Contribution to the knowledge of the oribatid mite genus Kalloia (Acari, Oribatida, Carabodidae), with description of a new species from Indonesia
Ermilov, Sergey G.1 ; Sandmann, Dorothee2 and Scheu, Stefan3
1✉ Tyumen State University, Tyumen, Russia.
2University of Göttingen, JF Blumenbach Institute of Zoology and Anthropology, Göttingen, Germany.
3University of Göttingen, JF Blumenbach Institute of Zoology and Anthropology, Göttingen, Germany & University of Göttingen, Centre of Biodiversity and Sustainable Land Use, Göttingen, Germany.
2019 - Volume: 59 Issue: 3 pages: 323-334
https://doi.org/10.24349/acarologia/20194334ZooBank LSID: 634891AD-AEA4-4AD4-B188-BA609DDCB287
Original research
Keywords
Abstract
Introduction
The oribatid mite genus Kalloia of the family Carabodidae (Acari, Oribatida) was proposed by Mahunka (1985) with Kalloia simpliseta Mahunka, 1985 as type species from the Caribbean. He listed the following generic traits: ``Prodorsum normal, lamellae simple, running marginally. Lamellar and interlamellar setae thin, simple. Sensillus slightly lanceolate. Dorsosejugal suture present, but a deep hollow lying between prodorsum and notogaster. Latter with some very highly extruding tubercles. Fifteen pairs of thin notogastral setae. Epimeral setal formula: 3–1–3–3. Four pairs of genital, one pair of aggenital, two pairs of anal and three pairs of adanal setae, ad3 in preanal, ad1 and ad2 in postanal position'' (Mahunka 1985). According to Subías's catalogue (2019), the genus is included as subgenus in the genus Gibbicepheus Balogh, 1958, and comprises three species (K. simpliseta, Kalloia mahunkai Pérez-Íñigo and Baggio, 1989 and Machadocepheus foveolatus Mahunka, 1978).
In the course of the study of carabodid mites from Indonesia, we found a new species of Kalloia. This paper aims to describe and illustrate this new species under the name K. gerdweigmanni n. sp., to update the generic diagnosis, to discuss the taxonomic status of Kalloia and the systematic placement of K. mahunkai and M. foveolatus (to include both species in Gibbicepheus), and to present data on the ecology and distribution of Kalloia species.
Material and methods
Material
This study was carried out within the framework of the interdisciplinary project ``Ecological and socioeconomic functions of tropical lowland rainforest transformation systems (Sumatra, Indonesia)'' – EFForTS on Sumatra Island (http://www.uni-goettingen.de/en/310995.html). See the Material examined section for detailed location data of the new species.
In order to study the morphology of the genus Kalloia, besides the new species, the specimens of Kalloia simpliseta from S.G. Ermilov's collection, from Cuba (Ermilov 2016) and the Caribbean (Ermilov and Smit 2017), were used.
Methods
Specimens were mounted in lactic acid on temporary cavity slides for measurement and illustration of the new species. Body length was measured in lateral view, from the tip of the rostrum to the posterior edge of the notogaster. Notogastral width refers to the maximum width of notogaster in dorsal view. Lengths of body setae were measured in lateral aspect. All body measurements are presented in micrometer. Formulas for leg setation are given in parentheses according to the sequence trochanter–femur–genu–tibia–tarsus (famulus included). Formulas for leg solenidia are given in square brackets according to the sequence genu–tibia–tarsus.
Drawings were made with a camera lucida using a Leica transmission light microscope ``Leica DM 2500''. SEM photos were made with the aid of a FEI Quanta 250 SEM microscope.
The general morphological terminology used in this paper mostly follows that of F. Grandjean: see Travé and Vachon (1975) for references, Norton (1977) for leg setal nomenclature, and Norton and Behan-Pelletier (2009) for overview.
The following abbreviations are used: lam = lamella; tlam = translamella; r = ridge; dep = depression; tu = tutorium; ro, le, in, bs = rostral, lamellar, interlamellar and bothridial setae, respectively; bo = bothridium; hlp = hump-like process; c, la, lm, lp, h, p = notogastral setae; ia, im, ip, ih, ips = notogastral lyrifissures; gla = opisthonotal gland opening; a, m, h = subcapitular setae; rbr = rutellar brush; v, l, d, cm, acm, ul, sul, vt, lt = palp setae; ω = palp and leg solenidion; cha, chb = cheliceral setae; Tg = Trägårdh's organ; Pd I, Pd II = pedotecta I and II, respectively; 1a, 1b, 1c, 2a, 3a, 3b, 3c, 4a, 4b, 4c = epimeral setae; dis = discidium; g, ag, an, ad = genital, aggenital, anal and adanal setae, respectively; iad = adanal lyrifissure; p.o. = preanal organ; Tr, Fe, Ge, Ti, Ta = leg trochanter, femur, genu, tibia and tarsus, respectively; p.a. = porose area; σ, φ = leg solenidia; ɛ = leg famulus; v, ev, bv, l, d, ft, tc, it, p, u, a, s, pv = leg setae.
Systematics
Generic diagnosis of Kalloia
Adult — With character states of Carabodidae (e.g., Mahunka 1986; Norton and Behan-Pelletier 2009). Body size: Medium to large (length 500-800). Body form: Body slightly pear-shaped, notogaster dilated posteriorly, wider than prodorsum and anterior part of notogaster. Body ratio (length/width) ≈ 1.6-1.7. Integument: Body surface microtuberculate, notogaster sparsely foveolate. Prodorsum: Rostral margin rounded. Rostrum with median internal light semi-oval structure. Lamellae long, broad, well separated, rounded or triangular distally, located dorsolaterally. Translamella and transverse mediodistal ridge on lamellae present or absent. Interlamellar hump-like processes absent. Tutoria strong, ridge-like. Rostral setae thick or phylliform, located ventrolateral on lamellae. Lamellar setae setiform or thickened, located in anteromedial parts of lamellae. Interlamellar setae setiform, located on prodorsal surface in interbothridial region. Bothridial setae thickened, heavily barbed. Bothridia cup-shaped, closed, not connected with margins of the lamellae. Notogaster: Narrow furrow between notogaster and prodorsum present or not observed. Humeral shoulders slightly developed, rectangular. Dorsoanterior and dorsoposterior parts of notogaster deeply depressed. Dorsocentral part of notogaster with high hump-like process. Fifteen pairs of medium size, setiform notogastral setae widely spaced. Setae c1 and c2 located in anterior notogastral depression; c3 on humeral shoulders; da, dm, dp, la, lm, lp and h1 on tubercles/ridges of notogastral hump-like process; five pairs of setae (h2, h3, p1–p3) in posterior position of notogaster; posterior notogastral depression without setae. Gnathosoma. Subcapitulum diarthric. Adoral setae and their alveoli absent. Palps with setation 0–2–1–3–9(+ω). Solenidion of palptarsi long, bacilliform, not attached to acm. Axillary saccule absent. Chelicerae chelate-dentate. Lateral podosomal and epimeral regions: Pedotecta I represented by large lamina, pedotecta II by small lamina. Discidia well developed. Epimeral setal formula 3–1–3–3; setae 1a, 1c, 2a and 3a short, spiniform, others setiform. Anogenital region: With depressions. Four pairs of genital, one pair of aggenital and three pairs of adanal setae setiform; ad1 posterior, ad2 posterolateral, ad3 anterolateral to anal plates. Two pairs of anal setae spiniform. Adanal lyrifissures removed from anal aperture. Legs: Tarsi IV with incomplete setation (11 setae; pv'' absent). Setae u on all tarsi thick mediobasally and setiform distally. Genua I with two setae (l', l''), genua II with three setae (l', l'', v'). Porose area present on all femora.
Juvenile instars — Not known.
Kalloia gerdweigmanni n. sp.
ZOOBANK: 04B80C22-9764-485E-86FB-550D98448991 ![]()
(Figures 1–6)



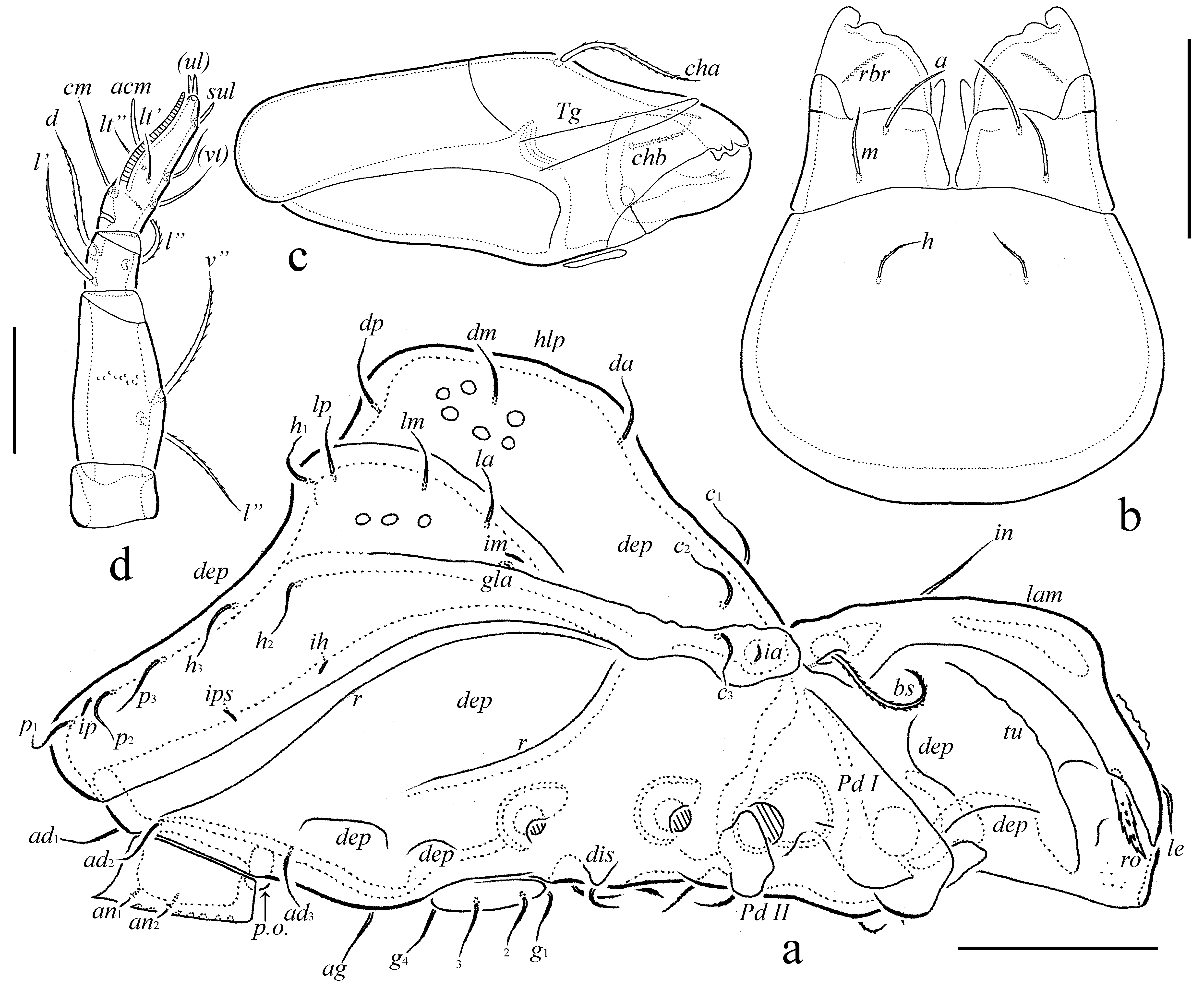














Diagnosis — Body size: 531–564 × 298–332. Lamellae mediodistally abruptly bent ventrad, with thicker cuticle appearing as a transverse ridge. Lamellar ends with anterolateral triangular projection. Translamella present. Rostral setae of medium size, thick, spinose. Lamellar setae comparatively short, slightly thickened. Interlamellar setae of medium size, setiform, slightly barbed. Bothridial setae long, setiform, densely barbed. Notogastral setae of medium size, setiform, slightly barbed. Notogastral hump-like process with two medioanterior convergent ridges, forming a triangular structure, bearing setae da and dm; with one pair of medioposterior tubercles, bearing setae dp; with one pair of large, elongate posterolateral tubercles bearing setae la, lm, lp and h1. Epimeral and anogenital setae (except minute 1a, 1c, 2a and 3a and anal setae) setiform, barbed.
Description — Measurements – Body length: 531 (holotype: female), 531–564 (four paratypes: all females); notogaster width: 307 (holotype), 298–332 (four paratypes).
Integument (Figs 1a, 2a, 3c, 3d, 4a, 4b, 5a, 5c, 6a) – Body color light brown to brown reddish and dark brown. Body covered by thick layer of gel-like cerotegument. Body surface (including subcapitular mentum and genae, genital and anal plates) microtuberculate (diameter of tubercles less than 1). Notogaster and dorsoantiaxial part of leg femora III, IV and trochanters III, IV sparsely foveolate (diameter of foveoles up to 12). Projecting parts of lamellae and tutoria slightly foveate.
Prodorsum (Figs 1a, 1b, 2a, 4a, 4b, 5a, 5b, 6a) – Rostrum broadly rounded. Lamellae long (slightly shorter than prodorsum), mediodistally abruptly bent ventrad, with thicker cuticle appearing as a transverse ridge (these regions of lamellae slightly convex, illusory forming the unclear hump-like processes – Figs 4a, 5a, which are really absent – see Figs 4b, 6a), with lateral triangular projection. Translamella broad. Tutoria (3/4 length of prodorsum) strong, ridge-like. With elongate depression between lamellae and tutoria, and two depressions ventrally to tutoria. Rostral setae (41–45) thick, with numerous spines. Lamellar setae (28–32) slightly thickened, roughened, located on translamella. Interlamellar setae (53–61) setiform, thin, slightly barbed. Bothridial setae (77–82) thickened, heavily barbed, curved semiovally in mediodistal part. Exobothridial setae and their alveoli not observed. Interlamellar region slightly depressed.
Notogaster (Figs 1a, 1b, 2a, 4a, 4b, 5b, 5c, 5a) – Anterior notogastral margin straight. Anterior and posterior notogastral depressions and dorsocentral hump-like process well-developed. Medioanterior region of notogastral hump-like process with two thick, diagonal, convergent ridges, forming triangular structure directed in anterior notogastral depression. Medioposterior region of notogastral hump-like process with one pair of tubercles, located behind diagonal ridges; these ridges and tubercles fused or indistinctly connected. Posterolateral regions of notogastral hump-like process with one pair of large, elongate tubercles. Fifteen pairs of notogastral setae (32–36) setiform, thin, slightly barbed; of these, da and dm located on diagonal notogastral ridges; dp on medioposterior tubercles; la, lm, lp and h1 on posterolateral tubercles. Lyrifissures and opisthonotal gland openings well visible; ia located on humeral shoulders, im and gla close to each other and anterolateral to posterolateral tubercles, ip between p1 and p2, ips and ih on lateral sides of notogaster.
Gnathosoma (Figs 2b-d) – Subcapitulum longer than wide (114–123 × 98–106). Subcapitular setae (a, 18–20; m, 16–18; h, 12–14) setiform, slightly barbed, h thinnest. Postpalpal setae (4) spiniform. Palps (71–73) with setation 0–2–1–3–9(+ω). Solenidion of palptarsi long, bacilliform. Chelicerae (123–135) with two setiform, barbed setae, cha (41) longer than chb (16–18). Trägårdh's organ of chelicerae elongate triangular.
Lateral podosomal and epimeral regions (Figs 1b, 2a, 4b, 5b, 6a) – Pedotecta II rounded in ventral view. Discidia triangular, rounded distally. Two depressions behind acetabula IV. One pair of large depressions bordered by a strong diagonal ridge. With typical epimeral setation: 3-1-3-3. Epimeral setae 1a, 1c, 2a and 3a minute (4), spiniform, 1b, 3b, 3c, 4a, 4b and 4b (32–36) setiform, barbed; 4b thickest.
Anogenital region (Figs 1b, 2a, 5b, 6b, 5c) – With one pair of long, longitudinal ridges lateral to genital aperture and posterior to epimere IV and several anogenital depressions (one large depression between genital and anal apertures; one pair of small depressions close and posterolateral to genital aperture; one pair of small depressions bearing aggenital setae; one pair of large and indistinct depressions lateral to anal aperture; two pairs of medium depressions posterior to acetabula IV). Usually with three poorly visible short, thin, parallel diagonal furrows lateral to genital aperture. Four pairs of genital, one pair of aggenital and three pairs of adanal setae similar in length (32–36), setiform, slightly barbed. Two pairs of anal setae (8) spiniform. Adanal lyrifissures removed from anal aperture and located lateral to ad3. Circumventral ridge poorly developed, interrupted posteriorly.
Legs (Figs 3a-d, 5b, 6a) – Claw of each leg strong, sparsely barbed dorsally and with tooth ventrobasally. Porose area distinct on all femora, not observed on trochanters III, IV. Formulas of leg setation and solenidia: I (1–4–2–4–16) [1–2–2], II (1–4–3–3–15) [1–1–2], III (2–3–1–2–15) [1–1–0], IV (1–2–2–2–11) [0–1–0]; homology of setae and solenidia indicated in Table 1. Famulus of tarsi I short, erect, blunt-ended, inserted posterior to solenidion ω1. Solenidion φ1 on tibiae I very long, setiform; ω2 on tarsi I and φ2 on tibiae I comparatively long, thickened, blunt-ended; other solenidia short (except long ω2 on tarsi I), bacilliform. Dorsoanterior apophysis of tibiae I (bearing φ1) slightly developed.
Material examined — Holotype (female) and two paratypes (two females): Indonesia, Sumatra, Bukit Duabelas landscape, oil palm plantation, research site BO3a, 02°04'15.2''S, 102°47'30.6''E, litter, November 2013 (B. Klarner). Two paratypes (two females): Indonesia, Sumatra, Harapan landscape, jungle rubber agroforest, research site HJ4a, 01°47'07.3''S, 103°16'36.9''E, litter, November 2013 (B. Klarner).
Type deposition — The holotype is deposited in the collection of LIPI (Indonesian Institute of Science) Cibinong, Indonesia; one paratype is deposited in the collection of the Senckenberg Museum of Natural History, Görlitz, Germany; three paratypes are deposited in the collection of the Tyumen State University Museum of Zoology, Tyumen, Russia. All in ethanol with a drop of glycerol. Additional pictures are available in the online repository www.ecotaxonomy.org.
Etymology — The species name is dedicated to our colleague, the well-known acarologist Prof. Dr. Gerd Weigmann (Free University of Berlin, Institute of Zoology, Berlin, Germany), for his extensive contributions to our knowledge of oribatid mites.
Remarks — Kalloia gerdweigmanni n. sp. differs from the type species of the genus — Kalloia simpliseta Mahunka, 1985 — by the presence of a transverse ridge in the mediodistal part of lamellae (versus ridge on lamellae absent), translamella (versus translamella absent) and two thick, diagonal, convergent ridges, forming a triangular structure in the medioanterior part of the notogaster (versus notogastral ridges absent), and the localization of each pair of notogastral setae da and dm on one ridge (versus setae located on separate tubercles) and la, lm, lp and h1 on one large elongate tubercle (versus la and lm on one small tubercle; lp and h1 on another small tubercle).



Discussion
Mahunka (1985) proposed the monotypic genus Kalloia with Kalloia simpliseta from the Caribbean. Later, he confirmed the generic status (e.g., Mahunka 1986, 1998), and this was also supported by other authors (Balogh and Balogh 1988, 1992, 2002; Fujikawa 1991; Ermilov 2016; Ermilov and Smit 2017; Ermilov and N'Dri 2018). Subías (2004) included Kalloia as subgenus in Diplobodes Aoki, 1958 and later (2016) in Gibbicepheus; Fernandez et al. (2014) included it in Machadocepheus (Kalloia) Balogh, 1958. The genus Kalloia is morphologically similar to Diplobodes, Gibbicepheus and Machadocepheus, however it differs from Diplobodes and Gibbicepheus by the distinctly depressed anterior part of the notogaster and by the presence of a centrodorsal notogastral hump-like process (versus anterior part of notogaster not depressed; centrodorsal part of notogaster without hump-like process); and from Machadocepheus by the absence of a centrodorsal prodorsal hump-like processes on which the interlamellar setae are located.
Also, the genus Kalloia is morphologically similar to the genera Tuberocepheus Balogh and Mahunka, 1969 and Mangabebodes Fernández, Theron, Leiva, Rollard and Tiedt, 2014 in having especially a centrodorsal notogastral hump-like process and distinctly depressed anterior and posterior parts of the notogaster. However, it differs from both by the presence of 15 pairs of notogastral setae, including the presence of notogastral setae c1–c3 and their localization in the depressed anterior part of the notogaster (versus 12 pairs of notogastral setae, setae c1–c3 absent, depressed anterior part of the notogaster without setae).
According to recent studies of Carabodidae, the presence or absence of prodorsal and notogastral depressions and hump-like processes and localization of interlamellar and notogastral setae are important morphological traits on generic level (Fernandez et al. 2013, 2016, 2018; Ermilov 2018; Ermilov and Starý 2018), therefore we support the generic status of Kalloia.
Pérez-Íñigo and Baggio (1989) described the second representative of Kalloia, Kalloia mahunkai from Brazil. In addition, Subías (2004) included the species Machadocepheus foveolatus from Mauritius in Diplobodes (Kalloia) and later (2016) in Gibbicepheus (Kalloia), implying that this species is the third representative of Kalloia. However, K. mahunkai and M. foveolatus do not have anterior notogastral depression and no centrodorsal notogastral hump-like process (generic traits of Kalloia) and correspond to generic traits of the genus Gibbicepheus, therefore we suggest to exclude these two species from the genus Kalloia, and to combine them in Gibbicepheus.
Distribution and ecology of Kalloia
At present, Kalloia simpliseta has been recorded from the Neotropical region (Mahunka 1985, 1998; Vázquez-González et al. 2015; Ermilov 2016; Ermilov and Smit 2017) and Côte d'Ivoire (Ermilov and N'Dri 2018); Kalloia gerdweigmanni n. sp. is known only from Indonesia (this study). Thus, the genus is distributed in the Neotropical, Ethiopian and Oriental regions.
Kalloia simpliseta is widely distributed in the Caribbean. It was described from several localities in Saint Lucia (Mahunka 1985): litter with underlying soil from a natural forest in Micoud, Mahaut, Quilesse Reserve; under bark of coastal trees, accumulated rotten material at tree bases in Anse La Raye, Pilori Pt.; sifted litter, wooden debris from various forest sites in Vigie. Later, the species was recorded by Mahunka (1998) in Saint Lucia from forests in the vicinity of Halcyon Sands Hotel in Vigie, and by Ermilov and Smit (2017) from litter and soil from different Caribbean islands (Antigua, Grenada, Saint-Barthelémy, Trinidad).
Also, K. simpliseta was recorded from tropical ecosystems in Cozumel, Quintana Roo, Mexico (Vázquez-González et al. 2015), from leaf litter of forests in Parque Nacional Alejandro de Humboldt (Ermilov 2016), and from ferralitic soil of primary forests in Goulikao, Oumé region, Côte d'Ivoire (Ermilov and N'Dri 2018).
Kalloia gerdweigmanni n. sp. is recorded from litter of oil palm plantations and jungle rubber agroforests in Bukit Duabelas and Harapan landscapes, Sumatra, Indonesia.
Acknowledgements
We cordially thank Dr. Julia Baumann (University of Graz, Graz, Austria) and two anonymous reviewers for valuable comments; Dr. Rahayu Widyastuti (Institute Pertanian Bogor, Bogor, Indonesia) for her collaboration in this project; Dr. Bernhard Klarner (University of Göttingen, Göttingen, Germany) for collecting samples; Kristina Richter (University of Göttingen, Göttingen, Germany) for help in building up the Indonesian oribatid mite morphospecies collection and database; Dr. Christian Fischer for help in preparing the SEM photos; the State Ministry of Research and Technology of Indonesia (RISTEK) for the research permit and the Indonesian Institute of Science (LIPI) and Ministry of Forestry (PHKA) for the collection permit; the village heads, local site owners, PT REKI and Bukit Duabelas National Park for granting access to their properties; and the many colleagues and helpers for support in the field.
Samples collected were based on collection permit no. S.07/KKH-2/2013 issued by the Indonesian Ministry of Forestry. Sample exportation was supported by the Indonesian Institute of Science (register file no. 24/SI/MZB/IV/2014) and based on permit no. 125/KKH-5/TRP/2014 issued by the Ministry of Forestry of the Republic of Indonesia.
References
Aoki J. 1958. Eine neue Gattung von Carabodidae aus der Insel Hachijo, Japan. Zool. Mag., 67(12): 390-392.
Balogh J. 1958. Oribatides nouvelles de l'Afrique tropicale. Rev. Zool. Bot. Afr., 58(1-2): 1-34.
Balogh J., Balogh P. 1988. Oribatid mites of the Neotropical region. I. Budapest: Elsevier Science Publishers, Amsterdam, The Netherlands and Akademiai Kiado. pp. 335.
Balogh, J., Balogh, P. 1992. The oribatid mite genera of the World. Vol. 1. Budapest: Hungarian National Museum Press. pp. 263.
Balogh J., Balogh P. 2002. Identification keys to the oribatid mites of the Extra-Holarctic regions. Vol. 1. Miskolc: Well-Press Publishing Limited. pp. 453.
Balogh J., Mahunka S. 1969. The scientific results of the Hungarian soil zoological expeditions to South America. 10. Acari: Oribatids, collected by the second expedition. I. Acta Zool. Acad. Sci. Hung., 15(1-2): 1-21.
Ermilov S.G. 2016. Contribution to the knowledge of carabodid oribatid mites (Acari, Oribatida, Carabodidae) of Cuba. Acarologia, 56(1): 33-43. doi:10.1051/acarologia/20162191 ![]()
Ermilov S.G. 2018. Contribution to the knowledge of the oribatid mite genus Aokiella (Acari, Oribatida, Carabodidae). Syst. Appl. Acar., 23(4): 643-651. doi:10.11158/saa.23.4.6 ![]()
Ermilov S.G., N'Dri J.K. 2018. Oribatid mites (Acari, Oribatida) from the Oumé region (Côte d'Ivoire): list of taxa, new findings, description of a new species. Biologia, 73(9): 867-873. doi:10.2478/s11756-018-0094-6 ![]()
Ermilov S.G., Smit H. 2017. Additions to the oribatid mite fauna of the Caribbean, with a description of a new species of Epidamaeus (Acari, Oribatida, Damaeidae). Acarologia, 57(4): 791-804. doi:10.1051/acarologia/20174156 ![]()
Ermilov S.G., Starý J. 2018. Camcarabodes korupensis gen. nov., sp. nov. (Acari, Oribatida, Carabodidae) from Cameroon. Syst. Appl. Acar., 23(3): 532-538. doi:10.11158/saa.23.3.11 ![]()
Fernandez N., Theron P., Rollard C. 2013. The family Carabodidae (Acari: Oribatida) I. Description of a new genus, Bovicarabodes with three new species, and the redescription of Hardybodes mirabilis Balogh, 1970. Int. J. Acar., 39(1): 26-57. doi:10.1080/01647954.2012.741144 ![]()
Fernandez N., Theron P., Rollard C., Leiva S. 2014. The family Carabodidae (Acari, Oribatida) VIII. The genus Machadocepheus (first part) Machadocepheus leoneae sp. n. and Machadocepheus rachii sp. n. from Gabon. ZooKeys, 456: 1-28. doi:10.3897/zookeys.456.8570 ![]()
Fernandez N., Theron P., Leiva S., Rollard C., Tiedt L. 2014. Revision of the family Carabodidae (Acari: Oribatida) VI. Mangabebodes kymatismosi gen. nov., sp. nov. and Antongilibodes paulae gen. nov., sp. nov. from Madagascar. Int. J. Acar., 40(4): 296-319. doi:10.1080/01647954.2014.914972 ![]()
Fernandez N., Theron P., Rollard C., Leiva S., Tiedt L. 2016. Revision of the family Carabodidae (Acari: Oribatida) X. Bovicarabodes jacquelinae sp. nov., redefinition of the genus Tuberocepheus Balogh & Mahunka, 1969 and redescription of Tuberocepheus longus (Balogh, 1962). Int. J. Acar., 42(2): 79-91. doi:10.1080/01647954.2015.1124920 ![]()
Fernandez N., Theron P., Leiva S., Jordaan A. 2018. Revision of the family Carabodidae (Acari: Oribatida) XVI. Synkrotima tsalakpmenoi sp. nov. from Zimbabwe and Kenya, and Congocepheus thailandae sp. nov. from Thailand, including a complementary study of Cavaecarabodes hauseri (Mahunka 1989). Zootaxa, 4504(3): 371-389. doi:10.11646/zootaxa.4504.3.4 ![]()
Fujikawa T. 1991. List of oribatid families and genera of the World. Edaphologia, 46: 1-132.
Mahunka S. 1978. Neue und interessante Milben aus dem Genfer Museum XXXIV. A compendium of the oribatid (Acari) fauna of Mauritius, Reunion and the Seychelles Is. II. Rev. Suisse Zool., 85(2): 307-340. doi:10.5962/bhl.part.82234 ![]()
Mahunka S. 1985. Mites (Acari) from St. Lucia (Antilles). 2. Oribatida. Acta Zool. Hung., 31(1-3): 119-178.
Mahunka S. 1986. A survey of the family Carabodidae C.L. Koch, 1836 (Acari: Oribatida). Acta Zool. Hung., 32(1-2): 73-135.
Mahunka S. 1998. New data on oribatids (Acari: Oribatida) from St. Lucia (Antilles). Acarologica Genevensia LXXXIX. Rev. Suisse Zool., 105(4): 839-877. doi:10.5962/bhl.part.80061 ![]()
Norton R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei (Acari) and its application to the family Damaeidae. In: Dindal D.L. (Ed.). Biology of oribatid mites. Syracuse: SUNY College of Environmental Science and Forestry. pp. 33-61.
Norton R.A., Behan-Pelletier V.M. 2009. Oribatida. Chapter 15. In: Krantz G.W., Walter D.E. (Eds.). A Manual of Acarology. Lubbock: Texas Tech University Press. pp. 430-564.
Pérez-Iñigo C., Baggio D. 1989. Oribates édafiques du Brésil (V). Oribates de l'État de São Paulo (Deuxieme partie). Acarologia, 30(3): 261-274.
Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Graellsia, 60 (número extraordinario): 3-305. doi:10.3989/graellsia.2004.v60.iExtra.218 ![]()
Subías L.S. 2016. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Online version accessed in February 2016, 593 pp.
Subías L.S. 2019. Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). Online version accessed in March 2019, 536 pp.; http://bba.bioucm.es/cont/docs/RO\_1.pdf ![]()
Travé J., Vachon M. 1975. François Grandjean. 1882-1975 (Notice biographique et bibliographique). Acarologia, 17(1): 1-19.
Vázquez-González M.M., May Uicab D.A., Alamilla Pastrana E.B. 2015. Riquesa específica y biodiversidad de Cozumel, Quintana Roo, México. Teorìa y Praxis, 19: 137-171. doi:10.22403/UQROOMX/TYP19/07 ![]()



2019-06-05
Date accepted:
2019-07-22
Date published:
2019-08-02
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2019 Ermilov, Sergey G.; Sandmann, Dorothee and Scheu, Stefan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




