Revision of the family Dolichocybidae (Acari: Heterostigmata) from the collection of V.D. Sevastianov
Khaustov, Alexander A.1 and Trach, Viacheslav A.2
1✉ Tyumen State University, Tyumen, Russia.
2Odessa I.I. Mechnikov National University, Odessa, Ukraine & Ukrainian I.I. Mechnikov Anti-Plague Research Institute, Odessa, Ukraine.
2018 - Volume: 58 Issue: 4 pages: 897-918
https://doi.org/10.24349/acarologia/20184295ZooBank LSID: 3C207D47-8CE2-41A8-89E4-AFEAF392C621
Keywords
Abstract
The family Dolichocybidae is a small group of early-derivative heterostigmatic mites that currently includes 2 subfamilies, 6 genera and 45 species (Hajiqanbar and Khaustov 2010; Rahiminejad et al. 2011; Zhang et al. 2011; Loghmani et al. 2013; Katlav et al. 2014; Bahramian et al. 2015; Mortazavi et al. 2015; Sobhi et al. 2017; Khaustov and Frolov 2017, 2018a, b; Khaustov and Trach 2017; Khaustov 2017). Little is known about the way of life of dolichocybid mites, but all of them are probably fungivorous (Rack 1967; Magowski 1988; Kaliszewski et al. 1995). Some species are important pests of edible mushrooms (Lan et al. 2017).
The identification of many dolichocybid mites is difficult or even impossible due to their incomplete and inadequate descriptions. All species described by V.D. Sevastianov and his co-authors belong to this “problematic” group. The list of dolichocybids described by V.D. Sevastianov and his co-authors includes 6 species, namely Formicomotes octipes Sevastianov, 1980, Pavania riparia Sevastianov, 1980, P. tadjikistanica Sevastianov, 1980, P. protracta Sevastianov, 1980, P. tahanae Sevastianov and Abo-Korah, 1985, and Dolichocybe firjusae Sevastianov and Chydyrov, 1994 (Sevastianov 1980; Sevastianov & Abo-Korah 1985; Sevastianov et al. 1994). Only Formicomotes octipes was recently redescribed (Khaustov and Frolov 2018a), while other species remain unstudied since the times of their original descriptions.
The main aim of this article is to provide redescriptions of dolichocybid mites described by V.D. Sevastianov and co-authors based on type and additional material.
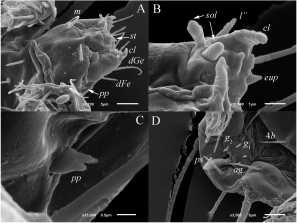
The type materials of dolichocybid mites were loaned from the collection of the Odessa I. I. Mechnikov National University Museum of Zoology, Odessa, Ukraine. Additional material of Pavania riparia was collected by junior author on the dung beetle Copris lunaris and mounted in Hoyer’s medium. One non type material slide of P. riparia from the collection of the junior author was remounted and several specimens were removed, dusted with gold and scanned with aid of a JEOL–JSM-6510LV SEM microscope.
Mite morphology was studied using a Carl Zeiss AxioImager A2 compound microscope with phase contrast and DIC objectives. Photomicrographs were taken with an AxioCam ICc5 digital camera. The terminology of the idiosoma and legs follows Lindquist (1986); the nomenclature of subcapitular setae follows Grandjean (1944). All measurements are given in micrometers (μm). For leg chaetotaxy the number of solenidia is given in parentheses.
Type species: Pavania fusiformis Lombardini, 1949, by original designation.
Pavania riparia Sevastianov, 1980, p.1457, Figs 1–3.
(Figs 1–4)
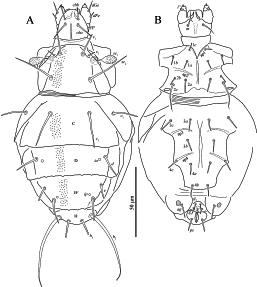










Female (Figs 1–4)
Body oval, weakly sclerotized. Length of idiosoma 125–130, width 74–86.
Gnathosoma (Figs 4A–C) — Gnathosomal capsule, excluding palps, almost round, its length 22, width 22–24. Dorsally with two pairs of weakly barbed and blunt-ended cheliceral setae (cha, chb). Setae cha 13–14 distinctly longer than chb 9–10. Dorsal median apodeme well developed. Postpalpal setae (pp) short, divided distally (Fig. 4C). Venter of gnathosoma with one pair of smooth, pointed subcapitular setae m 12. Palps with smooth setae dFe and dGe dorsolaterally, setae dGe 10–11 pointed, more than two times longer than blunt-ended dFe 4. Palps ventrally with two solenidia (sol). Inner solenidion slightly shorter than outer one. Palp tibiotarsus with short, blunt-ended ventrolateral seta (probably tibial l”) and distal eupathidium-like tiny seta (eup) (Fig. 4B). Palps terminate with well-developed tibial claw (cl) (Fig. 4A, B). Cheliceral stylets strong, curved. Pharynx thin-walled, elongate, with weak lateral projections.
Idiosomal dorsum (Figs 1A, 3A) — All dorsal shields with small sparsely distributed dimples (Fig. 3A). Prodorsal shield with three pairs of setae (v 1, v 2, sc 2) and a pair of clavate, barbed trichobothria sc 1 with rounded apex. All dorsal setae blunt-ended. Most of dorsal setae smooth or with very small barbs and only setae h 1 with distinct barbs. Tips of setae h 2 thickened into tiny clubs. Cupules ia on tergite D and ip on tergite EF small, round; other cupules not evident. Posterior margins of tergites C, D, and EF with several weak projections. Lengths of dorsal setae: v 1 16, v 2 6, sc 2 30–32, c 1 23–27, c 2 18, d 17, e 10–12, f 17–18, h 1 13–14, h 2 54–63. Distances between setae: v 1–v 1 16–17, v 2 –v 2 24–26, sc 2 –sc 2 28, c 1–c 1 24–28, d–d 48–49, e–e 40–41, f–f 28–29, h 1–h 1 10, h 1–h 2 7–8.
Idiosomal venter (Figs 1B, 3B, 4D) — All ventral plates smooth. All ventral setae smooth; setae 2c pointed, other setae blunt-ended. Apodemes 1 (ap1) and apodemes 2 (ap2) well developed; prosternal apodeme not evident; apodemes 3 (ap3) and 4 (ap4) well developed. Coxal fields I-IV each with three pairs of setae. Lengths of ventral setae: 1a 6, 1b 7, 1c 6, 2a 10, 2b 6, 2c 19, 3a 9, 3b 8–9, 3c 10–11, 4a 8, 4b 8–9, 4c 9, ag 9–10, g 1 4–5, g 2 4, ps 9.
Legs (Fig. 2) — Leg I (Fig. 2A). Setal formula: 0–4–2–6(2) –11(2). Tarsus with two small claws and semioval empodium. All leg setae smooth. Setae l’ of femur, l’, v’ of genu, k and v’ of tibia blunt-ended; other leg setae (except eupathidia) pointed. Trochanter dorsally with four spine-like projections. Tarsus I with ventrodistal membranous flange. Lengths of solenidia ω 1 5, ω 2 3, φ 1 6, φ 2 4; Solenidia φ 2 and ω 2 weakly clavate, solenidion φ 1 distinctly clavate, solenidion ω 1 finger-shaped. Leg II (Fig. 2B). Setal formula: 0–2–1–4(1)–6(1). Tarsal claws simple, hooked; empodium large. Solenidion ω 4 finger-shaped, solenidion φ 2–3 clavate. Trochanter dorsally with two spine-like projections. Setae tc” and u’ of tarsus with flattened and weakly sclerotized blunt-ended tips. Other leg setae pointed. Setae v” of femur, l’, v” of tibia and tc’, pl” of tarsus weakly barbed, other setae smooth. Leg III (Fig. 2C). Setal formula: 0–1–1–4–5. Claws and empodium of same shape as on tarsus II. Setae (tc) of tarsus with flattened and weakly sclerotized blunt-ended tips, other leg setae pointed. Seta pl”of tarsus weakly barbed; other leg setae smooth. Leg IV (Fig. 2D). Setal formula: 0–1–1–4–5. Claws and empodium of same shape as on tarsus III. Seta d of femur blunt-ended, seta tc’ of tarsus weakly blunt-ended, other leg setae pointed. Seta tc’ of tarsus smooth; other leg setae weakly barbed.
Male unknown.
Female holotype, and 10 female paratypes, slide No. D-T-2, Ukraine, vicinity of Odessa, coast of Kuyalnik Liman, on Copris lunaris (Coleoptera, Scarabaeidae), 22 May 1960, Sevastianov V.D. leg.; 10 females, Ukraine, Odessa Prov., Razdelnaya District, vicinity of settlement Kolontaevka (46°43' N, 30°18' E), on C. lunaris, 20 Sept. 2009, Trach V.A. leg.; 8 females, Ukraine, Kherson Prov., Kalanchak District., vicinity of settlement Preobrazhenka (46°11' N, 33°36' E), on C. lunaris, 2-4 May 2011, Trach V.A. leg.
The holotype and paratypes available for study are in poor condition and redescription is based on additional material collected by the junior author.
Pavania tadjikistanica Sevastianov, 1980, p.1457, Figs 4–7.
(Figs 5–7)
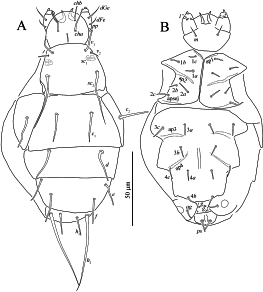








Female (Figs 5–7)
The holotype and two paratypes available for this study are in rather bad condition (Fig. 7) and some tiny structures like cupules, dimples of dorsal plates and weak barbs on setae are not discernible. Length of idiosoma 120, width 80.
Gnathosoma — Gnathosomal capsule, excluding palps, almost round, its length 27, width 31. Dorsally with two pairs of blunt-ended cheliceral setae (cha, chb). Setae cha 15 distinctly longer than chb 10. Dorsal median apodeme well developed. Postpalpal setae (pp) short, peg-like. Venter of gnathosoma with one pair of pointed subcapitular setae m 9. Palps with smooth and pointed setae dFe and dGe dorsolaterally, setae dGe 11 almost twice longer than dFe 6. Palps ventrally with two solenidia. Inner solenidion much shorter than outer one. Palp tibiotarsus with short, blunt-ended ventrolateral seta (probably tibial l”). Palps terminated with well-developed tibial claw. Cheliceral stylets strong, falcate. Pharynx not discernible.
Idiosomal dorsum (Figs 5A, 7A) — Prodorsal shield with three pairs of setae (v 1, v 2, sc 2) and a pair of broken trichobothria sc 1. Setae sc 2 pointed, other dorsal setae blunt-ended. Tips of setae h 2 thickened into tiny clubs. Posterior margins of tergites without projections. Lengths of dorsal setae: v 1 14, v 2 6, sc 2 36, c 1 20, c 2 20, d 22, e 16, f 17, h 1 13, h 2 53. Distances between setae: v 1–v 1 22, v 2 –v 2 29, sc 2 –sc 2 34, c 1–c 1 24, d–d 34, e–e 43, f–f 29, h 1–h 1 12, h 1–h 2 6.
Idiosomal venter (Figs 5B, 7B) — All ventral setae blunt-ended. Ap1 and ap2 well developed; prosternal apodeme not evident; sejugal apodeme (apsej) weakly developed laterally; ap3 and ap4 well developed. Coxal fields I-IV each with three pairs of setae. Genital setae (g 1, g 2) very small, vestigial. Lengths of ventral setae: 1a 6, 1b 6, 1c 5, 2a 8, 2b 5, 2c 8, 3a 7, 3b 7, 3c 9, 4a 6, 4b 9, 4c 8, ag 7, g 1 1, g 2 1, ps 9.
Legs (Fig. 6) — Leg setation as in previous species. Leg I (Fig. 6A). Tarsus with two small claws and semioval empodium. Setae l’ of femur, l’, v’ of genu, k, l’ and v’ of tibia blunt-ended; other leg setae (except eupathidia) pointed. Trochanter dorsally probably without spine-like projections. Lengths of solenidia ω 1 6, ω 2 3, φ 1 6, φ 2 5; solenidia ω 1, φ 2 and ω 2 weakly clavate, solenidion φ 1 distinctly clavate. Leg II (Fig. 6B). Tarsal claws simple, hooked; empodium large. Solenidia ω 5 and φ 3 weakly clavate. Trochanter probably without spine-like projections. All leg setae pointed. Leg III (Fig. 6C). Claws and empodium of same shape as on tarsus II. Seta d of femur blunt-ended, other leg setae pointed. Leg IV (Fig. 6D). Claws and empodium of same shape as on tarsus III. Seta d of femur blunt-ended, other leg setae pointed.
Male unknown.
Female holotype, and 2 female paratypes, slide No. T-D-3, Tadjikistan, vicinity of Dushanbe, under elytra of Onthophagus sp. (Coleoptera, Scarabaeidae), 25 Aug. 1969, Ilyasov I.N. leg.
Pavania protracta Sevastianov, 1980, p.1459, Figs 8–10.
(Figs 8–10)









Female (Figs 8–10)
Length of idiosoma 150, width 75.
Gnathosoma — Gnathosomal capsule, excluding palps, almost round, rather small, its length 18, width 19. Dorsally with two pairs of weakly barbed cheliceral setae (cha, chb). Setae cha 9 pointed, distinctly longer than blunt-ended chb 5. Dorsal median apodeme weakly developed. Postpalpal setae (pp) short, peg-like. Venter of gnathosoma with one pair of pointed and smooth subcapitular setae m 11. Palps with smooth setae dFe and dGe dorsolaterally, setae dGe 8 pointed, two times longer than blunt-ended dFe 4. Palps ventrally with two solenidia. Inner solenidion much shorter than outer one. Palp tibiotarsus with short, blunt-ended ventrolateral seta (probably tibial l”). Palps terminated with well-developed tibial claw. Cheliceral stylets relatively small, curved. Pharynx not discernible.
Idiosomal dorsum (Figs 8A, 10A) — All dorsal shields smooth. Prodorsal shield with three pairs of setae (v 1, v 2, sc 2) and a pair of clavate and distinctly barbed trichobothria sc 1. All dorsal setae weakly barbed. Setae v 2 blunt-ended, other dorsal setae pointed. Posterior margins of tergites without projections. Cupules not evident. Lengths of dorsal setae: v 1 18, v 2 7, sc 2 28, c 1 20, c 2 26, d 21, e 20, f 21, h 1 16, h 2 36. Distances between setae: v 1–v 1 9, v 2 –v 2 18, sc 2 –sc 2 20, c 1–c 1 21, d–d 37, e–e 38, f–f 25, h 1–h 1 12, h 1–h 2 8.
Idiosomal venter (Figs 8B, 10B) — All ventral plates smooth. Setae 1a, 1b, 1c, 2b, 2c, and g 1 blunt-ended; other ventral setae pointed. Setae 1a, 1b, 1c, 2b, and 2c weakly barbed; other ventral setae smooth. Only ap2 and ap3 present, weakly developed; other apodemes not evident. Coxal fields I-IV each with three pairs of setae. Lengths of ventral setae: 1a 5, 1b 3, 1c 5, 2a 9, 2b 4, 2c 8, 3a 8, 3b 8, 3c 10, 4a 8, 4b 9, 4c 10, ag 8, g 1 5, g 2 9, ps 11.
Legs (Fig. 9)— Leg setation as in previous species. Leg I (Fig. 9A). Tarsus with two small claws and very short empodium. Setae v’ of genu, k and v’ of tibia blunt-ended; other leg setae (except eupathidia) pointed. Setae v”, (l) of femur, k of tibia, pl’, (u) and all eupathidia of tarsus smooth; other leg setae weakly barbed. Trochanter dorsally with three spine-like projections; central projection distinctly smaller than lateral ones. Lengths of solenidia ω 1 9, ω 2 4, φ 1 8, φ 2 5; Solenidia ω 1 and φ 2 finger-shaped; solenidion ω 2 weakly clavate, solenidion φ 1 distinctly clavate. Leg II (Fig. 9B). Tarsal claws simple, hooked; empodium small, semioval. Solenidia ω 5 finger-shaped and φ 3 weakly clavate. Trochanter with one spine-like projection. All leg setae pointed and weakly barbed. Leg III (Fig. 9C). Claws and empodium of same shape as on tarsus II. Seta pv’ of tarsus thickened, spiniform, blunt-ended; other leg setae pointed. Setae (pv) and tc” of tarsus smooth; other leg setae weakly barbed. Leg IV (Fig. 9D). Claws and empodium of same shape as on tarsus III. Seta pv’ of tarsus thickened, spiniform, blunt-ended; other leg setae pointed. Setae pv’ and tc” of tarsus smooth; other leg setae weakly barbed.
Male unknown.
Female holotype, and 1 female paratype, slide No. T-D-4, Russia, Tatarstan, vicinity of Kazan, soil under maize, 27 Aug. 1968, Artemjeva T.I. leg.
The holotype is in rather bad condition (Fig. 13). One female paratype was remounted and description is based on this paratype specimen.
Pavania tahanae Sevastianov and Abo-Korah, 1985, p.35, Figs 1–4.
(Figs 11–13)
Female (Figs 11–13)









Length of idiosoma 135, width 80.
Gnathosoma — Gnathosomal capsule, excluding palps, almost round, its length 19, width 20. Dorsally with two pairs of smooth and blunt-ended cheliceral setae (cha, chb). Setae cha 14 distinctly longer than chb 11. Dorsal median apodeme indistinct. Postpalpal setae (pp) short, peg-like. Venter of gnathosoma with one pair of pointed and smooth subcapitular setae m 11. Palps with smooth setae dFe and dGe dorsolaterally, setae dGe 12 pointed, almost two times longer than blunt-ended dFe 7. Palps ventrally with two subequal solenidia. Palp tibiotarsus with short, blunt-ended ventrolateral seta (probably tibial l”). Palps terminated with well-developed tibial claw. Cheliceral stylets rather small, curved. Pharynx not discernible.
Idiosomal dorsum (Figs 11A, 13A) — All dorsal shields smooth. Prodorsal shield with three pairs of setae (v 1, v 2, sc 2) and a pair of clavate and weakly barbed trichobothria sc 1. Setae e weakly barbed, other dorsal setae apparently smooth. Setae v 1 and v 2 blunt-ended, other dorsal setae pointed. Posterior margins of tergites without projections. Cupules not evident. Lengths of dorsal setae: v 1 18, v 2 9, sc 2 32, c 1 22, c 2 29, d 23, e 25, f 28, h 1 18, h 2 35. Distances between setae: v 1–v 1 11, v 2 –v 2 20, sc 2 –sc 2 28, c 1–c 1 37, d–d 53, e–e 46, f–f 42, h 1–h 1 11, h 1–h 2 9.
Idiosomal venter (Figs 11B, 13B) — All ventral plates smooth. All ventral setae smooth. Setae g 1 and g 2 blunt-ended, other ventral setae pointed. Only ap2 and ap3 clearly discernible; other apodemes not evident. Coxal fields I-V each with three pairs of setae. Lengths of ventral setae: 1a 7, 1b 8, 1c 6, 2a 13, 2b 6, 2c 15, 3a 9, 3b 7, 3c 13, 4a 7, 4b 8, 4c 8, ag 11, g 1 4, g 2 4, ps 10.
Legs (Fig. 12) — Leg setation as in previous species. Leg I (Fig. 12A). Tarsus with two small claws, empodium not evident. All leg setae smooth. Seta k of tibia and all eupathidia of tarsus blunt-ended; other leg setae pointed. Trochanter dorsally without spine-like projections. Lengths of solenidia ω 1 9, ω 2 5, φ 1 8, φ 2 3; solenidion ω 1 finger-shaped; solenidion ω 2 baculiform, solenidia φ 1 and φ 2 distinctly clavate. Leg II (Fig. 12B). Tarsal claws simple, hooked; empodium very small, semioval. Solenidia ω 8 finger-shaped and φ 2 weakly clavate. Trochanter without spine-like projections. All leg setae pointed and smooth. Leg III (Fig. 12C). Claws and empodium of same shape as on tarsus II. Solenidion φ 2 weakly clavate. All leg setae pointed and smooth. Leg IV (Fig. 12D). Claws and empodium of same shape as on tarsus II. Solenidion φ 2 weakly clavate. All leg setae pointed and smooth.
Male unknown.
Female holotype, slide No. 704, Egypt, vicinity of Shibin El Kom, soil under cotton, 1 Sept. 1980, Abo-Korah leg.
1. Setae sc
1 absent
...... 2
— Setae sc
1 present
...... 5
2. Setae 1c and 2c present
...... 3
— Setae 1c and 2c absent
...... P. neotropica Khaustov and Frolov, 2017 (Brazil)
3. Setae v
1 shorter than distance between their bases; setae cha less than three times longer than chb; setae e never longer than f; setae h
2 at most seven times longer than h
1
...... 4
— Setae v
1 longer than distance between their bases; setae cha three times longer than chb; setae e longer than f; setae h
2 15 times longer than h
1
...... P. gymnopleuri Hajiqanbar and Khaustov, 2010 (Iran)
4. Genu I with one seta (v’); dorsal idiosomal setae smooth; setae c
1 longer than c
2; setae c
1 and d pointed
...... P. sabzevarensis Hajiqanbar and Khaustov, 2010 (Iran)
— Genu I with two setae (v’, l’); dorsal idiosomal setae weakly barbed; setae c
2 longer than c
1; setae c
1 and d distinctly blunt-ended
...... P. onthophagi Hajiqanbar and Khaustov, 2010 (Iran)
5. Setae sc
1 capitate
...... 6
— Setae sc
1 seta-like
...... P. setiformis Loghmani and Hajiqanbar, 2013 (Iran)
6. Setae (u) and (pv) of tarsus I not lanceolate
...... 7
— Setae (u) and (pv) of tarsus I lanceolate
...... P. lanceolata Bahramian and Hajiqanbar, 2015 (Iran)
7. Coxal fields II with 3 pairs of setae
...... 8
— Coxal fields II with 2 pairs of setae
...... P. equisetosa Mahunka, 1975 (Ghana)
8. Empodium on tarsi II-IV small, not reaching beyond tips of claws, seta pv’ on tarsi III and IV simple
...... 9
— Empodium on tarsi II-IV large, reaching beyond tips of claws
...... 10
9. Seta pv’ on tarsi III and IV thickened, spiniform and blunt-ended, solenidia on tibiae III and IV absent
...... P. rotracta Sevastianov, 1980 (Russia)
— Seta pv’ on tarsi III and IV simple, solenidia on tibiae III and IV present
...... P. tahanae Sevastianov and Abo-Korah, 1985 (Egypt)
10. Setae h
2 less than 3.5 times longer than h
1
...... 11
— Setae h
2 more than 3.5 times longer than h
1
...... 15
11. Setae c
1 never reaching beyond bases of setae f; setae c
1 shorter than h
2; setae d shorter than h
2
...... 12
— Setae c
1 reaching beyond bases of setae f; setae c
1 longer than h
2; setae d and h
2 subequal
...... P. perhirsuta Mahunka, 1973 (Ghana)
12. Setae sc
2 subequal to distance between their bases
...... 13
— Setae sc
2 distinctly longer than distance between their bases
...... P. luisiae Mahunka, 1974 (Ghana)
13. Setae c
1, d, e and f blunt-ended
...... 14
— Setae c
1, d, e and f pointed
...... P. bembidii Khaustov, 2005 (Crimea)
14. Setae h
1 almost three times longer than ps, solenidion φ
2 with rounded tip
...... P. carabidophila Khaustov, 2005 (Russia: Krasnodarskiy Kray, Primorskiy Kray)
— Setae h
1 almost subequal with ps, solenidion φ
2 with attenuated tip
...... P. africana Khaustov and Frolov, 2018b (South Africa)
15. Setae h
2 more than six times longer than h
1
...... 16
— Setae h
2 less than six times longer than h
1
...... 19
16. Setae sc
2 less than 2.5 times longer than v
1; setae f less than twice as long as e; setae e shorter than v
1
...... 17
— Setae sc
2 at least 3.5 times longer than v
1; setae f more than twice as long as e; setae e longer than v
1
...... P. endroedyi Mahunka, 1975 (Ghana)
17. Setae sc
2 more than twice as long as v
1; setae f and d subequal; setae c
1 never reaching beyond posterior border of tergite C
...... 18
— Setae sc
2 less than twice as long as v
1; setae f longer than d; setae c
1 reaching beyond posterior border of tergite C
...... P. brasiliensis Mahunka, 1970b (Brazil)
18. Setae 2a as long as 2c and both longer than c
1, d and f; setae m protruding beyond anterior border of gnathosoma
...... P. elongata Hajiqanbar and Khaustov, 2010 (Iran)
— Setae 2a longer than 2c and both shorter than c
1, d and f; setae m never protruding beyond anterior border of gnathosoma
...... P. simplex Mahunka, 1973 (Ghana)
19. Setae f distinctly longer than e; setae e and h
1 subequal
...... 20
— Setae e and f subequal; setae e longer than h
1
...... P. tadjikistanica Sevastianov, 1980 (Tadjikistan, Iran)
20. Setae 2c subequal with 2a
...... 21
— Setae 2c about two times longer than 2a
...... P. riparia Sevastianov, 1980 (Ukraine, Slovakia)
21. Setae f more than two times longer than e
...... 22
— Setae f less than 1.5 times longer than e
...... P. khiavensis Sobhi and Hajiqanbar, 2017 (in Sobhi et al. 2017) (Iran)
22. Most dorsal idiosomal setae weakly barbed and blunt-ended; setae c
1 longer than c
2; setae sc
2 less than twice as long as c
1
...... P. kamalii Hajiqanbar and Khaustov, 2010 (Iran)
— Dorsal idiosomal setae smooth and pointed; setae c
2 longer than c
1; setae sc
2 more than twice as long as c
1
...... P. fusiformis Lombardini, 1949 (Italy, Iran)
Type species: Dolichocybe keiferi Krantz, 1957, by original designation.
Dolichocybe firjusae Sevastianov et al. 1994, p.3, Figs 1(1–4).
(Figs 14–16)
Female (Figs 14–16)









Body elongate, weakly sclerotized. Length of idiosoma 180, width 77.
Gnathosoma — Gnathosomal capsule, excluding palps, almost oval, its length 20, width 15. Dorsally with two pairs of smooth and pointed cheliceral setae (cha, chb). Setae cha 12 distinctly longer than chb 6. Dorsal median apodeme indistinct. Postpalpal setae (pp) rod-like. Venter of gnathosoma with one pair of smooth, pointed subcapitular setae m 17. Palps freely articulated to gnathosomal capsule, with smooth and pointed setae dFe and dGe dorsolaterally, setae dGe 12 pointed, more than two times longer than blunt-ended dFe 5. Palps ventrally with two subequal solenidia. Palp tibiotarsus with short ventrolateral seta (probably tibial l”) and distal eupathidium-like tiny seta. Palps terminated with well-developed tibial claw. Cheliceral stylets small, indistinct. Pharynx thin-walled, elongate, with weak lateral projections.
Idiosomal dorsum (Figs 14A, 16) — All dorsal shields smooth. Prodorsal shield with three pairs of setae (v 1, v 2, sc 2) and a pair of clavate, smooth trichobothria sc 1 with rounded apex; setae v 1 and v 2 located on the same transverse line. Setae v 1, v 2, and c 1 blunt-ended, other dorsal setae pointed. Setae h 1 and h 2 smooth, other dorsal setae weakly barbed. Cupules ia on tergite D and ip on tergite EF small, round; other cupules not evident. Lengths of dorsal setae: v 1 14, v 2 23, sc 2 44, c 1 13, c 2 25, d 18, e 19, f 25, h 1 13, h 2 76. Distances between setae: v 1–v 1 10, v 2 –v 2 22, sc 2 –sc 2 20, c 1–c 1 30, d–d 36, e–e 36, f–f 30, h 1–h 1 13, h 1–h 2 5.
Idiosomal venter (Figs 14B, 16) — All ventral plates smooth. Setae 3a, 3c, and ag weakly barbed, other ventral setae smooth; setae g 1 and g 2 blunt-ended, other ventral setae pointed. Only ap3 and ap4 weakly developed, other apodemes indistinct. Coxal fields I-IV each with three pairs of setae. Lengths of ventral setae: 1a 13, 1b 11, 1c 10, 2a 17, 2b 11, 2c 23, 3a 12, 3b 13, 3c 15, 4a 9, 4b 10, 4c 12, ag 14, g 1 3, g 2 4, ps 7.
Legs (Fig. 15) — Leg setation as in previous species. Leg I (Fig. 15A). Tarsus with two small claws; empodium indistinct. Setae d of femur, l’ of genu, d of tibia, tc’ and pl” of tarsus weakly barbed; over leg setae smooth. Seta k and eupathidia blunt-ended, other leg setae pointed. Trochanter dorsally with one large spine-like projection. Lengths of solenidia ω 1 3, ω 2 2, φ 1 7, φ 2 2; solenidia φ 2 and ω 2 peg-like, solenidion φ 1 distinctly clavate, solenidion ω 1 finger-shaped. Leg II (Fig. 15B). Tarsal claws simple, hooked; empodium large, flipper-like. Solenidion ω 4 finger-shaped, solenidion φ 1 very small, peg-like, difficult to discern. Trochanter dorsally without spine-like projections. All leg setae pointed. Setae tc”, u” and (pv) of tarsus smooth, other setae weakly barbed. Leg III (Fig. 15C). Claws and empodium of same shape as on tarsus II. All leg setae pointed. Seta d of tibia and all tarsal setae smooth, other leg setae weakly barbed. Leg IV (Fig. 15D). Claws and empodium of same shape as on tarsus III. All leg setae pointed. Seta d of tibia and all tarsal setae smooth, other leg setae weakly barbed.
Male unknown.
Female holotype, slide No. 908, Turkmenistan, Chardzhou Prov., vicinity of settlement Karabekaul, soil on cotton field, 24 Sept. 1982, Chydyrov P.R. leg.
The main differences between the genera Dolichocybe and Pavania according to Cross (1965) are the shape of cheliceral stylets (falcate and well developed in Pavania and small, indistinct in Dolichocybe) and position of palps (palps arising ventrolaterally in Pavania and laterally in Dolichocybe). In fact, the position of palps is rather uniform in all dolichocybid mites and can not be used as a generic-level character. The shape and size of stylets are also highly variable. In typical Pavania (like most species associated with dung beetles), stylets are really large and falcate. In typical Dolichocybe (D. keiferi, D. subcorticalis Khaustov, 2006, D. sibiriensis Khaustov, 2017, D. firjusae) stylets are small and indistinct. However, at least in Pavania protracta and P. tahanae, size of the cheliceral stylets is intermediate between typical for Pavania and for Dolichocybe. Therefore, the shape of cheliceral stylets is a vague character, difficult to use in separation of the genera Dolichocybe and Pavania. Rahiminejad et al. (2011) redefined the genus Dolichocybe and used the following characters to separate Dolichocybe from Pavania: “gnathosoma apparently longer than wide; chelicerae small and indistinct; tarsus I with 10 or 11 setae; tibiae III and IV with one minute solenidion each; with deep constriction between propodosoma and hysterosoma separated by soft and transversely striated cuticle” (in Dolichocybe); “gnathosoma almost as long as wide; chelicerae large and distinct; tarsus I with 11 setae; tibiae III and IV with no solenidia; without deep constriction between propodosoma and hysterosoma” (in Pavania). Based on our study, the number of setae on tarsus I is the same in Pavania and Dolichocybe and can not be used in separation of these genera. The presence of solenidia on tibiae III and IV in Dolichocybe is also problematic character. In fact, solenidia on tibiae III and IV are apparently absent in D. keiferi (type species) (Cross 1965), D. hippocastani Rack, 1967, D. picea Rack, 1967 (Rack 1967), and absent in D. subcorticalis, D. sibiriensis (Khaustov 2006, 2017) and D. firjusae (present study). Potentially solenidia on tibiae III and IV are present in Pavania brasiliensis Mahunka, 1970, at least Mahunka (1970) depicted a tiny solenidion on the tibia III. Therefore, the presence of solenidia on tibiae III and IV also can not be used for separation of these genera. It is also difficult to understand the extent of constriction between propodosomal and hysterosoma.
Two redescribed herein species, Pavania protracta and P. tahanae, have intermediate characters between Dolichocybe and Pavania: body shape is similar to that in Pavania (not strongly elongate), gnathosoma is rather small (as in Dolichocybe), cheliceral stylets are of medium size (intermediate), constriction between propodosomal and hysterosoma is not deep (as in Pavania). In addition, both of these species have unusually short — considerably shorter than tarsal claws — empodia on tarsi II-IV. Pavania tahanae also has solenidia on tibiae III and IV (like some “Dolichocybe”). Both species were collected in soil (not phoretic on insects) and potentially could represent a non-phoretic form of Pavania/Dolichocybe complex. At present female dimorphism has been evident only in the genus Formicomotes Sevastianov, 1980. Non-phoretic form of F. heteromorphus Magowski, 1988 has much shorter empodia than phoretic form (Magowski 1988). In this paper, we retain all species in the genera in which they were originally described. The redefinitions of the genera Dolichocybe and Pavania are necessary based on redescription of their type species and more discoveries of new species in these two close genera (particularly Dolichocybe) are required (phoretic or in soil) to see what characters are more variable.
The authors are grateful to Dr. Vladimir A. Lobkov and Yuri V. Suvorov, of the Odessa I. I. Mechnikov National University Museum of Zoology (Odessa, Ukraine) for access to the type material of V.D. Sevastianov.
The study was supported by the Russian Foundation for Basic Research (RFBR), research project No. 18-04-01092A.
Bahramian M., Hajiqanbar H., Talebi A.A. 2015. Two heterostigmatic mite species (Acari: Dolichocybidae, Podapolipidae) associated with Scarabaeus pius (Coleoptera: Scarabaeidae) from Iran. Acta Zool. Acad. Sci. Hung., 61: 25-32. doi:10.17109/AZH.61.1.25.2015 ![]()
Cross E.A. 1965. The generic relationships of the family Pyemotidae (Acarina, Trombidiformes). Univ. Kans.Sci. Bull., 45: 29-215.
Grandjean F. 1944. Observations sur les Acariens de la famille des Stigmaeidae. Arch. Sci. Phys. Nat., 26: 103-131.
Hajiqanbar H., Khaustov A.A. 2010. A new species group and five new species of the genus Pavania (Acari: Dolichocybidae) associated with insects, with notes on leg chaetotaxy and the distribution of genera. Europ. J. Entomol., 107: 441-453. doi:10.14411/eje.2010.051 ![]()
Kaliszewski M., Athias-Binche F., Lindquist E.E. 1995. Parasitism and parasitoidism in Tarsonemina (Acari: Heterostigmata) and evolutionary considerations. Adv. Parasitol., 35: 335-367. doi:10.1016/S0065-308X(08)60074-3 ![]()
Katlav A., Hajiqanbar H., Talebi, A. 2014. First record of the genus Acanthomastix Mahunka, 1972 (Acari: Dolichocybidae) from Asia, with the description of a new species. Internat. J. Acarol., 40: 7–14.
Khaustov A.A. 2005. Two new mite species of the genus Pavania (Heterostigmata, Dolichocybidae) from the Crimea and southern European Russia. Zool. Zh., 84: 1515-1521. [in Russian]
Khaustov A.A. 2006. A new species of mites of the genus Dolichocybe (Acari, Heterostigmata, Dolichocybidae) from the Crimea. Zool. Zh., 6: 772-774. [in Russian]
Khaustov A.A. 2017. A new species of Dolichocybe (Acari: Dolichocybidae) from Western Siberia, Russia. Syst. Appl. Acarol., 22: 1678-1687. doi:10.11158/saa.22.10.9 ![]()
Khaustov A.A., Frolov A.V. 2017. New species of heterostigmatic mites (Acari: Heterostigmata: Athyreacaridae, Dolichocybidae, Pygmephoridae) associated with scarab beetles (Coleoptera: Geotrupidae, Scarabaeidae) from Brazil. Zootaxa, 4294: 501-521. doi:10.11646/zootaxa.4294.5.1 ![]()
Khaustov A.A., Frolov A.V. 2018a. A new species of Formicomotes Sevastianov (Acari: Heterostigmata: Dolichocybidae) associated with termites (Isoptera: Termitidae) from Brazil, with redescription of Formicomotes octipes Sevastianov, 1980. Zootaxa, 4382: 393-400. doi:10.11646/zootaxa.4382.2.10 ![]()
Khaustov A.A., Frolov A.V. 2018b. A new species of Pavania (Acari: Heterostigmata: Dolichocybidae) associated with Frankenbergerius gomesi (Coleoptera: Scarabaeidae) from South Africa. Acarina, 26(1): 133-140.
Khaustov A.A., Trach V.A. 2017. On the phoresy and morphology of Pavania carabidophila Khaustov, 2005 (Acari: Dolichocybidae). Acarina, 25(1): 25-28. doi:10.21684/0132-8077-2017-25-1-25-28 ![]()
Krantz G.W. 1957. Dolichocybe keiferi, a new genus and species of pyemotid mite, with a description of a new species of Siteroptes (Acarina: Pyemotidae). Ann. Entomol. Soc. Am., 50: 259-264. doi:10.1093/aesa/50.3.259 ![]()
Lan Q., Lu Zh., Ke B., Liao J., Fan, Q.-H. 2017. Temperature and humidity effects on physogastric development and reproduction of the mushroom mite Dolichocybe perniciosa (Acari: Dolichocybidae). Syst. Appl. Acarol., 22(11): 1843-1848. doi:10.11158/saa.22.11.5 ![]()
Lindquist E.E. 1986. The world genera of Tarsonemidae (Acari: Heterostigmata): a morphological, phylogenetic, and systematic revision, with a reclassification of family-group taxa in the Heterostigmata. Mem. Entomol. Soc. Can., 118: 1-517. doi:10.4039/entm118136fv ![]()
Loghmani A., Hajiqanbar H., Talebi, A.A. 2013. A new species group and species of the genus Pavania (Acari: Dolichocybidae), phoretic on Onthophagus vitulus (Coleoptera: Scarabaeidae) from Iran. Zootaxa, 3693: 320-328. doi:10.11646/zootaxa.3693.3.2 ![]()
Lombardini G. 1949. Acari nuovi. Redia, 34: 67-74.
Magowski W.Ł. 1988. Description of a new species of Formicomotes Sevastianov, 1980 (Acari: Dolichocybidae) with notes on the female dimorphism within this genus. Mitt. Hamb. Zool. Mus. Inst., 85: 163-182.
Mahunka S. 1970a. Considerations on the systematics of the Tarsonemina and the description of new European taxa (Acari: Trombidiformes). Acta Zool. Acad. Sci. Hung., 16: 137-174.
Mahunka S. 1970b. The scientific results of the Hungarian soil zoological expeditions to South America. 21. Acari: Tarsonemine species from Brazil. Acta Zool. Acad. Sci. Hung., 16(3-4): 371-408.
Mahunka S. 1973. Auf Insecten lebende Milben (Acari: Acarida, Tarsonemida) aus Africa II. Acta Zool. Acad. Sci. Hung., 19: 289-337.
Mahunka S. 1974. Auf Insecten lebende Milben (Acari: Acarida, Tarsonemida) aus Africa IV. Acta Zool. Acad. Sci. Hung., 20: 367-402.
Mahunka S. 1975. Auf Insecten lebende Milben (Acari: Acarida und Tarsonemida) aus Africa V. Acta Zool. Acad. Sci. Hung., 21: 39-72.
Mortazavi A., Hajiqanbar H., Kamali, K. 2015. A new species of the family Dolichocybidae Mahunka, 1970 (Acari: Heterostigmata) associated with Sinoxylon pugnax Lesne (Coleoptera: Bostrichidae) from Iran. Syst. Appl. Acarol., 20: 441–448. doi:10.11158/saa.20.4.9 ![]()
Rack V.G. 1967. Untersuchungen über die biologie von Dolichocybe Krantz, 1957 und beschreibung von zwei neuen Arten (Acarina, Pyemotidae). Mitt. Hamb. Zool. Mus. Inst., 64: 29-42.
Rahiminejad V., Hajiqanbar H., Fathipour, Y. 2011. Redefinition of the genus Dolichocybe (Acari: Doli¬chocybidae), with description of two new species associated with insects. Ann. Entomol. Soc. Am., 104: 627-635. doi:10.1603/AN11006 ![]()
Sevastianov V.D. 1980. New taxa of mites of the family Dolichocybidae (Trombidiformes, Tarsonemina) and phylogenetic relations of its subfamilies. Zool. Zh., 59: 1453-1462. [in Russian]
Sevastianov V.D, Abo-Korah S.M. 1985. New mite species of the cohort Tarsonemina (Trombidiformes) from agrocoenoses of Egypt. Vestn. Zool., 19(4): 35-41. [in Russian]
Sevastianov V.D., Chydyrov P.R., Marroch, T.N. 1994. New mite species of the cohort Tarsonemina (Trombidiformes) from Turkmenistan, Ukraine and Russian Federation. Vestn. Zool., 28: 3-10. [in Russian]
Sobhi M., Hajiqanbar H., Mortazavi A. 2017. New species and records of heterostigmatic mites (Acari: Prostigmata: Heterostigmata) phoretic on scarabaeid dung beetles (Coleoptera: Scarabaeidae) from northwestern Iran. Zootaxa, 4276: 427-434. doi:10.11646/zootaxa.4276.3.7 ![]()
Zhang Z.-Q., Fan Q.-H., Pesic V., Smit H., Bochkov A.V., Khaustov A.A., Baker A., Wohltmann A., Wen T.-H., Amrine J.W., Beron P., Lin J.-Z., Gabrys G., Husband R. 2011. Order Trombidiformes Reuter, 1909. In: Zhang Z-Q. (ed.) Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 129-138.



2018-09-26
Date accepted:
2018-10-29
Date published:
2018-11-09
Edited by:
Sidorchuk, Ekaterina

This work is licensed under a Creative Commons Attribution 4.0 International License
2018 Khaustov, Alexander A. and Trach, Viacheslav A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



