Effect of pollen of different plant species on the oviposition of two phytoseiid mites (Acari: Phytoseiidae) commonly found in citrus orchards in the Brazilian Amazonia
Ferreira, Camila Tavares1 ; Krug, Cristiane2 and Moraes, Gilberto José de3
1✉ Depto. de Entomologia e Acarologia, Escola Superior de Agricultura Luiz de Queiroz (ESALQ), Universidade de São-Paulo (USP), Piracicaba, SP, Brazil.
2Depto. de Entomologia, Empresa Brasileira de Pesquisa Agropecuária (Embrapa Amazônia Ocidental), Manaus, AM, Brazil.
3Depto. de Entomologia e Acarologia, Escola Superior de Agricultura Luiz de Queiroz (ESALQ), Universidade de São-Paulo (USP), Piracicaba, SP, Brazil.
2020 - Volume: 60 Issue: 1 pages: 22-29
https://doi.org/10.24349/acarologia/20204360Original research
Keywords
Abstract
Introduction
Phytoseiids are important biological control agents of several pests in different crops. This large family contains more than 2,700 species (Demite et al. 2017), some of which have been commercially used for pest control. They are mostly found on plants, feeding mainly on phytophagous mites, but some species also feed on pollen or small insects (McMurtry et al. 2013). In fact, Euseius and Iphiseiodes species can be found in high abundance when the only food source available is pollen (McMurtry et al. 2013).
In a study conducted in Manaus region, of the Brazilian Amazonia, Bobot et al. (2011) reported ten species of phytophagous mites from citrus plants, belonging to the families Eriophyidae, Tenuipalpidae and Tetranychidae. In a more recent study conducted in the same region, six species of phytophagous mites were reported by Ferreira et al. (2018), all of which also reported by Bobot et al. (2011).
Several species of predatory mites were collected in the study conducted by Ferreira et al. (2018), including eight phytoseiid species. The most numerous species were Amblyseius aerialis Muma and Iphiseiodes zuluagai Denmark & Muma, corroborating the results of Bobot et al. (2011).
Phytophagous mites are still not considered pests in citrus orchards in the Brazilian State of Amazonas, which could be due to of the effectiveness of the prevailing predatory mites, although other factors may also play an important role. Pollen from native plants of Amazonia could potentially support phytoseiid populations in citrus orchards in Manaus region.
Pollen has been used as a supplement to feed predators on crops after their preventive releases. The presence of natural vegetation in the surroundings of orchards might favour the maintenance of those predators, by naturally supplying complementary food (Tixier et al. 2000; Duso et al. 2004; Maoz et al. 2014). Thus, more diverse agricultural systems may provide better resources to natural enemies, favouring their performance (Kennett et al. 1979; Tixier et al. 2000; Duso et al. 2004).
The suitability of the use of pollen to foster the control of citrus pests by facilitating the maintenance of predatory mites in the area has been extensively studied. The aim of this study was to evaluate the effect of pollen of different plant species, most of which commonly found in Amazon region, on the oviposition of A. aerialis and I. zuluagai.
Material and methods
Origin of mites and rearings
The phytoseiid species evaluated were collected from citrus orchards in Manaus a few months before initiating the experiment. Colonies were established in the laboratory, each onto a piece of resin plate (Paviflex®, 10 x 15 cm) placed onto a piece of polyethylene foam (2 cm thick) kept moist by daily additions of distilled water, inside a plastic tray (16 x 22 x 7 cm). A cover slip was placed onto cotton fibers to serve as a shelter and oviposition sites for the predator. The colonies were fed a mixture of cattail (Typha domingensis) pollen and immatures of Aleuroglyphus ovatus (Tropeau) (Acari: Acaridae). Colonies and experimental units were maintained in a rearing chamber at 25 ± 1 ° C, 70 ± 10% relative humidity and 12 h of daily photophase.
Pollen sources
Pollen of 13 plant species were evaluated, including 12 species common in that region (Agave sp., Amaryllis sp., Citrus sinensis, Cocos nucifera, Elaeis guineensis, Elaeis oleifera, Euterpe oleracea, Helianthus annuus, Montrichardia linifera, Oenocarpus bacaba, Passiflora edulis and Turnera ulmifolia) and one species not found in that region, but available commercially (cattail, T. domingensis). Pollen was obtained from plants available in Manaus or Belém (both in the Brazilian Amazonia), except for Amaryllis sp., Helianthus sp. and T. domingensis, whose pollen was obtained from other parts of Brazil. For comparison, commercially available cattail pollen (of T. domingensis, ECOPolen, supplied by ''Empresa ECOntrole'', Brazil), and commercially available honeybee (Apis mellifera: Apidae) harvested pollen, as well as field collected Melipona seminigra merrillae (Apidae) harvested pollen were also evaluated.
To remove pollen from Arecaceae (C. nucifera, E. guineensis, E. oleifera, E. oleracea and O. bacaba), whose flowers were already open when collected in the field, inflorescences were collected in fabric bags, taken to air conditioned room (about 18°C) for drying overnight, and then processed according to Cunha et al. (2007). For most other pollen types, the flowers were still not open; hence, flowers were collected in paper bags and transported to the laboratory, where their peduncles were maintained in a vial with water placed onto a piece of white paper, waiting for pollen grains to fall. These were then sieved to remove impurities and packed in a vial for storage in a refrigerator at -20 °C. Pollen of C. sinensis and T. ulmifolia were offered fresh to the mites, inside the anthers, due to the difficulty in separating them from other flower structures.
Experimental procedure
Each experimental unit corresponded to a plastic Petri dish (2 cm high x 3 cm in diameter) whose base was covered with two filter paper discs overlaid by a citrus leaf disc with the abaxial surface facing up. The filter paper disks were kept moist by daily addition of distilled water.
A gravid and seemingly healthy female predator was transferred with the use of a thin brush from the stock colony to an experimental unit containing pollen grains of one of types to be evaluated onto a piece of coverslip, totalling 20 females of each predator species per treatment. The amount of pollen to be supplied was determined in preliminary tests, to ensure availability of a surplus amount to the predator. Every 24 h, the old food was replaced by new food, which prevented mould incidence. The units were closed with a PVC (Magipack®) film and examined daily under a stereomicroscope to determine the number of eggs laid by the predator, in a period of 11 days. Data from the first day were not considered in the calculations to reduce the influence of previous feeding. Each female was transferred to a new unit every five days, also with the use of a thin brush.
The effect of treatments on oviposition and survivorship were compared by Kruskal-Wallis nonparametric test, as data did not have normal distribution (Shapiro Wilk's test) or stable variance (Bartlett test). Analysis was conducted using R software (R CORE TEAM 2016).
Pollen morphology
Pollen grains was examined from photographs taken under fluorescence optical (Axio Imager M2- ZEISS) and scanning electron (LEO 435-VP-ZEISS) microscopes. In the preparation of the samples for light microscopy examination, the grains were placed in a vial containing glycerin (50% glycerin and 50% water) according to Wodehouse (1933). The material was homogenized, transferring a drop with a pipette onto a slide and covering it with a coverslip. The slide was placed in a heating plate for about 30 seconds, waiting for the grains to spread on the slide, to facilitate examination. Images were taken to measure the major axis of each grain and to evaluate the purity, based on their shape. Ten pollen grains of each sample were measured and the grains were classified as very small (< 10 μm), small (10-25 μm), median (25-50 μm), large (50-100 μm), very large (100-200 μm) and giant (\textgreater 200 μm) (Halbritter et al. 2018). For scanning electron microscopy examination, small amounts of pollen were fixed to the upper end of a stub with the use of double-sided adhesive tape. The grains were covered with a gold layer (conductive layer) to improve contrast of the image. Photographs were then taken to examine grain size and ornamentation (Ybert et al. 2012; Halbritter et al. 2018).
Results
Oviposition and survival
For each predator species, significant differences were observed for daily oviposition rates and survivorship on the different pollen types. Oviposition rates ranged from zero to 1.6 eggs per female, with a gap between 0.4 and 0.9 egg/ female/ day for both species (except pollen of P. edulis for I. zuluagai, 0.6 egg/ female/ day). Few pollen types promoted the production of at least 0.9 egg a day (Table 1). For A. aerialis, the highest oviposition rates were obtained on pollen of E. guineensis, field collected T. domingensis, C. nucifera and the commercial pollen of T. dominguensis (respectively 1.5, 1.0, 0.9 and 0.9 egg/ female/day). For I. zuluagai, the highest levels were obtained on pollen of E. guineensis, C. nucifera, field collected T. domingensis and E. oleifera (respectively 1.6, 1.5, 1.2 and 0.9 egg/ female/ day). For both predators, oviposition was at most 0.4 egg/ female/ day on other pollen types.
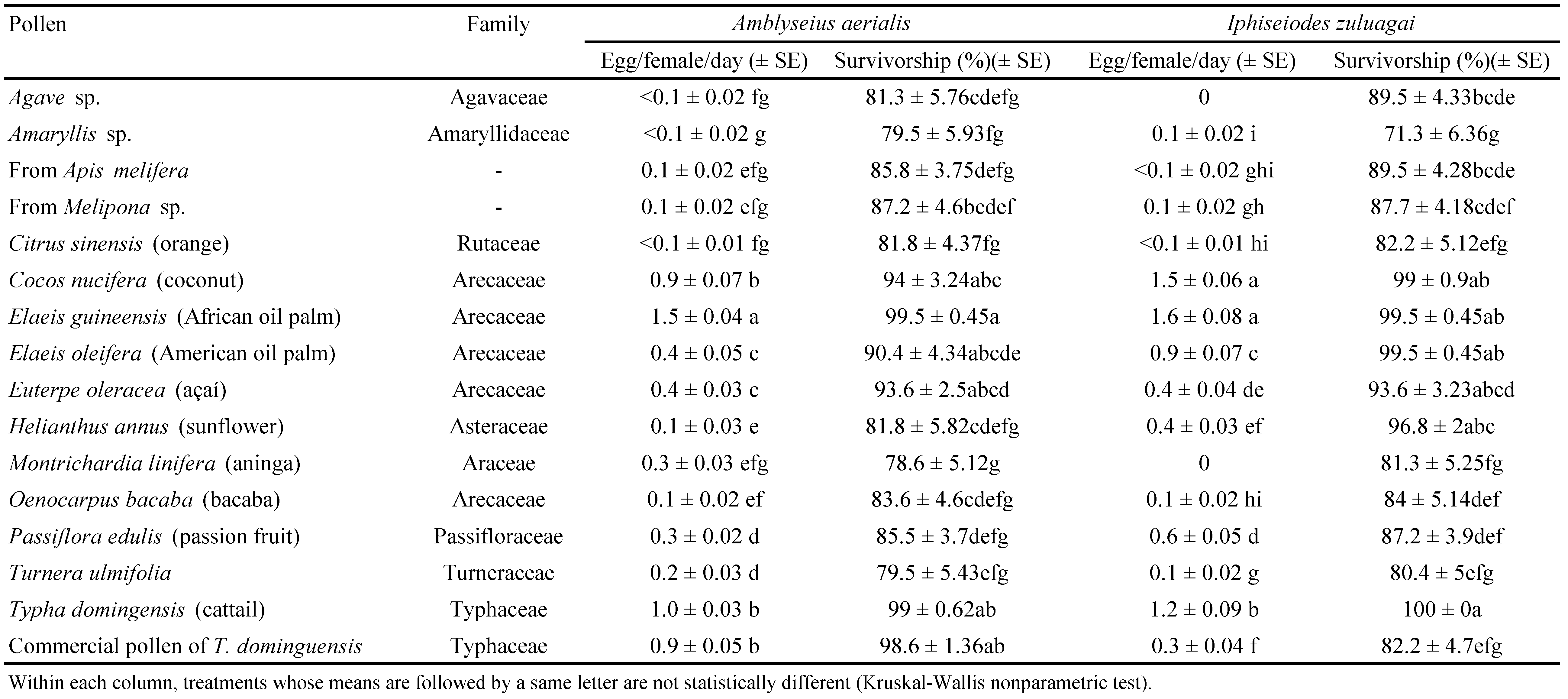


Survivorship of A. aerialis was at least 90% when fed pollen of C. nucifera, E. guineensis, E. oleifera, E. oleracea, field collected T. domingensis and commercial pollen of T. dominguensis (Table 1). Survivorship of I. zuluagai was also at least 90% when fed pollen of C. nucifera, E. guineensis, E. oleifera, E. oleracea, H. annuus, field collected T. domingensis.
Pollen morphology
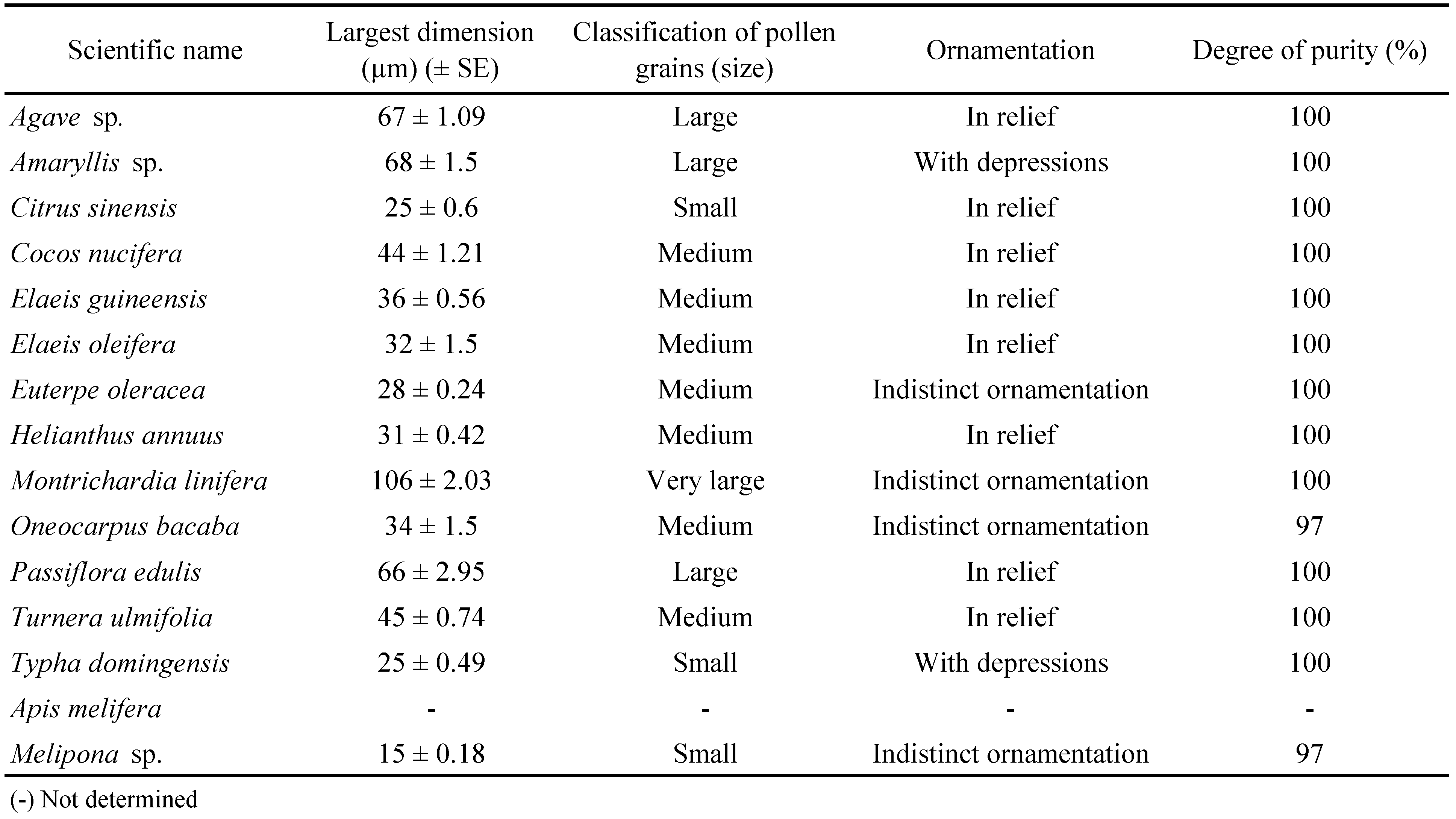
Great variation in grain size and ornamentation was observed (Table 2). Sizes ranged from small to very large, but grains of palm plants were the least variable, all classified as medium sized, although they were quite variable in ornamentation. Grains collected by A. mellifera consisted of pollen of more than eight plant species.


Discussion
The highest level of daily oviposition of A. aerialis in the present study was similar or slightly higher than that obtained by Castillo and Noronha (2008), when the predator was fed pollen of Typha angustifolia (1.3 eggs/female). This was expected, considering the numerous reports on the suitability of pollen of plants of this genus to phytoseiid species (McMurtry and Croft 1997; Furtado and Moraes 1998; Van Rijn and Tanigoshi 1999; Ferla and Moraes 2003; Lofego and Moraes 2005; Bellini et al. 2010). For I. zuluagai the highest level of daily oviposition was also slightly higher than that obtained by Reis et al. (1998) and Marques et al. (2014) when fed with castor bean pollen (Ricinus communis L.) (0.7 and 0.6 egg/female respectively).
Survivorship in the course of the experiment was rather variable between treatments, but for both predators the types of pollen promoting highest oviposition rates also promoted highest survivorship rates.
Some hypothesis could be raised in relation to the performance of the predators on some pollen types. The shared features of pollen types allowing comparatively higher daily oviposition rates (at least 0.9 egg/female) were: average to small sizes and irregular surfaces (either depressions or elevations) (according to the classification of Ybert et al. 2012). In pollen of H. annuus, embossment of the exine with spicules seems to have hindered exine rupturing by both predators. During the observations, it could be noticed that predators moved quickly away from those pollen grains soon after contacting them. In a study on the effect of different pollen types on the development of Amblyseius swirskii Athias-Henriot, the presence of very long spines on pollen of Hibiscus syriacus (Malvaceae) seemed to be one of the factors preventing feeding (Goleva and Zebitz 2013). In the same study, the authors classified sunflower pollen as not suitable for A. swirskii, but did not report whether the presence of spicules interfered in the feeding process. In the present study, oviposition rates were low on all unornamented pollen types, but presence of ornamentation was not always associated with pollen suitability.
The highest levels of oviposition could also be related to the nutritional value of each pollen type, but not much can be mentioned in relation to the nutritive quality of the pollen types used in this study. Most of the information in this regard available in the literature refers to pollen collected by bees, probably because of the relative ease in collecting large amount of those pollen types. Because of the difficulty in collecting sufficient amounts, only pollen of E. guineensis could be analyzed in this study. In the analysis conducted by our request by Laboratório de Bromatologia, Departamento de Zootecnia ESALQ-USP, the composition of E. guineensis pollen (the pollen type providing the best results for both predator species in the present study) was exactly the same reported by Figueiredo et al. (2018). In fact, difference in nutritional value could be one of the main reasons for the differences in oviposition rates reported for different phytoseiids on different pollen types.
The content of crude protein is considered an adequate parameter to infer pollen nutritional quality for bees; the proportion required for their development is 20 to 23% (Pernal and Currie 2000; Somerville 2005). The proportion found in E. guineensis pollen (38 %) is much higher than that. Massaro et al. (2016) reported oviposition of 0.9 and 1.1 eggs/female/day by the phytoseiids Amblyseius tamatavensis Blommers and Euseius concordis (Chant), on pollen of E. guineensis. However, the study of Figueiredo et al. (2018) offering the same type of pollen did not confirm its suitability for the maintenance of a colony of E. concordis. Pollen of C. nucifera also favoured the oviposition of both predators in this study, and this could also be related to its high crude protein (33.5%, according to Moura 2014).
One of the common characteristics for most pollen types promoting low oviposition levels (Agave sp., Amaryllis sp., C. sinenses, H. annuus, P. edulis and pollen gathered by A. mellifera) was the water condensation on the surface of the grains during the experiment. This may have hampered mite feeding. However, the observed condensation could have happened as a consequence of the method used in this experiment in which the experimental units were closed to prevent mites from escaping. It is conceivable that under natural conditions, with pollen grains totally exposed onto plant leaves, grains could be drier in periods of no rain.
In conclusion, of the nine pollen types obtained from plants present in or around citrus orchards in Manaus region, four (C. nucifera, E. guineensis, E.oleifera and P. edulis) promoted daily oviposition of at least 0.6 egg/ female by at least one of the predator species, supporting the hypothesis that pollen from plants found in the Amazonian region can help to maintain predatory mites in citrus orchards, theoretically enhancing their effect as natural enemies of phytophagous mites.
The mite species considered in this study are commonly found in citrus orchards throughout the country (Bittencourt and da Cruz, 1988; Sato et al., 1994; Reis et al., 2000; Albuquerque and Moraes, 2008; Silva et al., 2013; Noronha, 2013). Regarding the distribution of plants providing the best results, E. guineensis is mainly grown in northern Brazil and in the Bahia State (northeast), producing abundant pollen (Cunha et al. 2007). Typha domingensis and C. nucifera can be found in several regions of Brazil, but T. domingensis is not common in the north. However, by being pollen of T. domingensis and E. guineensis commercially available and readily acceptable by those predators, they could eventually be used as alternative food sources for the maintenance of predators in citrus orchards, as suggested for other predators and pollen types on the same crop (Aguilar-Fenollosa et al. 2011; Maoz et al. 2014; Jacas et al. 2015). Complementary field studies are needed to investigate the technical and economic feasibility of using pollen provisioning in that region, for the control of pest mites.
Acknowledgements
This work was partially financed by the following projects: Research and technology transfer for the development of citriculture in Amazonas State, Embrapa Western Amazon (Fundação de Amparo à Pesquisa do Estado do Amazonas–FAPEAM), and Plant viruses transmitted by Brevipalpus sp. (Acari: Tenuipalpidae) - VTB: survey, identification, molecular characterization, phylogeny; Virus/vector/host relationships; Biology, taxonomy and vector management (Fundação de Apoio à Pesquisa do Estado de São Paulo-FAPESP).
References
Aguilar-Fenollosa E., Ibáñez-Gual M.V, Pascual-Ruiz S., Hurtado M., Jacas J.A. 2011. Effect of ground-cover management on spider mites and their phytoseiid natural enemies in clementine mandarin orchards (I): Bottom-up regulation mechanisms. Biol. Control, 59(2): 158-170. doi:10.1016/j.biocontrol.2011.06.013 ![]()
Albuquerque F.A. de, Moraes G.J. de 2008. Perspectivas para a criação massal de Iphiseiodes zuluagai Denmark & Muma (Acari: Phytoseiidae). Neotrop. Entomol., 37: 328-333. doi:10.1590/S1519-566X2008000300013 ![]()
Bellini M.R., Araujo R.V. de, Silva E.S., Moraes G.J. de, Berti Filho E. 2010. Ciclo de vida de Proprioseiopsis cannaensis (Muma) (Acari: Phytoseiidae) com diferentes tipos de alimentos. Neotrop. Entomol., 39: 360-364. doi:10.1590/S1519-566X2010000300008 ![]()
Bittencourt M.A.L., da Cruz F.Z. 1988 . Toxicidade de produtos químicos sobre ácaros predadores (Acari: Phytoseiidae) em citros. An. Soc. Entomol. Bras., 17: 249-261.
Bobot T. da E., Franklin E., Navia D., Gasnier T.R.J., Lofego A.C., Oliveira B.M. de 2011. Mites (Arachnida, Acari) on Citrus sinensis L. Osbeck orange trees in the state of Amazonas, Northern Brazil. Acta Amaz., 41: 557-566. doi:10.1590/S0044-59672011000400013 ![]()
Castillo A.B., Noronha A.C. da S. 2008. Estudio de los aspectos fundamentales de la biología de Amblyseius aerialis (Muma)(Acari: Phytoseiidae) en condiciones de laboratorio. CitriFrut, 25(1): 45-52.
Cunha R.N.V., Lopes R., Dantas J.C.R.., Rocha R.N.C. 2007. Procedimentos para produção de sementes comerciais de dendezeiro na Embrapa Amazônia Ocidental. Manaus: Embrapa Amazônia Ocidental, 2007. 34 pp.
Demite P.R., Moraes G.J.D., McMurtry, J.A.; Denmark H.A.., Castilho R.C. 2017. Phytoseiidae Database [access September 15, 2017]. Available from: http://www.lea.esalq.usp.br/phytoseiidae/ ![]()
Duso C., Malagnini V., Paganelli A., Aldegheri L., Bottini M., Otto S. (2004). Pollen availability and abundance of predatory phytoseiid mites on natural and secondary hedgerows. BioControl, 49(4): 397-415. doi:10.1023/B:BICO.0000034601.95956.89 ![]()
Erdtman G. 1986 . Pollen morphology and plant taxonomy: Angiosperms. Brill Archive. p. 553. doi:10.1126/science.117.3030.86 ![]()
Ferla N.J., Moraes G.J. de 2003. Oviposição dos ácaros predadores Agistemus floridanus Gonzalez, Euseius concordis (Chant) e Neoseiulus anonymus (Chant & Baker) (Acari) em resposta a diferentes tipos de alimento. Rev. Bras. Zool., 20: 153-155. doi:10.1590/S0101-81752003000100019 ![]()
Ferreira C.T., Krug C., Garcia M.V.B., Moraes G.J. 2018. Leprosis mite and other mite species (Acari) associated to orange groves in Brazilian Central Amazon. Syst. Appl. Acarol., 23(3): 449-462. doi:10.11158/saa.23.3.4 ![]()
Figueiredo E.S. de, Massaro M., Carmo S. do, Moraes G.J. de 2018. Rearing system for the predatory phytoseiid Euseius concordis (Acari: Phytoseiidae). Exp. Appl. Acarol., 74(1): 13-23. doi:10.1007/s10493-018-0212-8 ![]()
Furtado I.P., Moraes G. 1998. Biology of Euseius citrifolius, a candidate for the biological control of Mononychellus tanajoa (Acari: Phytoseiidae, Tetranychidae). Syst. Appl. Acarol., 3: 43. doi:10.11158/saa.3.1.6 ![]()
Goleva I., Zebitz C.P.W. 2013. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol., 61(3): 259-283. doi:10.1007/s10493-013-9700-z ![]()
Halbritter H., Ulrich S., Grímsson F., Weber M., Zetter R., Hesse M., Bruchner R., Svojtka M., Frosch-Radivo A. 2018. Illustrated Pollen Terminology. Springer International Publishing. 483 pp. doi:10.1007/978-3-319-71365-6 ![]()
Jacas J., Aguilar-Fenollosa E., Hurtado M., Pina T. 2015. Food Web Engineering to Enhance Biological Control of Tetranychus urticae by Phytoseiid Mites (Tetranychidae: Phytoseiidae) in Citrus. In: Prospect. Biol. Control Plant Feed. Mites Other Harmful Org.: 251-269. doi:10.1007/978-3-319-15042-0\_10 ![]()
Kennett C.E., Flaherty D.L., Hoffmann R.W. 1979. Effect of wind-borne pollens on the population dynamics of Amblyseius hibisci [Acarina: Phytoseiidae]. Entomophaga, 24(1): 83-98. doi:10.1007/BF02377513 ![]()
Lofego A.C., Moraes G.J. de 2005. Taxa de oviposição dos predadores Amblyseius acalyphus e Amblyseius neochiapensis (Acari: Phytoseiidae) com diferentes tipos de alimento. Arq. Inst. Biol. (Sao. Paulo), 72: 379-382.
Maoz Y., Gal S., Argov Y., Domeratzky S., Melamed E., Gan-Mor S., Coll M., Palevsky E. 2014. Efficacy of indigenous predatory mites (Acari: Phytoseiidae) against the citrus rust mite Phyllocoptruta oleivora (Acari: Eriophyidae): augmentation and conservation biological control in Israeli citrus orchards. Exp. Appl. Acarol., 63(3): 295-312. doi:10.1007/s10493-014-9786-y ![]()
Massaro M., Martin J.P.I., Moraes G.J. de 2016. Factitious food for mass production of predaceous phytoseiid mites (Acari: Phytoseiidae) commonly found in Brazil. Exp. Appl. Acarol., 70(4): 411-420. doi:10.1007/s10493-016-0087-5 ![]()
Marques R.V., Sarmento R.A., Ferreira V.A., Venzon M., Lemos F.,Pedro-Neto M., Erasmo E.A.L., Pallini A. 2014. Alternative food sources to predatory mites (Acari) in a Jatropha curcas (Euphorbiaceae) crop. Rev. Colombiana Entomol., 40: 74-79.
McMurtry J.A., Croft B.A. 1997. Life-styles of Phytoseiid mites and their roles in biological control. Annu. Rev. Entomol., 42: 291-321. doi:10.1146/annurev.ento.42.1.291 ![]()
McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18(4): 24. doi:10.11158/saa.18.4.1 ![]()
Moura J.T. de 2014. Importância e valor nutricional do pólen de Cocos nucifera para abelhas africanizadas cultivadas no litoral alagoano. Universidade Federal de Alagoas, 56 pp.
Noronha A.C. da S. 2013. Ácaros em fruteiras na Amazônia Oriental. In: Embrapa Amaz. Orient. em An. Congr., In: Simposio Brasileiro de Acarologia, 4., 2013, Bento Gonçalves.
Pernal S.F., Currie R.W. 2000. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie, 31(3): 387-409. doi:10.1051/apido:2000130 ![]()
R Core Team. 2016. R: a language and environment for statistical computing [Access June 25, 2016]. Available from: www.r-project.org/
Reis P.R., Chiavegato L.G., Alves E.B., Sousa E.O. 2000. Ácaros da família Phytoseiidae associados aos citros no município de Lavras, Sul de Minas Gerais. An. da Soc. Entomológica do Bras., 29: 95-104. doi:10.1590/S0301-80592000000100012 ![]()
Reis P.R., Chiavegato L.G., Alves E.B. 1998. Biologia de Iphiseiodes zuluagai Denmark & Muma (Acari: Phytoseiidae). An. Soc. Entomol. Brasil 27: 185-191. doi:10.1590/S0301-80591998000200003 ![]()
Van Rijn P.C.J., Tanigoshi L.K. 1999. Pollen as food for the predatory mites Iphiseius degenerans and Neoseiulus cucumeris (Acari: Phytoseiidae): dietary range and life history. Exp. Appl. Acarol., 23(10): 785-802. doi:10.1023/A:1006227704122 ![]()
Sato M.E., Raga A., Cerávolo L.C., Rossi A.C., Potenza M.R. 1994. Ácaros predadores em pomar cítrico de Presidente Prudente, Estado de São Paulo. An. Soc. Entomol. Bras., 23(3): 435-441.
Silva R.R., Silva M.J.S., Silva E.A., Serra A., Reis G.P.R. 2013. Acarofauna em pomar cítrico com ênfase em Phytoseiidae. Magistra, 25(3/4): 197-203.
Somerville D. 2005. Fat Bees Skinny Bees: A Manual on Honey Bee Nutrition for Beekeepers: a Report for the Rural Industries Research and Development Corporation. Rural Industries Research and Development Corporation.
Tixier M.-S., Kreiter S., Auger P. 2000. Colonization of vineyards by phytoseiid mites: their dispersal patterns in the plot and their fate. Exp. Appl. Acarol., 24(3): 191-211. doi:10.1023/A:1006332422638 ![]()
Wodehouse R.P. 1933. Preparation of pollen for microscopic examination. Bull. Torrey Bot. Club,: 417-421. doi:10.2307/2480494 ![]()
Ybert J.-P., Carvalho M. de, Scheel-Ybert R., Rezende R.C. de 2012. Dicionário temático de morfologia esporopolínica. Museu Nacional. 100 pp.



2019-03-05
Date accepted:
2019-12-03
Date published:
2020-01-17
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Ferreira, Camila Tavares; Krug, Cristiane and Moraes, Gilberto José de
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




