New data to the knowledge of Gaeolaelaps mites (Acari: Mesostigmata: Laelapidae)
Nemati, Alireza1 ; Gwiazdowicz, Dariusz J.2 and Khalili-Moghadam, Arsalan3
1✉ Plant Protection Department, Agricultural College, Shahrekord University, Shahrekord, Iran.
2Poznan University of Life Sciences, Forestry Faculty, Wojska Polskiego 71C, 60–625 Poznań, Poland.
3Plant Protection Department, Agricultural College, Shahrekord University, Shahrekord, Iran.
2018 - Volume: 58 Issue: 3 pages: 710-734
https://doi.org/10.24349/acarologia/20184266Keywords
Abstract
The mite family Laelapidae includes hundreds of species that are free-living predators in soil, as well as many others that have varying degrees of association with other animals, both vertebrates and invertebrates (Faraji and Halliday 2009). The Genus Gaeolaelaps Evans and Till, 1966 is currently one of the largest genus of the family Laelapidae Berlese (Beaulieu 2009, Kazemi et al. 2014, Vatankhah et al. 2016). The known representatives of this genus are active predator of small invertebrates such as other mites, insect eggs and nematodes (Lindquist et al. 2009). Beaulieu (2009) and Kazemi et al. (2014) have studied the characteristics of the genus, which contains more than 100 species. In recent years, several species of this genus have been described, which seems to have increased the number of species to more than one hundred (Kavianpour et al. 2013, Nemati and Kavianpour 2013, Nemati and Mohseni 2013, Kavianpour and Nemati 2014, Kazemi et al. 2014, Saeidi et al. 2016, Vatankhah et al. 2016). The systematic situation of some species of this genus is unclear. There are still some species that may be considered especially in Hypoaspis s. lat. genus or other genera and need to study and transfer to their proper genus. The authors of the present article have begun to examine different species of this family in collections of different European museums. In this article and the articles that will be published later, the results of this research will be presented. We have also looked for the possible synonymies in Laelapidae family. Currently, some species of this family such as Hypoaspis tripodiger Berlese, 1916, G. angustus, G. queenslandicus, Androlaelaps trifurcatoides and A. trifurcatus are studied in this article. Various morphological features of these species were studied in the collections in Germany (Museum für Naturkunde Berlin and Zoologische Staatssammlung München), Italy (Istituto Sperimentale per la Zoologia Agraria, Firenze) and various specimens in the Acarological Laboratory, Plant Protection Department, Agricultural College, Shahrekord University (APAS) in Iran and also a few specimens which have been collected from Australia. In this paper, the important characters to differentiate G. angustus and G. queenslandicus are discussed and the validity of mentioned species are presented at the end.
Morphological features of Hypoaspis (Hypoaspis) angustus and Hypoaspis tripodiger were studied from specimens examined at the Museum für Naturkunde Berlin, Zoologische Staatssammlung München (Germany) and Istituto Sperimentale per la Zoologia Agraria, Firenze (Italy). Additional specimens were collected from Australia and from soil or material taken from ant nests in different parts of Iran. Mites were extracted from samples using Berlese funnels, placed in lactic acid at 55°C for clearing and then mounted in Hoyer’s medium on permanent microslides for examination under compound microscope. Specimens were deposited at the Acarological Laboratory, Agricultural College, Shahrekord University (APAS). Line drawings were made with the use of a drawing tube and figures were performed with Corel X–draw software, based on the scanned line drawings. Measurements of structures expressed as minimum-maximum ranges in micrometers (μm) which were obtained using scaled ocular lens of Olympus BX–43 equipped with phase-contrast and Digimizer Software. Notation of the dorsal setae follows that of Lindquist and Evans (1965). Terminology for idiosomal adenotaxy and poroidotaxy are based on Kazemi et al. (2014). Leg and palp setal notation and chaetotactic formulae are based on Evans (1963a; b).
Length of the dorsal shield is the distance from its anteromedian edge anterior to bases of setae j1 to its posteromedian edge posterior to bases of setae Z5; width of dorsal shield is measured at widest part; length of the sternal shield is measured along midline from anterior edge to its posterior margin, width measured between coxae II-III (widest point) and slightly above the insertion of st2 (narrowest point); the length of anal shield is midline from the anterior margin to the posterior edge of the cribrum, and width was measured at widest point. Setae were measured at level of insertions to their tips and distance between setae as the distance between their insertions. For curved setae and other morphological features that are bent or aligned in the Z axis, high-quality microscopic photographs were taken and then the length of the curved setae/feature were measured by calibrated Digimizer software (version 4.6.1 MedCalc Software). Lengths of leg segments were measured dorsomedially, and tarsi were measured without the stalk and pretarsus. In the text of this paper angustus-like specimens means, those specimens with straight opisthonotal margins and queenslandicus-like specimens are those with different kinds of concave margins.
Australia: four microslides of female G. queenslandicus collected in Sydney from soil, coll., M. Ameri, 2016. Italy (specimens of Hypoaspis tripodiger deposited in Instituto Sperimentale per la Zoologia Agraria, Florence): microslides 9 myrm. /1–2, t, female, Nidi di Acromyrmex lundi, la Plata, Bruck, 208/46, female, terriccio Castagno, Firenze. Germany [specimens designated as Hypoaspis angustus (all determined by W. Karg) deposited in Museum für Naturkunde Berlin; holotype and six paratypes]: microslides: ZMB Nr. 39835, female, holotype, Grasaussaat (grass-seed), Stahnsdorf b. Berlin, A10, Brandenburg, Potsdam-Mittelmark, Stahnsdorf, Sammler (collector): W. Karg, 8/10/1957, slide no. 3714; ZMB Nr. 39836, female, paratypus, Grasaussaat, Stahnsdorf b. Berlin, DDR, Germany, Brandenburg, Potsdam-Mittelmark, Stahnsdorf, Sammler: W. Karg, 1/20/1958, slide no. 3715; ZMB Nr. 39841, female, Einsendung, Zürich, Institut für Pflanzenwiss. Phytomedizin, U Habersaat, Switzerland, Zürich, Sammler: H. Habersaat, 8/15/1988, slide no. 3720; ZMB Nr. 39842, female, Einsendung, Zürich, Institut für Pflanzenwiss. Phytomedizin, U Habersaat, Switzerland, Zürich, Sammler: H. Habersaat, 8/15/1988, slide no. 3721; ZMB Nr. 39843, female, Einsendung, Zürich, Institut für Pflanzenwiss. Phytomedizin, U Habersaat, Switzerland, Zürich, Sammler: H. Habersaat, 8/15/1988, slide no. 3722; ZMB Nr. 39844, female, Einsendung, Zürich, Institut für Pflanzenwiss. Phytomedizin, U Habersaat, Switzerland, Zürich, Sammler: H. Habersaat, 8/15/1988, slide no. 3723; ZMB Nr. 39845, slide no. 3724. Iran (specimens deposited in APAS): Chaharmahal va Bakhtiari Province: Shahrekord, soil and unknown ant nest, 21 females, coll., A. Nemati, 2006-2015; Shahrak, soil, three females, coll., S. Babaeian, 2009, Laleh and Tehlijan Parks, soil and ant nest, 18 females, coll., E. Mosharaf, 2008; different places in Shahrekord environs from ant nest, 10 females, coll., H. Maleki, 2008; Nafch, soil, 11 females, coll., A. Nemati, 2010, Ben, soil, six females and two males, coll. A. Nemati, 2010; Zayanderood verge from ant nest, 16 females and six males, coll., A. Khalili-Moghadam, 2014; Saman, soil in almond garden, three females, S.H. Noorbakhsh, 2010; Borujen and Naghan, soil, eight females and one male, coll., M. Mohseni, 2013; Lordegan from different habitats, 19 females and seven males, coll., A. Nemati, 2015.
During this survey and in 2016–2017, based on above explanation concerning the procedure of sampling, 75 females and 11 males (10 females and two males of G. queenslandicus and 65 females and 9 males of G. angustus) were collected from soil and ant nest in different parts of Chaharmahal va Bakhtiari Province and examined.
Khuzestan Province: Izeh, Ghaletol and Baghmalek environs from soil and ant nest, 21 females, coll., A. Nemati, 2012–2014; Ahvaz, ant nest in Jondishahpour University, three females, F. Vatankhah, 2014; Andimeshk, ant nest, five females, coll., K. Siheii, 2014; Masjedsoleiman, ant nest, 31 females and 10 males, coll., M. Nemati, 2013; Dezfoul, soil, ant nest in citrus garden, nine females, coll., M. Nemati, 2013; Roodzard, soil., two females, coll., M. Nemati, 2013; Barangerd, ant nest in pomegranate garden, four females and two males, coll., M. Nemati, 2013; Esfahan Province: Shahreza, soil, 17 females and six males, coll., M. Kavianpour, 2010. In the course of study on some mesostigmatid mites in Esfahan by F. Kadkhodaei-Eliaderani during 2010-2012 in different habitats, 106 females of both species were collected which all of them examined herein. The collection data is as follows: 2011/March/25, Khomeinishahr; 2011/March/19, Bostan Kodak park, 2011/March/24, Atash street; 2011/March/27, Marnan bridge, 2011/August/14, Bostan moshtagh; 2011/July/10, Bishe-habib park; 2011/Agust/10, Bostan melat; 2011/Agust/30, Bostan mirzakochakkhan, 2012/March/12, Bagh-daryache street; 2012/March/17, Vahid street; 2012/March/19, Khayyam street. Zanjan Province: different parts of Zanjan city in soil and ant nest, 22 females, coll., H. Rahmani, 2014; West Azerbaijan Province: Urmia, soil and ant nest, 19 females, coll., M. Kavianpour, 2015; Golestan Province: Gorgan, soil, eight females, coll., S. Malekshah-Koohi, 2011; Boushehr Province: Borazjan, soil and ant nest, five females, coll., F. Sohrabi, 2015; Eilam Province: Eilam, soil, four females, coll., A. Barfi, 2014.
Hypoaspis (Geolaelaps). — Bregetova 1977: 499; Karg 1979: 79; Karg 1982: 237; Karg 1989: 107; Karg 1993a: 136.
Gaeolaelaps. — Casanueva 1993: 40; Farrier and Hennessey 1993; Beaulieu 2009: 35; Kazemi et al. 2014: 504. Geolaelaps. — Rosario 1981: 46; Walter and Oliver 1989: 295; Hunter 1993: 23; Karg and Schorlemmer 2013: 203.
Type species: Laelaps aculeifer Canestrini, 1884, by original designation (Evans and Till 1966).
Androlaelaps queenslandicus Womersley, 1956: 577.
Androlaelaps queenslandicus. — Wang and Li 1965: 239.
Geolaelaps queenslandicus. — Ryke 1963: 13; Walter and Oliver 1989: 295; Farrier and Hennessey 1993: 73.
Gaeolaelaps queenslandicus. — Hyatt 1964: 472; Beaulieu 2009: 37; Kavianpour et al. 2013: 8; Nemati and Kavianpour 2013: 71; Kavianpour and Nemati 2014: 321.
Hypoaspis queenslandicus. — Costa 1966: 141; Zeman 1982: 233. Nasr and Nawar 1989: 70; Nawar et al. 1993: 347.
Hypoaspis (Hypoaspis) queenslandicus. — Aswegen and Loots 1970: 190; Hafez et al. 1982: 4.
Hypoaspis (Geolaelaps) queenslandicus. — Karg 1979: 81; Tenorio 1982: 265; Karg 1993a: 141; Karg 1993b: 266.
Hypoaspis (Gaeolaelaps) queenslandica. — Faraji et al. 2008: 207.
Gaeolaelaps queenslandica. — Trach 2012: 162.
Hypoaspis tripodiger Berlese, 1916: 167. New synonymy.
Androlaelaps trifurcatus Wang and Li, 1965: 238. New synonymy.
Androlaelaps trifurcatus. — Kazemi et al. 2014: 519; Li et al. 1998: 266; Moreira 2014: 185; Ren and Guo 2008: 328; Wang and Liao 2000: 27.
Androlaelaps trifurcatoides Yan and Ma, 1999. New synonymy.
Androlaelaps trifurcatoides. — Moreira 2014: 185; Ren and Guo 2008: 328; Yan and Ma 1999: 149.
Hypoaspis (Hypoaspis) angustus Karg, 1965: 274. New synonymy.
Hypoaspis angustus. — Costa 1966: 145; Zeman 1982: 233; Ma 1996: 51.
Hypoaspis (Hypoaspis) angustus. — Karg 1971: 171; Karg 1978: 15.
Hypoaspis (Geolaelaps) angustus. — Bregetova 1977: 504.
Hypoaspis (Geolaelaps) angustus. — Karg 1979: 81; Karg 1982: 239; Karg 1993a: 141; Ruf and Koehler 1993: 197.
Geolaelaps angustus. — Walter and Oliver 1989: 295.
Hypoaspis angustus. — Farrier and Hennessey 1993: 77; Ruf and Koehler 1993: 197.
Gaeolaelaps angustus. — Beaulieu 2009: 36.
Gaeolaelaps angustus. — Kavianpour et al. 2013: 7; Nemati and Kavianpour 2013: 70; Kavianpour and Nemati 2014: 321.
Berlese (1916) described Hypoaspis tripodiger that collected from Cl. Bruck "La Plata" in nest of Acromyrmex lundi (Guérin-Méneville). Some of the morphological characters that explained concerning this species by him are as follows: “idiosoma elongated, dorsal shield covered with setae of varied length. Sternal shield in the shape of a bowl, and rounded at the height of legs II. Epigynal shield expands posteriorly to leg IV. Anal shield small, oval, elongated. Jugularia are narrow. Epistome arched with fine teeth. Legs II are much thicker than the other legs. Femur armed with spine-like setae, the genu is weak, and the tibia and tarsus have spine-like setae. Legs II and IV are also armed with spine-like setae like Hypoaspis aculeifer. Length of idiosoma is 600 μm and width 280 μm”. We’ve examined specimens at the Berlese Collection identified as Hypoaspis tripodiger (see material examined). One slide of “co-types” of H. tripodiger (myrmec. 9/1, cotipi) contains specimens, but was unfortunately deemed not suitable for study. However, the specimen that was apparently identified as the “type” by Berlese (myrmec. Tipico, nidi: Acromyrmex lundi, Laplata), which we therefore consider it as the holotype, was in sufficiently adequate shape to make some measurements (Table 4), and the following observations. Dorsal shield possesses 37 pairs of smooth acicular setae. The lengths of lateral dorsal shield setae are slightly longer than the other setae on the shield. Epistome is deeply denticulate. Chelicerae chelate dentate, moveable digit with two and fixed digit multidentate (difficult for determination of teeth number). Presternal shields with lineate reticulate, granulated and similar to those in G. angustus. Sternal shield is longer than wide and reticulated in most surface, with three smooth acicular setae and two pairs of lyrifissures (iv1–2). Metasternal setae and iv3 located posterior of shield on soft skin. General appearance of well-reticulated epigynal shield is similar to G. angustus. The data of some dorsal shield setae and morphological characters appeared in Tables (1, 4). The legs chaetotaxy of this species is identical to those in Figures (13A, B). Palp-tarsal claw is three-tined.
The oldest available name for this species is Gaeolaelaps tripodiger (Berlese, 1916). However, that name is almost completely unknown, and to use it as valid now would cause taxonomic confusion. We continue to use the widely known name Gaeolaelaps queenslandicus (Womersley, 1956) for this species, in the interests of stability.
This species was collected from the body of Rattus losea exiguous in Fukien, China (Wang and Li 1965) and described as a member of Androlaelaps while the morphological traits do not fit the characteristics of the genus and consistent with the Gaeolaelaps genus as also suspected by Kazemi et al. (2014). Androlaelaps mites have membranous flap-like epistome more or less rounded anteriorly and never denticulate. Cheliceral fixed digit in female usually shorter than movable one and each with various situation of dentation, pilus dentilis with different shapes and in any case it has grown to varying degrees. Palp tarsal-claw is two-tined. Sternal shield usually wider than long. Male usually has special fixed and movable digits equipped with spermadactyle, which has some variation in shape and length in the genus. Fixed digit in the male markedly reduced, edentate; movable digit usually edentate and partially fused with the elongated and grooved spermadactyle.
The morphological traits of G. trifurcatus new comb., including the dorsal shield and its chaetotaxy, as well as the presented figures of ventral idiosomal characters such as presternal, sternal, epigynal shields and also leg II (Figures 12 and 13 sensu Wang and Li 1965), all indicate its similarity with G. angustus.
This species described and reported from China (Yan and Ma 1999; Ren and Guo 2008). The morphological traits are more similar to those of the genus Gaeolaelaps than those of Androlaelaps and should be assigned to it. In this species, epistome denticulate (smooth in Androlaelaps and never denticulate), presternal shield distinct and granulate (Androlaelaps with no definite presternal shields and usually contiguous with sternal shield and not granulate), sternal shield longer than wide (usually wider than long in Androlaelaps), the male fixed cheliceral digit multidentate, spermadactyle finger-like and curved distally (see above explanation concerning male chelicera). These characters show the consistency of this species with Gaeolaelaps genus. On the other hand, the various traits of this species are very similar to the related characters of G. angustus. Male and female cheliceral characters, number and distribution of dorsal shield setae; leg chaetotaxy as far as the relevant article is concerned: spine, spur-like and elongate setae on legs II and IV perfectly compatible with what exists in G. angustus [see the following explanation concerning G. angustus and G. queenslandicus characters and leg chaetotaxy appeared in (Figures 13A and 13B) herein]. By comparing the descriptions and figures of these species, we could find no distinguishing authentic morphological differences, therefore, G. trifurcatoides and G. angustus considered to be synonyms.
Gaeolaelaps queenslandicus (Womersley, 1956) originally described as a species of Androlaelaps, based on a single specimen collected from leaf debris in Taringa, South Queensland, Australia. Womersley (1956) described it as a rather small species with dorsal shield not covering entire idiosomal dorsum, bears about 46 setae longer on posterior margin. Based on Womersley’s (1956) figure 48–B (p. 578), the left lateral margin of the dorsal shield presents a small inward curvature at level of Z3 seta, while the margin is almost straight on the opposite side. Karg (1965) described Hypoaspis (Hypoaspis) angustus based on specimens collected from meadow soil, Berlin, Kleinmachnow, which have dorsal shield with straight lateral margins in the opisthonotal region. Many morphological characters not described in original descriptions of Karg (1965) and Womersley 1956). Ten years after the original description of Hypoaspis queenslandicus, Costa (1966) redescribed it and rectified some morphological characters of this species based on type material and Israel specimens, especially concerning the dorsal shield margins. Redrawn figures (Figure 1 p. 142) of Costa (1966) based on mentioned specimens of G. queenslandicus show a dorsal shield with lateral margins that are clearly curved inwards at level of Z3– S3, and angled posteriorly at level of Z4– S4 (Figures 2A, B). Costa (1966) pointed out the morphological differences between G. queenslandicus and G. angustus, and presented these two species as distinct based on the following differences: 1) Opisthonotal region of dorsal shield with lateral margins concave (G. queenslandicus) vs straight (G. angustus).
2) The distribution of dorsal shield setae.
3) Relative lengths of dorsal shield setae (longer in the first species).
4) The shape of deutosternal groove (G. queenslandicus with discontinuous lateral margins of deutosternal groove while the second one with contiguous lateral margins) and their relative sizes (longer in the first one).
5) The smaller number of teeth on the cheliceral fixed digit in G. angustus.
All mentioned characters and some other morphological traits examined as follow.
The lateral margins of the dorsal shield in G. angustus (based on type materials and Iranian specimens with straight opisthonotal margins) tend to be converged in podonotal region at level of r3–r4 as an almost straight line and without curvature along it with nearly rounded posterior end of the shield (Figures 1A-B, 6 and 8).



Podonotal lateral margins of dorsal shield in G. queenslandicus sensu Costa (1966) and examined Australian specimens are similar to those in G. angustus, but the margins of opisthonotal part with concave at the level of Z3– S4, then tapered near the level of Z4– S5 as a straight line in both sides of shield with posterior end rounded (Figures 2A, B).
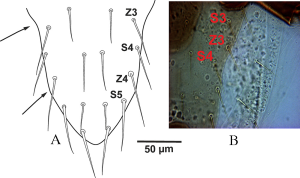


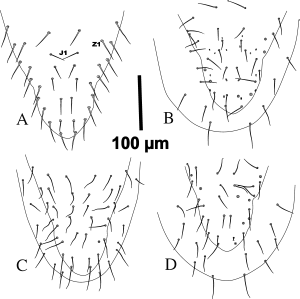


The above mentioned variations in lateral margins of dorsal shield in opisthonotal part shows that this character with inconstancy condition and worthless taxonomically.
The number of dorsal shield setae in G. queenslandicus and G. angustus (as in literature and the most examined specimens in this survey) normally is 37 pairs of simple acicular setae with similar distribution. However, dorsal shield setae also vary in number in both species (Figures 4A, 5B, 6 and 7B) based on some Iranian specimens. Some specimens with one pair of PX3 setae between J3– J4 with totally 38 pairs of dorsal shield setae (Figures 4A, 5B and 6), some with one PX3 in right or left side at the same place (Figure 6).












The distribution of dorsal shield setae of G. angustus holotype is the same as Figure (8). The most specimens of intermediate forms, with some characters intermediate between G. angustus holotype and G. queenslandicus description by Costa (1966) which were checked in Iran and all specimens which considered as G. angustus based on straight lateral margins of dorsal shield in opisthonotal part in Germany (Karg collection, Berlin) have such situation of setae distribution with 37 pairs of setae on dorsal shield (Figure 8). Note that Costa (1966) considered z2 missing on the dorsal shield of G. angustus (Figure 13 p. 145). The seta z3 typically appears at the deutonymphal stage in Gamasina, and z2 appears at the larval stage. The theory is that setae appearing later during development have higher probabilities of being missing (i.e. and not appearing at all) in the adult stage. In other words, larval setae present in the larval stage tend to be the most stable (i.e. are not often lost later on), and setae appearing at the protonymphal stage are more stable than setae added in the deutonymphal and adult stages (Evans and Till 1965; Lindquist and Evans 1965; Faraji and Halliday 2009). Therefore if there is a need for suppress, z3 is most often considered missing because it appears during development later than z2 and seta z2 has theoretically more chances of being present. Overall setae mentioned as z3, s3, r3, r4, r5 and r6 on dorsal shield of G. angustus in mentioned figure of Costa (1966) should be replaced with z2, z3, s3, r3, r4 and r5 respectively. Seta r6 is located on soft integument in ventral side, slightly posteriorly to s6. Based on above explanation we do not agree with the statement of Costa (1966) concerning the difference between dorsal setae distribution between G. angustus and G. queenslandicus.



As mentioned before, different variations have been observed in populations of G. angustus and G. queenslandicus and intermediate forms which were collected from different parts of Iran and Australia. The lengths of dorsal shield setae in these specimens in addition of G. angustus sensu Costa (1966) and holotype and G. tripodiger shown in Tables (1, 4). The ranges of these measurements for nearly all dorsal shield setae are similar except for z1, s3 and r2 which perhaps with more specimens those also would overlaps.












The deutosternal groove of G. angustus and G. queenslandicus are quite similar (Figure 9), both having the three posterior rows of denticles narrower than the three anterior rows. However, the difference between anterior and posterior rows is stronger for the illustration of G. queenslandicus [as illustrated by Costa (1966)]. Our observations indicate that such difference is not dichotomic among specimens examined, and it is not correlated with other characters previously assigned to G. angustus or G. queenslandicus, such as the shape of the lateral margins of the dorsal shield.Hypostomal setae (h1-3) vary within groups and locations, and broadly overlap between them as in Table (3). According to the specimens, which have been studied herein, these characters states overlap.



The chelicerae of all samples examined, including types of G. angustus, and all specimens from Iran, Australia and Italy, shared the following characteristics (Fig 10): chelate-dentate, with fixed digit bearing 11–13 teeth including a small proximal tooth followed by an enlarged one, then by 6–8 small teeth, ending up with the largest tooth at level of pilus dentilis and two teeth subapically including the offset and most distal tooth (gabelzhan). It bears terminal hook similar to thumb nail (Figures 10A, B), including well-developed gabelzhan. The number and shape of small teeth between the two large teeth on fixed digit varies from six (Figures 10B, C) to eight (Figure 10D) within each groups of angustus-like and queenslandicus-like specimens [based on opisthonotal lateral margins and deutosternal grooves (sensu Costa, 1966)] from Iran and Australia. In some specimens, the number of fixed digit teeth varies between left and right chelicerae (Figures 10B, C). Movable digit of chelicera with two enlarges teeth.



Karg (1979) considered the Gaeolaelaps genus as a subgenus of Hypoaspis s. lat. and divided it into four species-groups based on various attributes. Hypoaspis (Geolaelaps) angustus-Group with posterior end of dorsal shield resembles wedge shape, opisthonotal lacks Zx setae and legs II in female possess spur or spine-like setae, including: H. (G.) queenslandicus, H. (G.) angustus, H. (G.) elongata and H. (G.) angustiscutatus. The last one has conspicuous knob at basal part of dorsal setae and transferred to Cosmolaelaps genus by Nemati and Gwiazdowicz (2016). Karg (1979) in a key to the species of this group separated G. queenslandicus and G. angustus according to the length of their first legs relative to their idiosomal length. So that the first legs of the first species are longer than the length of the idiosoma while in the second one those are shorter. For this purpose we studied some other morphological characters including the sizes of different parts of body.
Presternal area with narrow granulate stripe adjacent to anterior margin of sternal shield and two presternal plates close together basally and with granulated surfaces. Sternal shield surface reticulated throughout, except small medio-posterior portion, anterior margin with variations and in some specimens medially concave, straight or tend to concave antero-laterally.
Some morphological character measurements of Gaeolaelaps angustus (type materials), G. queenslandicus (specimens from Australia), G. angustus-like from Iran, G. queenslandicus-like from Iran, G. tripodiger (type) and male specimens from Iran have been shown in Table (4).
The length and width of sternal shield in G. angustus-like and G. queenslandicus-like populations (specimens from Iran) are larger than the others. The sizes of other characters do not differ among populations.
Epigynal shield in different specimens of G. queenslandicus-like (with different variation in lateral margins of dorsal shield as in Figures 3-5) with different shape and reticulation (Figure 11). Abnormality observed in one specimen with small plate at posterolateral part (Figure 11D). Epigynal shield measurements shown in Table (4).



Palp chaetotaxy is normal for Gamasida (sensu Evans 1963b), palp tarsal claw three-tined. Palp segments and setation were similar in all specimens, which studied here. Anterior margin of epistome is densely denticulate in all members. The length of denticles and the extensions of teeth vary among members of G. queenslandicus-like and G. angustus-like in Iran. In some specimens, there are two long projected teeth with different shapes and different numbers of teeth at apex at lateral margins of epistome (Figure 12). Some specimens have a small protuberance in median part of epistome (Figure 12C).



The corniculi well sclerotised, horn-like with different length [in specimens which were considered as G. angustus and angustus-like (53–60) and for G. queenslandicus and queenslandicus-like (55–69)]; internal malae with two pairs of separated median fringed projections extended beyond the tip of curniculi and one pair of lateral projections fringed and smaller than curniculi. Pilose labrum is longer than median internal malae projections.
Legs Chaetotaxy. The leg chaetotaxy is essentially identical for all specimens that we examined including G. tripodiger type material. The situation of legs II and IV chaetotaxy could be seen in Figure (13) respectively.



The formulae and some explanation concerning all leg chaetotaxy of mentioned species and all relevant forms in Iran are as follows: Leg I: coxa 0 0/1 /1 0; trochanter 1 0/2 1/1 1; femur 2 2/1 3/3 2 (pd3 slightly thicker than the others); genu 2 3/2 3/1 2; tibia 2 3/2 3/1 2. Leg II (Figure 13A): coxa 0 0/1 0/1 0; trochanter 1 0/2 0/1 1; femur 2 3/1 2/2 1(pv1 spine-like and thick); genu 2 3/1 2/1 2 (av slightly thicker than other setae on the segment); tibia 2 2/1 2/1 2 (av and pv thicker than other setae on the segment); tarsus 3 3/2 3/2 3 + mv, md (pl1, al1, pv2, av1–2, md and mv thicker than other setae on the segment). Leg III: coxa 0 0/1 0/1 0; trochanter 1 0/2 0/1 1; femur 1 2/1 1/0 1 (setae pd and pl thicker than the others); genu 2 2/1 2/1 1 (setae av and pv thicker than the others); tibia 2 1/1 2/1 1 (setae av and pv thicker than the others); tarsus 3 3/2 3/2 3 + mv, md (setae mv, av1–2, pv1–2, md, pd2, al1 and pl1 thicker than the others). Leg IV (Figure 13B): coxa 0 0/1 0/0 0; trochanter 1 0/2 0/1 1 (av2 slightly thicker than the other setae on the segment) ; femur 1 2/1 1/0 1 (pd slightly thicker); genu 2 2/1 3/0 1 (av thicker than other setae on segment); tibia 2 1/1 3/1 2 (av, pv and pl2 thicker than other setae on the segment); tarsus 3 3/2 3/2 3 + mv, md (setae md, ad2 and pd3 slightly longer than the others on the segment; al1, pl2, av1–2, pv1–2, mv, md, al3 and pl3 slightly thicker than other setae on the segment). All setae fine and needle-like unless otherwise noted.
Male (Figure 14). Short description of G. queenslandicus male was given by Ryke (1963) and Yan and Ma (1999). In this study, males were collected with different samples from Iran (see materials examined). The morphological characters of males were similar between populations, and that here is a description of the main characters of the male that differ from those of the female.



Idiosoma smaller than in female: dorsal shield length (405-450), dorsal idiosomal length (418-457), dorsal shield width (226-253). Dorsal shield in all male specimens without curvature. This situation could be observed in male specimens, which have been collected with female specimens of G. queenslandicus and queenslandicus-like population. In other words, the changes in the female dorsal shield, seen in different G. queenslandicus-like population, cannot be seen in male specimens. The length of dorsal, some ventral idiosomal setae and hypostomal setae of Gaeolaelaps queenslandicus (Womersley) male shown in Table (5). Sternitogenital, anal and endopodal shields fused in a well-developed holoventral shield (378-396), with anterior margin well defined, prominent at level of genital opening (Figure 14A). Holoventral shield well reticulated, bears 10 pairs of smooth acicular setae (st1–st5, Zv1–Zv2, Jv1–Jv3), including para and post-anal setae. The width of shield at level of st1 setae 86-91, at level of st2 89–91, between coxae II-III 154–156, at level of st3 98–100, widest part slightly posterior to coxae IV 135–170, the distances between st1–st1 (54–61), st2–st2 (64–66), st3–st3 (83–85), the lengths of ventral setae: Zv1–Zv2, Jv1–Jv3 (24–30), post-anal seta (27–30). Movable digit of chelicera with one large tooth, arched spermatodactyl finger-like, longer than movable digit and with rounded tip; fixed digit multidentate (Figures 14C, D). Other morphological characters including Hypostome (Figure 14E), epistome (Figure 14F), legs and palp chaetotaxy as in female.



Our taxonomical studies and analysis on various specimens in Iran which have been previously considered as G. angustus (sensu Karg, 1965) and G. queenslandicus (sensu Costa, 1966), the type materials of G. angustus (Karg collection, Berlin), the information presented in Costa, 1966 concerning holotype of G. queenslandicus resulted in the absence of significant differences in the important morphological traits between these species and, using this information, those (G. angustus sensu Karg, 1965 and G. queenslandicus sensu Costa, 1966) cannot be considered as distinct and valid species. Therefore, these species are considered here as synonyms. Our observations on different morphological characters of type species of G. tripodiger showed no difference with G. angustus. From all this information, we conclude that the two species G. angustus and G. queenslandicus are junior synonyms of G. tripodiger. The original descriptions of G. trifurcatus and G. trifurcatoides show no tangible differences with specimens of G. tripodiger (including specimens previously identified as G. angustus and G. queenslandicus), and therefore are herein considered as junior synonyms.
According to the International Commission on Zoological Nomenclature (1999) Article 23.9, two conditions must be met in order to continue usage of the younger name, i.e., G. queenslandicus. First, the senior synonym must not have been used as valid name after 1899 (Art. 23.9.1). Clearly, this condition is not met for G. tripodiger (Berlese, 1916). Second, the junior synonym has been used as the valid name in at least 25 publications by ten or more authors “in the immediately preceding 50 years and encompassing a span of not less than 10 years” (Art. 23.9.2). This condition has been met for G. queenslandicus. In its Article 23.9.3, the ICZN (1999) states that “If the conditions of 23.9.1 (23.9.1.1 and 23.9.1.2) are not met but nevertheless an author considers that the use of the older synonym or homonym would threaten stability or universality or cause confusion, and so wishes to maintain use of the younger synonym or homonym, he or she must refer the matter to the Commission for a ruling under the plenary power [Art. 81]. While the case is under consideration use of the junior name is to be maintained [Art. 82]. Using the name G. tripodiger for this species would threaten stability, universality or cause confusion (Article 23.9.3). (1) The name G. tripodiger has not been used as valid since its original description; (2) no new taxonomic information about the species has not been used as valid since its original description except in catalogues; (3) no new specimens have been identified as G. tripodiger; (4) the name G. queenslandicus has been used as valid at least 25 times since 1956; (5) Gaeolaelaps queenslandicus has been redescribed very thoroughly (Costa, 1966); (6) many new specimens of G. queenslandicus have been collected from all over the world (Kazemi and Rajaei, 2013; Nemati et al., 2018; for others see taxonomic literature under its name); (7) the name G. queenslandicus has been used for a possible biological control agent (Milne, 1977; Saito, 2013; Saito & Takaku, 2013); (8) apart from G. tripodiger, G. queenslandicus is the oldest available name for this species, so we continue to use G. queenslandicus as the valid name in the interests of stability.
We would like to thank our friends: Dr. Jason A. Dunlop (Museum für Naturkude, Berlin, Germany), Stefan Friedrich and Dr. Roland Melzer from Zoologische Staatssammlung München, and also Dr. Roberto Nannelli and Dr. Sauro Simoni from Istituto Sperimentale per la Zoologia Agraria in Firenze, for making available the type material and M. Ameri from Australia for providing G. queenslandicus specimens. Deeply thanks to Dr. Bruce Halliday (Australia) for his several comments on previous version of this article and two anonymous reviewers for their numeral constructive comments.
Beaulieu F. 2009. Review of the mite genus Gaeolaelaps Evans & Till (Acari: Laelapidae), and description of a new species from North America, G. gillespiei n. sp. Zootaxa, 2158: 33-49.
Berlese A. 1916. Centuria seconda di Acari nuovi. Redia, 12: 125-177.
Bregetova N.G. 1977. Family Laelaptidae Berlese, 1882. In: Ghilyarov M.S., Bregetova N.G. (Eds.), Key to the Soil Inhabiting Mites. Mesostigmata. Leningrad: Akademia Nauka. p. 486–556.
Canestrini G. 1884. Acari nuovi o poco noti. I. Acari Italiani. II. Acari dellAustralia. Atti ist. Veneto, 2(6): 693-724.
Casanueva M.E. 1993. Phylogenetic studies of the free-living and arthropod associated Laelapidae (Acari: Mesostigmata). Gayana Zool., 57(1): 21-46.
Costa M. 1966. A redescription of Hypoaspis queenslandicus Womersley, 1956, comb. nov. Acari Mesostigmata with notes on the genus Hypoaspis Canestrini. Isr. J. Zool., 15: 141-147.
Evans G.O. 1963a. Observations on the chaetotaxy of the legs in the free-living Gamasina (Acari: Mesostigmata). Bull. Br. Mus. (Nat. Hist.), Zool., 10(5): 277-303.
Evans G.O. 1963b. Some observations on the chaetotaxy of the pedipalps in the Mesostigmata (Acari). Ann. Mag. Nat. Hist. (Ser. 13), 6: 513-527. doi:10.1080/00222936308651393 ![]()
Evans G.O., Till W.M. 1965. Studies on the British Dermanyssidae (Acari: Mesostigmata). Part 1. External morphology. Bull. Br. Mus. (Nat. Hist.), Zool., 13: 247-294.
Evans G.O., Till W.M. 1966. Studies on the British Dermanyssidae (Acari: Mesostigmata). Part II. Classification. Bull. Br. Mus. (Nat. Hist.), Zool., 14(5): 109-370.
Evans G.O., Till W.M. 1979. Mesostigmatic mites of Britain and Ireland (Chelicerata: Acari-Parasitiformes). An introduction to the external morphology and classification. Trans. Zool. Soc. Lond., 35: 139-270. http://dx.doi.org/10.1111/j.1096-3642.1979.tb00059.
Faraji F., Abedi L., Ostovan H. 2008. A new species of Hypoaspis Canestrini from Iran with a key to the Iranian species of Hypoaspis (Acari, Gamasina, Hypoaspididae). Zoosystematics Evol., 84; 205-209. doi:10.1002/zoos.200800005 ![]()
Faraji F., Halliday B. 2009. Five new species of mites (Acari: Laelapidae) associated with large Australian cockroaches (Blattodea: Blaberidae). Internat. J. Acarol, 35: 245-265. doi:10.1080/01647950903059445 ![]()
Farrier M.H., Hennessey M.K. 1993. Soil-inhabiting and free-living Mesostigmata (Acari-Parasitiformes) from North America. An annotated checklist with bibliography and index. North Carolina Agricultural Research Service Technical Bulletin, 302: 1-408.
Hafez S.M., Elbadry E.A., Nasr A.K. 1982. Soil Mites of the Family Laelapidae from Egypt (Acari: Mesostigmata). Res. Bull. Ain Shams Univ. Faculty of Agriculture, 1759: 1-14.
Hunter P.E. 1993. Mites associated with new world passalid beetles (Coleoptera: Passalidae). Acta Zool. Mex. Nueva Ser., 58: 1-37.
Hyatt K.H. 1964. A collection of Mesostigmata (Acari) associated with Coleoptera and Hemiptera in Venezuela. Bull. Br. Mus. (Nat. Hist.), Zool., 11: 465-509.
ICZN. 1999. International Code of Zoological Nomenclature. Fourth edition, adopted by the International Union of Biological Sciences. International Trust for Zoological Nomenclature, The Natural History Museum, London.
Karg W. 1965. Larvalsystematische und phylogenetische Untersuchung sowie Revision des Systems der Gamasina Leach, 1915 (Acarina, Parasitiformes). Mitt. Zool. Mus. Berl., 41: 193-340. doi:10.1002/mmnz.19650410207 ![]()
Karg W. 1971. Acari (Acarina), Milben. Unterordung Anachtinichaeta (Parasitiformes). Die freilebenden Gamasina (Gamasides), Raubmilben. In: Dahl, F., Peus,, F. Die Tierwelt Deutschlands. G. Fisher, Jena, 1-475.
Karg W. 1978. Zur Kenntnis der Milbengattungen Hypoaspis, Androlaelaps und Reticulolaelaps (Acarina, Parasitiformes, Dermanyssidae). Zool. Jahrb. Syst., 105: 1-32.
Karg W. 1979. Die Gattung Hypoaspis Canestrini, 1884 (Acarina, Parasitiformes). Zool. Jahrb. Syst., 106: 65-104.
Karg W. 1982. Zur Kenntnis der Raubmilbengattung Hypoaspis Canestrini, 1884 (Acarina, Parasitiformes). Mitt. Zool. Mus. Berl., 58: 233-256.
Karg W. 1989. Zur Kenntnis der Untergattungen Geolaelaps, Alloparasitus und Laelaspis der Raubmilbengattung Hypoaspis Canestrini, 1884 (Acarina, Parasitiformes). Mitt. Zool. Mus. Berl., 65: 115-126. doi:10.1002/mmnz.19890650103 ![]()
Karg W. 1993a. Acari (Acarina), Milben Parasitiformes (Anactinochaeta) Cohors Gamasina Leach, Raubmilben. Die Tierwelt Deutschlands, 59: 1-523.
Karg W. 1993b. Raubmilben der Hypoaspididae, Laelapidae und Phytoseiidae auf dem Galapagos-Archipel (Acarina, Parasitiformes). Mitt. Zool. Mus. Berl., 69(2): 261-284. doi:10.1002/mmnz.19930690207 ![]()
Karg W., Schorlemmer A. 2013. Origin of five unique mite-genera in geological periods com¬pared to other group of Gamasina (Acarina, Parasitiformes) and description of two new species of Rykellus Lee and Oloopticus Karg. Zoosystematics Evol., 89: 193-207. doi:10.1002/zoos.201300006 ![]()
Kavianpour M., Nemati A. 2014. Gaeolaelaps (Acari: Laelapidae) mites of Iran with description of a new species. J. Crop. Prot., 3(3): 315-325.
Kavianpour M., Nemati A., Gwiazdowicz D.J., Kocheili F. 2013. A new species of the genus Gaeolaelaps (Acari, Mesostigmata, Laelapidae) from Iran. ZooKeys, 277: 1-11.
Kazemi Sh., Rajaei A. 2013. An annotated checklist of Iranian Mesostigmata (Acari), excluding the family Phytoseiidae. Persian J. Acarol., 2(1), 63-158.
Kazemi Sh., Rajaei A., Beaulieu F. 2014. Two new species of Gaeolaelaps (Acari: Mesostigmata: Laelapidae) from Iran, with a revised generic concept and notes on significant morphological characters in the genus. Zootaxa, 3861(6): 501-530. doi:10.11646/zootaxa.3861.6.1 ![]()
Li C., Yang X.Z., Zhang W.S. 1998. A new species of Androlaelaps from Qinghai Province, China (Acari: Laelapidae). Acta. Zootaxon. Sin, 23(3): 264-266.
Lindquist E.E., Evans G.O. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Can., 47: 1-64. doi:10.4039/entm9747fv ![]()
Lindquist E.E., Krantz G.W., Walter D.E. 2009. Order Mesostigmata. In : Krantz G.W., Walter D.E. (Eds.). A manual of Acarology (3rd ed.). Texas: Texas Tech University Press. p. 12-233.
Ma L. 1996. Three new species of the genus Hypoaspis from Jilin Province, China (Acari: Laelapidae). Acta. Zootaxon. Sin., 21: 48-54.
Milne D.L. 1977. Biological control of citrus thrips, Scirtothrips aurantii: what are the prospects? Citrus and Subtropical Fruit Journal, 16: 16-14.
Moreira G.F. 2014. Taxonomic Studies of Laelapid Mites (Acari: Mesostigmata: Laelapidae) and Their Use in Combination with Entomopathogenic Nematodes (Rhabditida: Steinernematidae, Heterorhabditidae) to Control Frankliniella occidentalis (Thysanoptera: Thripidae). Ph.D. thesis, Universidade Estadual Paulista – Unesp Campus de Jaboticabal. 522 pp.
Nasr A.K., Nawar M.S. 1989. Hypoaspis cucumerus, a new species of laelapid mites from Egypt (Acari, Mesostigmata: Laelapidae). Bull. Soc. Entomol., 68: 69-74.
Nawar M.S., Shereef G.M., Ahmed M.A. 1993. Influence of food on development and reproduction of Hypoaspis solimani (Acari: Laelapidae). Insect Sci. Appl., 14: 343-349. doi:10.1017/S1742758400014831 ![]()
Nemati A., Gwiazdowicz D.J. 2016. Description of a new species of Cosmolaelaps Berlese and the male of C. brevipedestra (Karg) from Iran, with notes on some other species of Cosmolaelaps Berlese (Acari: Laelapidae). Zootaxa, 4066(5): 535-551. doi:10.11646/zootaxa.4066.5.2 ![]()
Nemati A., Kavianpour M. 2013. A new species of Laelapidae (Acari: Mesostigmata) from Iran. J. Crop. Prot., 2(1): 63-73.
Nemati A., Mohseni M. 2013. Two new species of Gaeolaelaps (Acari: Laelapidae) from Iran. Zootaxa, 3750(1): 71-82. doi: 10.11646/zootaxa.3750.1.5 doi:10.11646/zootaxa.3750.1.5 ![]()
Nemati A., Riahi E., Khalili-Moghadam A., Gwiazdowicz D.J. 2018. Catalogue of the Iranian Mesostigmata (Acari): the additions and updates of the previous catalogue. Persian J. Acarol., 7(2): 115-191. doi.org/10.22073/pja.v7i2.36985
Ren T-G., Guo X-G. 2008. Preliminary study on Laelapidae fauna in China (Acari: Gamasida: Laelapidae). Chin. J. Vector Bio. & Control, 19(4): 322-326.
Rosario R.M.T. 1981. Philippine Hypoaspidinae (Acarina: Mesostigmata: Laelapidae). Philip. Entomol. 5: 23-82.
Ruf A., Koehler H. 1993. Hypoaspis fishtowni sp. nov. (Acari, Mesostigmata, Laelapidae): a new predatory mite. Acarologia, 34: 193-198.
Ryke P.A.J. 1963. Some free-living Hypoaspidinae (Acari: Mesostigmata) from South Africa. Rev. Biol., 5: 1-15.
Saeidi Z., Nemati A., Khalili-Moghadam A. 2016. Description of a new species of Gaeolaelaps (Acari: Laelapidae) from Iran. ZooKeys, 612: 31-40. doi:10.3897/zookeys.612.9678 ![]()
Saito M. 2013. Studies of Techniques to Control the Acarid Mite, Tyrophagus similis Volgin (Acari: Acaridae) by Using Indigenous Predatory Mites (Acari: Gamasina) in Spinach Greenhouses. Report of Hokkaido Research Organization Agricultural Experiment Station, 1-81.
Saito M., Takaku G. 2013. Predation of Tyrophagus similis Volgin (Acari: Acaridae) by indigenous predatory mites (Acari: Gamasina) found in spinach fields. J. Acarol. Soc. Japan, 22, 37-43. doi:10.2300/acari.22.37 ![]()
Tenorio J.M. 1982. Hypoaspidinae (Acari: Gamasida: Laelapidae) of the Hawaiian Islands. Pacific Insects, 24: 259-274.
Trach V.A. 2012. Gaeolaelaps carabidophilus n. sp., a new mite species (Acari: Mesostigmata: Laelapidae) from carabid beetles (Coleoptera: Carabidae) from southern Ukraine. Acarologia, 52(2): 157-163. doi:10.1051/acarologia/20122045 ![]()
Van Aswegen P.I.M., Loots G.C. 1970. A taxonomic study of the genus Hypoaspis Canestrini sens. lat. (Acari: Laelapidae) in the Ethiopian Region. Publicações Culturais da Companhia de Diamentes de Angola, 82: 169-213.
Vatankhah F., Nemati A., Esfandiari M., Shishehbor P. 2016. Description of a new species of Gaeolaelaps (Acari: Laelapidae) from Iran, with a key to world species of the genus with short peritremes. Zootaxa, 4121(5): 566-574. doi:10.11646/zootaxa.4121.5.6 ![]()
Walter D.E., Oliver J.H. 1989. Geolaelaps oreithyiae, n. sp. (Acari: Laelapidae), a thelytokous predator of arthropods and nematodes, and a discussion of clonal reproduction in the Mesostigmata. Acarologia, 30: 293-303.
Wang D.C., Li K.C. 1965. On some new species of Gamasoidea. Act. Zootaxon. Sin., 2: 233-242.
Wang D-Q., Liao H-R. 2000. Dermanyssoidea (Acari: Mesostigmata). In: Fauna of Insects, Fujian Province of China. Fujian Scientific and Technical Press, Fuzhou, 1-45.
Womersley H. 1956. On some new Acarina–Mesostigmata from Australia, New Zealand and New Guinea. J. Linn. Soc. Lond. Zool., 42: 505-599. doi:10.1111/j.1096-3642.1956.tb02218.x ![]()
Yan J.Z., Ma L.M. 1999. A new species of the genus Androlaelaps from Hubei, China (Acari: Gamasina: Laelapidae). Act. Zootaxon. Sin. 24(2): 149-152.
Zeman P. 1982. Two new species of Hypoaspidinae (Acari: Mesostigmata: Dermanyssidae) associated with ants. Vestn. Cesk. Spol. Zool., 46: 231-237.



2017-12-21
Date accepted:
2018-05-02
Date published:
2018-07-24
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2018 Nemati, Alireza; Gwiazdowicz, Dariusz J. and Khalili-Moghadam, Arsalan
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



