Functional response of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) to Eotetranychus frosti (Tetranychidae) and Cenopalpus irani (Tenuipalpidae)
Bazgir, Fereshteh1 ; Shakarami, Jahanshir2 and Jafari, Shahriar3
1Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khoramabad, Iran.
2✉ Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khoramabad, Iran.
3Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khoramabad, Iran.
2020 - Volume: 60 Issue: 1 pages: 30-39
https://doi.org/10.24349/acarologia/20204359Original research
Keywords
Abstract
Introduction
Eotetranychus frosti (McGregor) (Acari: Tetranychidae) and Cenopalpus irani Dosse (Acari: Tenuipalpidae) are phytophagous mites that were reported on various plant species, especially Rosaceae in Western regions of Iran (Kamali et al., 2001; Mehrnejad, 2001; Khanjani et al., 2012). Occasional outbreaks of these pests have caused serious concerns about their detrimental effects on different plants (Darbemamieh, 2008; Khodayari et al., 2010; Bazgir et al., 2015a; Bazgir et al., 2015b). The population of these pests may reach high densities during the summer and cause significant damages to apple trees (Jafari and Bazgir, 2015). The feeding of both adults and immatures of these pests may result in significant plant damage such as low fruit quality, low plant vigor, defoliation, and significant losses in apple yield in the following season (Jafari et al., 2014).
The use of predatory mites of the family Phytoseiidae (Acari: Mesostigmata) is a reliable strategy to minimize pesticide usage, reducing environmental pollution, and protecting beneficial insect and mite species (Badii et al., 2004; Patel and Zhang, 2017). The predatory mite Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) is one of the most efficient biological control agents of mites of several families such as Eriophyidae, Tenuipalpidae, and Tetranychidae and insect pests such as thrips and whiteflies (Buitenhuis et al., 2015; Calvo et al., 2015).
It has been commercially used in various countries around the world as a biological control agent (Arthurs et al., 2009; Park et al., 2011; Calvo et al., 2015; Fathipour et al., 2017b; Knapp et al., 2017). The development and reproduction capacity of this species on various kinds of pollen before the appearance of pests on the plant (Calvo et al., 2015; Fathipour et al., 2017b; Riahi et al., 2017a) and also its ability to control several pests simultaneously (Buitenhuis et al., 2015; Janssen and Sabelis, 2015) are important factors of this predator's success in biological control programs.
The investigation of functional response characteristics of natural enemies is one of the crucial methods to evaluate their effective role in regulating pest populations (Fantinou et al., 2012). Among the functional response types reported by Holling (1959), predators with type III functional response are usually regarded as efficient in regulating prey populations; while natural enemies with type II functional response have been successfully used as biological control agents and have considerably gained the researchers' attention (Xiao and Fadamiro, 2010; Ali et al., 2011; Carrillo and Pena, 2012; Ganjisaffar and Perring, 2015; Fathipour et al., 2017a,b; Fathipour et al., 2019). The functional response type and the value of its parameters may be affected by various factors such as prey species, prey stage, age of predator, the characteristics of host plant, temperature and humidty (Escudero and Ferragut, 2005; Ahn et al., 2010; Farazmand et al., 2012; Doker et al., 2016; Song et al., 2016; Fathipour et al., 2017a,b).
Though exploring the functional responses of A. swirskii feeding on Tetranychus urticae Koch has gained noticeable attention over recently (Xiao et al., 2013; Fathipour et al., 2017b; Fathipour et al., 2019), no information is available about its predator–prey interactions with other tetranychid and tenuipalpid mites.
In an earlier study, it was shown that A. swirskii has a high capacity of development and population growth when fed on the two pest species; E. frosti and C. irani (Bazgir et al., 2018). A critical step in determining the ability of A. swirskii to regulate E. frosti and C. irani is to assess its functional response and consumption rate when offered these preys. The objectives of this study were to determine the functional response type and parameters and feeding capacity of the predatory mite A. swirskii for varying densities of life stages of E. frosti and C. irani.
Material and methods
Stock cultures of prey and predator
Eotetranychus frosti and C. irani were collected from apple orchards in Chaghalvandi region in the vicinity of Khorramabad, Lorestan province, southwestern Iran, and reared on apple leaf discs at 25 ± 1 °C, 60 ± 10 % RH and 16:8 h (L: D) photoperiod.
Initial populations of A. swirskii were obtained from Koppert Biological Systems, the Netherlands. The predatory mites were extracted from the bran carrier material and transferred to the leaf disks with a fine paintbrush. The predator was reared on apple leaves infested with mixed stages of each prey separately under the aforementioned laboratory conditions before the experiments, for several generations.
Functional response experiments
Experimental arenas consisted of a piece of apple leaf (4 cm in diameter) placed upside down on a wet cotton layer in a Petri dish (6 cm diameter) with a 0.5 cm hole drilled in its center. This Petri dish was placed in the middle of a larger Petri dish (9 cm) containing water to keep the leaves fresh. To prevent escaping predator and prey, a water-saturated cotton strip was placed around the leaf margin. Before each test, 4-day old mated females of the predator were placed individually in experimental arenas and starved for 24 hours. To determine the functional response of the predator on each of the prey species (E. frosti and C. irani), a 24 h starved female predator was exposed to seven densities (2, 4, 8, 16, 32, 64 and 128 newly-emerged individuals) of different prey stages (egg, larvae, protonymph, and deutonymph). The prey mites were transferred onto leaf discs with a fine paintbrush (number 0000). After 24 h, the predators were removed and the number of prey individuals eaten was recorded by counting intact eggs and the carcasses of dead larvae, protonymphs or deutonymphs. Sixteen replicates were prepared for each prey density. All experiments were conducted in the laboratory at constant temperature 25 ± 1 °C, 60 ± 10 % RH and 16:8 h L: D photoperiod in an incubator.
Data analysis
The data on functional response were analyzed in two steps (Juliano, 2001). First, the logistic regression of the proportion of prey consumed as a function of initial density was used to determine the shape of the functional response curve of A. swirskii to different stages of E. frosti and C. irani:
Ne / N0 = (exp(P0 + P1N0 + P2N02 + P3N03)) / (1 + exp(P0 + P1N0 + P2N02 + P3N03))
where Ne is the number of prey consumed, N0 is the initial prey density, (Ne/ N0) is the probability of prey consumption, and P0, P1, P2 and P3 are the intercept, linear, quadratic, and cubic coefficients, respectively, estimated using the maximum likelihood method.
The type of functional response was determined by the signs of P1 and P2. If the linear coefficient is negative ( P1 < 0), it describes a type II functional response because the proportion of prey consumed declines monotonically with the initial prey density (Juliano, 2001).
In the next step, the handling time ( Th) and the attack rate (α) coefficients of a type II response were estimated using the Rogers' random predator equation (Rogers, 1972):
Ne = N0[1 - exp(α(ThNe - T))]
where Ne is the number of prey consumed, N0 is the initial prey density, T is the searching time (1 day), α is the attack rate, and Th is the handling time. Nonlinear regression was used to estimate the attack rate and the handling time parameters (Proc NLIN, SAS Institute 2003).
The data on prey consumption was evaluated by three factors: the effect of prey species with two levels, prey stage with four levels and prey density with seven levels using 23 factorial analysis by SAS v.9.1 software (SAS Institute 2003). Differences between treatments were compared by Tukey's test (α = 0.05).
Results
Results of the logistic regression analysis showed significantly negative linear coefficient ( P1 < 0) and positive quadratic coefficient ( P2 \textgreater 0) for all prey stages, indicating that the percentage of prey consumed for each prey stage declined as prey density increased (Table 1). Thus, the predator, A. swirskii showed a type II functional response on all immature stages of both E. frosti and C. irani (Figure 1).
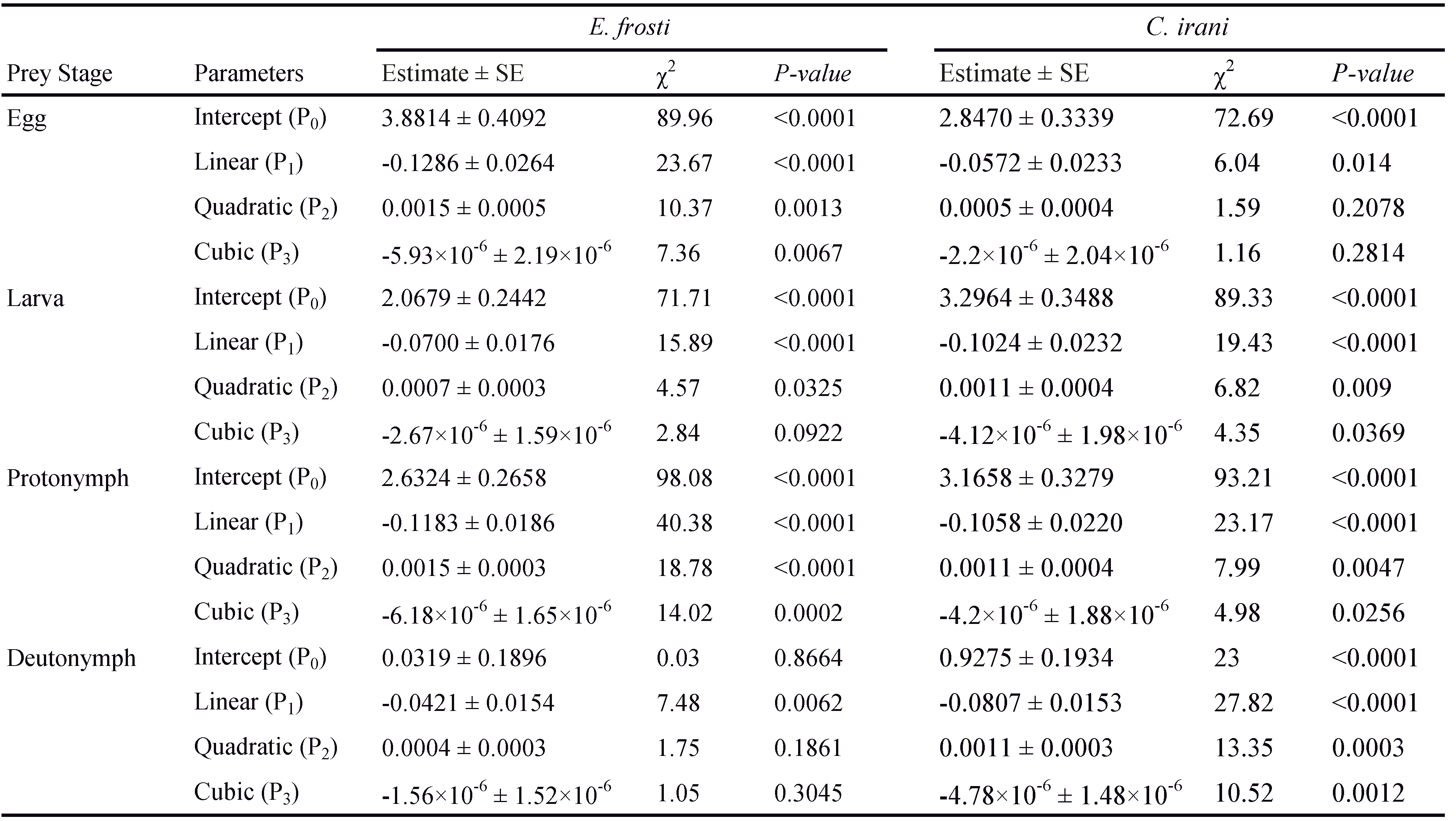





Amblyseius swirskii had a higher attack rate coefficient (α) on immature stages of C. irani than E. frosti. The attack rate (α) was the highest for predators feeding on eggs and estimated to be 0.1142 and 0.1404 h-1 on E. frosti and C. irani, respectively. The value of handling time ( Th) of A. swirskii was much shorter on immature stages of C. irani than E. frosti. The shortest handling time on both species was recorded for eggs (0.4858 and 0.3819 h on E. frosti and C. irani, respectively), followed by larvae, protonymphs and deutonymphs, respectively (Table 2).



The interaction between prey species and density was significant for the number of prey eaten (F = 26.19; df = 6; P < 0.001). The consumption rate of both prey species gradually increased with an increase in prey density. Nearly 90% of eggs, larvae, and protonymphs of C. irani and about 70% of the same stages of E. frosti were consumed at the lowest densities (2, 4, 8 and 16). Then, proportion of prey consumption for each prey stage by A. swirskii females decreased with increasing prey density (Figure 1).
The interaction between prey stage and different densities of prey was significant (F = 73.51; df =18; P < 0.001). With increasing density of prey, the consumption rate was highest on eggs followed by larvae and protonymphs whereas the consumption rate of deutonymphs was lowest (Table 3). When each stage of two preys was offered to the predator, the interaction between prey species and prey stage was not significant (F = 0.75; df = 3; P = 0.525).
There was a significant effect of species (F = 149.51; df = 1; P < 0.001) on the number of prey consumed. Amblyseius swirskii attacked more C. irani than E. frosti. There was also a significant difference between the different prey densities (F = 1711.70; df = 6; P < 0.001) and between the life stages (F = 519.63; df = 3; P < 0.001) of the number of preys eaten. Average prey consumption at each density decreased with increasing size of the life stage. For example, the lowest mean number of prey consumed, at the density of 128, was estimated to be 14.1 and 19.2 prey/day for deutonymphs of E. frosti and C. irani, respectively (Table 3).



Discussion
In general, the predator's consumption rate is inversely related to the size of life stage (Ali et al., 2011). In this study, the consumption rate of A. swirskii on eggs of E. frosti and C. irani was the highest, followed by the larvae and protonymphs, and the consumption rate of deutonymphs was the lowest. Soleymani et al. (2016) also reported higher consumption rates on eggs compared to other stages for A. swirskii feeding on T. urticae. The higher consumption rate on eggs compared to the other stages might be due to the fact that the weight of eggs is lower than other stages and thus predators need to feed on a greater number of eggs to get the same amount of nutrients (Ganjisaffar and Perring, 2015).
On the other hand, the mouthparts of A. swirskii may be better adapted to penetrate the egg chorion than the sclerotized cuticle of other stages. Usually, the predator successfully penetrated the egg chorion in a single attack attempt. But for feeding on nymphs and adults, the predator often required several attack attempts to penetrate the sclerotized cuticle of the mite, consequently a longer handling time on these stages was recorded (Carrillo and Pena, 2012; Ganjisaffar and Perring, 2015).
Amblyseius swirskii exhibited a type II functional response on all life stages of E. frosti and C. irani. The proportions of prey consumed by A. swirskii were higher at lower densities for all stages of E. frosti and C. irani. The proportion of killed prey decreased at high population densities likely due to satiation or interference on their predation capacity related to prey density (Carrillo and Pena, 2012). This pattern suggests that this predator could be more efficient at low or moderate densities of E. frosti and C. irani.
Our results are in line with Xiao et al. (2013) who reported that A. swirskii showed Type II functional response to T. urticae eggs. However, Fathipour et al. (2019) reported that the functional response of this predator fed on T. urticae eggs and nymphs changed to a Type III response when pollen was added as additional food source to the experimental arenas.
The Type II functional response is common in many phytoseiid species, including Phytoseiulus persimilis Athias-Henriot (Skirvin and Fenlon, 2003), Euseius hibisci (Chant) (Badii et al., 2004), Neoseiulus californicus (McGregor) (Xiao and Fadamiro, 2010; Farazmand et al., 2012; Song et al., 2016), Neoseiulus womersleyi (Schicha) (Ali et al., 2011), Kampimodromus aberrans (Oudemans) (Kasap and Atlihan, 2011), Typhlodromus (Anthoseius) bagdasarjani Wainstein and Arutunjan (Farazmand et al., 2012), and Metaseiulus flumenis (Chant) (Ganjisaffar and Perring, 2015).
Tetranychid mites have been utilized as prey in most of these studies; in contrast, the functional response of phytoseiid mites preying on tenuipalpid mites has been investigated in very few studies. Carrillo and Pena (2012) studied the functional response of Amblyseius largoensis (Muma) on Raoiella indica Hirst (Acari: Tenuipalpidae) and found a type II functional response. This type of response has also been observed in two other phytoseiid species namely, Euseius mesembrinus (Dean) and Amblyseius herbicolus (Chant), when feeding on Brevipalpus californicus (Banks) and Brevipalpus phoenicis (Geijskes), respectively (Badii et al., 1993; Reis et al., 2007). Although phytoseiid mites usually exhibit a type II functional response to their prey, this may change depending on physical characteristics of host plant (Skirvin and Fenlon, 2003), prey stage (Ganjisaffar and Perring, 2015), and different prey species (Escudero and Ferragut, 2005).
Two parameters that help to determine the magnitude of functional responses are the attack rate and the handling time (Pervez and Omkar, 2006). As an important indicator of the consumption rate and the predator efficiency, the handling time reflects the cumulative time needed for capturing, killing, and digesting the prey (Veeravel and Baskaran, 1997). Different factors can impinge on handling time such as the speed of the predator, movement of prey, and the time spent subduing individual prey (Hassell, 1978), which could be related to behavioral and structural prey defense mechanisms (Ali et al., 2011).
In our study, A. swirskii had a shorter handling time ( Th) on eggs than other stages. This value was lower when compared to A. swirskii feeding on eggs of T. urticae (Xiao et al., 2013; Fathipour et al., 2019); suggesting that this predator had a much stronger predation response on E. frosti and C. irani. Ganjisaffar and Perring (2015) reported that G. flumenis had a lower handling time on eggs than on other stages when consuming Oligonychus pratensis Banks. Attack rate coefficients (α) varied between life stages of both pest species (E. frosti and C. irani) and in general reduced as prey size increased. This can be attributed to the immobility of eggs and their incapacity to defend themselves. The attack rate on the deutonymph stage was much lower than on the other stages; these findings concurred with reports for G. flumenis (Ganjisaffar and Perring, 2015), N. womersleyi (Ali et al., 2011), and K. aberrans (Kasap and Atlihan, 2011).
In our previous study, life history and predation parameters demonstrated that A. swirskii is able to feed and complete its development on E. frosti and C. irani; likewise, this predator can exhibit a high potential for population increase when fed on both pest mites (Bazgir et al., 2018). Results of the present study confirmed this. Also, higher proportions of prey were consumed by A. swirskii at lower densities of all stages of E. frosti and C. irani, suggesting a high searching ability of A. swirskii, and implying that this predator could be more efficient at regulating low densities of all stages of these pests.
Although functional response studies in laboratory condition provide some insight into the predator-prey interaction, this has been criticized for ignoring the environmental complexities and multispecies prey and predator systems that occur in the field (Ganjisaffar and Perring, 2015). For example, no or very little spider mite webbing was produced in laboratory experiments, however, in the field all stages of prey are surrounded by webbing which maybe a limiting factor of predation efficiency. Thus, field studies are required to determine the efficiency of A. swirskii and the improvement of management tactics for the control of E. frosti and C. irani under more realistic conditions.
Acknowledgments
We are grateful to the Department of Plant Protection, Lorestan University, Khoramabad, Iran for supporting this project.
References
Ahn J., Kim K., Lee J.H. 2010. Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J. Appl. Entomol., 134: 98-104. doi:10.1111/j.1439-0418.2009.01440.x ![]()
Ali M., Naif A.A., Huang D. 2011. Prey consumption and functional response of a phytoseiid predator, Neoseiulus womersleyi, feeding on spider mite, Tetranychus macfarlanei. J. Insect Sci., 11: 167. doi:10.1093/jis/11.1.167 ![]()
Arthurs S., McKenzie C.L., Chen J., Dogramaci M., Brennan M., Houben K., Osborne L. 2009. Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol. control, 49: 91-96. doi:10.1016/j.biocontrol.2009.01.002 ![]()
Badii M., Hernandez E., Flores S. 1993. Functional response of Euseius mesembrinus (Dean) as a function of the density of Brevipalpus californicus (Banks)(Acari: Phytoseiidae, Tenuipalpidae). Southwest. Entomol., 18: 301-304.
Badii M.H., Hernández-Ortiz E., Flores A.E., Landeros J. 2004. Prey stage preference and functional response of Euseius hibisci to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp. Appl. Acarol., 34: 263-273. doi:10.1023/B:APPA.0000049222.65883.77 ![]()
Bazgir F., Jafari S., Shakarami J. 2015a. Influence of temperature on life table parameters of Iranian false spider mite, Cenopalpus irani Dosse (Tenuipalpidae) on apple leaves. Int. J. Acarol., 41: 1-9. doi:10.1080/01647954.2014.983164 ![]()
Bazgir F., Jafari S., Shakarami J., Bahirae F. 2015b. Effect of temperature on the reproductive parameters and survival of Cenopalpus irani Dosse (Tenuipalpidae). Acarina, 23: 181-187.
Bazgir F., Shakarami J., Jafari S. 2018. Life table and predation rate of Amblyseius swirskii (Acari: Phytoseiidae) fed on Eotetranychus frosti (Tetranychidae) and Cenopalpus irani (Tenuipalpidae). Syst. Appl. Acarol., 23: 1614-1627. doi:10.11158/saa.23.8.11 ![]()
Buitenhuis R., Murphy G., Shipp L., Scott-Dupree C. 2015. Amblyseius swirskii in greenhouse production systems: a floricultural perspective. Exp. Appl. Acarol., 65: 451-464. doi:10.1007/s10493-014-9869-9 ![]()
Calvo F.J., Knapp M., van Houten Y.M., Hoogerbrugge H., Belda J.E. 2015. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol., 65: 419-433. doi:10.1007/s10493-014-9873-0 ![]()
Carrillo D., Pena J.E. 2012. Prey-stage preferences and functional and numerical responses of Amblyseius largoensis (Acari: Phytoseiidae) to Raoiella indica (Acari: Tenuipalpidae). Exp. Appl. Acarol., 57: 361-372. doi:10.1007/s10493-011-9488-7 ![]()
Darbemamieh M. 2008. Tetranychoid mites and their acarid predators in Kermanshah orchards; Spatial distribution and population dynamics of some species on apples [MSC Thesis]. Tehran: Tarbiat Modares University. pp. 116. (in Persian)
Doker I., Kazak C., Karut K. 2016. Functional response and fecundity of a native Neoseiulus californicus population to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae) at extreme humidity conditions. Syst. Appl. Acarol., 21: 1463-1472. doi:10.11158/saa.21.11.3 ![]()
Escudero L., Ferragut F. 2005. Life-history of predatory mites Neoseiulus californicus and Phytoseiulus persimilis (Acari: Phytoseiidae) on four spider mite species as prey, with special reference to Tetranychus evansi (Acari: Tetranychidae). Biol. Control, 32: 378-384. doi:10.1016/j.biocontrol.2004.12.010 ![]()
Fantinou A., Baxevani A., Drizou F., Labropoulos P., Perdikis D., Papadoulis G. 2012. Consumption rate, functional response and preference of the predaceous mite Iphiseius degenerans to Tetranychus urticae and Eutetranychus orientalis. Exp. Appl. Acarol., 58: 133-144. doi:10.1007/s10493-012-9557-6 ![]()
Farazmand A., Fathipour Y., Kamali K. 2012. Functional response and mutual interference of Neoseiulus californicus and Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol., 38: 369-376. doi:10.1080/01647954.2012.655310 ![]()
Fathipour Y., Karimi M., Farazmand A., Ali A.T. 2017a. Age-specific functional response and predation capacity of Phytoseiulus persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia, 58: 31-40. doi:10.24349/acarologia/20184225 ![]()
Fathipour Y., Karimi M., Farazmand A., Talebi A.A. 2017b. Age-specific functional response and predation rate of Amblyseius swirskii (Phytoseiidae) on two-spotted spider mite. Syst. Appl. Acarol., 22: 159-170. doi:10.11158/saa.22.2.1 ![]()
Fathipour Y., Maleknia B., Soufbaf M., Reddy G.V.P. 2019. Functional and numerical responses, mutual interference and resource switching of Amblyseius swirskii on two-spotted spider mite. Entomol. Exp. Appl., (in press).
Ganjisaffar F., Perring T.M. 2015. Prey stage preference and functional response of the predatory mite Galendromus flumenis to Oligonychus pratensis. Biol. Control, 82: 40-45. doi:10.1016/j.biocontrol.2014.12.004 ![]()
Hassell M.P. 1978. The dynamics of arthropod predator-prey systems. Princeton University Press. pp. 248.
Holling C.S. 1959. Some characteristics of simple types of predation and parasitism. Canad. Entomol., 91: 385-398. doi:10.4039/Ent91385-7 ![]()
Jafari S., Bazgir F. 2015. Life history traits of predatory mite Typhlodromus (Anthoseius) bagdasarjani (Phytoseiidae) fed on Cenopalpus irani (Tenuipalpidae) under laboratory conditions. Syst. Appl. Acarol., 20: 366-375. doi:10.11158/saa.20.4.2 ![]()
Jafari S., Rahmati M., Bahirae F. 2014. Spatial and temporal distribution of Eotetranychus frosti and Cenopalpus irani and their predator Typhlodromus bagdasarjani in an unsprayed apple orchard at Khorramabad, Western Iran. Persian J. Acarol., 3: 51-61.
Janssen A., Sabelis M.W. 2015. Alternative food and biological control by generalist predatory mites: the case of Amblyseius swirskii. Exp. Appl. Acarol., 65:413-418. doi:10.1007/s10493-015-9901-8 ![]()
Juliano S.A. 2001. Non-linear curve fitting: predation and functional response curve. Design and Analysis of Ecological Experiments. New York, Oxford University Press. p.178-196.
Kamali K., Ostovan H., Atamehr A. 2001. A catalog of mites and ticks (Acari) of Iran. Islamic Azad University Scientific Publication Center.pp. 192.
Kasap I., Atlihan R. 2011. Consumption rate and functional response of the predaceous mite Kampimodromus aberrans to two-spotted spider mite Tetranychus urticae in the laboratory. Exp. Appl. Acarol., 53: 253-261. doi:10.1007/s10493-010-9400-x ![]()
Khanjani M., Khanjani M., Saboori A., Seeman O.D. 2012. The false spider mites of the genus Cenopalpus Pritchard & Baker (Acari: Tenuipalpidae) from Iran. Zootaxa, 3433: 1-59. doi:10.11646/zootaxa.3433.1.1 ![]()
Khodayari S., Fathipour Y., Kamali K., Naseri B. 2010. Seasonal activity of Zetzellia mali (Stigmaeidae) and its preys Eotetranychus frosti (Tetranychidae) and Tydeus longisetosus (Tydeidae) in unsprayed apple orchards of Maragheh, northwestern of Iran. J. Agric. Sci. Technol., 12: 549- 558. (in Persian)
Knapp M., Van Houten Y., Van Baal E., Groot T. 2017. Use of predatory mites in commercial biocontrol: current status and future prospects. Acarologia, 58: 72-82. doi:10.24349/acarologia/20184275 ![]()
Mehrnejad M.R. 2001. Mites (Arthropoda, Acari) associated with pistachio trees (Anacardiaceae) in Iran (I). Syst. Appl. Acarol., 6: 13-20. doi:10.11158/saasp.6.1.1 ![]()
Park H.-H., Shipp L., Buitenhuis R., Ahn J.J. 2011. Life history parameters of a commercially available Amblyseius swirskii (Acari: Phytoseiidae) fed on cattail (Typha latifolia) pollen and tomato russet mite (Aculops lycopersici). J. Asia Pac. Entomol., 14: 497-501. doi:10.1016/j.aspen.2011.07.010 ![]()
Patel K., Zhang Z.-Q. 2017. Functional and numerical responses of Amblydromalus limonicus and Neoseiulus cucumeris to eggs and first instar nymph of tomato/potato psyllid (Bactericera cockerelli). Syst. Appl. Acarol., 22: 1476-1489. doi:10.11158/saa.22.9.12 ![]()
Pervez A., Omkar. 2006. Ecology and biological control application of multicoloured Asian ladybird, Harmonia axyridis: A review. Biocontrol Sci. Technol., 16: 111-128. doi:10.1080/09583150500335350 ![]()
Reis P.R., Teodoro A.V., Pedro Neto M., Silva E.A.d. 2007. Life history of Amblyseius herbicolus (Chant)(Acari: Phytoseiidae) on coffee plants. Neotrop. Entomol., 36: 282-287. doi:10.1590/S1519-566X2007000200016 ![]()
Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017a. Linking life table and consumption rate of Amblyseius swirskii (Acari: Phytoseiidae) in presence and absence of different pollens. Ann. Entomol. Soc. Am., 110: 244-253. doi:10.1093/aesa/saw091 ![]()
Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017b. Natural diets versus factitious prey: comparative effects on development, fecundity and life table of Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol., 22: 711-724. doi:10.11158/saa.22.5.10 ![]()
Rogers D. 1972. Random search and insect population models. J. Anim. Ecol., 41: 353-360. doi:10.2307/3474 ![]()
Skirvin D.J., Fenlon J.S. 2003. The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol., 31: 37-49. doi:10.1023/B:APPA.0000005107.97373.87 ![]()
Soleymani S., Hakimitabar M., Seiedy M. 2016. Prey preference of predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae) and Bemisia tabaci (Hemiptera: Aleyrodidae). Biocontrol Sci. Technol., 26: 562-569. doi:10.1080/09583157.2015.1133808 ![]()
Song Z.-W., Zheng Y., Zhang B.-X., Li D.-S. 2016. Prey consumption and functional response of Neoseiulus californicus and Neoseiulus longispinosus (Acari: Phytoseiidae) on Tetranychus urticae and Tetranychus kanzawai (Acari: Tetranychidae). Syst. Appl. Acarol., 21: 936-947. doi:10.11158/saa.21.7.7 ![]()
Veeravel R., Baskaran P. 1997. Functional and numerical Responses of Coccinella transversalis Fab. and Cheilomenes sexmaculatus Fab. feeding on the Melon Aphid, Aphis gossypii Glov. Int. J.Trop. Insect Sci., 17: 335-339. doi:10.1017/S1742758400019159 ![]()
Xiao Y., Fadamiro H.Y. 2010. Functional responses and prey-stage preferences of three species of predacious mites (Acari: Phytoseiidae) on citrus red mite, Panonychus citri (Acari: Tetranychidae). Biol. Control, 53: 345-352. doi:10.1016/j.biocontrol.2010.03.001 ![]()
Xiao Y., Osborne L.S., Chen J., McKenzie C.L. 2013. Functional responses and prey-stage preferences of a predatory gall midge and two predacious mites with twospotted spider mites, Tetranychus urticae, as host. J. Insect Sci., 13: 8. doi:10.1673/031.013.0801 ![]()



2019-03-30
Date accepted:
2019-12-03
Date published:
2020-01-17
Edited by:
Kreiter, Serge

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Bazgir, Fereshteh; Shakarami, Jahanshir and Jafari, Shahriar
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



