The Tenuipalpidae (Acari: Trombidiformes) of Israel
Ueckermann, Edward A. 1 ; Palevsky, Eric2 ; Gerson, Uri3 ; Recht, Eitan4 and Theron, Pieter D.5
1✉ Research Unit for Environmental Sciences and Management, North-West University, Potchefstroom Campus 2520, South Africa.
2Department of Entomology, Newe-Ya’ar Reseach Center, Agricultural Research Organization (ARO), P.O. Box 1012, 30095 Ramat Yishay, Israel.
3Department of Entomology, The Robert H. Smith Faculty of Agriculture, Food and Environment, POB 12, Rehovot 76100, Israel.
4Department of Diagnosis and Identification of Pests and Diseases, Plant Protection and Inspection Services, POB 78 Bet Dagan 50250, Israel.
5Research Unit for Environmental Sciences and Management, North-West University, Potchefstroom Campus 2520, South Africa.
2018 - Volume: 58 Issue: 2 pages: 483-525
https://doi.org/10.24349/acarologia/20184255ZooBank LSID: AABAF96C-DA66-4BF7-BE62-9596C4FFE347
Keywords
Abstract
The flat mite family Tenuipalpidae (Trombidiformes) contains many species that are world-wide major plant pests (Mesa et al., 2009). Studies of the flat mites of Israel were initiated by Bodenheimer (1930, 1937), reporting the first two tenuipalpids, Cenopalpus pulcher (Canestrini & Fanzago) (= Tenuipalpus bodenheimeri Berlese) and Cenopalpus spinosus Donnadieu (= Tenuipalpus geisenheyeri Ruebsaamen) from Palestine. Twenty-one years later four more species were added, namely Brevipalpus californicus (Banks) (= B. browning Baker), B. obovatus Donnadieu, Obdulia tamaricis Pritchard & Baker and Tenuipalpus punicae Pritchard & Baker (Pritchard & Baker, 1958). Bytinski-Salz (1966) reported two new species to the Israel fauna, namely Brevipalpus phoenicis (Geijskes) and Brevipalpus lewisi (McGregor). A year later Sternlicht & Golan (1967) recorded Tenuipalpus granati Sayed from Israel. Dosse (1974) reported Cenopalpus lineola (Canestrini & Fanzago) from Haifa. After 1980 sixteen species were described or reported as new to the Israel fauna: Aegyptobia eremia Meyer & Gerson, A. salixi Zaher & Yousef, Brevipalpus oleae Baker, B. olearius Sayed, B. recki Livschits & Mitrofanov, B. yothersi Baker, Cenopalpus halperni Castagnoli, C. lanceolatisetae (Attiah), C. wainsteini (Livschits & Mitrofanov), Capedulia maritima Gerson & Smith Meyer, Dolichotetranychus australianus (Womersley), Phyllotetranychus aegyptiacus Sayed, Tenuipalpus cupressoides Smith Meyer & Gerson, T. dubinini Reck and T. pareriophyoides Smith Meyer & Gerson (Gerson & Smith Meyer, 1980; Smith Meyer & Gerson,1981; Halperin et al., 1989; Klein & Zarabi, 2011; Castagnoli, 1987; Beard et al., 2015). Brevipalpus yothersi Baker, synonymized by Pritchard & Baker (1952) with B. phoenicis, was one of two species resurrected and redescribed by Beard et al. (2015a) after examining two females on guava fruit from Israel, intercepted in Washington DC, in 1985 and in Chicago, USA (no date).
The number of flat mite species recognized in Israel has grown over the past 35 years, due to the increase in the importation of exotic plant material, along with the introduction and establishment of new crops and of new varieties of traditional crops. Of the local species about 70% are exotics, and 30% are pests of local crops.
This paper presents an annotated list of 26 flat mite species identified from agricultural systems and indigenous plants in Israel. Hosts, distributional data, and biological notes are appended, and a key to the Israeli species is provided.
General information from abroad is based on Mesa et al. (2009), whereas additional data on the local fauna is based on Klein & Zarabi (2011). These observations and the fact that many of the indigenous shrubs and trees have not yet been surveyed for tenuipalpids suggest that the species listed here represent only a part of the local flat mite fauna. The species are listed alphabetically, genus and species.



Seventeen of the 26 species noted here were figured from specimens in the National Collection of the Biosystematics Division, ARC-Plant Protection Research Pretoria, South Africa, namely Aegyptobia eremica, Brevipalpus californicus, B. lewisi, B. obovatus, B. phoenicis, B. yothersi, Capedulia maritima, Cenopalpus lanceolatisetae, C. pulcher, C. spinosus, Dolichotetranychus australianus, Raoiella indica, Tenuipalpus cupressoides, T. granati, T. pareriophyoides and T. punicae by the senior author while still in their employ. Some of the latter and B. lewisi, B. olearius and C. lineola were also based on material used for a study on the Turkish Tenuipalpidae (Çobanoğlu et al., 2016). The rest of the species’ figures are reconstructions from the original descriptions. Beard et al. (2015b) were also consulted to verify the identity of some species. For all figures photos of specimens and scans of figures from descriptions were taken and illustrated with a Zeiss AxioskopTM Research microscope equipped with a Zen Soft Imaging System. The photos included here are of specimens collected in Israel and where taken at the Plant Protection and Inspection Services, Israel using both a Hirox RH-2000 digital and an Olympus BX62 optical microscope.
Prostigmata, Kramer, 1877: 219
Tetranychoidea, Donnadieu, 1875: 9
Tenuipalpidae, Berlese, 1913: 17
Aegyptobia Sayed, 1950
Both this genus and Phytoptipalpus Trägardh have 13 pairs of opisthosomal setae (rarely 12), 3 pairs of dorsocentral (c1, d1, e1), 4 pairs of dorsosublateral (c2, d2, e2, f2) (Fig 2A) and 6 pairs of marginal setae (c3, d3, e3, f3, h2, h1). However, Phytoptipalpus differs from Aegyptobia only in that the former has 2 pairs of anal setae (ps1-2) (Fig 2C) instead of 3 pairs and in that its adult members have 3-4 pairs of legs, 4 pairs in Aegyptobia. Palp 5-segmented (Fig 1H). Leg tarsi with claws either uncinate or padlike. Ventral shields not clearly outlined.
Diagnosis (Female) — Dorsum reticulated, anteriorly indented; all dorsal setae smooth, broad and with obscure transverse subdivisions, opisthosoma with one pair of pores posterior to setae d2 (Figs 2A-B); rostrum reaches to about mid-genu I; femora I-III and genua I-II each with one lanceolate seta, rest setiform; ventral setae 3a and 4a short, less than half the distance separating them (Fig 2C); legs with true claws uncinated.



Deutonymph — Dorsal setae as in female; prodorsum with indistinct shield; opisthosoma with transverse striae on anterior half and longitudinal striae on posterior half.
Hosts and locality. Described from Hammada scoporia (Pomel) Iljin and Salsola sp. (Chenopodiaceae), Yeroham, and Nahal Boqer, Israel.
Symptoms — Unknown.
Diagnosis (Female) — Prodorsum with more or less even reticulations medially, rounded anteriorly, opisthosoma provided with a few transverse striae; rostrum extends to about distal end of genu I; dorsal body setae are broadly lanceolate and serrate (Fig 3); femora I-II each with one lanceolate serrate seta as long as width of segment; legs with true claws uncinated.



Host and localities — Described from Salix alba L. (Salicaceae), Cairo, Giza, El-Sharkia El-Menia, Egypt; Israel, Lake Galilee, (Smith Meyer & Gerson,1981), same host.
Symptoms — Unknown.
Members of this genus have 3 pairs of prodorsal setae (v2, sc1, sc2), 7-10 pairs of opisthosomal setae consisting of: 1-3 pairs of dorsocentral setae (c1, d1, e1) and 6-7 pairs of dorsolateral setae (c3, d3, e3, f2, f3, h2, h1, with f2 absent or present), sublateral setae c2 absent (Fig 4A). Setae h2 not exceptionally long. Tarsus II with one or 2 solenidia distally (Fig 4D). Palp 4-segmented (Fig 1G). Ventral shields clearly demarcated (Fig 4B).
Diagnosis (Female) — Prodorsum with even reticulations and short serrate setae, opisthosoma between setae c1 and d1 and d1 and e1 wrinkled with a few V-shaped folds behind setae e1, reticulations mediodorsally, with 7 pairs of short, serrate marginal setae (including f2) and 3 pairs of dorsocentral setae apparently smooth (Fig 4A); rostrum extends to about middle of femur I; tarsus II with 2 solenidia distally (Fig 4D); genital shield with large reticulations, connected to form transverse bands, and ventral shield with smaller reticulations also forming transverse bands (Fig 4B); spermatheca terminates into a round vesicle with a crown of short finger-like projections, vesicle with a semi-lunar “bubble” internally (Fig 4C).



Host and localities — From a wide range of hosts, but mainly citrus from: Australia, Ecuador, Israel, Italy, Kenya, Mexico, Nepal, Palestine, Peru, South Africa, Spain, Sri Lanka, Thailand, USA (Beard et al., 2015b).
Symptoms — Can cause chlorosis, blistering, or necrotic areas on leaves. In the New World, it is strongly associated with the nuclear citrus leprosis viruses, citrus leprosis virus (Roy et al., 2015).
Diagnosis (Female) — Prodorsum weakly to strongly wrinkled or folded medially, can also appear like areolae, laterally with elongated cells forming a reticulation, opisthosoma smooth or wrinkled between setae c1-c1 and e1-e1 with weak to strong V-shaped folds posterior to e1-e1, 3 pairs of dorsocentral setae different in shape to dorsolateral setae (f2 present) (Fig 5A); spermatheca terminating into a small rounded vesicle with a series of short projections and clear internal “bubble” (Fig 5C); genital and ventral shields with reticulations forming transverse bands (Fig 5B); palp femur seta slender, tapered and barbed; tarsus II with one solenidion (Beard et al., 2015b; Hao et al., 2016).



Deutonymph — Prodorsum with setae sc1-2 broadly lanceolate. Opisthosoma with setae c3 and the 4 pairs of caudolateral setae (f2, f3, h2, h1) broadly lanceolate and about as long as intervals between them, rest minute and smooth or slightly serrate (Fig 5D) (Beard et al., 2015b).
Hosts and localities — In Israel it was collected from “Blue Serbian vines”, at Nachshonim (Bytinski-Salez, 1966), but could not be found again till recently in Mizpe Ramon on petit vardo leaves. Other overseas hosts are: lemon, walnut, Boston ivy, grape, pistachio, pomegranate, Metasequoia (Dawn Redwood) and many ornamental plants (Jeppson et al., 1975). It was also reported from Algeria, Australia, Bulgaria, Canada, China, Egypt, Greece, India, Iran, Japan, Lebanon, Mexico, Taiwan, Turkey, USA and former Yugoslavia (Attiah, 1956; Baker, 1949; Baker & Tuttle,1964, 1987; Ehara, 1956; Hatzinikolis, 1982, 1986, 1987; Khosrowshahi & Arbabi,1997; Ma & Yuan,1977; McGregor,1949; Smith Meyer,1979; Mitrofanov & Strunkova,1979; Pritchard & Baker,1952, 1958; Sadana,1997; Smiley & Gerson,1995; Tseng,1974; Hao et al., 2013).
Symptoms — This mite is an important pest of oranges, tangerines and lemons in California (USA) and Japan. Serious injury has not been recorded on grape fruit. The mites lay their red, oval eggs singly in depressions and crevices of fruit, twigs and leaves, apparently preferring any stage of fruit to twigs or leaves. They seem to prefer the stem end of citrus fruit near or under the fruit button and fruit depressions and cause a silvering of the fruit. In central California, they overwinter in the adult stage. Peak populations are reached during the warmest months. Feeding on fruit produces scab-like scars, starting from fruit depressions or from depressions produced by leafhoppers or other insects, or by any injury that ruptures the oil sacs in the citrus peels. As the mites continue to feed, the scabbed area may increase to cover most of the fruit. Damage results in reducing the grade of fruit (Reuther, 1989; Jeppson et al., 1975; Elmer & Jeppson, 1957). In Greece, it is a pest of citrus, grapes, and pomegranates (Hatzinikolis, 1986). Bytinski-Salez (1966) reported it from “Blue Serbiam” vines in 1952 in Israel, causing severe leaf chlorosis. It was recently collected again from under side of petit vardo leaves infested with Tetranychus turkestani in Mizpe Ramon. It is not known as a vector of viruses (Elmer & Jeppson, 1957).
Biology and Ecology — Overwinters in adult stage on deciduous host plants such as grapes in California and is active throughout the year on citrus. Peak populations occur during warmest months (Rice & Weinberger, 1981). Number of annual generations is related to the type of host, for example 4 on vines in Bulgaria and California. It reproduces by thelytoky, as no males were found (Buchanan et al., 1980).
Diagnosis (Female) — Prodorsum mostly smooth centrally, strongly reticulate laterally with large cells posteriorly and smaller cells anteriorly, setae short, serrate; opisthosoma between setae c1 and e1 weakly reticulate to weakly wrinkled, cuticle between and posterior to setae e1 with short transverse folds, setae f2 absent, with marginal setae short, serrate and 3 pairs of dorsocentral setae apparently smooth (Fig 6A); genital and ventral shields colliculate (having small elevations) (Fig 6B); rostrum extends to about middle of femur I; palp tarsus II with one solenidion distally; spermatheca terminates into a round vesicle with short finger-like projections around entire perimeter (Fig 6C).



Deutonymph — Prodorsum with setae sc1-2 short but clearly longer than v2, opisthosoma also with setae short but c1, d1, d3 and e1 shorter than c3, e3, f3, h1-2 (Fig 6D).
Hosts and localities — On a wide range of hosts, but mainly citrus from: Argentina, Australia, Brazil, Canada, Chile, China, Colombia, Costa Rica, Democratic Republic of Congo, Ecuador, France, Germany, Hungary, India, Iran, Italy, Jamaica, Japan, Korea, Mauritius, Mexico, Pakistan, Papua New Guinea, Thailand, The Philippines, South Africa, Taiwan, USA, Venezuela (Beard et al., 2015b). In Israel on many host plants, from Nes Ziona, Miqwe Israel, Jerusalem and Urim (Smith Meyer & Gerson, 1981).
Symptoms — This species may cause chlorosis, blistering, or necrotic areas on citrus leaves. It is strongly associated with the nuclear citrus leprosis viruses in the New World, citrus leprosis virus N (CiLV-N) and citrus necrotic spot virus (CiNSV) (Roy et al. 2015).
Diagnosis (Female) — Dorsum rugose-reticulate medially and striate-granular laterally. The dorsal setae short, narrowly lanceolate and serrate. Three pairs of dorsocentral setae and f2 present (Fig 7A). Venter smooth medially, from ventral shield to gnathosoma but laterally with reticulations and granules. Ventral, genital, and anal shields striate (Fig 7B). Dorsal setae on genua I-II and femora I-III broadly lanceolate and serrate. Spermatheca a very long, slender, coiling tube terminating into a large, prominent hairy bulb (Fig 7C); tarsus II with one solenidion distally; two setae on trochanter III and one seta on trochanter IV; rostrum reaches to distal end of genu I. This species and B. olearius are similar but differs only in trochanters III and IV with 2 and one setae, respectively, in B. oleae, as opposed to one and without setae in B. olearius.



Deutonymph — Dorsal setae serrate and short, except for setae sc2, c3, f3 and h1 which are clearly longer and lanceolate (Fig 7D). The deutonymphs of this species and that of B. olearius also differ as depicted (Figs 7D, 8D).
Hosts and localities — Olea europaea L. (Oleaceae). Described from Morocco (Baker, 1949), but also reported from Portugal, Greece, Italy and Israel (Klein & Zarabi, 2011).
Symptoms — Feeds on the bark of olive trees (Baker, 1949)
Diagnosis (Female) — Resembles B. oleae in all respects except for the number of setae on trochanters III and IV and the dorsal setal arrangements of the deutonymphs. Dorsum irregularly reticulate-striate medially and striate-granular laterally. Dorsal body setae short, narrowly lanceolate and serrate. Three pairs of dorsocentral setae and f2 present (Fig 8A). Venter smooth medially from ventral shield to gnathosoma, but laterally with reticulations and granules. Ventral, genital, and anal shields striate (Fig 8B). Dorsal setae on genua I-II and femora I-III broadly lanceolate and serrate. Spermatheca a very long, slender, coiling tube terminating into a large, prominent hairy bulb (Fig 8C); tarsus II with one solenidion distally; one seta on trochanter III and trochanter IV without setae; rostrum reaches to distal end of genu I.



Deutonymph — Setae sc2, c3, d3, e3, f3 and h1 longest, broadly lanceolate, and serrate; setae v2, sc1, c1, d1, e1, f2, h2 much shorter and serrate (Fig 8D).
Hosts and localities — Olea europaea L. (Oleaceae). Described from Egypt by Sayed, 1950, but also reported from Crimea, Ukraine, Greece, Iran, Italy, Libya, Turkey, and Israel (Castagnoli & Pegazzano, 1979; Hatzinikolis, 1986; Smith Meyer & Gerson, 1981; Khanjani et al., 2013).
Symptoms — Unknown.
Diagnosis (Female) — Prodorsum centrally with strong areolae, mediolateral reticulations with large round cells posteriorly, small round cells anteriorly, but smooth near anterior margin, setae short serrate; opisthosoma between setae c1 and d1 strongly wrinkled to weakly areolate, cuticle between d1 and e1 strongly wrinkled with few transverse folds, between e1 and h1 with transverse folds, mediolateral reticulations with large round cells; transverse folds behind setae e1, setae f2 absent, marginal and 3 pairs of dorsocentral setae short, serrate (Fig 9A); rostrum extends to about middle of femur I; venter verrucose laterally behind 4a with transverse bands centrally becoming weaker to smooth towards 4a, ventral shield with cells forming transverse bands, genital shield covered with large cells (Fig 9B); palp tarsus II with 2 solenidia distally, palp femorogenu with dorsal setae broad, flat, serrate; spermatheca terminates into a membranous bulb (Fig 9C).



Deutonymph — Prodorsal setae v2 short and sc1-2 broadly lanceolate; opisthosomal setae c1, d1, d3, e1, e3 minute, setae c3, f3, h1, h2 large and broadly lanceolate (Fig 9 D).
Host and localities — Material for the designation of a neotype was collected from Phoenix canariensis Chabaud (Arecaceae), The Netherlands. However, it is also present in Turkey, New Zealand, Brazil, and South Africa. Its presence in Israel remains to be determined (Personal communication between Dr. Jenny Beard and Prof. Uri Gerson).
Symptoms — Its main economic damage is to smaller orange fruits. In Italy, its feeding resulted in grayish scabby patches and in medial cracks on the apical epidermis of mandarins. Many lesions were located on the oil glands, which were emptied and dried; affected oranges show rounded reddish patches. In heavy infestations, the mite attacks all above-ground citrus parts, causing the leaves to turn brownish, dry and drop (Gerson, 2017).
Diagnosis (Female) — Dorsum reticulated, prodorsum with setae, broadly lanceolate, serrate and covered with large cells; opisthosoma with one pair of broadly lanceolate dorsocentral setae (c1), setae d1, e1 and f2 absent; dorsolaterals c3 and d3 broadly lanceolate, rest setiform, opisthosoma mostly reticulate with large cells, cuticle medially with short transverse folds (Fig 10A); solenidia on tarsi I and II longer or about as long as width of segments, each with only one solenidion (Fig 10B); rostrum reaches to middle of femur I; palp femur seta thin, tapered, weakly barbed.



Deutonymph — Prodorsum with setae v2, sc1-2 broadly lanceolate; opisthosoma with all setae broadly lanceolate, except for h1 which are very short (Fig 10C).
Hosts and localities — Quercus ithaburensis Decaisne (Fagaceae), Benyamina, Israel (Smith Meyer & Gerson, 1981). Described from beech and Inula conyza (Griess.) DC (Asteraceae) (formerly known as Inula vulgaris), from the Ukraine (Livschitz & Mitrofanov, 1967). Also reported from Greece (Hatzinikolis, 1987).
Symptoms — Unknown.
Diagnosis (Female) — Prodorsum centrally with strong areolae, posteriorly reticulation with large cells, anteriorly weakly reticulate, with setae short and serrate, setae v2 shortest; opisthosoma between setae c1 and d1 smooth to weakly reticulate, cuticle between d1 and e1 weakly reticulate or wrinkled, between e1 and h1 with strong V-shaped folds, mediolateral with reticulations, transverse folds behind setae e1; setae f2 absent with marginal setae short, serrate, 3 pairs of short, serrate dorsocentral setae (Fig 11A); ventral and genital shields verrucose and verrucose-reticulate, respectively (Fig. 11B); rostrum extends to about middle of femur I; palp tarsus II with 2 solenidia distally; palp femorogenu with dorsal seta narrow, serrate; spermathecal terminates into a sclerotised oval vesicle with a thick distal stipe (Fig 11C).
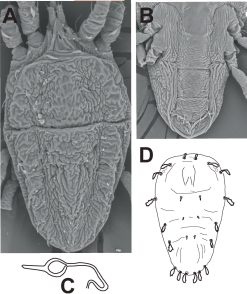


Deutonymph — Prodorsal setae v2 and sc1-2 broadly lanceolate, but with v2 short; opisthosomal setae c1, d1, e1 minute, setae c3, d3, e3, f3, h1, h2 large and broadly lanceolate, but setae c3, d3 and e3 can vary from short to large (Fig 11D).
Host and localities — From a wide range of hosts, but mainly citrus from: Argentina, Australia, Bangladesh, Brazil, Burma, China, Colombia, Costa Rica, Cuba, Democratic Republic of Congo, Dominican Republic, Ecuador, El Salvador, Ethiopia, France, Guatemala, Honduras, India, Indonesia, Israel (passion fruit), Malaysia, Mexico, Nigeria, Pakistan, Puerto Rico, Spain, Sri Lanka, Thailand, The Philippines, Trinidad, South Africa (probably, not confirm yet), Venezuela (Beard et al. 2015a). Beard et al. (2015a) examined 2 females on guava fruit from Israel that were intercepted in Washington DC, in 1985 and in Chicago (no date).
Symptoms — Large numbers found on passion fruit in Israel, causing substantial epidermal blemishing to fruits and stems.
This genus resembles the genera Obdulia Pritchard & Baker, Larvacarus Baker & Pritchard and Obuloides Baker & Tuttle in the reduction of palpi (Fig 1A). Capedulia and Obdulia are more closely related but the former differs from the latter in that setae c2 are present (Fig 12); 3 pairs of prodorsal setae (v2, sc1, sc2), opisthosoma with 10 pairs of setae, comprising 3 pairs of dorsocentral (c1, d1, e1), 6 pairs of lateral (c3, d3, e3, f3, h2, h1) and one pair of sublateral setae (c2); leg tarsi with padlike claws; palp one-segmented (Fig 1A). Ventral shields indistinct.
Diagnosis (Female) — All dorsal setae are very long and serrate; dorsum coarsely striate, most striae with small tubercles (Fig 12); venter with genital shield with transverse striae and 2 pairs of setae (g1-2), one pair of aggenital setae (ag) anterior to this shield, and anal shield with 2 pairs of setae (ps); rostrum reaches to middle of tibia I.



Symptoms — Unknown.
Members of this genus bear 3 pairs of prodorsal setae (v2, sc1, sc2), 10-11 pairs of opisthosomal setae, comprising 3 pairs of dorsocentral (c1, d1, e1), 6-7 pairs of dorsolateral (c3, d3, e3, f2, f3, h2, h1, f2 absent or present) and one pair of sublateral setae (c2); setae h2 not exceptionally long (Fig 13A). Palp 4-segmented (Fig 1G). Ventral shields clearly demarcated.
Diagnosis (Female) — All dorsal setae, long, lanceolate, serrate, setae f2 present; dorsum reticulated mediolaterally, reticulations transverse medially (Fig 13A); tarsus II with one solenidion distally; rostrum reaching to distal margin of genu I, palp tibia with 2 setae; spermatheca terminating in a round bulb (Fig 13B).Deutonymph — Dorsal setae long and serrate, except for setae v2, sc1, c1-2, d1, e1 and h1 which are clearly shorter (Fig 13C).



Hosts and localities — This species was described from Pinus halepensis Miller and P. brutia Ten (Pinaceae), in Israel at Mount Carmel and En Zetim, respectively. In Italy it was collected from P. pinaster Eiton, Mount Lu Pinu (Castagnoli, 1987).
Symptoms — Unknown.
Diagnosis (Female) — Prodorsum with setae longest and broadly lanceolate, serrate; opisthosoma with dorsolaterals broadly lanceolate, but gradually decrease in size caudally, c2 also broadly lanceolate, serrate, f2 present; dorsum reticulated (Fig 14A); tarsus II with one solenidion distally; rostrum reaching to middle of genu I, palp tibia with 2 setae; venter with area between 3a and 4a smooth, but with fine transverse striae, posterior to 4a with large cells laterally but smooth centrally with fine transverse striae, ventral shield anterior smooth but posteriorly with medium cells, genital shield with large rounded cells (Fig 14B).



Deutonymph — All dorsal setae long, broadly lanceolate, and serrate, except for setae d1, e1 and h1 which are very short (Fig 14C).
Hosts and localities — This species was described from Prunus domestica L., P. armeniaca L., Pyrus malus L. and P. communis L. (Rosaceae), Egypt (Attiah, 1956). In Israel it was reported from: P. domestica L., P. malus, Cotoneaster sp. and Crataegus azarolus L. (Rosaceae), Tal Shahar, Qiryat Shemona and Mt. Meron. P. malus and P. communis, Iraq; Armenia; Cydonia oblonga Mill. Prunus insititia L., apricot, pomegranate, Greece; Prunus salicina Lindl., P. armeniaca, Malus sylvestris (L.) Mill. and Pyrus malus L., Iran, Cyprus, England, Jordan, Lebanon, Libya and Portugal (Smith Meyer & Gerson, 1981; Al-Gboory, 1987; Bagdasarian, 1962; Hatzinikolis & Emmanouel, 1987; Hatzinikolis et al., 1999; Khosrowshahi & Arbabi, 1997; Khanjani et al., 2012).
Symptoms — In Iraq and Egypt this species is a pest of apples and pears and occurs on young shoots, buds and on both sides of leaves (Al-Gboory, 1987; Hatzinikolis & Emmanouel, 1987; Wafa et al., 1968-1969).
Diagnosis (Female) — Dorsum coarsely striate, prodorsal setae broadly lanceolate, lateral setae lanceolate, serrate, dorsocentral setae d1 and e1 very short and slightly serrate, c1 more than twice the length of d1 and e1, setae f2 present; rostral shield deeply notched (Fig 15A); tarsi I-II each with one solenidion distally; rostrum reaching to middle of femur I; setae 4a not reaching setae 3a; palp tibia with one seta.



Deutonymph — All dorsal setae long and lanceolate, except setae d1 and e1 which are very short (Fig 15B).
Hosts and localities — This species was described from Pinus spp., Italy (Canestrini & Fanzago, 1876) and from: Pinus spp., Algeria, Armenia, France, Georgia (SSR), Iran, Israel, Japan, Lebanon, Libya, Morocco, Netherlands, The Philippines, Poland, and Portugal (Mitrofanov & Strunkova, 1979; Baker & Pritchard, 1952; Reck, 1951; Gutierrez et al., 1989; Ehara, 1966; Hatzinikolis, 1970; Dosse, 1974).
Symptoms — A pest of pine trees in The Netherlands, Italy, and Georgia, SSR (Jeppson et al., 1975).
Diagnosis (Female) — Prodorsum with 3 pairs of long, narrowly lanceolate, serrate setae, longer than opisthosomal setae, evenly reticulate with large cells; opisthosoma with f2 present and covered with large regular cells between c1-d1, between d1-h1 reticulation becoming series of short transverse folds, mediolaterally with even reticulation of large cells (Fig 16A); tarsus II with one solenidion distally; rostrum reaching to middle of genu I, palp tibia with 2 setae. Opisthogaster with cuticle between 3a and 4a smooth, but with fine striae, posterior to 4a with even large round cells laterally forming weak transverse bands or become smooth centrally, ventral shield with large round cells often transversely elongate, genital shield with large transverse cells (Fig 16B).



Deutonymph — Opisthosomal setae c1, c2, d1, e1, f2, h1 and h2 minute, rest of setae long (Fig 16C).
Hosts and localities — Hosts mainly of the Rosaceae family from: Algeria, Afghanistan, Argentina, Austria, Bulgaria, China, Croatia, Greece, Cyprus, Denmark, Egypt, England, France, Germany, Greece, India, Iran, Israel, Italy, Lebanon, Morocco, The Netherlands, Pakistan, Portugal, Romania, former Soviet Union USSR, Syria, Turkey, USA, Wales, Yugoslavia (Beard et al., 2013; Hatzinikolis et al., 1999; Hatzinikolis & Emmanouel,1987; Khanjani et al., 2012; Pritchard & Baker,1958)
Symptoms — Unknown.
Diagnosis (Female) — Prodorsum with 3 pairs of long, strongly barbed setae, longer than opisthosomal setae, most also strongly barbed, evenly reticulate with small to medium cells, opisthosoma with f2 present and cuticle with large irregular cells between c1-d1, between d1-h1 reticulation becoming series of short transverse folds, mediolaterally with even reticulation of small cells (Fig 17A); tarsus II with one solenidion distally; rostrum reaching to middle of genu I, palp tibia with 2 setae. Opisthogaster with cuticle between 3a-3a smooth but with fine striae, between 4a-4a with weak transverse bands, ventral and genital shields with medium to large rounded cells and genital shield (Fig 17B).



Deutonymph — Opisthosomal setae c1, d1, e1, h1 and h2 minute, rest of setae long (Fig 17C).
Hosts and localities — Hosts mainly of the Rosaceae family from: Algeria, France, Germany, Greece, India, Iran, Israel, Morocco (Beard et al. 2013; Hatzinikolis et al., 1999; Hatzinikolis & Emmanouel, 1987; Khanjani et al., 2012; Pritchard & Baker, 1958).
Symptoms — This species prefers the underside of host leaves and causes yellow to dark spots when feeding on roses (Hatzinikolis & Emmanouel, 1987).
Diagnosis (Female) — All dorsal setae, lanceolate, serrate, opisthosoma with 3 pairs of dorsocentrals gradually becoming shorter posteriorly, c1 subequal or slightly longer than d1, setae f2 present; dorsum coarsely striate and rostral shield slightly indented (Fig 18); setae 4a reaching pass setae 3a; palp tibia with one seta; tarsus II with one solenidion distally; rostrum reaching just past distal margin of femur I.



Deutonymph — Hatzinikolis & Emmanouel (1987) described the deutonymph but excluded setae f2, while present in the female. Therefore we decided to omit it till it can be proofed otherwise.
Hosts and localities — This species was described from Pinus sp. in the Ukraine (Livschitz & Mitrofanov, 1967). In Israel, it was reported from ornamentals by Halperin et al., (1989). Arabuli & Kvavadze (2013) redescribed C. wainsteini from Ficus carica L. (Moraceae), Philadelphus caucasicus Koehne (Hydrangeaceae) and Styphnolobium japonicum Schott (Fabaceae) Caucasia and Georgia. Other records are from: Giza, Egypt and Gaza Strip (Hatzinikolis 1983, 1987; Hatzinikolis & Emmanouel, 1987).
Symptoms — Distorted needles that eventually dry out (Hatzinikolis & Emmanouel, 1987).
Idiosoma elongate, evenly rounded, without dorsal plates, striated. Anterior margin of prodorsum without rostral shield, projections, or notch; opisthosomal setae mainly short, setiform, smooth or with few barbs, with 9 pairs of setae: 2 pairs of dorsocentral (c1, d1), 6 pairs of dorsolateral (c3, d3, e3, f3, h2, h1) and one pair of sublateral setae (c2); setae d3 and e3 usually inserted in submedial position; setae e1–2, f2 absent (Fig 19A). Ventral shields not distinct with one or 2 pairs of anal setae (ps1-2). One or two pairs of genital setae (Fig 19B). Palp 3-segmented (Fig 1F). Tibia I–II with four setae. Genua III–IV bare. Tarsal claws uncinate or highly reduced. Male with posterior opisthosoma elongated, narrowing to a blunt point, modified pseudanal setae ps1 in membranous tubercle (Seeman et al., 2016).
Diagnosis (Female) — Body elongate-oval, opisthosoma with dorsolaterals h1, h2 and f3 clearly longest, all dorsal setae serrate; dorsum covered with longitudinal striae (Fig 19A); spermatheca a long tube terminating in a small vesicle; ventrally genital shield with longitudinal, smooth striae and 2 pairs of setae, one pair of aggenital (ag) setae anterior to this shield, anal shield with 2 pairs of setae (ps) (Fig 19B); setae 3a four times longer than 4a (Fig 19C). Tarsal claws with small hooks (Fig 19D).



Hosts and localities — Collected from Cynodon dactylon (L.) Pers. (Graminaceae) at Elot, Israel (Smith Meyer & Gerson, 1981). Womersley (1943) described this species from C. dactylon, Australia. It was also recorded from C. magennisii Hurcombe in South Africa, from C. dactylon, Egypt and Zimbabwe (Baker & Pritchard, 1956; Wafa & Yousef, 1968-1969; Goldsmid, 1962). Recently it was also reported from Bangladesh and the Seychelles (Woods, 2010).
Symptoms — Dolichotetranychus australianus is a serious pest of turf grasses in Australia and is often found together with the eriophyid Aceria cynodoniensis Sayed. The symptoms of these two species differ in that A. cynodoniensis causes a “witches-broom” response at the nodes, whereas the feeding of D. australianus results in thinning and weakening of stands; severe infestations can virtually turn the whole sward brown and die; the symptoms can be described as pinetree-like growths (Seeman et al., 2016).
Idiosoma roundish; prodorsum with 3 pairs of setae (v2, sc1, sc2); opisthosoma with 11 pairs of setae consisting of 3 pairs of dorsocentral (c1, d1, e1), 6 pairs of dorsolaterals (c3, d3, e3, f3, h2, h1) and 2 pairs sublateral setae (c2, d2), setae f2 absent (Fig 20); palp one-segmented (Fig 1A); tarsi with true claws uncinated.
Diagnosis (Female) — Body broadly oval, prodorsum smoothly rounded anteriorly, all dorsal setae short, setiform and serrate; dorsum striate (Fig 20); ventral and genital shields not outlined, 2 pairs of genital, one pair of aggenital (ag) and 2 pairs anal setae (ps) present; palp with one segment with 2 setae and one solenidion.



Hosts and localities — From Tamarix maris-mortui Gutm. (Tamaricaceae), Wadi Fukra, Israel (Pritchard & Baker, 1958). Smith Meyer & Gerson (1981) reported that it occurred all along the Arava, from the Dead Sea to the Red Sea. It was also reported from Greece (Hatzinikolis, 1987) and Iran (Khanjani et al., 2013).
Symptoms — Cause twig galls.
Idiosoma oval, bearing large fan-shaped, veined dorsal setae: 3 pairs of prodorsal (v2, sc1, sc2), opisthosoma with 7 pairs of dorsolateral (c3, d3, e3, f2, f3, h2, h1), 3 pairs of sublateral (c2, d2, e2) and 3 pairs of dorsocentral setae (c1, d1, e1); palp two-segmented (Fig 1E).
Diagnosis (Female) — Body oval and dorsally covered with fan-shaped, veined setae, prodorsum with setae v2 very broad near middle and distally pointed (Fig 21). Differs from second species in this genus, P. romaine Pritchard & Baker, only in having setae v2 elongate-elliptical. Palp two-segmented.



Hosts and localities — In Israel this species was collected from Phoenix dactylifera L. (Arecaceae), Arava Valley (Smith Meyer & Gerson, 1981). It was originally described from P. dactylifera from Egypt (Sayed, 1938) and also reported from Iran (Khanjani et al., 2013).
Symptoms — Sucks plant sap with chlorophyll from leaves resulting in infested areas turning yellowish. In heavy infestations the affected areas change to dirty whitish blotches which result from the aggregation of the mites with their whitish fan-like setae, and the leaves eventually turn dark brown and die (Zaher et al., 1969). In Israel, no damage has ever been reported in association with this species (Palevsky, unpublished, Blumberg, 2008).
Idiosoma round; prodorsum with 3 pairs of setae (v2, sc1, sc2); opisthosoma with 13 pairs of setae consisting of 3 pairs of dorsocentral, (c1, d1, e1), 6 pairs of dorsolateral (c3, d3, e3, f3, h2, h1) and 4 pairs sublateral setae (c2, d2, e2, f2) (Fig 22A); palp 2-segmented (Fig 1E); tarsi with true claws uncinated.
Diagnosis (Female) — Body round, prodorsum with 3 pairs setae and smoothly rounded anteriorly, dorsal setae long and with clavate tips, setae c1, d1, e1 weakly spatulate (e1 often tapered), seta h1 subequal in length to h2, seta h2 setiform, with finely tapered tip, seta f2 shorter than f3 (Fig 22A); coxae III-IV nude, femur II with 4 setae, genua I-II with 3 setae, tarsi I-II with companion seta obviously longer than solenidion (Fig 22B); palp with distal segment with one setiform eupathidium, one seta and one solenidion.



Hosts and localities — In Israel this species was collected from Phoenix dactylifera L. (Arecaceae), Arava Valley (Smith Meyer & Gerson, 1981). Originally described from Cocos nucifera L. (Arecaceae), in India (Hirst, 1924), it has since then been collected from many palm species world-wide (Dowling et al., 2012). After its introduction into the Americas this pest has expanded its host plant range to 96 plant species, including bananas (Amaro & De Morais, 2013).
Symptoms — The symptoms in coconut plants start as small yellow spots on the abaxial leaf surface. Feeding damage causes the two sides of leaflets to fold onto each other. Continued feeding probably is responsible for the curling and drying of the leaflet tips. As feeding progresses the green leaves turn pale green, then yellow and finally copper-brown (Rodrigues et al., 2007). This species is not a pest in Israel but a severe pest in Central America and southern USA.
This genus can be defined as follows: prodorsum with 3 pairs of setae (v2, sc1, sc2) (rarely 2 pairs); 8-10 pairs of opisthosomal setae: 1-3 pairs of dorsocentral (c1, d1, e1) and 6-7 pairs of dorsolateral c3, d3, e3, f2 absent or present, f3, h2, h1; setae c2 absent; setae h2 flagellate and elongate; opisthosoma tapers behind legs IV; metapodosoma with 1-3 pairs of 3a (3a always present; 3a2, 3a3 present or absent) and 1-5 pairs of 4a setae (4a always present; 4a2, 4a3, 4a4, 4a5 present or absent); palp one to 3 segmented (Figs. 1B,1C,1D); ventral and genital shields usually fused and not developed.
Diagnosis (Female) — Prodorsum with 2 Y-shaped ridges stretching from eyes posteriorly, first 2 pairs of setae (v2 and sc1) minute and spatulate, setae sc2 much longer and broadly lanceolate, rostral shield deeply bifurcate; opisthosoma with 10 pairs of setae and constricted and covered with folds and irregular coarse striae: 3 pairs of spatulate dorsocentrals, with c1 and d1 much bigger than minute e1, 7 pairs of dorsolaterals varying from spatulate to broadly lanceolate, setae d3 are minute and h2 long, flagellate; one pair of lateral projections associated with setae c3 (Fig 23A); venter with one pair of setae 3a and 4a, ventral and genital shields indistinct with one pair of aggenital and 2 pairs of genital setae, respectively; anal shield with 2 pairs of setae (Fig 23B); tarsal claws and empodia padlike; palp with 3 segments; spermatheca a short tube terminating in a knob (Fig 23C).



Hosts and localities — Described from Cupressus sempervirens L. (Cupressaceae), at Kabri and Maabarot, Israel (Smith Meyer & Gerson (1981).
Symptoms — Unknown.
Diagnosis (Male) — Dorsum striate, most setae very short and serrate, except for very long setae h2, setae sc2, e3, f3 and h1 also longer than rest of setae, rostral shield deeply bifurcate, opisthosoma with 9 pairs of setae, 2 large pores: 3 pairs of dorsocentrals, 6 pairs of dorsolaterals (Fig 24A); venter with 2 pairs of 3a and 5 pairs of 4a setae, ventral and genital shields indistinct with a pair of aggenital and 2 pairs of genital setae, respectively; anal shield with 2 pairs of setae (Fig 24B). Palp 3-segmented.



Hosts and localities — Reported from Ephedra campylopoda C.A.Mey (Ephedraceae), Hiram, Israel (Smith Meyer & Gerson, 1981). Reck (1951) described it from E. procera Fisch. & Mey, Georgia, SSR.
Symptoms — Unknown.
Diagnosis (Female) — Dorsum with irregular striae, longitudinal laterally on opisthosoma, most setae very short and serrate, except for setae sc2, f2, f3, e3, h1, which are much longer; h2 long and flagellate; rostral shield deeply bifurcate, opisthosoma with 8 pairs of setae, 2 large pores and constricted behind coxae IV: one pair of dorsocentrals (c1 present, d1 and e1 absent), 7 pairs of dorsolaterals (Fig 25A); venter with mainly transverse striae, with one pair of 3a and 2 pairs of 4a setae, ventral and genital shields indistinct with one pair of aggenital and 2 pairs of genital setae, respectively; anal shield with 2 pairs of setae (Fig 25B). Spermatheca with basal half bulged but anterior half a slender tube, terminating blunt (Fig 25C). Palp 3-segmented.



Hosts and localities — This species was described from pomegranate Punica granatum L. (Lythraceae) in Egypt (Sayed, 1946) and reported from Israel, the former USSR, Greece, Cyprus, India and Iran (Sternlicht & Golan, 1967; Channabasavanna & Lakkundi, 1977; Hatzinikolis, 1986; Khosrowshahi & Arbabi, 1997).
Symptoms — A pest of pomegranates and grapes in the Middle East (Gerson 2017) and Greece (Hatzinikolis, 1986), but has not recently been reported on pomegranates in Israel (Tselila Ben-David unpublished).
Diagnosis (Female) — Dorsum striate, most setae very short and serrate, except for very long setae h2, rostral shield deeply bifurcate, opisthosoma with 10 pairs of setae, 2 large pores: 3 pairs of dorsocentrals, 7 pairs of dorsolaterals (Fig 26A); venter with one pair of 3a and 4 pairs of 4a setae overreaching genital setae, ventral striae anastomosing and broken, ventral and genital shields indistinct with one pair of aggenital and 2 pairs of genital; anal shield with 2 pairs of setae, all barbed (Fig 26B). Palp 3-segmented.



Symptoms — Unknown.
Diagnosis (Female) — Dorsum medially and laterally reticulate; prodorsum with oblique striae and folds laterally; opisthosoma with longitudinal striae laterally. Prodorsal setae v2, sc1 minute and sc2 longer, lanceolate and serrate; dorsal opisthosomal setae c1, d1, e1, f2 present.; opisthosoma with dorsocentral setae c1, d1, e1 short and slightly serrate; lateral setae longer, weakly lanceolate and serrate; setae h2 flagelliform (Fig 27A). Venter mostly smooth with weak striae laterally and posteriorly (Fig 27B). Palp 3-segmented. Spermathecal duct narrows to form proximal constriction, prior to forming a membranous bulge and narrows again into a slender tube terminating into a small bulb distally (Fig 27C).



Hosts and localities — Reported from Punica granatum L. (Lythraceae) in Greece, India, Iraq, Iran, Israel, Jordan, Spain, Turkey and Russia (Pritchard & Baker, 1958; Wainstein, 1960; Livschits & Mitrofanov, 1967; Hatzinikolis, 1986; Khanjani et al., 2013).
Symptoms — Serious pest of pomegranates (Zaher & Yousef, 1972; Al-Gboory & El-Haidari, 1989) and is the prevalent species on pomegranates in Israel (Tselila Ben-David unpublished).
1. Dorsal opisthosoma with any of sublateral setae c2, d2, e2 and f2 present
...... 2
— Dorsal opisthosoma setae c2, d2, e2 absent, f2 absent or present
...... 14
2. Dorsal opisthosoma with two or all pairs of setae c2, d2, e2, f2 present; ventral and genital shields not developed to weakly developed; ventral shield not framed by distinct striae; palp 4–5-segmented (Figs 1G-H)
...... 10
— Dorsal opisthosoma with setae c2 present and setae d2, e2 absent
...... 3
3. Palp 1 or 3-segmented (Fig 1 A, F)
...... 4
— Palp 4-segmented (Fig 1G); setae f2 present or absent; ventral and genital shields distinct and separate; ventral shield framed by distinct striae (= genus Cenopalpus Pritchard & Baker)
...... 5
4. Palp 3-segmented (Fig 1F); Idiosoma elongate, more than 1.5 times as long as wide, slender, parallel-sided, tapering posteriorly; d3 and e3 usually inserted in submedial region; rostral shield absent; opisthosomal setae usually setiform or serrate, not ramose or lanceolate nor longer than half width of idiosoma; posterior end of idiosoma with 4 pairs of dorsal setae, f2 absent; genu IV without setae (= genus Dolichotetranychus Sayed), setae v2 less than half as long as distance between them, setae h1, h2 and f3 3-4 times longer than other dorsal setae; setae 3a 4 times longer than 4a (Figs 19 A-D)
...... D. australianus (Womersley)
— Palp one-segmented (Fig 1A); Idiosoma oval; opisthosoma with 10 pairs of dorsal setae; paired claws and empodium short and padlike, each claw with 2 short inner tenant hairs and a long outer hair; empodium with 2 rows of short tenant hairs (= genus Capedulia Smith Meyer), all dorsal setae very long (Fig 12)
...... C. maritima Gerson & Smith Meyer
5. Dorsum striate; palp tibia with one seta
...... 6
— Dorsum mainly covered with reticulations; palp tibia with 2 setae
...... 7
6. Rostral shield deeply incised, rostrum reaches to middle of femur I; setae c1 longer than half the length of d1 (Figs 15 A-B)
...... C. lineola (Canestrini & Fanzago)
— Rostral shield shallowly incised, rostrum reaches past anterior margin of femur I; setae c1 subequal or slightly longer than d1 (Fig 18)
...... C. wainsteini (Livschitz & Mitrofanov)
7. Prodorsal setae narrowly lanceolate to setiform, serrate
...... 8
— Prodorsal setae broadly lanceolate to spatulate, strongly pilose
...... 9
8. Dorsal opisthosomal cuticle with distinct series of short transverse bands between setae d1-e1; prodorsum with small irregular elements in reticulation; ventral cuticle between setae 4a and ventral shield covered in small irregular reticulations; deutonymph with dorsal opisthosomal setae very long except for setae c1, d1, e1, h1 and h2, very short (Figs 17 A-C)
...... C. spinosus (Donnadieu)
— Dorsal opisthosoma cuticle without distinct series of short transverse bands between setae d1-e1; prodorsum with large regular elements in reticulation; ventral cuticle between setae 4a and ventral plate with large regular reticulation, strongest laterally becoming weak to absent centrally (i.e. smooth centrally); deutonymph with dorsal opisthosomal setae c3, d3, e3 and f3 very long and c1, c2, d1, f2, h1 and h2 minute (Figs 16 A-C)
...... C. pulcher (Canestrini & Fanzago)
9. Prodorsum reticulate laterally, with transverse reticulations medially; rostrum extending to anterior margin of genu I; deutonymph with setae sc2, d3, e3, f2, f3 and h2 clearly longest, rest of dorsal setae clearly shorter (Figs 13 A-C)
...... C. halperini Castagnoli
— Prodorsum entirely reticulated with medial elements larger; rostrum reaching pass distal end of femur I; deutonymph with most dorsal setae long except for d1, e1, and h1 which are minute (Figs 14 A-C)
...... C. lanceolatisetae (Attiah)
10. Four pairs of sublateral setae present (c2, d2, e2, f2)
...... 11
— Two pairs of sublateral setae (c2, d2) present; idiosoma roundish; opisthosoma with 11 pairs of setae; e2 absent and d2 present (= genus Obdulia Baker & Pritchard), dorsal body setae setiform, minutely serrate (Fig 20)
...... O. tamaricis Baker & Pritchard
11. Palp 5-segmented (Fig 1H) (= genus Aegyptobia Sayed)
...... 12
— Palp 2-segmented
...... 13
12. Prodorsum with reticulations medially only; dorsal setae broadly lanceolate and coarsely serrate (Fig 3)
...... A. salixi Zaher & Yousef
— Dorsum completely covered with reticulations; dorsal setae broadly club-shaped and smooth (Figs 2 A-C)
...... A. eremia Smith Meyer & Gerson
13. Dorsal setae long and rodlike, with clavate tips; idiosoma round (= genus Raoiella Hirst), dorsal body setae long with small knobs distally, seta h1 subequal in length to h2, seta h2 setiform, with finely tapered tip, seta f2 shorter than f3 (Figs 22 A-C)
...... R. indica Hirst
— Dorsal setae broadly palmate with extensive venation (=Phyllotetranychus Sayed), dorsal setae large, fan-shaped and veined, with setae v2 very broad near middle (Fig 21)
...... P. aegyptiacus Sayed
14. Setae h2 not long and attenuate, almost similar in length to other opisthosomal setae; genital and ventral shields separated, former elliptical, wider than long, latter rectangular, usually with striate cuticle forming a frame around plate; palp 4-segmented (Fig 1G); lateral margin of opisthosoma with 6 or 7 pairs of setae (= genus Brevipalpus Donnadieu)
...... 15
— Setae h2 long and whip-like, at least 4 times longer than other dorsal opisthosomal setae, opisthosoma tapered with 6–10 pairs of setae; ventral shields indistinctly outlined; palp usually 1-3-segmented (Figs 1A-D) (= genus Tenuipalpus Donnadieu)
...... 22
15. Opisthosoma with 6 pairs of dorsolateral setae (c3, d3, e3, f3, h2 and h1; setae
f2 absent)
...... 16
— Opisthosoma with 7 pairs of dorsolateral setae (f2 present)
...... 18
16. Tarsus II with a single solenidion distally; prodorsum with even reticulations laterally but devoid of median reticulations; spermatheca terminates into a round vesicle with short finger-like projections around entire perimeter; deutonymph with setae c3, d3, e3 shorter than f3, h1-2 (Figs 6 A-D)
...... B. obovatus Donnadieu
— Tarsus II with 2 solenidia distally
...... 17
17. Prodorsum centrally with strong areolae, posterior reticulation with large round cells, small round cells medially, anteriorly mostly smooth; palp femorogenu with dorsal seta broad, flat, serrate; spermatheca terminates into a membranous bulb; deutonymph with prodorsal setae v2 short, opisthosomal setae c1, d1, d3, e1, e3 minute, setae c3, f3, h1, h2 large and broadly lanceolate (Figs 9 A-D)
...... B. phoenicis (Geijskes)
— Prodorsum centrally with strong areolae, posterior reticulation with large cells, anteriorly weakly reticulate; palp femorogenu with dorsal seta narrow, serrate; spermathecal terminates into a sclerotised oval vesicle with a thick distal stipe; deutonymph with prodorsal setae v2 lanceolate, opisthosomal setae c1, d1, e1 minute, setae f3, h1, h2 large and broadly lanceolate, setae c3, d3 and e3 vary from short to large (like f3, h1, h2) (Figs 11 A-D)
...... B. yothersi Baker
18. Tarsus II with one single solenidion distally
...... 19
— Tarsus II with two solenidia distally; Prodorsum evenly reticulated; 3 pairs of dorsocentral setae very short, setiform, equally long; all dorsal setae very short, setae d1, e1 and f2 present (Figs 4 A-D)
...... B. californicus (Banks)
19. Solenidia on tarsi I and II shorter than width of segments
...... 20
— Solenidia on tarsi I and II longer or as long as width of segments; most dorsal setae broadly lanceolate, marginal setae slightly longer and serrate; nymph with setae sc1, sc2, f2, f3, h2 and h1 broadly lanceolate, serrate, except for h1, which are clearly shortest (Figs 10 A-C)
...... B. recki Livschits & Mitrofanov
20. Rostrum extending beyond distal end of femur
...... 21
— Rostrum not extending beyond distal end of femur; spermatheca terminating into a small rounded vesicle with a series of short projections and clear internal “bubble”; deutonymph with setae sc1, sc2, c3, f2, f3, h2 and h1 longest, rest short (Figs 5 A-D)
...... B. lewisi McGregor
21. Trochanter III with one seta, trochanter IV without setae; spermatheca a very long, slender, coiling tube terminating into a large, prominent hairy bulb; deutonymph with setae sc2, c3, d3, e3, f3 and h1 longest, broadly lanceolate and serrate; setae v2, sc1, c1, d1, e1, f2, h2 much shorter and serrate (Figs 8 A-D)
...... B. olearius Sayed
— Trochanter III with 2 setae and trochanter IV with one seta; spermatheca a very long, slender, coiling tube terminating into a large, prominent hairy bulb; deutonymph with setae sc2, c3, f3 and h1 longest, broadly lanceolate, and serrate; setae v2, sc1, c1, d1, d3, e1, e3, f2, h2 much shorter and serrate (Figs7 A-D)
...... B. oleae Baker
22. Venter with two to five pairs of setae 4a
...... 23
— Venter with one pair of setae 4a
...... 25
23. Venter with two or four pairs of setae 4a
...... 24
— Venter with five pairs of setae 4a (Fig 24 B)
...... T. dubinini Reck
24. Venter with four pairs of setae 4a (Fig 26 B), overreaching aggenital setae (ag); 3 pairs of
dorsocentral setae present, dorsum with complete or incomplete reticulations (Figs 26 A-B)
...... T. pareriophyoides Smith Meyer & Gerson
— Venter with two pairs of setae 4a (Fig 24 B); one (rarely 2) pairs of dorsocentral setae resent, dorsum nearly smooth (Figs 25 A-C)
...... T. granati Sayed
25. Most dorsal setae minute except for sc2, c3, e3, f2, f3 and h1 which are slightly longer and serrate; dorsum covered with strong, irregular reticulations (Figs 27 A-C)
...... T. punicae Pritchard & Baker
— Setae v2, sc2, d3 and e1 minute and spatulate, rest larger and spatulate or broadly lanceolate; dorsum striate (Figs 23 A-C)
...... T. cupressoides Smith Meyer & Gerson
Seven of the 26 tenuipalpid species known from Israel can be regarded as pests, the rest have either no, or little, economic impact, or else are controlled by their natural enemies. Brevipalpus californicus, B. lewisi, B. phoenicis and B. yothersi infest citrus. B. lewisi was reported from “Blue Serbian” vines, causing severe leaf sclerosis, at Nachshonim in 1952 (Bytinski-Salez, 1966), but never found again till during this study when it was found on under side of petit vardo leaves in Mizpe Ramon. Tenuipalpus granati is a pest of pomegranates and grapes, and T. punicae can be a very significant pest of pomegranates. However the palm-infesting Phyllotetranychus aegypticus, and Tenuipalpus pareriophyoides do not seem to affect their hosts. Nor does Raoiella indica, another palm-infesting species, despite being a major pest of palms (and banana) in Central America (Amaro & De Morais, 2013; Dowling et al., 2012). A review of the Tenuipalpidae fauna of Turkey (Çobanoğlu et al., 2016) revealed almost the same number of species (28) as in Israel (26). Both faunas shared 11 species (Cenopalpus lineola, C. spinosus, C. pulcher, C. lanceolatisetae, T. granati, T. punicae. B. phoenicis, B. obovatus, B. californicus, B. lewisi and B. olearius). Turkey has four endemic species (Cenopalpus bakeri Duzgunes, C. pritchardi Duzgunes, Pseudoleptus zelihae Pritchard & Baker and Aegyptobia juniper Çobanoğlu et al.), oppose to five in Israel (A. eremia, C. halperni, C. maritima, T. cupressoides and T. pareriophyoides). The Israel fauna consists of six Cenopalpus, eight Brevipalpus, five Tenuipalpus, two Aegyptobia spp., one Capedulia, one Dolichotetranychus, one Phyllotetranychus, one Obdulia and one Raoiella sp. The Turkish fauna comprises seven Cenopalpus, seven Brevipalpus, two Tenuipalpus, six Aegyptobia, three Pentamerismus spp., one Raoiellana, one Pseudoleptus and one Phytoptipalpus sp.
We thank the Israel Taxonomy Initiative for making the course which this paper is based on possible. This work is also based on research supported in part by the National Research Foundation of South Africa (UID) 85288. Any opinion, findings and conclusions or recommendations expressed in the material are those of the authors and therefore the NRF does not accept any liability in regard thereto.
Al-Gboory I. 1987. Taxonomic studies of false spider mites (Acari: Tenuipalpidae) in central Iraq. Ph.D. Thesis. Institut für angewandte Zoologie der Rheinischen Friedrich-Wilhelms-Universitat Bonn: 205 pp.
Al-Gboory I., El-Haidari H. 1989. Studies on some factors affecting the pomegranate false spider mite, Tenuipalpus punicae (Acari: Tenuipalpidae) in Iraq. In: Channabasavanna, G.P. & Viraktamath C.A (Eds.), Progress in Acarology; VII International Congress of Acarology, Bangalore, India. p 67-72.
Amaro G., De Morais E.G.F. 2013. Potential geographical distribution of the red palm mite in South America. Experimental and Applied Acarology, 60: 343-355. doi:10.1007/s10493-012-9651-9 ![]()
Arabuli T., Kvavadze E. 2013.New record for Caucasus fauna: Cenopalpus wainsteini Livschitz & Mitrofanov, 1967 (Acari: Tenuipalpidae), additional description and three new host plants. International Journal of Acarology, 39(7): 538-541. doi:10.1080/01647954.2013.837961 ![]()
Attiah H.H. 1956. The genus Brevipalpus in Egypt (Acarina: Tenuipalpidae). Bulletin de la Société Entomologique d'Egypte, 40: 433-477.
Bagdasarian A.T. 1962. Contribution to the fauna of false spider mites from Armenia. Izvestiia Akademii Nauk SSR, Moscow, 15: 49-58.
Baker E.W. 1949. The genus Brevipalpus (Acarina: Pseudoleptidae). The American Midland Naturalist, 42(2): 350-402. doi:10.2307/2422013 ![]()
Baker E.W., Pritchard E.A. 1956. False spider mites of the genus Dolichotetranychus (Acarina: Tenuipalpidae). Hilgardia, 24(13): 359-381. doi:10.3733/hilg.v24n13p357 ![]()
Baker E.W., Tuttle D.M. 1964. The false spider mite of Arizona (Acarina: Tenuipalpidae). Agricultural Experiment Station, University Arizona, Technical Bulletin, 163: 1-80.
Baker E.W., Tuttle D.M. 1987. The false spider mite of Mexico (Tenuipalpidae: Acari). United States Department of Agriculture, Agricultural Research Services, Technical Bulletin, 1706: 1-236.
Banks N. 1904. Four new species of injurious mites. Journal of the New York Entomological Society, 12: 53-56.
Beard J.J., Ochoa R., Braswell W.E., Bauchan G.R. 2015a. Brevipalpus phoenicis (Geijskes) species complex (Acari: Tenuipalpidae) – a closer look. Zootaxa, 3944: 1-67. doi:10.11646/zootaxa.3944.1.1 ![]()
Beard J.J., Ochoa R., Bauchan G.R., Trice M.D., Redford A.J., Walters T.W., Mitter C. 2015b. Flat Mites of the World. Available from: http://idtools.org/id/mites/flatmites/ (Accessed on Feb 2015) ![]()
Blumberg D. 2008. Review: Date Palm Arthropod Pest and their management in Israel. Phytoparasitica, 36(5): 411-448 doi:10.1007/BF03020290 ![]()
Bodenheimer F.S. 1930. Die Schadlungsfauna Palastinas. Berlin. Paul Parey. 439
Bodenheimer F.S. 1937. Prodromus Faunae Palastinae. Essai sur les éléments zoogéographiques et historiques du sud-ouest, du sous-règne paléarctique. Mémoires de I'institut d'Egypte, 33, pp 286.
Buchanan G.A., Begston M., Exley E.M. 1980. Population growth of Brevipalpus lewisi McGregor (Acarina: Tenuipalpidae) on grapevines. Australian Journal of Agricultural Research, 31: 957-965. doi:10.1071/AR9800957 ![]()
Bytinski-Salez H. 1966. An annotated list of insects and mites introduced into Israel. Israel Journal of Entomology, 1: 15-48.
Çobanoğlu S., Ueckermann E.A., Sağlam H.D. 2016. The Tenuipalpidae of Turkey, with a key to species (Acari: Trombidiformes). Zootaxa, 4097(2): 151-186. doi:10.11646/zootaxa.4097.2.1 ![]()
Canestrini G., Fanzago F. 1876. Nuovi acari Italiani. Atti Academia Cientifico Veneto, Trentino, Istriana, Padua, Italy, 5: 130-142.
Castagnoli M. 1987. Due nuove specie di Tenuipalpidi delle conifer: Cenopalpus (Cenopalpus) halperini e C. (C.) pegazzanoae (Acari: Tenuipalpidae). Redia, 70: 105-119.
Castagnoli M., Pegazzano F. 1979. Rassegna degli Acari Tenuipalpidi dell'olivo con descrizione di Hystripalpus rotai sp.nov. Redia, 62: 281-297.
Channabasavanna G.P., Lakkundi N.H. 1977. Tenuipalpus lalbaghensis (Acarina: Tenuipalpidae), a new species from Southern India. Indian Journal of Acarology, 1: 19-22.
Donnadieu A.L. 1875. Recherches pour servir à l'histoire des Tétranyques. Thèse. Faculté des Sciences de Lyon-France: pp. 131.
Dosse G. 1974. Uber schadliche Milbenarten an Pinus (Acari). Zeitschrift für Pflanzenkrankheiten und Pflanzenschutz, 81(6-7): 364-371.
Dowling A.P.G., Ochoa R., Beard J.J., Welbourn W.C., Ueckermann E.A. 2012. Phylogenetic investigation of the genus Raoiella (Prostigmata: Tenuipalpidae): diversity, distribution, and world invasions. Experimental and Applied Acarology, 57: 257-269. doi:10.1007/s10493-011-9483-z ![]()
Ehara S. 1956. Two false spider mites from Japanese orchards (Phytoptipalpidae). Annotationes Zoologicae Japonensis, 29 (4): 234-238.
Ehara S. 1966. The Tetranychoid mites of Okinawa Island (Acarina: Prostigmata). Journal of the Faculty of Science Hokkaido University, Series VI Zoology, 16(1): 1-22.
Elmer H.S., Jeppson L.R. 1957. Biology and control of the citrus flat mite. Elmer & Jeppson, 1957. Journal of Economic Entomology, 50(5): 566-570.
Gejskes D.C. 1939. Beitrӓge zur kenntnis der europӓischen spinnmilben (Acari, Tetranychidae), mit bensonderer berücktigung der Niederländischen arten. Mededeelingen van de Landbouwhoogeschool-Wageningen, 42: 1-68
Gerson U., Smith Meyer M.K.P. 1980. A new species of Capedulia (Acari: Tenuipalpidae) from a marine habitat in Israel. Israel Journal of Entomology, 14: 9-12.
Gerson U. 2017. Plant Pests of the Middle East .Available from: http://www.agri.huji.ac.il/mepests/ ![]()
Goldsmid J.M. 1962. The mites (Acarina) of the Federation of Rhodesia and Nyasaland. Bulletin of the Federal Ministry of Agriculture, Rhodesia, Nyasaland, 2162: 1-11.
Gutierrez J., Kreiter S., Bolland H.R., Cotton D. 1989. Cinq espèces de Tenuipalpidae (Acari: Tetranychoidea) vivant en France sur conifers et trois de leurs prédateurs caryotype d'Oligomerismus oregonensis. Acarologia, 30(1): 51-58.
Halperin J., Brosh S., Asad N. 1989. Damages in ornamental plants in Israel. 96 pp. (in Hebrew).
Hao D.-J., Fan B.-Q., Su P., Liu Q., Wang Y. 2013. The flat mite Brevipalpus lewisi (Acari: Tenuipalpidae) infesting the Dawn Redwood Metasequoia glyptostroboides. Systematic and Applied Acarology, 18(2): 197-199. doi:10.11158/saa.18.2.12 ![]()
Hao D.-J., Su P., Pfammatter J., Liu Q., Fan B.-Q., Wang Y., Gu T.Z. 2016. Morphological and genetic characteristics of Brevipalpus lewisi (Acari: Tenuipalpidae) and comparison with other Brevipalpus species. International Journal of Acarology, 42(1): 34-40. doi:10.1080/01647954.2015.1114022 ![]()
Hatzinikolis E.N.1970. Acariens de la famille des Tenuipalpidae observés sur des plantes cultivées en Grêce. Annales Institut Phytopathology Benaki, 9: 242-244.
Hatzinikolis E.N. 1982. New phytophagous mites found in Greece. Agricultural Research, 6: 67-76.
Hatzinikolis E.N. 1983. A new species of Hystripalpus (Acari:Tenuipalpidae). Entomologia Hellenica, 1(2):71-75.
Hatzinikolis E.N. 1986. The genus Brevipalpus in Greece (Acari: Tenuipalpidae). Entomologia Hellenica, 4: 37-48. doi:10.12681/eh.13931 ![]()
Hatzinikolis E.N. 1987. A revision of tenuipalpid mites of Greece (Acarina:Tenuipalpidae). Entomologia Hellenica, 5(2): 47-60.
Hatzinikolis E.N., Emmanouel N.G. 1987. A revision of the genus Cenopalpus in Greece (Acari: Tenuipalpidae). Entomologia Hellenica, 5: 13-26. doi:10.12681/eh.13943 ![]()
Hatzinikolis E.N., Panou H.N., Papadoulis G.TH. 1999. Three new species of Cenopalpus Pritchard & Baker (Acari: Tenuipalpidae) from Rubus in Greece. International Journal of Acarology, 25(4): 275-287. doi:10.1080/01647959908684165 ![]()
Hirst S. 1924. On some new species of red spider. Annals and Magazine of Natural History Serie, 9(14): 522-527. doi:10.1080/00222932408633151 ![]()
Jeppson L.R., Keifer H.H., Baker E.W. 1975. Mites injurious to economic plants. University of California Press, Berkley. pp 614.
Khanjani M., Khanjani M., Saboori A., Seeman O. 2012. The false spider mites of the genus Cenopalpus Pritchard & Baker (Acari: Tenuipalpidae) from Iran. Zootaxa, 3433: 1-59.
Khanjani M., Farsan S., Asadi M., Khanjani M. 2013. Checklist of the flat mites (Acari: Trombidiformes: Tenuipalpidae) of Iran. Persian Journal of Acarology, 2(2): 235-251.
Khosrowshahi M., Arbabi M. 1997. The Tenuipalpidae (Acari) of Iran with introduction of new species for the world fauna and Iran. Ministry of Agriculture, Agriculture Research, Education and Extension Organization Plant Pests and Diseases Research Institute, Tehran, 1-19.
Klein Z., Zarabi L. 2011. Mites and ticks of Israel. Plant Protection and Inspection Services, Ministry of Agriculture and Rural Development, Israel. pp 344.
Kramer P. 1877. Grundzüge zur Systematik der Milben. Archiv für Naturgeschichte, 2: 215-247
Krantz G.W., Walter D.E. 2009. A manual of Acarology. Third Edition. Texas Tech University Press. pp 807.
Livshitz I.Z., Mitrofanov V.I. 1967. Materials to the cognition of the Acariformes: Tenuipalpidaes fauna. Proceedings Nikitsky Botanic Garden, 39: 1-72. (In Russian, English Summary)
Ma E.P., Yuan Y. 1977. Uber Tenuipalpus and Brevipalpus in China. Bulletin University Jiangxi, 1: 119-123.
Mcgregor E.A. 1949. Nearctic mites of the family Pseudoleptidae. Memoir of the Southern California Academy of Sciences, 3(2): 1-45.
Mesa N.C., Ochoa R., WelbournW.C., Evans G.A., De Moraes G.J. 2009. A catalog of the Tenuipalpidae (Acari) of the world with a key to genera. Zootaxa, 2098, pp. 185
Mitrofanov V.I., Strunkova Z.I. 1979. A key to false spider mites. Operdelitl Kleshchei-ploskotelok, USSR, 148: 1-148.
Pritchard E.A., Baker E.W. 1952. The false spider mites of California (Acarina: Phytoptipalpidae). University of California Publications in Entomology, 9: 1-94.
Pritchard E.A., Baker E.W. 1958. The false spider mites (Acarina: Tenuipalpidae). University of California Publications in Entomology, 14: 175-274.
Reck G. 1951. Kleshchirodov Tenuipalpus, Brevipalpus e Brevipalpoides (Trichadenidae, Acarina) po materalam iz grunzii. Trudy Instituta Zoologii Akademiya Nauk Gruz SSR, 10: 289-297.
Reuther E. 1909. Zur Morphologie und Ontogenie der Acariden mit besonderer Berücksichtigung von Pediculopsis graminum. Acta Societatis Scientiarum Fennicae, Helskinki, 36: 1-288.
Reuther W. 1989. The Citrus Industry: Crop protection, postharvest technology and early history of citrus research in California. Vol 5. Ed: W. Reuther, E.C. Calavan, G.E. Carman. University of California, Division: pp 383.
Rice R., Weinberger G.B. 1981. Citrus flat mite on pistachios in California. California Agriculture, 35(7):25-26.
Rodrigues J.C.V., Ochoa R., Kane E.C. 2007. First report of Raoiella indica Hirst (Acari: Tenuipalpidae) and its damage to coconut palms in Puerto Rico and Culebra Island. International Journal of Acarology, 33(1): 3-5. doi:10.1080/01647950708684493 ![]()
Roy A., Hartung J.S., Schneider W.L., Shao J., Leon M.G., Melzer M.J., Beard J.J., Otero-Colina G., Bauchan G.R., Ochoa R., Brlansky R.H. 2015. Role bending: complex relationships between viruses, hosts and vectors related to citrus leprosies, an emerging disease. Phytopathology, 105(7): 1013-1025. doi:10.1094/PHYTO-12-14-0375-FI ![]()
Sadana G.L. 1997. False spider mites infesting crops in India. Kalyani Publishers, New Delhi, pp 194.
Sayed M.T. 1938. Sur une nouvelle sous-famille et deux nouveau genres de Tétranyques (Acariens). Bulletin du Muséum National d'Histoire Naturelle, Paris (série 2), 10: 601-610.
Sayed M.T.1946. Description of Tenuipalpus granati nov. sp. and Brevipalpus pyri nov. sp. (Acarina: Trichadenidae). Bulletin of the Society Fouad Ier. Entomology, 30, 99-103.
Sayed M.T. 1950. Description of a new genus and two new species of the family Tenuipalpidae Sayed (Acarina). Proceedings of the 8th International Congress of Entomology, Stockholm, Sweden: 1018-1021.
Seeman O.D., Loch D.S., Mcmaugh P.E. 2016. Redescription of Dolichotetranychus australianus (Trombidiformes: Tenuipalpidae), a pest of bermudagrass, Cynodon dactylon (Poaceae). International Journal of Acarology, 42(4): 193-205. doi:10.1080/01647954.2016.1153143 ![]()
Smiley R.I., Gerson U. 1995. A review of the Tenuipalpidae (Acari: Prostigmata) of Australia with descriptions of two new genera and four new species. International Journal of Acarology, 21(1): 33-45. doi:10.1080/01647959508684041 ![]()
Smith Meyer M.K.P. 1979. The Tenuipalpidae (Acari) of Africa with keys to the world fauna. Entomology Memoir, Department of Agriculture Republic of South Africa, Pretoria, 50: 1-133.
Smith Meyer M.K.P., Gerson U. 1981. Some false spider mites (Prostigmata: Tenuipalpidae) from Israel. Israel Journal of Entomology, 15: 67-81.
Sternlicht M., Golan J. 1967. Trials to control the flat mite on vines. Hassadeh, 47: 92-95 (In Hebrew).
Tseng Y.-H. 1974. Systematics and distribution of phytophagous and predatory mites on grape in Taiwan. Part I. Phytophagous mites. Journal of Agricultural Association of China, 88: 57-72.
Wainstein B.A. 1960. Tetranychoid mites of Kazakhstan (with revision of the family). Kasakhstan Akademia Selskokhoziaistvikh nauk Trudy Naukho-IsslelovaInstituta Zashchity Rastenii, 5: 1-276.
Wafa A.K., Zaher M.A., Yousef A.A. 1968-69. Survey of the tenuipalpid mites in U.A.R. (Acarina: Tenuipalpidae). Bulletin of the Zoological Society of Egypt, 22: 52-59.
Womersley H. 1943. Australian acarina of the family Trichadenidae Oudemans, 1938. Records South Australia Museum, Queensland, 7: 245-248.
Woods M. 2010. Mites, a corpse plant, and native grasses [cited 2015 Dec. 7). Available from: http://turfdiseases.blogspot.com.au/2010/06/mites-corpse-plant-and-native-grasses.html. ![]()
Zaher M.A., Yousef A.A. 1969. Three genera of family Tenuipalpidae (Acarina) in the U.A.R. with descriptions of three new species. Acarologia, 11(2): 272-280.
Zaher M.A., Wafa A.K., Yousef A.A. 1969. Biological studies on Raoiella indica Hirst and Phyllotetranychus aegyptiacus Sayed infesting date palm trees in U.A.R. (Acarina – Tenuipalpidae). Journal of Applied Entomology, 63(1-4): 406-411.
Zaher M.A., Yousef A.A. 1972. Biology of the false spider mites Tenuipalpus punicae P. & B. in U.A.R. (Acarina – Tenuipalpidae). Zeitschrift für Angewandte Entomologie, 21: 23-29.



2017-09-26
Date accepted:
2017-12-12
Date published:
2018-04-12
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2018 Ueckermann, Edward A. ; Palevsky, Eric; Gerson, Uri; Recht, Eitan and Theron, Pieter D.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



