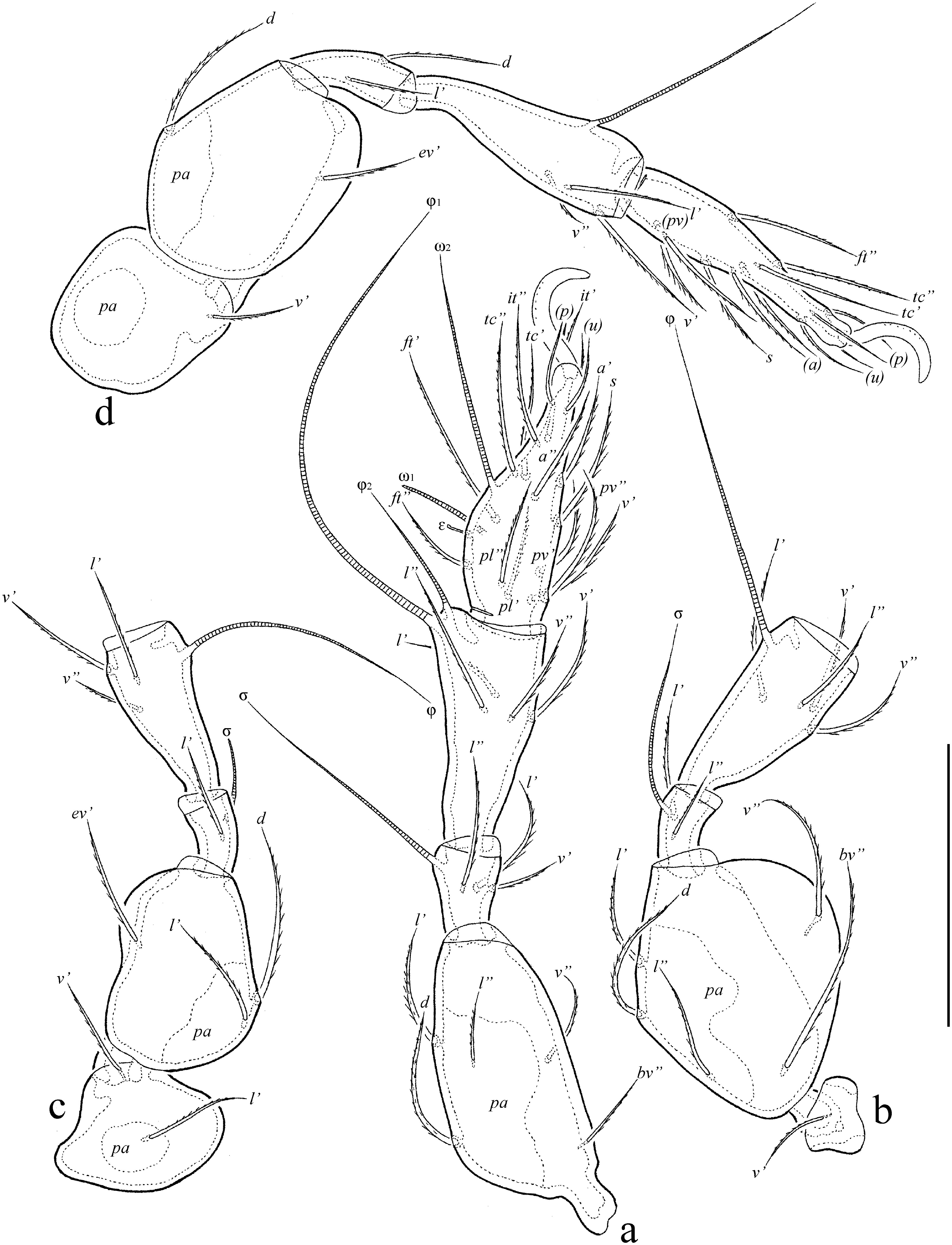


Legs (Figs 6a-d) – Claw of leg pretarsus sparsely barbed on dorsal side. Porose area on femora I–IV and on trochanters III, IV slightly visible; ventral porose area in basal part of tarsus and distal part of tibia not observed. Formulas of leg setation and solenidia: I (1-5-3-4-19) [1-2-2], II (1-5-2-4-15) [1-1-2], III (2-3-1-3-15) [1-1-0], IV (1-2-2-3-12) [0-1-0]; homology of setae and solenidia indicated in Table 2. Seta it present on all tarsi. Famulus of tarsus I short, erect, slightly swollen distally, inserted between solenidion ω1 and seta ft''. Solenidion ω1 on tarsus I, ω1 and ω2 on tarsus II and σ on genu III bacilliform, other solenidia setiform.
Material examined — Holotype (female) and three paratypes (one male and two females): U.S.A., Michigan, Emmet Co., Wilderness State Park, 45°44'N, 84°55'W, phoretic on specimens of Phellopsis obcordata (Kirby, 1873) (Coleoptera, Zopheridae), May 1981 (J. Kukor). Host specimen in UMMZ bearing the voucher label, ''Mites removed, B.M. OConnor \#81-0604-001.'' Mites were found on 3 specimens of P. obcordata collected from a fruting body of the polypore fungus, Porodaedalea pini (Brot.) Murrill, 1905 (=Fomes pini, = Phellinus pini), growing on red pine, Pinus resinosa Alton, 1789. Mites were found in pits on the dorsal surface of the elytra. The habitat consists of around 4050 hectares of dense coniferous and mixed hardwood forest on the northeast shore of Lake Michigan. The host beetle is associated with several species of polypore fungi in dense boreal forests in Eastern North America (Evans 2014), suggesting that the normal habitat of this mite species is either on or in the fungal fruiting bodies or in the mycelium growing within the wood of the host tree.



Type deposition — The holotype and two paratypes are deposited in the collection of the University of Michigan Museum of Zoology, Ann Arbor, MI, USA; one paratype is deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia. All specimens are stored in ethanol with a drop of glycerol.
Etymology — The species name parakontumensis refers to the similarity between the new species and Perscheloribates kontumensis Ermilov & Frolov, 2019.
Remarks — In the presence of pointed rostrum, lanceolate bothridial head with long setiform apex and developed notogastral setae, and in the absence of prolamella, the new species is similar to Perscheloribates kontumensis Ermilov & Frolov, 2019 from Vietnam (see Ermilov & Frolov 2019a), but differs from the latter by the presence of simple notogastral setae (versus with flexible tip in P. kontumensis) and the absence of body sculpturing (versus simple in P. kontumensis) and setae it on leg tarsi I–III (versus absent in P. kontumensis).
General remarks
As previously noted (Norton 1980; Ermilov 2019; Ermilov & Frolov 2019a, b), some oribatid mites are actively phoretic on insects, having morphological adaptations for attachment to the host (for example, gripping setae of beetles between the rostrum of the aspis and the anterior portion of the genital plates in ptyctimous mites, or specifically curved leg claws for holding onto setae of the host as in Siculobata (Paraleius)) (Norton 1980).
However, other oribatid species (including P. paracuriosus n. sp. and P. parakontumensis n. sp.) have no visible adaptations, so mostly hold the host's surface by hiding in various depressions and grooves of the host body and using the force of the pretarsal claws for attaching to the surface.
Beetles of the family Passalidae are one of the more common insect hosts actively used by oribatid mites for phoresy (Ermilov 2019), however, phoretic oribatid mites had not been previously reported from A. lamellatus, nor from any beetles of the family Zopheridae. Hence, our findings (P. paracuriosus n. sp. on A. lamellatus and P. parakontumensis n. sp. on P. obcordata) are the first records of the use of A. lamellatus and zopherid beetles by oribatid mites for phoresy.
Acknowledgements
We thank Prof. Dr. Roy A. Norton (State University of New York, Syracuse, USA) for many years of cooperation and collaboration and Dr. Julia Baumann (University of Graz, Graz, Austria) and two anonymous reviewers for valuable comments. We also are grateful to the late Alan Gillogly who identified the specimen of Aceraius lamellatus.
References
Ahadiyat A., Akrami M.A. 2015. Oribatid mites (Acari: Oribatida) associated with bark beetles (Coleoptera: Curculionidae: Scolytinae) in Iran, with a review on Paraleius leontonychus (Berlese) and a list of bark beetles in association with this species. Pers. J. Acar., 4(4): 355-371.
Balogh J., Balogh P. 1990. Oribatid mites of the Neotropical region. II. Budapest: Akadémiai Kiadó Press. 333 p.
Balogh J., Balogh P. 1992. The oribatid mites genera of the World. Vol. 1. Budapest: Hungarian National Museum Press. 263 p.
Balogh J., Balogh P. 2002. Identification keys to the oribatid mites of the Extra-Holarctic regions. Vol. 1. Miskolc: Well-Press Publishing Limited. 453 p.
Berlese A. 1910. Brevi diagnosi di generi e species nuovi di Acari. Redia, 6: 346-388.
Corpuz-Raros L. 1980. Philippine Oribatei (Acarina) V. Scheloribates Berlese and related genera (Oribatulidae). Kalikasan, Philipp. J. Biol., 9(2-3): 169-245.
Ermilov S.G. 2019. Oribatid mites (Acari: Oribatida) phoretic on passalid beetles (Coleoptera: Passalidae), with description of a new species from Indonesia. Ecol. Mont., 22: 90-96.
Ermilov S.G., Frolov A.V. 2019a. New and interesting oribatid mites (Acari, Oribatida) phoretic on Aceraius grandis (Coleoptera, Passalidae) from Vietnam. Syst. Appl. Acarol., 24(5): 945-961. doi:10.11158/saa.24.5.15 ![]()
Ermilov S.G., Frolov A.V. 2019b. Ramusella (Dosangoppia) bochkovi (Acari, Oribatida, Oppiidae), a new subgenus and species of oribatid mites phoretic on Ceratophyus polyceros (Pallas, 1771) (Coleoptera, Geotrupidae) from Russia. Syst. Appl. Acarol., 24(2): 209-221. doi:10.11158/saa.24.2.4 ![]()
Ermilov S.G., Martens, J. 2014. A new species of Perscheloribates (Acari, Oribatida, Scheloribatidae) from Nepal with a key to all species of the genus from the Oriental region. Acarina, 22(1): 14-19. doi:10.3897/zookeys.424.7990 ![]()
Ermilov S.G., Starý J. 2018. A new species of Perscheloribates (Acari, Oribatida, Scheloribatidae) from Vietnam, with notes on the genus records in the country. Spixiana, 41(2): 189-196.
Ermilov S.G., Tolstikov A.V. 2016. A new species of oribatid mites of the genus Perscheloribates (Acari, Oribatida, Scheloribatidae) from Cuba. Acarina, 24(1): 27-32. doi:10.21684/0132-8077.2016.24.1.27.32 ![]()
Ermilov S.G. Mokrousov M.V., Dmitrieva I.N. 2008. Acarofauna of curculionoid beetles (Сoleoptera, Curculionoidea). Povol. Ecol. Zh., 1: 65-68. [In Russian]
Ermilov S.G., Rybalov L.B., Franke K. 2011. Ethiopian oribatid mites of the family Scheloribatidae (Acari: Oribatida). Afr. Inverteb., 52(2): 311-322. doi:10.5733/afin.052.0207 ![]()
Evans A.V. 2014. Beetles of Eastern North America. Princeton: Princeton University Press. 560 pp. doi:10.1515/9781400851829 ![]()
Franklin E., Woas S. 1992. Some oribatid mites of the family Oppiidae (Acari, Oribatei) from Amazonia. Andrias, 9: 5-56.
Knee W., Forbes M.R., Beaulieu F. 2013. Diversity and host use of mites (Acari: Mesostigmata, Oribatida) phoretic on bark beetles (Coleoptera: Scolytinae): Global generalists, local specialists? Ann. Ent. Soc. Amer., 106(3): 339-350. doi:10.1603/AN12092 ![]()
Knee W. 2017. A new Paraleius species (Acari, Oribatida, Scheloribatidae) associated with bark beetles (Curculionidae, Scolytinae) in Canada. ZooKeys, 667: 51-65. doi:10.3897/zookeys.667.12104 ![]()
Kunst M. 1958. Euscheloribates samsinaki n. g., n. sp., eine neue Moosmilbe aus der Tschechoslowakei (Acarina - Oribatei). Cas. opic. Ceskoslov. spol. ent., 55(1): 67-70.
Hammer M. 1973. Oribatids from Tongatapu and Eua, the Tonga Islands, and from Upolu, Western Samoa. Det Kong. Dansk. Vidensk. Selsk. Biol. Skr., 20(3): 1-70.
Michael A.D. 1885. New British Oribatidae. Roy. Micros. Soc., series 2, 2: 385-397. doi:10.1111/j.1365-2818.1885.tb05787.x ![]()
Niedbała W. 1981. Mesoplophora subtilis sp. n. de Pérou (Acari, Oribatida, Mesoplophoridae). Polsk. Pismo Ent., 51: 511-517.
Niedbała W. 1985. Essai critique sur Mesoplophora (Acari, Oribatida, Mesoplophoridae). Ann. Zool., 39(4): 93-117.
Niedbała W. 2000. The ptyctimous mites fauna of the Oriental and Australian regions and their centers of origin (Acari: Oribatida). Genus (Supplement): 1-493.
Norton R.A. 1973. Phoretic mites associated with the hermit flower beetle, Osmoderma eremicola Knoch (Coleoptera: Scarabaeidae). Amer. Mid. Nat., 90(2): 447-449. doi:10.2307/2424466 ![]()
Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal D.L. (Ed.). Biology of oribatid mites. Syracuse: SUNY College of Environmental Science and Forestry. pp. 33-61.
Norton R.A. 1980. Observations on phoresy by oribatid mites (Acari: Oribatei). Int. J. Acarol., 6(2): 121-130. doi:10.1080/01647958008683206 ![]()
Norton R.A., Behan-Pelletier V.M. 2009. Oribatida. Chapter 15. In: Krantz G.W., Walter D.E. (Eds.). A Manual of Acarology. Lubbock: Texas Tech University Press. pp. 430-564.
Trägårdh I. 1910. Acariden aus dem Sarekgebirge. Nat. Unt. Sarek. Schwed.-Lap., Zool., Stockholm, 4: 375-586.
Travé J., Vachon M. 1975. François Grandjean. 1882-1975 (Notice biographique et bibliographique). Acarologia, 17(1): 1-19.
Oudemans A.C. 1911. Acarologische Aanteekeningen XXXVII. Ent. Berich., 61(3): 165-175.
Woolley T.A. 1969. A new and phoretic oribatid mite (Acarina: Cryptostigmata: Licnodamaeidae). Proc. Ent. Soc. Washington, 71(4): 476-481.



2019-12-15
Date accepted:
2020-03-18
Date published:
2020-03-23
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Ermilov, Sergey G. and OConnor, Barry M.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



