Phytoseiid mites of Martinique, with redescription of four species and new records (Acari: Mesostigmata)
Kreiter, Serge1 ; Zriki, Ghais2 ; Ryckewaert, Philippe3 ; Pancarte, Clovel4 ; Douin, Martial5 and Tixier, Marie-Stéphane6
1✉ CBGP, Montpellier SupAgro, INRA, CIRAD, IRD, Univ Montpellier, Montpellier, France.
2CBGP, Montpellier SupAgro, INRA, CIRAD, IRD, Univ Montpellier, Montpellier, France.
3CIRAD, Campus Agro-environnemental Caraïbe, BP 214, 97285 Le Lamentin cedex 2, Martinique, France.
4CIRAD, Campus Agro-environnemental Caraïbe, BP 214, 97285 Le Lamentin cedex 2, Martinique, France.
5CBGP, Montpellier SupAgro, INRA, CIRAD, IRD, Univ Montpellier, Montpellier, France.
6CBGP, Montpellier SupAgro, INRA, CIRAD, IRD, Univ Montpellier, Montpellier, France.
2018 - Volume: 58 Issue: 2 pages: 366-407
https://doi.org/10.24349/acarologia/20184248ZooBank LSID: 2787B6F9-EDC7-427B-A64E-F5C1E3A8078E
Keywords
Abstract
Several species in the family Phytoseiidae are important natural enemies controlling phytophagous mite and small insects in natural areas and crops all around the world (McMurtry and Croft 1997; McMurtry et al. 2013). This family is widespread all over the world and consists of 2,479 valid species dispatched in three subfamilies and 94 genera (Demite et al. 2017).
The Caribbean area constitutes one of the world’s hotspots of biodiversity. The hotspot of biodiversity concept was defined by Myers (1988) in order to identify the most immediately important areas for conservation of biodiversity. These hotspots hold high endemism levels and have lost at least 70 % of their original natural vegetation (Myers et al. 2000). The characterization of the phytoseiid mite diversity in the Caribbean area is thus contributing to this general topic of conservation.Nine species of phytoseiid mites were found in a first survey conducted in various locations in Guadeloupe and Martinique (Kreiter and Moraes 1997). In a second survey, 41 additional species were recorded from all islands of the French Antilles (Moraes et al. 2000), including three new species to science [Neoseiulus martinicensis Moraes & Kreiter, Amblyseius neoarcus (Moraes & Kreiter), and Metaseiulus (Metaseiulus) neoflumenis Moraes & Kreiter]. In a third survey, conducted mainly in Guadeloupe and Martinique, six additional species were added to the French Antilles fauna, including a new species to science, Neoseiulus cecileae Kreiter (Kreiter et al. 2006). The known number of species from the French Antilles was then of 56. Eleven new species for French Antilles were found from April 2008 to February 2011 during a fourth survey and a new species to Science, Transeius mariae-angeae Kreiter was described (Mailloux et al. 2010; Kreiter et al. 2013).
In conclusion, a total of 67 species belonging to 22 genera were thus known at the beginning of the year 2011 from the French Antilles after these four surveys. These species belong to the three subfamilies: Amblyseiinae with 51 species, Phytoseiinae with 4 species and Typhlodrominae with 12 species.
This paper focuses on results of a fifth survey carried out in Martinique from May 2011 to September 2013 mainly on plants used as cover-crop in citrus orchards in a framework of fruits diversification in the context of agroecology method enhancements.
The study took place in Martinique between May 2011 and September 2013. Plant inhabiting mites were collected from plants used as cover-crops and tested with the aim to evaluate potentialities of these plants to harbour and to release phytoseiid mites in citrus orchards. These plants [Neonotonia wightii (Wight & Arn.) J.A. Lackey, Pueraria phaseoloides (Roxb.) Benth., Macroptilium atropurpureum (DC.) Urb. (the three plants belongs to the Fabaceae); Paspalum notatum Flügge cv. Pensacola (Poaceae)] were planted in experimental plots in CIRAD station, Le Lamentin (Martinique). Mites were collected on these plants and in some orchards on citrus trees and some of these plants when present. Depending on the plants considered, mites were directly collected on leaves or by using the leaf “dipping-shaking-washing-filtering” method of Boller (1984). Mites were then transferred with a fine brush into small plastic vials containing 70 % ethanol.
Plant species were identified according to the nomenclature developed in Fournet (2002).
Mites were then mounted on slides using Hoyer’s medium and identified using a phase and interferential contrast microscope (Leica DMLB, Leica Microsystèmes SAS, Nanterre, France).
Specimens collected in fields in Martinique within this survey were all identified and some type or additional material have been borrowed and studied:- the holotypes of Amblyseius terminatus Chant & Baker and of Typhlodromalus peregrinus (Muma) at the Smithsonian Institution, National Museum of Natural History, Washington DC, USA;- additional material of A. terminatus, the Canadian Collection of Insects, Ottawa, Canada;- the holotype of Typhlodromalus aripo De Leon at the Museum of Comparative Zoology, Invertebrate Zoology Collection in Harvard University, Cambridge, USA;- and the holotypes of Neoseiulus tunus (De Leon) and of N. neotunus (Denmark & Muma) and paratypes of T. aripo, the Florida Department of Agriculture, Gainesville, Florida, USA.
Characters of specimens of 14 species from Martinique and type and additional materials borrowed were measured using a graduate eyepiece (Leica, see above). Drawings of four species were made using a drawing tube attached to the microscope (Leica, see above).
Chant and McMurtry’s (1994, 2007) concepts of the taxonomy of the family Phytoseiidae and the world catalogue database of Demite et al. (2017) were used for faunistical and biogeographical aspects. The chaetotaxy terminologies used in this paper followed those proposed by Lindquist and Evans (1965) as adapted by Rowell et al. (1978) for Phytoseiidae for dorsal and by Chant and Yoshida-Shaul (1991) for ventral idiosomal setae, respectively. Adenotaxy and poroidotaxy terminologies are those proposed by Athias-Henriot (1975).
Numbers of teeth on the fixed and movable cheliceral digits do not include the respective apical teeth. Setae not mentioned in the Results section should be considered as absent.
All measurements are given in micrometers (μm) and presented as the mean in bold followed by the range in parenthesis and if available, the measurement of holotype in italics.
According to Tixier (2012), at least 10 individuals when available were measured in order to have a good assessment of the variability.
Specimens of each species are deposited in the mite collections of Montpellier SupAgro conserved in UMR CBGP INRA/IRD/CIRAD/SupAgro.
The following abbreviations are used in this paper for morphological characters: dsl = dorsal shield length; dsw = dorsal shield width; lisl = Largest inguinal sigilla (= “metapodal plate”) length; lisw = Largest inguinal sigilla (= “metapodal plate”) width; sisl = smallest inguinal sigilla (= “metapodal plate”) length; vsl = ventrianal shield length; vsw ZV2 and anus = ventrianal shield width at ZV2 level and at anus level; scl = spermatheca cervix length; scw = spermatheca cervix width; fdl = fixed digit length; mdl = movable digit length.
The following abbreviations are used in this paper for institutions: CBGP = Centre de Biologie pour la Gestion des Populations; CIRAD = Centre International de Recherche Agronomique pour le Développement; CAEC = Campus Agro-environnemental Caraïbe; INRA = Institut National de la Recherche Agronomique; MSA = Montpellier SupAgro, France; UMR = Unité Mixte de Recherche.
A total of 22 species were found from May 2011 to September 2013 in these surveys. Eight species were already very well-known, very common in French West Indies, already recorded and sometimes re-described in previous papers (Kreiter and Moraes 1997; Moraes et al. 2000; Kreiter et al. 2006, 2013): Arrenoseius urquharti (Yoshida-Shaul & Chant), Amblyseius aerialis (Muma), A. largoensis (Muma), A. tamatavensis Blommers, Euseius ovaloides (Blommers), Paraphytoseius orientalis (Narayanan, Kaur & Ghai), Phytoseiulus macropilis (Banks) and Phytoseius rex De Leon. These species are very common everywhere in French West Indies and giving a very long list of new records has no interest. Measurements of individuals of these eight species are very close to those of original descriptions, of measurements given by several authors and especially very close to those already published in Kreiter and Moraes (1997), Moraes et al. (2000) and Kreiter et al. (2006, 2013).
A catalogue of the 14 remaining species is completed by the available information on the biology and the distribution, along with taxonomical data.
Six species are already known among which four [Neoseiulus longispinosus (Evans), Neoseiulus tunus (De Leon), Proprioseiopsis mexicanus (Garman), and P. ovatus (Garman)] are rather common but some interesting new data and new discussions are provided.
New locations for the two remaining very rarely collected species in the French Antilles (Phytoseius bennetti De Leon and Typhlodromina subtropica Muma & Denmark) are provided and P. bennetti is re-described.
Seven species are more remarkable. Three species are new for French West Indies [Neoseiulus benjamini (Schicha), Neoseiulus paraibensis (Moraes & McMurtry), Transeius terminatus (Chant & Baker) new. comb.]. Four others [Amblyseius collaris Karg, Euseius sibelius (De Leon), Transeius aciculus (De Leon) and Typhlodromalus peregrinus (Muma)] were already known from some islands but are mentioned from Martinique for the first time. Transeius aciculus, T. terminatus and A. collaris are re-described.
And finally, one species (Phytoseius sp.) probably new to Science is unfortunately not described in this paper because only one female was collected and with some broken legs, especially the two leg IV, which prohibit to our point of view any possible description of a new species (but many characters, especially spermatheca and ventrianal shield are totally original among the subfamily).
Amblyseiinae Muma, 1961: 273.
Amblyseiini, Muma, 1961: 68.
Amblyseiina Muma, 1961: 69.
Transeius Chant & McMurtry, 2004: 181.
Typhlodromips aciculus De Leon, 1967: 28; Moraes et al., 1986: 135; Moraes et al., 2004b: 205. Amblyseius aciculus, Moraes et al., 1991: 122; Transeius aciculus, Chant & McMurtry, 2004: 185; Chant & McMurtry, 2007: 71.
This species was described as a Typhlodromips and mentioned in this genus by Moraes et al. (2000) from various islands of French West Indies but not from Martinique. This species was then mentioned as a Transeius (Kreiter et al. 2006) but still from Guadeloupe. Only few specimens of T. aciculus were recorded during previous surveys. This is the first record of T. aciculus from Martinique and the first survey with a lot of specimens collected. It is found in the low vegetation, grasses, especially on companion plants in citrus orchards (Dubois, 2009; Mailloux et al. 2010). Species of the genus Transeius are considered as type III (generalist predators) by McMurtry et al. (2013) but the biology of that species remains totally unknown.The description of De Leon (1967) includes minute drawings, a poor description and is difficult to use for an accurate identification. There are no re-description with new details and new drawings for that species. We re-describe here Transeius aciculus (De Leon) with new measurements, new details on shapes of characters and new drawings.
Description (Table 1 and Figs. 1-2)
Adult Female (Table 1 and Fig. 1) (n = 13)









Dorsum (Fig. 1) — Dorsal shield 278 (263 – 310) long and 157 (138 – 168) wide, slightly reticulated on the anterior dorsum, with 5 solenostomes (gd2, 5, 6, 8 and 9), 7 pairs of poroids, 17 pairs of dorsal setae and 2 pairs of sub-lateral setae: j1 21 (20 – 23), j3 30 (25 – 33), j4 14 (13 – 15), j5 11 (10 – 13), j6 11 (10 – 13), J2 13 (10 – 13), J5 6 (5 – 8), z2 19 (18 – 20), z4 29 (28 – 35), z5 8, Z1 13 (10 – 15), Z4 56 (50 – 60), Z5 65 (60 – 70), s4 61 (58 – 63), S2 28 (25 – 33), S4 11 (10 – 13), S5 9 (8 – 10), r3 28 (25 – 30), R1 13 (10 – 15). All setae smooth except Z4 and Z5 which are serrated.
Peritreme (Fig. 1A) — Extending to the level of j1.
Venter (Fig. 1B) — All ventral shields smooth. Sternal shield not very large, with 3 pairs of setae and 2 pairs of pores; 1 pair (st4) out of the sternal shield, on a small metasternal shield with one pair of pores; posterior margin slightly concave. Distances between st1-st1 53 (50 – 55), st1-st3 56 (55 – 58), st2-st2 63 (60 – 65), st3-st3 69 (68 – 73), st4-st4 87 (73 – 100), st5-st5 56 (50 – 60). Two pairs of inguinal sigilla (called also “metapodal shields”) 17 (15 – 18), long and 5, wide for the largest, 12 (10 – 13), long and very thin for the smallest one. Ventrianal shield almost rectangular with 3 pairs of pre-anal setae (JV1, JV2 and ZV2) and one pair of large elliptical pre-anal solenostomes. Membrane surrounding ventrianal shield with 4 pairs of setae (ZV1, ZV3, JV4 and JV5) and 6 pairs of poroids (called also sometimes “platelets”); ventrianal shield long and smooth 93 (88 – 100) long, 57 (50 – 60) wide at level of anterior corners (ZV2) and 54 (50 – 60) wide at level of anus. JV5 47 (40 – 50).
Chelicera (Fig. 1C) — Fixed digit 27 (25 – 28), with 9 teeth and movable digit 30 (29 – 30) with 3 teeth. Pilus dentilis not visible.
Spermatheca (Fig. 1D) — Spermatheca (called also “insemination apparatus”) pocular, 10 (8 – 13) long and 8 (7 – 8) large. Minor and major ducts visible on almost all specimens.
Legs (Fig. 1E) — Macrosetae on leg IV as other species of Transeius: SgeIV 36 (33 – 38), StiIV 23 (20 – 25), StIV 42 (38 – 45). All macrosetae whip-like. Chaetotactic formula of genu II: 2-2/0, 2/1-1; genu III: 1-2/1, 2/0-1.
Adult Male (Table 1 and Fig. 2) (n = 3). In italics bold , measurements of a paratype male (in Moraes et al. 1991).
Dorsum (Fig. 2A). Dorsal shield pattern similar to female (ornamentation, solenostomes and poroids), 216 (213 – 220) 223 long and 132 (125 – 138) 134 wide. Setae j1 18 (15 – 20) 17 , j3 27 (25 – 28) 29 , j4 14 (13 – 15) 12 , j5 13 10 , j6 10 (8 – 13) 10 , J2 9 (8 – 10) 10 , J5 5 2 , z2 17 (15 – 18) 14 , z4 27 (25 – 28) 29 , z5 8 5 , Z1 11 (10 – 13) 10 , Z4 39 (38 – 40) 36 , Z5 46 (43 – 48) 43 , s4 53 (50 – 55) 48 , S2 21 (18 – 23) 22 , S4 10 10 , S5 8 7 , r3 21 (20 – 23) 24 , R1 11 (10 – 13) ? . Setae r3 and R1 inserted on the lateral cuticle. All setae smooth except Z4 and Z5 which are serrated.
Peritreme (Fig. 2A) — Extending anteriorly to the level of j1.
Venter (Fig. 2B) — Sternogenital shield smooth, with 5 pairs of setae and 2 pairs of lyrifissures. Distances between st1-st1 41 (40 – 43), st1-st5 99 (95 – 103), st2-st2 54 (53 – 55) 53 , st3-st3 50, st4-st4 39 (38 – 40), st5-st5 30. Ventrianal shield sub-triangular and slightly reticulated, 93 (88 – 95) long, 94 (80 – 105) wide at level of anterior corners and 35 at level of anus, with 3 pairs of pre-anal setae (JV1, JV2 and ZV2), 1 pair of pre-anal solenostomes and 1 pair of poroids; ventrianal shield not fused with the peritremal shields. Membrane surrounding the ventrianal shield with only one pair of setae (JV5) and no pore; JV5 30 (28 – 35) long, smooth.
Chelicera (Fig. 2C) — Fixed digit 20 (25 – 28), with 9 teeth and movable digit 21 (20 – 23) with 3 teeth. Shaft of the spermatodactyl 12 (10 – 13). Pilus dentilis not visible.
Legs — No macrosetae on the three first legs. Macrosetae only on leg IV as other species of Transeius: SgeIV 28 24 , StiIV 18 14 , StIV 37 (35-38) 36 . All macrosetae whip-like. Chaetotactic formula of genu II and genu III as females.
Specimens examined — 70 ♀♀ + 3 ♂♂ in total (13 ♀♀ + 3 ♂♂ measured). Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 48 ♀♀ + 2 ♂♂ on Neonotonia wightii (Wight & Arn.) J.A. Lackey, 12 ♀♀ on Pueraria phaseoloides (Roxb.) Benth., 9 ♀♀ + 1 ♂ on Macroptilium atropurpureum (DC.) Urb.; 1 ♀ collected on Paspalum notatum Flügge cv. Pensacola; all mites collected between 08-01 and 18-09-2013.
Previous record — Brazil, Colombia, Guadeloupe, Jamaica, Marie-Galante, Mexico, Panama, Dominican Republic, Saint-Martin and Trinidad.
Remarks — The specimens collected in Martinique (Table 1) are very similar to those collected in Guadeloupe except from some slightly greater setae (J2, Z5, S4). They are also very similar to the one of the type specimens but setae are all slightly longer. Specimens from Colombia reported by Moraes et al. (1991) show a shorter S2 than the types.
Amblyseius terminatus Chant & Baker, 1965: 25; Chant & McMurtry, 2004: 197; Chant & McMurtry, 2007: 81.
Typhlodromalus terminatus, Moraes et al., 1986: 135; Denmark et al., 1999: 63 ; Moraes et al., 1986: 135; Moraes et al., 2004b: 205.
This species was described as an Amblyseius by Chant and Baker (1965), reported in this genus in the Phytoseiidae Database of Demite et al. (2017). We propose in this paper a new combination. This is the first record of this species for French Caribbean Islands. The description of Chant and Baker (1965) is now old, poor, with very minute drawings, difficult to use and there are no redescription with new drawings for that species. We re-describe here Transeius terminatus (Chant & McMurtry) new. comb. with new measurements, new details on the shape of some characters and new drawings. Species of the genus Transeius are considered as type III (generalist predators) by McMurtry et al. (2013) but the biology of this species remains totally unknown.
Description
Adult Female (Fig. 3) (n = 5). In italics bold, measurements of the holotype.



Dorsum (Fig. 3A) — Dorsal shield 325 (322 – 350) 320 long and 199 (183 – 213) 200 wide, smooth, with no solenostomes, 6 pairs of poroids (identical in the holotype ), 17 pairs of dorsal setae and 2 pairs of sub-lateral setae: j1 23 (21 – 23) 23 , j3 31 (28 – 33) 28 , j4 5 ? , j5 5 ? , j6 7 (5 – 8) ? , J2 7 (5 – 8) ? , J5 10 (9 – 10) 8 , z2 12 (10 – 13) ? , z4 28 (23 – 33) 30 , z5 5 ? , Z1 11 (10 – 13) 10 , Z4 59 (55 – 60) 62 , Z5 72 (68 – 75) 70 , s4 50 (48 – 50) 50 , S2 24 (20 – 25) 23 , S4 12 (10 – 13) 10 , S5 13 (10 – 15) 13 , r3 26 (25 – 28) ? , R1 19 (18 – 20) 20 . All setae smooth except Z4 and Z5 which are serrated (identical in the holotype ).
Peritreme (Fig. 3A) — Extending to the level of j1 (identical in the holotype).
Venter (Fig. 3B) — All ventral shields slightly reticulated. Sternal shield not very large, with 3 pairs of setae and 2 pairs of pores; 1 pair (st4) out of the sternal shield, on a small metasternal shield with one pore; posterior margin slightly concave (identical in the holotype). Distances between st1-st1 54 (50 – 56) 53 , st1-st3 56 (48 – 60) 60 , st2-st2 63 (63 – 65) 65 , st3-st3 75 (73 – 78) 73 , st4-st4 72 (65 – 75) 68 , st5-st5 71 (65 – 75) 68 . Two pairs of inguinal sigilla (called also “metapodal shields”) 22 (20 – 23) 23 long and 3 3 wide for the largest, 10 (8 – 15) 13 long and very thin for the smallest one. Ventrianal shield pentagonal with 3 pairs of pre-anal setae, (JV1, JV2 and ZV2) and one pair of large elliptical pre-anal solenostomes. Membrane surrounding ventrianal shield with 4 pairs of setae (ZV1, ZV3, JV4 and JV5) and 5 pairs of poroids (called also “platelets”), the last one near the anus not visible on the same focus and thus not drawn on Figure 10; ventrianal shield 107 (100 – 113) 108 long, 94 (93 – 95) 90 wide at level of anterior corners and 69 (65 – 75) 70 wide at level of anus. JV5 53 (50 – 55) 50 long and smooth.
Chelicera (Fig. 3C) — Fixed digit 32 (30 – 33) 33 with 4 teeth 4 and movable digit 33 33 with 1 tooth 1 . Pilus dentilis not visible.
Spermatheca (Fig. 3D) — Spermatheca (called also “insemination apparatus”) saccular, 19 (15 – 20) 20 long and 5 5 large. Minor and major ducts visible on few specimens.
Legs (Fig. 3E) — Macrosetae on legs IV: SgeIV 41 (40 – 43) 40 , StiIV 23 (20 – 25) 23 , StIV 51 (50 – 53) 53 . All macrosetae whip-like. Chaetotactic formula of genu II: 2-2/0, 2/1-0; genu III: 1-2/1, 2/0-1 (identical in the holotype).
Adult male. Unknown before and not collected during this study.
Specimens examined — Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 1 ♀ on N. wightii, 06-03-2013; Sainte-Anne, Conseil Général (long. 14°26′N, lat. 60°52′O, alt. 26 m), 2 ♀♀ N. wightii, 9-X-2012; Saint-Esprit, Mrs Solis’ farm (long. 14°33′N, lat. 60°55′O, alt. 46 m), 1 ♀ on lime trees [C. latifolia (Tanaka ex Yu.Tanaka) Tanaka, family Rutaceae], 20-10-2011; Le Lorrain, Mr. Trepon’s farm (long. 14°49′N, lat. 61°50′O, alt. 117 m), 1 ♀ on various weeds in a citrus orchard, 25-11-2012; Saint-Pierre, Habitation Parnasse (long. 14°75′N, lat. 61°94′O, alt. 284 m), 1 ♀ on various weeds in a citrus orchard [Citrus sinensis (L.) Osbeck, Rutaceae], 30-11-2011.
We have also examined: one holotype female on one slide with label: Managa, San Pedro, Honduras, 01-02-1959 on Baltimora recta L., J.G. Matthysse (USNM n° 30008), 3 ♀♀ (and additionally: 1 ♂ of Typhlodromalus aripo De Leon, 2 Astigmatina and 3 Thysanoptera on the same slide) borrowed to the National Museum of Natural History; 2 ♀♀ on two slides with label: Belize, Toledo district, near Upper Bladen Branch Rivers, sapling, climax forest, 11-11-1965, borrowed to the Canadian National Collection. Notice that they were identified as T. terminatus but are actually 2 ♀♀ of the genus Amblyseius, of the species group chiapensis, probably cupulus Denmark & Muma but this must be confirmed.
Previous record — Honduras.
Remarks — The measurements of the five specimens collected in Martinique are very close to the measurements of original specimens collected in Manaca, San Pedro, Honduras, on Baltimora recta L. (Asteraceae) by Chant and Baker (1965). This species was mentioned in the Moraes et al. (1986)’s catalogue of the family Phytoseiidae and in the Denmark et al. (1999)’s catalogue of Phytoseiidae of Central America as Typhlodromalus terminatus and in Chant and McMurtry (2004, 2007), in Prasad (2012) and in the phytoseiid Database of Demite et al. (2017) as Amblyseius terminatus. Examination of the holotype has shown that this species is actually neither an Amblyseius nor a Typhlodromalus but a real Transeius sensu Chant and McMurtry (2004) and that our specimens are similar to the holotype and belong to this genus. Macrosetae are actually not present on all legs but only on leg IV and the ratio length seta s4 / length S2 is much less than 2.7. We are proposing a new combination, Transeius terminatus (Chant and Baker) new comb.
Amblyseius Berlese, 1914: 143.
Amblyseius collaris Karg, 1983: 317; Moraes et al., 1986: 11; Denmark & Muma, 1989: 48; Moraes et al., 2004b: 20; Chant & McMurtry, 2004: 201; Chant & McMurtry, 2007: 78.
This species was already known from Guadeloupe but only recorded with one female (Moraes et al. 2000). This is the first record of A. collaris from Martinique. Species of the genus Amblyseius are considered as type III (generalist predators) by McMurtry et al. (2013) but the biology of that species remains totally unknown. The description of Karg (1983) and of Denmark and Muma (1989) are quite poor in details, difficult to use for an accurate identification and there are no re-description with new drawings and new details for that species. We re-describe here Amblyseius collaris Karg with new measurements, new details on the shape of some characters and new drawings.
Description (Table 2 and Fig. 4)
Adult Female (Fig. 4) (n = 3)
Dorsum (Fig. 4A) — Dorsal shield 371 (364 – 375) long and 272 (262 – 280) wide, smooth, with no visible solenostomes and pairs of poroids, 17 pairs of dorsal setae and 2 pairs of sub-lateral setae: j1 29 (28 – 30), j3 37 (36 – 37), j4 5, j5 5, j6 5, J2 9 (6 – 10), J5 8, z2 13, z4 5, z5 5, Z1 8, Z4 176 (175 – 178), Z5 356 (346-362), s4 133 (130 – 135), S2 6 (5 – 8), S4 12 (12 – 13), S5 6 (5 – 8), r3 10, R1 15 (10 – 20). All setae smooth.






Peritreme (Fig. 4A) — Extending to the level of j1.
Venter (Fig. 4B) — All ventral shields smooth. Sternal shield not very large, with 3 pairs of setae and 2 pairs of pores; 1 pair (st4) out of the sternal shield, on a small metasternal shield with one pore; posterior margin straight. Distances between st1-st1 62 (60 – 63), st1-st3 65 (63 – 67), st2-st2 75, st3-st3 83 (80 – 85), st4-st4 84 (78 – 93), st5-st5 78 (75 – 80). Two pairs of inguinal sigilla (called also “metapodal shields”) 22 long and 5 wide for the largest, 18 (15 – 20) long and 2 for the smallest one. Ventrianal shield pentagonal with 3 pairs of pre-anal setae (JV1, JV2 and ZV2) and one pair of elliptical pre-anal solenostomes. Membrane surrounding ventrianal shield with 3 pairs of setae (ZV1, ZV3, JV4 and JV5) and 5 pairs of poroids (called also “platelets”), the last one near the anus not visible on the same focus and thus not drawn on Figure 15; ventrianal shield 116 (113 – 120) long, 94 (92 – 95) wide at level of anterior corners and 75 wide at level of anus. JV5 74 (72 – 75) long and smooth.
Chelicera (Fig. 4C) — Fixed digit 29 (28 – 30) with 15 teeth and movable digit 35 (33 – 36) with 3 teeth. Pilus dentilis not visible.
Spermatheca (Fig. 4D) — Spermatheca (called also “insemination apparatus”) fundibular, 24 (22 – 25) long and 5 (3 – 5) wide. Minor and major ducts visible on the three specimens.
Legs (Fig. 4E) — Macrosetae on all legs: SgeI 47 (45 – 48), SgeII 36 (35 – 37), SgeIII 64 (63 – 65), StiIII 45 (43 – 48), SgeIV 150 (144 – 155), StiIV 96 (94 – 98), StIV 72 (70 – 75). All macrosetae whip-like. Genua 2 and 3 with seven setae. Chaetotactic formula of genu II: 2-2/0, 0/2-1; genu III: 1-2/1, 2/0-1.
Adult male — Unknown and no males were collected during the survey.
Specimens examined — Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 1 ♀ on P. phaseoloides, 4-12-2012; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 1 ♀ on possibly (not sure) Teramnus labialis (L. f.) Spreng. (Fabaceae), 20-10-2011; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 1 ♀ on Citrus leaves (C. sinensis et C. latifolia), 17-07-2012.
Previous record — Brazil (Amazonas), Costa Rica, Guadeloupe, USA (Florida), Venezuela.
Remarks — Measurements of the three females collected (Table 2) fit very well with the measurements of the holotype female (Karg 1983; Denmark and Muma 1989) except for slightly longer s4, Z4 and Z5 and SgeIV and StiIV. These are the longest setae and variations are always more important (Tixier 2012). Measurements (Table 2) fit also very well with measurements of specimens collected in Costa Rica (Castro et al. 2010) except in this case shorter s4, Z4 and Z5 and SgeIV and StiIV in specimens from Martinique. These long setae may be very variable for the genus Amblyseius and at least for that species.
Proprioseiopsina Chant & McMurtry, 2004: 219.
Proprioseiopsis Muma, 1961: 277.
Amblyseiopsis mexicanus Garman, 1958: 75.
Amblyseius mexicanus, Moraes & McMurtry, 1983: 134.
Proprioseiopsis mexicanus, Muma & Denmark, 1970: 48; Denmark & Muma, 1973: 237; Moraes et al., 1986: 118; Kreiter & Moraes, 1997: 379; Moraes et al., 2004b: 181; Chant & McMurtry, 2005a: 13; Chant & McMurtry, 2007: 89.
This species was already known from all islands of French West Indies (Kreiter & Moraes 1997; Moraes et al. 2000, Kreiter et al. 2006; Mailloux et al. 2010) but it was found only in very large number during a previous study on companion plant in Guadeloupe (Mailloux et al. 2010) and in an actual study in La Réunion (Le Bellec, unpublished data). This species seems to be very abundant on weeds in the lower vegetation. Phytoseiid mites of the genus Proprioseiopsis have been found mainly in ground surface, humus, litter, soil, moss or on grass (Muma and Denmark 1970; McMurtry et al. 2015). Proprioseiopsis mexicanus population increase when fed Tetranychus urticae Koch eggs (Mégevand et al. 1993) and this species seems to be a good predator of thrips (Kreiter, unpublished data). It is one of the prevailing phytoseiid species on citrus orchards in Alabama (Fadamiro et al. 2009). Denmark and Evans (2011) mentioned that the species can be reared on T. urticae and Oligonychus pratensis (Banks) and is associated with Bryobia praetiosa Koch, Bryobia sp. and Panonychus ulmi (Koch). It was also found in association with Tetranychus evansi Baker & Pritchard (Furtado et al. 2014) but mentioned as a poor predator of that species. The biology of this species is however almost unknown.
Specimens examined — 25 ♀♀ + 3 ♂♂ in total (12 ♀♀ + 3 ♂♂ measured). Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 4 ♀♀ + 2 ♂♂ on P. notatum, 11 ♀♀ + 1 ♂ on N. wightii, 1 ♀ on M. atropurpureum collected between 18-VI and 19-09-2012, and 1 ♀ on P. phaseoloides collected 20-08-2013; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 3 ♀♀ on citrus leaves (C. sinensis et C. latifolia), 1-07-2012; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 1 ♀ on possibly (not sure) T. labialis, 20-10-2011; Le Lorrain, Mr. Trepon’s farm (long. 14°49′N, lat. 61°50′O, alt. 117 m), 2 ♀♀ on various weeds in a citrus orchard, 25-11-2012; Saint-Pierre, Habitation Parnasse (long. 14°75′N, lat. 61°94′O, alt. 284 m), 1 ♀ on various weeds in a citrus orchard, 30-11-2011; Le François, Mr. Peronnet’s farm, La Digue François (long. 14°34′N, lat. 61°53′O, alt. 59 m), 1 ♀ on leaves of lime trees C. latifolia.
Previous record — Australia, Benin, Brazil (Bahia, Maranhão, Mato Grosso do Sul, Paraiba, Pernambuco, Rondonia, São Paulo), Canada (Northwest Territories, Ontario, Quebec), China (Jiangxi), Colombia, Costa Rica, Cuba, Galapagos, Ghana, Guadeloupe, Hawaii, Jamaica, Kenya, Madagascar, Martinique (only 2 ♀♀ in Kreiter et al. 2006), Mexico, New Zealand, Nicaragua, Panama, Peru, Réunion Island, Saudi Arabia, Taiwan, United Arab Emirates, USA (Alabama, Arizona, California, Florida, Georgia, Iowa, Kansas, Louisiana, Maryland, Minnesota, Missouri, New Jersey, North Carolina, Ohio, Pennsylvania, Texas, West Virginia, Wisconsin).
Remarks — Measurements of the twelve females (Table 3) fit very well with the those of the holotype female except for slightly longer s4, Z4 and Z5. Measurements of females (Table 3) fit also very well with those of the specimens collected in Peru (Guanilo et al. 2008a) except shorter j3 and longer Z5 in specimens from Martinique. This is the same for male measurements (Table 4) with additionally shorter s4, Z4 and Z5 in specimens from Martinique.






Amblyseius ovatus Garman, 1958: 78.
Proprioseiopsis ovatus, Moraes et al., 1986: 121; Moraes et al., 2004b: 184; Chant & McMurtry, 2005a: 15; Chant & McMurtry, 2007: 89.
Amblyseiulus cannaensis Muma, 1962: 4, synonymy according to Denmark & Evans, 2011: 214.
Amblyseius cannaensis, Moraes & McMurtry, 1983: 132; Moraes & Mesa, 1988: 77; Moraes et al., 1991: 126.
Proprioseiopsis cannaensis, Muma & Denmark, 1970: 38; Kreiter & Moraes, 1997: 379.



This species was already known from Guadeloupe, Marie-Galante and Martinique (Kreiter & Moraes 1997; Moraes et al. 2000; Mailloux et al. 2010) but misidentified as P. mexicanus. This species was found in very large number only during a previous study on companion plant in Guadeloupe (Mailloux et al. 2010) and in a recent study in La Réunion (Le Bellec, unpublished data). In other habitats, this species seems to be very rare. This species like P. mexicanus seems to be abundant on weeds in the lower vegetation. Denmark and Evans (2011) indicated that this species is associated with O. pratensis and Brevipalpus sp. It was found in association with T. evansi (Furtado et al. 2014) but mentioned as poor predator of that species. The biology of this species is totally unknown.
Specimens examined — 36 ♀♀ in total (11 measured). Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 10 ♀♀ on N. wightii, 1 ♀ on M. atropurpureum and 1 ♀ on P. phaseoloides collected between 18-06 and 19-09-2012, and 1 ♀ on M. atropurpureum collected 6-03-2013; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 9 ♀♀ collected 17-07-2012, 2 ♀♀ collected 25-07-2012 and 12 ♀♀ collected 3-05-2012 on citrus leaves (C. sinensis et C. latifolia).
Previous record — Argentina, Australia (New South Wales, Queensland), Brazil (Alagoas, Amazonas, Bahia, Goiás, Maranhão, Mato Grosso do Sul, Minas Gerais, Pernambuco, Piaui, Rio Grande do Sul, Roraima, São Paulo, Tocantins), Canada (British Columbia), China (Guangdong, Hainan), Columbia, Costa Rica, Cuba, DR Congo, Ecuador, Egypt, Fiji, Ghana, El Salvador, Guadeloupe, Guyana, Hawaii, Honduras, India (West Bengal), Lesotho, Madagascar, Malawi, Malaysia, Marie-Galante, Martinique, Mozambique, New Caledonia, Papua New Guinea, Paraguay, Peru, Philippines, Puerto Rico, Saudi Arabia, Sierra Leone, South Africa, Spain, Sri Lanka, Taiwan, Thailand, Turkey, USA (Arizona, California, Florida, Kansas, Louisiana, Minnesota, Missouri, New Mexico, North Carolina, Utah, Washington), Venezuela, Zaire, Zimbabwe.
Remarks — Measurements in Table 5 show great variations. Those of the 11 females from Martinique fit very well with the measurements of the holotype female except for slightly longer s4, Z4, Z5 and SgeIV and shorter StIV. Measurements of females fit also very well with those of specimens collected in Peru and Argentina (Guanilo et al. 2008a, b). Some measurements of the two specimens from Ecuador show differences (j3, z2, s4, Z4, Z5 far longer and Z1, S2, S4, r3 shorter). This might be an indication of another species of Proprioseiopsis involved.
Euseiini Chant & McMurtry, 2005b: 191.
Euseiina Chant & McMurtry, 2005b: 209.
Amblyseius (Amblyseius) section Euseius, Wainstein, 1962: 15; Euseius De Leon, 1967: 86.
Amblyseius (Typhlodromalus) sibelius De Leon, 1962: 21.
Euseius sibelius, Muma & Denmark, 1970: 98; Feres & Moraes, 1998: 128; Moraes et al., 1986: 54; Moraes et al., 2004b: 83; Chant & McMurtry, 2005b: 216; Chant & McMurtry, 2007: 123.
Euseius subalatus De Leon, 1965: 127 (synonymy according to Muma & Denmark, 1970).



This species was already known from Guadeloupe and Les Saintes (Moraes et al. 2000) but not from Martinique. This is the first record of this species from this island. This species seems to be rather rare on companion plants as it was collected in few numbers. It was also found in association with T. evansi (Moraes and McMurtry 1983) but probably an inefficient predator of that species. The biology of this species is totally unknown.
Specimens examined — 36 ♀♀ + 1 ♂ in total (12 ♀♀ measured but 1 ♂ in bad state not measured). Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 5 ♀♀ + 1 ♂ on N. wightii, 5 ♀♀ on M. atropurpureum and 26 ♀♀ on P. phaseoloides collected between 23-07-2012 and 18-09-2013.
Previous record — Brazil (Bahia, Ceará, Distrito Federal, Espirito Santo, Goiás, Mato Grosso do Sul, Minas Gerais, Paraiba, Paraná, Pernambuco, Piaui, Rio Grande do Sul, Santa Catarina, Sao Paulo), Colombia, Dominican Republic, El Salvador, Guadeloupe, Honduras, Jamaica, Les Saintes, Peru, Puerto Rico, USA (Florida), Venezuela.
Remarks — Measurements in the Table 6 show only slight variations. Measurements of the twelve females fit very well with those of the holotype females of E. sibelius and E. subalatus. Measurements of females fit also very well with measurements of specimens collected in Dominican Republic (Ferragut et al. 2011), Brazil and Peru (Guanilo et al. 2008a).
Typhlodromalina Chant & McMurtry, 2005b: 195.
Amblyseius (Typhlodromalus) Muma, 1961: 288; Typhlodromalus, De Leon, 1966: 87.
Typhlodromus peregrinus Muma, 1955: 270;
Typhlodromus (Amblyseius) peregrinus Chant, 1959: 97.
Typhlodromalus peregrinus, Muma & Denmark, 1970: 88; Moraes et al., 1986: 132; Moraes et al., 2004b: 202; Zacarias & Moraes, 2001: 582; Chant & McMurtry, 2005a: 199; Chant & McMurtry, 2007: 111. Amblyseius peregrinus, McMurtry, 1983: 255. Moraes et al., 1991: 130;
Typhlodromus (Amblyseius) robineae Chant, 1959: 98;
Typhlodromus (Amblyseius) evansi Chant, 1959: 99;
Typhlodromus (Amblyseius) primulae Chant, 1959: 99 (synonymies, according to Muma, 1964).
This species is very common on citrus (Muma 1955, 1967; Peña, 1992; Childers 1994; Villanueva and Childers 2004, 2005; Fadamiro et al. 2008, 2009) and solanaceous plants (McMurtry 1983; Fiaboe et al. 2007) in several countries and is very often reported as the most abundant species. Typhlodromalus peregrinus can be found at the underside of mature citrus leaves, inside tree canopy, under empty scale armour, clump and dead scale insects, whitefly exuvia, sooty mould and mines of Phyllocnistis citrella Stainton (Muma 1967; Childers 1994; Villanueva and Childers 2011). Muma (1969) reported that T. peregrinus was able to reproduce and develop on Panonychus citri (McGregor) but perform better on eggs and crawlers of Parlatoria pergandii Comstock, and Eotetranychus sexmaculatus (Riley). This phytoseiid was also reported to feed on Phyllocoptruta oleivora (Ashmead), with at least partial rust mite suppression on lime (Peña, 1992). Thus, T. peregrinus seems to be a generalist species with the ability to reproduce and develop on the two key pests of Guadeloupe and Martinique citrus, P. citri and P. oleivora and probably several occasional pests. Its optimal preys were evaluated as Aleyrodidae, Coccidae, and Tetranychidae by Muma (1971).
The following organisms were evaluated by Fouly et al. (1995) as suitable diet in the laboratory at 26°C: all stages of T. urticae; immature stages of P. citri; pollens of Malephora crocea (Jacquemin) Schwant., Quercus virginiana Miller, and Typha latifolia L.
The occurrence of high densities of this species on ground cover vegetation (weeds) of Alabama citrus orchards (Fadamiro et al. 2008, 2009) can be explained by the possibility that grasses may serve as overwintering sites and alternative food sources, which is probably the most important factors in French West Indies citrus orchards as there is no overwintering in citrus crop in this tropical area.
Typhlodromalus peregrinus was collected from 64 ground cover plants in Florida citrus fields (Childers and Denmark, 2011) with highest numbers found on the following plants: Bidens alba (L.) DC., Solanum americanum Miller (one plant of the ground cover on which T. peregrinus was collected previously in Guadeloupe), Amaranthus spinosus L., Gnaphalium pensylvanicum (Willdenow) Cabrera, Lantana camara L. and Dysphania ambrosioides (L.) Mosyakin & Clemants.
In Florida, the highest numbers of T. peregrinus in ground cover corresponded with peaks in thrips numbers, suggesting possible predation on one or more species of thrips occurring. Childers and Denmark (2011) suggest that this species should therefore be evaluated as a predator of thrips larvae and/or adults. Significant increases in numbers of T. peregrinus were also correlated with increased levels of several pollen species on citrus leaves (Villanueva and Childers 2004).
Thus, considering all these elements, it is possible that T. peregrinus may constitute a key species in citrus orchards in French West Indies: in Guadeloupe where it is abundant on companion plants in citrus orchard (Kreiter et al. 2013) and in Martinique apparently in the same way in the case of this study.
Specimens examined — 75 ♀♀ + 9 ♂♂ in total (13 ♀♀ + 6 ♂♂ measured). Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 44 ♀♀ + 4 ♂♂ on N. wightii, 23 ♀♀ + 5 ♂♂ on P. phaseoloides, 6 ♀♀ on M. atropurpureum and 1 ♀ on P. notatum collected between 20-08-2012 and 18-09-2013; Le Lorrain, Mr. Trepon’s farm (long. 14°49′N, lat. 61°50′O, alt. 117 m), 1♀ on various weeds in a citrus orchard, 25-11-2012.
We have also examined: one holotype and four paratype ♀♀ (all measured) and one paratype ♂ and six paratype immatures (not measured) of Typhlodromalus peregrinus (Muma) in one slide with label: Minneola, Florida, 23-01-1952, on scaly orange leaves, M.H. Muma coll., borrowed at the National Museum of Natural History in Washington DC, USA; one holotype ♀ (measured) of Typhlodromalus aripo (De Leon) borrowed at the Museum of Comparative Zoology in the University of Harvard, Cambridge, USA; and one paratype ♀ (measured) and one paratype nymph (not measured) of T. aripo, holotype and paratypes in three slides with the same label: Trinidad, Upper Aripo Valley, 6-10-1963 on Solanum stromonifolium, Bennett and De Leon (n° 2435-1c), for comparison with T. peregrinus.
Previous Records — Argentina, Brazil (Pernambuco, São Paulo), Colombia, Costa Rica, Cuba, Dominican Republic, Ecuador, Guadeloupe, Guatemala, Guyana, Hawaii, Honduras, Mexico, Nicaragua, Peru, Puerto Rico, Suriname, USA (District of Columbia, Florida, Georgia, Louisiana, Maryland, Massachusetts, Missouri, New Jersey, North Carolina, Oklahoma, Pennsylvania, South Carolina, Tennessee, Texas, Virginia), Venezuela.









Remarks — Measurements in the Table 7 show low variations. Measurements of the 13 females fit very well with all those of all other specimens from all other locations. The maximum divergence is observed with measurements of the holotype especially with the longer Z5 and StIV in the holotype and the longer j6, z4, Z1, s4, S2 and S4 in specimens from Martinique. All measurements obtained for males (Table 8) are very close. Muma and Denmark (1962) pointed out that T. peregrinus is a highly variable species in relation to dorsal setal lengths, shape of the ventrianal shield and leg macrosetae. McMurtry (1983) stated that T. peregrinus is very close to T. aripo (De Leon) and that detailed comparative studies were necessary in order to determine if these species are both valid or not. In the study of Moraes and Mesa (1988), T. peregrinus was separated from T. aripo based only on some differences in setal lengths. In T. peregrinus, z4 is nearly 20 % longer than z2, whereas in T. aripo z4 is nearly twice longer than z2. These authors considered that T. peregrinus showed generally shorter setae j3, z4, Z4, Z5 and longer j4, j5, j6 and J2. Looking at the table 9, j3 is equal for both species, Z5 is longer in T. peregrinus and not shorter, and all setae j-J mentioned as longer in T. peregrinus are actually shorter. If we compare our measurements to measurements of type material of both species, if more lengths correspond to T. peregrinus, some data are very confusing as they are closer to T. aripo. The synonymy between these two species is consequently suspected.
Neoseiulini Chant & McMurtry, 2003a: 6.
Neoseiulus Hughes, 1948: 141.
Amblyseius benjamini, Schicha, 1981; Amblyseius (Amblyseius) benjamini, Ueckermann & Loots 1988.
Neoseiulus benjamini, Moraes et al., 1986: 72; Moraes et al., 2004b: 108; Chant & McMurtry, 2003: 27; Chant & McMurtry, 2007: 25.
Neoseiulus benjamini (Schicha) was previously known from Australia and South Africa (Schicha 1981; Ueckermann and Loots 1988; Beard 2001) and found recently in the Neotropical area, in Brazil where it seems to be quite common in several states (Lofego et al. 2009; Rezende and Lofego 2011, 2012; Demite et al. 2011, 2012; Rezende et al. 2012). This is the first record of this species in the French Caribbean islands. Lofego et al. (2009) found a great variation in the number of teeth on both cheliceral digits, even between right and left chelicerae of the same individual. Neoseiulus benjamini belongs to paspalivorus species group (14 species) of the large genus Neoseiulus and it is more similar to N. mumai (Denmark), N. paspalivorus (De Leon) and N. baraki (Athias-Henriot). Neoseiulus paspalivorus was previously found in Guadeloupe in two locations (Moraes et al. 2000; Mailloux et al. 2010, specimens of N. paspalivorus misidentified as N. baraki; Kreiter et al. 2013, correct identification as N. paspalivorus) but not in Martinique. However N. paspalivorus differs from N. benjamini by having the pre-anal pores longitudinally aligned with the base of JV2 and setae Z4 and StIV shorter (12 and 18 μm respectively against 18 and 25-28 respectively for our two specimens). Whether these differences can really allow distinctions between different species and not variations of the same or disable to distinguish a significant number of cryptic species demands a further investigation. Molecular and other tools (Tixier et al. 2009; Famah Sourassou et al. 2012) would be of great help for not only a lot of phytoseiid mites identification but also for that species group in particular. This species was found on pineapples associated with Dolichotetranychus floridanus (Banks) and Bryobia tuberosa Meyer (Schicha 1987) but most of the biology of N. benjamini remains totally unknown.



Specimens examined — Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 1 ♀ on N. wightii, 15-VII-2013; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 1 ♀ on citrus leaves (C. sinensis et C. latifolia), 17-VII-2012.
Previous record — Australia (New South Wales, Queensland), Brazil (Distric Federal, Goiás, Minas Gerais, São Paulo, Tocantins), South Africa.
Remarks — The measurements reported in the table 10 of the two specimens collected during this study agree well with measurements of specimens of Lofego et al. (2009) from Brazil and even also with the holotype of Schicha (1981), with only very slight differences.
Typhlodromus longispinosus Evans, 1952: 413; Evans, 1953: 465; Womersley, 1954: 177; Ehara, 1958: 55; Typhlodromus (Amblyseius) longispinosus, Chant, 1959: 74;Amblyseius longispinosus, Corpuz and Rimando, 1966: 129; Schicha, 1975: 103;
Neoseiulus longispinosus, Moraes et al., 1986: 85; Moraes et al., 2000: 245; Moraes et al., 2004b: 129; Chant & McMurtry, 2003: 37; Chant & McMurtry, 2007: 29.
This species was already mentioned from Guadeloupe and other Islands of the French Antilles (Moraes et al. 2000; Mailloux et al. 2010; Kreiter et al. 2013) but only in very few localities on various host plants. It is distributed in many countries of the world, mainly in tropical areas.
The biology of this species has been studied for pest control purposes including side effects of acaricides (Bin Ibrahim and Tan 2000). The activity, feeding, development, predation, cannibalism, intra-guild predation and behaviour have been extensively studied by several authors (Schausberger and Croft 1999a, b; Croft et al. 1999a, b; Schausberger and Croft 2000a, b; Blackwood et al. 2001). It was found very rarely except in a study on companion plants in citrus orchards in Guadeloupe (Mailloux et al. 2010; Kreiter et al. 2013) and La Réunion (Le Bellec, unpublished data). This species seems to be more common on grasses of the lower vegetation, especially Fabaceae with populations of tetranychid mites.
Previous Records — Australia, China (Fujian, Guangdong, Guangxi, Hainan, Yunnan), Cuba, Dominican Republic, Guadeloupe, Egypt, Hawaii, Hong-Kong, India, Indonesia, Japan, Les Saintes, Malaysia, Marie-Galante, Martinique, New Zealand, Nicaragua, Pakistan, Papua New Guinea, Philippines, Russia (Primorsky Territory), South Korea, Sri Lanka, Taiwan, Thailand, USA (Florida), Vietnam.
Specimens examined — All 8 ♀♀ measured: Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 4 ♀♀ on P. phaseoloides collected 23-05-2012, 1 ♀ on N. wightii collected 7-02-2013, 2 ♀♀ on M. atropurpureum collected 6-03-2013; Saint-Joseph, Rivière Lézarde, CIRAD (long. 14°39′N, lat. 60°59′O, alt. 45 m), 1 ♀ on citrus leaves (C. sinensis et C. latifolia), 17-07-2012.



Remarks — Although showing some great variations, especially with the holotype from Indonesia re-described by Schicha (1975), all the measurements and description of the specimens collected in this study fit very well those concerning other populations given in the table 11, especially with those from specimens of the French Caribbean Islands (Moraes et al. 2000) and from specimens of the Dominican Republic (Abo-Shnaf et al. 2016).
Amblyseius paraibensis Moraes & McMurtry, 1983: 135; Moraes & Mesa, 1988: 76; Moraes et al., 1991: 126;
Neoseiulus paraibensis, Moraes et al., 1986: 92; Moraes et al., 2004b: 137; Chant & McMurtry, 2003: 23; Chant & McMurtry, 2007: 29.



This species is known from Brazil, Colombia, Costa Rica, Nicaragua, Panama and USA (Florida), so a wide area around the Caribbean Sea. This is however the first record of this species for the Caribbean Islands and so for French Caribbean Islands. It seems to be very rare. Moraes and McMurtry (1983) have collected and described this species from Musa sp. in Brazil. But then, Moraes et al. (1991) have recorded first this species from Colombia on Oriza sativa. Rodriguez et al. (2009) and Quiros-McIntire and Rodriguez (2010) have found it in Cuba and Panama respectively also on rice on which it was the more frequent and abundant predator, associated in great numbers with Steneotarsonemus spinki Smiley. However, most of its biology remains totally unknown.
Specimens examined — Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 2 ♀♀ on P. phaseoloides, 20-08 and 19-11-2012.
Previous Records — Brazil (Paraiba, Rio Grande do Sul, São Paulo), Colombia, Costa Rica, Cuba, Nicaragua, Panama, USA (Florida).
Remarks — Only two females were found. All measurements reported in the table 12 agree very well with those of the original description and other subsequent measurements of specimens of several locations given in the table 12.
Typhlodromips tunus De Leon, 1967: 29; Denmark & Muma, 1973: 253;
Amblyseius tunus, Feres & Moraes, 1998: 126.
Neoseiulus tunus, Moraes et al., 2004b: 148 ; Chant & McMurtry, 2003: 21; Chant & McMurtry, 2007: 31.
Neoseiulus tunus is one of the most frequently reported species in the Neotropical Region. This species was described briefly only from the holotype collected In Trinidad by De Leon in 1967. Soon after, another species, N. neotunus Denmark and Muma, was described on the basis of a single female and a male by Denmark and Muma in 1973 from Piracicaba, São Paulo State, Brazil. Neoseiulus tunus was then reported from other Caribbean islands and South America (Cavalcante et al. 2017). Measurements of the holotype of N. tunus were provided by Moraes et al. (2000) followed by complementary descriptions on specimens from French Caribbean Islands (Moraes et al. 2000) or from South America (for example Lofego et al. 2004; Guanilo et al. 2008a, b). The great similarity between N. tunus and N. neotunus has been outlined very early (Moraes and Mesa 1988; Lofego 1998). Denmark and Muma (1973) arguments for distinction between N. neotunus and N. tunus were based on setal ornamentation (all setae barbed except j5 and not all setae of the j-J serie smooth like in N. tunus), shape of the spermatheca (cervix fundibuliform and not cup-shaped like in N. tunus) and of leg IV macrosetae setiform (and not knobbed distally like in N. tunus).Our examination of both holotypes and of the huge number of specimens collected in this study (12 measured) let us to conclude like Cavalcante et al. (2017) that the differences mentioned in the original description of N. neotunu s correspond to intraspecific variations and to agree with Cavalcante et al. (2017) that N. neotunus is a junior synonym of N. tunus. Actually, some of our specimens have all setae barbed except j5 and J5 and with knobbed macrosetae and some specimens have setae j-J smooth with setaceous macrosetae.
As populations identified as N. tunus and as N. neotunus exist in several places and are available, and as it is possible to recover specimen for a posteriori identification after molecular extraction, the best solution in order to establish definitively this synonymy is to undertake a molecular study with several populations from South and Central America and Caribbean area.
Specimens examined — 126 ♀♀ + 1 ♂ in total (12 ♀♀ measured and 1 ♂ in very bad state not measured). Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 19 ♀♀ + 1 ♂ on N. wightii, 28 ♀♀ on M. atropurpureum, 76 ♀♀ on P. phaseoloides and 3 ♀♀ on P. notatum collected between 23-07-2012 and 18-09-2013.
We have also examined: one holotype ♀ (measured) of Neoseiulus tunus (De Leon) in one slide with label: Tunapuna, Trinidad, 16-10-1963, on Psidium guajava L., De Leon coll., borrowed at Florida Department of Agriculture and Consumer Services, Department of Plant Industry, Gainesville, USA; one holotype and one paratype ♀ (both measured) of N. neotunus (Denmark & Muma) in one slide with the label: Picacicaba, São Paulo, Brazil, 1-03-1967 Pothomorphe sidifolia (Link & Otto) Miq. (and not P. sidaesolia as labelled), which is a junior synonym of Piper umbellatum L. (Piperaceae), Flechtmann coll., for comparisons.



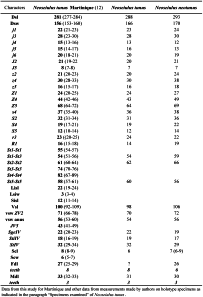


Previous Records — Argentina, Brazil (Bahia, Ceará, Goiás, Mato Grosso, Mato Grosso do Sul, Minas Gerais, Paraná, Rio Grande do Sul, Santa Catarina, São Paulo), Guadeloupe, Jamaica, Marie-Galante, Martinique, Peru, Trinidad.
Remarks — The females collected agree well with all measurements provided in the literature (Table 13) and with our measurements of holotypes of N. tunus and N. neotunus (Table 14).
Phytoseiini Berlese, 1913: 3; Phytoseiinae, Vitzthum, 1941: 768.
Phytoseius Ribaga, 1904: 177.
Phytoseius (Pennaseius) bennetti De Leon, 1965: 14;
Phytoseius (Phytoseius) bennetti, Denmark, 1966: 36.
Phytoseius bennettii, Moraes et al., 2004b: 233; Chant & McMurtry, 2007: 129.
This species is found in several locations around the Caribbean Sea. It seems to be quite rare in French West Indies as only one female was previously found and only in Martinique (Moraes et al. 2000). Its biology is totally unknown.
Description
Adult Female (Fig. 5 and Table 15) (n = 3)






Dorsum (Fig. 5A) — Dorsal shield 255 (250 – 260) long and 124 (123 – 125) wide, smooth, with 3 solenostomes (gd1, gd5 and gd9), 6 pairs of poroids, 17 pairs of dorsal setae and 2 pairs of sub-lateral setae: j1 19 (18 – 20), j3 31 (28 – 35), j4 8, j5 8, j6 9 (8 – 10), J2 9 (8 – 10), J5 6 (5 – 8), z2 11 (10 – 12), z3 41 (40 – 43), z4 16 (13 – 18), z5 8, Z4 56 (52 – 58), Z5 49 (48 – 50), s4 48 (47 – 48), s6 61(58 – 65), r3 35, R1 15 (13 – 17). All setae barbed except the j-J series and setae z2, z4, z5 and R1.
Peritreme (Fig. 5A) — Extending to the level of j1.
Venter (Fig. 5B) — All ventral shields smooth to very slightly reticulated. Sternal shield not very large, with 3 pairs of setae and 2 pairs of pores; 1 pair (st4) out of the sternal shield, on a small metasternal shield with one pore; posterior margin straight. Distances between st1- st1 50, st1-st3 55 (53 – 58), st2-st2 60, st3-st3 67 (65 – 70), st4-st4 78 (75 – 84), st5-st5 55 (50 – 62). Two pairs of inguinal sigilla (called also “metapodal shields”) 55 (50 – 62) long and very thin for the largest, not measurable for the smallest one. Ventrianal shield amphora-shaped with 3 pairs of pre-anal setae (JV1, JV2 and ZV2) and one pair of small lateral pre-anal pores in the middle. Membrane surrounding ventrianal shield with 3 pairs of setae (ZV1, ZV3 and JV5) and 5 pairs of poroids (called also “platelets”), the last one near the anus not visible on the same focus and thus not drawn on Figure 20; ventrianal shield 81 (75 – 85) long, 45 (42 – 47) wide at level of anterior corners and 51 (50 – 52) wide at level of anus. JV5 50 long and smooth.
Chelicera (Fig. 5C) — Fixed digit 20 with 3 – 4 teeth and movable digit 22 (20 – 23) with 1 tooth. Pilus dentilis not visible.
Spermatheca (Fig. 5D) — Spermatheca (called also “insemination apparatus”) saccular, 15 long and 9 (8 – 10) large. Minor and major ducts visible on few specimens.
Legs (Fig. 5E) — Macrosetae on legs IV, SgeIV 14 (12 – 15), StiIV 14 (12 – 15), StIV 21 (20 – 23). All macrosetae knobbed. Chaetotactic formula of genu II: 2-2/0, 2/1-0; genu III: 1-2/1, 2/0-1.
Adult male — Unknown and not collected in our study.
Specimens examined — Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 1 ♀ on M. atropurpureum collected 4-12-2012 and 1 ♀ on P. phaseoloides and 1 ♀ on M. atropurpureum collected 8-01-2013.
Previous Records — Dominican Republic, El Salvador, Honduras, Martinique, Puerto Rico and Trinidad.
Remarks — This species was already known from Martinique but only from a single female. Measurements of the three adult females (Table 15) agree well with measurements of three females of Guadeloupe (Table 15), better than with measurements of the 3 specimens collected in the close Dominican Republic by Ferragut et al. (2011). These specimens are smaller and have 10 to 20 % shorter setae (Table 15).
Specimens examined — Lamentin, CIRAD-CAEC station (long. 14°37′N, lat. 60°58′O, alt. 25 m), 1 ♀ broken, with missing legs and setae, on P. phaseoloides, 19-09-2012.
Typhlodromini Wainstein, 1962: 26; Typhlodrominae, Chant & McMurtry, 1994: 235.
Metaseiulini Chant & McMurtry, 1994: 258.
Typhlodromina Muma, 1961: 297.
Typhlodromina subtropica Muma & Denmark, 1969: 412; Muma & Denmark 1970: 132; Denmark & Muma, 1978: 16. Typhlodromus subtropicus, Chant & Yoshida-Shaul, 1983a: 1046.
Typhlodromina subtropica, Moraes et al., 1986: 240; Moraes et al., 2004b: 305; Chant & McMurtry, 2007: 169.
This species seems to be very rare in French West Indies as only some individuals were previously found (Moraes et al. 2000; Kreiter et al. 2006) and its biology is totally unknown.
Specimens examined — Le François, Mr. Peronnet’s farm, La Digue François (long. 14°34′N, lat. 61°53′O, alt. 59 m), 1 ♀ in bad state on leaves of lime trees C. latifolia.
Previous Records — Antigua, Brazil (Bahia, Espirito Santo, Maranhao, Pernambuco, Rio Grande do Sul, Roraima, São Paulo), Colombia, Costa Rica, Cuba, Dominican Republic, Fernado de Noronha Archipelago, Galapagos, Guadeloupe, Jamaica, Les Saintes, Marie-Galante, Martinique, Mexico, Nicaragua, Peru, Saint-Martin, USA (Florida, Georgia, Maryland, Tennessee, Texas), Venezuela.
Remarks — This species was already known from Martinique but only from one female (Moraes et al. 2000). The measurements of the single adult female collected in this study agree well with the measurements of the holotype given by Muma and Denmark (1969), by Chant & Yoshida-Shaul (1983a), and very well with those of the single female collected by Moraes et al. (2000). Measurements of the single female collected and identified in this study are not provided as it was in a bad state, with setae missing. However, distinctive characters of the genus according to Chant and McMurtry (2007) and of the species as provided by Chant and Yoshida-Shaul (1983) were accessible.
A total of 67 species belonging to 22 genera were known at the beginning of the year 2011 from the French Antilles after four surveys. After a fifth survey focused on plants tested in order to be used as cover-crops in citrus orchard and in some citrus orchards on trees and weeds in Martinique, the number of species for French Antilles is now reached to 70 with three new records: N. benjamini, N. paraibensis and T. terminatus new. comb.
These species belong to the three subfamilies: Amblyseiinae with 54 species, Phytoseiinae with 4 species and Typhlodrominae with 12 species.
Some of species collected during this survey have interesting potential for biological control, especially P. mexicanus, T. peregrinus, and N. longispinosus. This must be underlined as new regulations on importation of macro-organisms are proposed in a lot of countries and specifically for over-sea territories for countries like France that have very far tropical territories. Therefore it is impossible to import and of course to sell and use exotic species if they are not indigenous in the territory. An importation permit must be requested, but it is expensive and chances to obtain are generally very low (Kreiter et al. 2016). The knowledge of the biodiversity, especially of efficient biological control agents from overseas territories, not only for conversation purposes but for agricultural and economical ones, is so of a considerable importance.
Thanks are due to Dr Frédéric Beaulieu and Mr. Wayne Knee (The Canadian Collection of Insects, Ottawa, Canada) for the loan of additional material of A. terminatus; to Mrs. Laura Liebensperger (Museum of Comparative Zoology, Invertebrate Zoology Collection in Harvard University, Cambridge, USA) for the loan of the holotype of T. aripo; to Mr. Ronald A. Ochoa, Mrs. Debra Creel and Dr Patricia Gentili-Poole (The Smithsonian Institution, National Museum of Natural History in Washington DC, USA) for the loan of holotypes of A. terminatus and T. peregrinus; to Dr Calvin Welbourn (Florida Department of Agriculture in Gainesville, Florida, USA) for the loan of holotypes of Neoseiulus tunus and N. neotunus and paratypes of T. aripo. We are finally very grateful to the two anonyme reviewers for valuable comments on an earlier version of the manuscript and the great improvements allowed.
Abo-Shnaf R.I.A., Sánchez L., Moraes G.J. 2016. Plant inhabiting Gamasina mites (Acari: Mesostigmata) from the Dominican Republic, with descriptions of four new species of Lasioseius (Blattisociidae) and complementary descriptions of other species. Syst. Appl. Acarol. 21(5): 607-646. doi:10.11158/saa.21.5.5 ![]()
Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides, Phytoseiidae). Acarologia 27: 20-29.
Berlese A. 1913. Systema Acarorum genera in familiis suis disposita. Acaroteca Italica 1-2: 3-19.
Bin Ibrahim Y., Tan S.Y. 2000. Influence of sublethal exposure to abamectin on the biological performance of Neoseiulus longispinosus (Acari: Phytoseiidae). J. Econ. Entomol. 93(4): 1085-1089. doi:10.1603/0022-0493-93.4.1085 ![]()
Blackwood J.S., Schausberger P., Croft B.A. 2001. Prey stage preferences in generalist and specialist phytoseiid mites (Acari: Phytoseiidae) when offered Tetranychus urticae (Acari: Tetranychidae) eggs and larvae. Environ. Entomol. 30: 1103-1111. doi:10.1603/0046-225X-30.6.1103 ![]()
Boller H.F. 1984. Eine ainfache Ausschwemm-methode zur schellen Erfassung von Raumilben, Trips und anderen Kleinarthropoden imbWeinbau. Z. Obst- und Weinbau 120: 249-255.
Castro T.M.M.G. de, Moraes G.J. de, McMurtry J.A. 2010. New Phytoseiidae (Acari: Mesostigmata) from Costa Rica, with description of two new species and additional information on other species. Intern. J. Acarol. 36(1): 35-48. doi:10.1080/01647950903506718 ![]()
Cavalcante A.C.C., Demite P.R, Amaral F.S.R., Lofego A.C., Moraes G.J. de 2017. Complementary description of Neoseiulus tunus (De Leon) (Acari: Mesostigmata: Phytoseiidae) and observation on its reproductive strategy. Acarologia 57(3): 591-599. doi:10.24349/acarologia/20174178 ![]()
Chant D.A. 1959. Phytoseiid mites. Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of 38 new species. Can. Entomol. 91, suppl. 12: 1-166.
Chant D.A., Baker E.W. 1965. The Phytoseiidae (Acarina) of Central America. Mem. Entomol. Soc. Can. 41, 56 pp.
Chant D.A., McMurtry J.A. 1994. A review of the subfamilies Phytoseiinae and Typhlodrominae. Intern. J. Acarol. 20: 223-310. doi:10.1080/01647959408684022 ![]()
Chant D.A., McMurtry J.A. 2003. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part I. Neoseiulini new tribe. Intern. J. Acarol. 29(1): 3-46. doi:10.1080/01647950308684319 ![]()
Chant D.A., McMurtry J.A. 2004. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part III. The tibe Amblyseiini Wainstein, subtribe Amblyseiina n. subtribe. Intern. J. Acarol. 30(3): 171-228. doi:10.1080/01647950408684388 ![]()
Chant D.A., McMurtry J.A. 2005a. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part V. The tibe Amblyseiini Wainstein, subtribe Amblyseiina n. subtribe. Intern. J. Acarol. 31(1): 3-22. doi:10.1080/01647950508684412 ![]()
Chant D.A., McMurtry J.A. 2005b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part V. The tibe Euseiini n. tribe, subtribe Typhlodromalina n. subtribe, Euseiina n. subtribe, and Ricoseiina n. subtribe. Intern. J. Acarol. 31(3): 187-224. doi:10.1080/01647950508684424 ![]()
Chant D.A., McMurtry J.A. 2007. Illustrated keys and diagnoses for the genera and sub-genera of the Phytoseiidae of the World. Indira Publishing House, West Bloomfield, Michigan, USA, 220 pp.
Chant D.A., Yoshida-Shaul E. 1983. A world review of the simplex species group in the genus Typhlodromus Scheuten (Acarina: Phytoseiidae). Can. J. Zool. 61: 1142-1151. doi:10.1139/z83-151 ![]()
Chant D.A., Yoshida Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol. 17: 187-199. doi:10.1080/01647959108683906 ![]()
Childers C.C. 1994. Biological control of phytophagous mites on Florida citrus utilizing predatory arthropods. D. Rosen, F. Bennet, and J. Capinera [eds.], Pest management in the subtropics: biological control Florida perspective. Intercept, Andover, United Kingdom: 255-288.
Childers C.C., Denmark H.A. 2011. Phytoseiidae (Acari: Mesostigmata) within citrus orchards in Florida: species distribution, relative and seasonal abundance within trees, associated vines and ground cover plants. Exp. Appl. Acarol. 54: 331-371. doi:10.1007/s10493-011-9449-1 ![]()
Corpuz L.A., Rimando L. 1966. Some Philippine Amblyseiinae (Phytoseiidae: Acarina). Philipp. Agric. 50: 114-136.
Croft B.A., Luh H.-K., Schausberger P. 1999a. Larval size relative to larval feeding, cannibalism of larvae, egg, or adult female size and larval-adult setal patterns among thirteen phytoseiid mite species. Exp. Appl. Acarol. 23: 599-610. doi:10.1023/A:1006236310613 ![]()
Croft B.A., McMurtry J.A., Luh H.-K. 1999b. Do literature citation frequencies for six prey-food groups reflect feeding specialization and preferences among for Phytoseiid predation types?. Exp. Appl. Acarol. 23: 551-565. doi:10.1023/A:1006236310613 ![]()
De Leon D. 1962b. Twenty-three new phytoseiids, mostly from southeastern United States (Acarina: Phytoseiidae). Fla Entomol. 45(1): 11-27. doi:10.2307/3492899 ![]()
De Leon D. 1965a. Phytoseiid mites from Puerto Rico with descriptions of new species (Acarina: Mesostigmata). Fla Entomol. 48(2): 121-131. doi:10.2307/3493102 ![]()
De Leon D. 1966. Phytoseiidae of British Guyana with keys to species (Acarina: Mesostigmata). Studies on the Fauna of Suriname and other Guyanas 8: 81-102.
De Leon D. 1967. Some mites of the Caribbean Area. Part I. Acarina on Plants in Trinidad, West Indies. Allen Press Inc., Lawrence, Kansas, 66 pp.
Demite P.R., Lofego A.C., Feres R.J.F. 2011. Phytoseiidae (Acari) in forest fragments in the State of São Paulo, Brazil. Zootaxa 3086: 31-56.
Demite P.R., Lofego A.C., Feres R.J.F. 2012. Acarofauna de fragmentos florestais remanescentes na região noroeste do estado de São Paulo. In: Nechi Júnior, O.N. (Ed.), Fauna e flora de fragmentos florestais remanescentes da região noroeste do estado de São Paulo. Editora Holos, Ribeirão Preto, Brazil: 167-179.
Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2017. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae ![]()
Denmark H.A. 1966. Revision of the genus Phytoseius Ribaga, 1904 (Acarina: Phytoseiidae). Fla Dep. Agric. Bull. 6, 1-105.
Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, 451 pp.
Denmark H.A., Evans G.A., Aguilar H., Vargas C., Ochoa R. 1999. Phytoseiidae of Central America. Indira Publishing House, West Bloomfield, Michigan, USA, 125 pp.
Denmark H.A., Muma M.H. 1973. Phytoseiid mites of Brazil (Acarina: Phytoseiidae). Rev. Bras. Biol. 33(2): 235-276.
Denmark H.A., Muma M.H. 1978. Phytoseiidae of Jamaica, an annotated list (Acari: Mesostigmata). Intern. J. Acarol. 4(1): 1-22. doi:10.1080/01647957808683094 ![]()
Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occasional Papers of the Florida State Collection of Arthropods, USA, 4, 149 pp.
Dubois P. 2009. Impact de la gestion de l'enherbement sur les auxiliaires Phytoseiidae dans les vergers d'agrumes en Guadeloupe. MSc Thesis Agronomie Approfondie Ecole Supérieure d'Agriculture d'Angers, 89 pp.
Ehara S. 1958. Three predatory mites of the genus Typhlodromus from Japan (Phytoseiidae). Annot. Zool. Japonenses 31: 53-57.
Evans G.O. 1952. On a new predatory mite of economic importance. Bull. Entomol. Res. 43: 397-401. doi:10.1017/S0007485300040566 ![]()
Evans G.O. 1953. On some mites of the genus Typhlodromus Scheuten, 1857, from S. E. Asia. Ann. Mag. Nat. Hist. 6: 449-467. doi:10.1080/00222935308654444 ![]()
Fadamiro H.Y., Xiao Y., Hargroder T., Nesbitt M., Childers C.C. 2009. Diversity and seasonal abundance of predacious mites in Alabama Satsuma citrus. Ann. Entomol. Soc. Am. 102 (4): 617-628. doi:10.1603/008.102.0406 ![]()
Fadamiro H.Y., Xiao Y., Hargroder T., Nesbitt M., Umeh V., Childers C.C. 2008. Seasonal occurrence of key arthropod pests and associated natural enemies in Alabama satsuma citrus. Environ. Entomol. 2: 555-567. doi:10.1603/0046-225X(2008)37[555:SOOKAP]2.0.CO;2 ![]()
Famah Sourassou N., Hanna R., Zannou I., Moraes G.J. de, Breeuwer J.A.J., Sabelis M.W. 2012. Morphological, molecular and cross-breeding analysis of geographic populations of coconut-mite associated predatory mites identified as Neoseiulus baraki: evidence for cryptic species? Exp. Appl. Acarol. 57: 15-36. doi:10.1007/s10493-012-9534-0 ![]()
Feres R.J.F., Moraes G.J. de 1998. Phytoseiid mites (Acari: Phytoseiidae) from woody areas in the State of São Paulo, Brazil. Syst. Appl. Acarol. 3: 125-132. doi:10.11158/saa.3.1.20 ![]()
Ferragut F., Moraes G.J. de, Návia D. 2011. Phytoseiid mites (Acari: Phytoseiidae) of the Dominican Republic, with a re-definition of the genus Typhloseiopsis De Leon. Zootaxa 2997: 37-53.
Fiaboe K.K.M., Gondim M.G.C. Jr., Moraes G.J. de, Ogol C.K.P.O., Knapp. M. 2007. Surveys for natural enemies of the tomato red spider mite Tetranychus evansi (Acari: Tetranychidae) in the northeastern and southeastern Brazil. Zootaxa 1395: 33-58.
Fouly A.H., Abou-Setta M.M., Childers C.C. 1995. Effects of diets on the biology and life tables of Typhlodromalus peregrinus. Environ. Entomol. 24: 870-877. doi:10.1093/ee/24.4.870 ![]()
Fournet J. 2002. Flore illustrée des Phanérogames de Guadeloupe et Martinique. CIRAD + Gondwana éditions, Trinité, Martinique: 2538 pp.
Furtado I.P., Moraes G.J. de, Kreiter S., Flechtmann C.H.W., Tixier M.-S., Knapp M. 2014. Plant inhabiting phytoseiid predators of Midwestern Brazil, with emphasis on those associated with the tomato red spider mite, Tetranychus evansi (Acari: Phytoseiidae, Tetranychidae). Acarologia 54(4): 425-431 doi:10.1051/acarologia/20142138 ![]()
Garman P. 1958. New species belonging to the genera Amblyseius and Amblyseiopsis with keys to Amblyseius, Amblyseiopsis, and Phytoseiulus. Ann. Entomol. Soc. Am. 51: 69-79. doi:10.1093/aesa/51.1.69 ![]()
Guanilo A.D., Moraes G.J. de, Knapp M. 2008a. Phytoseiid mites (Acari: Phytoseiidae) of the subfamily Amblyseiinae Muma from Peru, with description of four new species. Zootaxa 1880: 1-47
Guanilo A.D., Moraes G.J. de, Toledo S., Knapp M. 2008b. Phytoseiid mites (Acari: Phytoseiidae) from Argentina, with description of a new species. Zootaxa 1884: 1-35.
Hughes A.M. 1948. The mites associated with stored food products. Ministry of Agriculture and Fisheries, H. M. Stationary Office, London, 168 pp.
Karg W. 1983. Systematische untersuchung der Gattungen und Untergattungen der Raubmilbenfamilie Phytoseiidae Berlese, 1916, mit der beschreibung von 8 neuen Arten. Mitt. Zool. Mus. Berl. 59(2): 293-328. doi:10.1002/mmnz.4830590203 ![]()
Kreiter S., Moraes G.J. de 1997. Phytoseiidae mites (Acari: Phytoseiidae) from Guadeloupe and Martinique. Fla. Entomol. 80: 376-382. doi:10.2307/3495770 ![]()
Kreiter S., Tixier M.-S., Etienne J. 2006. New records of phytoseiid mites (Acari: Mesostigmata) from the French Antilles, with description of Neoseiulus cecileae sp. nov. Zootaxa 1294: 1-27.
Kreiter S., Mailloux J., Tixier M.-S., Le Bellec F., Douin M., Guichou S., Etienne J. 2013. New phytoseiid mites of the French West Indies, with description of a new species, and new records (Acari: Mesostigmata). Acarologia 53(3): 285-303 doi:10.1051/acarologia/20132095 ![]()
Kreiter S., Vicente V., Tixier M.-S., Fontaine O. 2016. An unexpected occurrence of Amblyseius swirskii Athias-Henriot in La Reunion Island (Acari: Phytoseiidae). Acarologia 56(2): 175-181. doi:10.1051/acarologia/20162254 ![]()
Lindquist E., Evans G.W. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina Acarina: Mesostigmata. Mem. Entomol. Soc. Can., 47: 1-64. doi:10.4039/entm9747fv ![]()
Lofego A.C. 1998. Caracterização morfológica e distribuição geográfica das espécies de Amblyseiinae (Acari: Phytoseiidae) no Brasil. MSc Thesis, University of São Paulo, 167 pp + viii.
Lofego A.C., Demite P.R., Moraes G.J. de, Kishimoto R.G. 2009. Phytoseiid mites on grasses in Brazil (Acari: Phytoseiidae). Zootaxa 2240: 41-59.
Lofego A.C., Moraes G.J. de, Castro L.A.S. 2004. Phytoseiid mites (Acari: Phytoseiidae) on Myrtaceae in State of São Paulo, Brazil. Zootaxa 516: 1-18. doi:10.11646/zootaxa.516.1.1 ![]()
Mailloux J., Le Bellec F., Kreiter S., Tixier M.-S., Dubois P. 2010. Influence of ground cover management on diversity and density of phytoseiid mites (Acari: Phytoseiidae) in Guadeloupean citrus orchards. Exp. Appl. Acarol. 52: 275-290. doi:10.1007/s10493-010-9367-7 ![]()
McMurtry J.A. 1983. Phytoseiid mites from Guatemala, with descriptions of two new species and redefinitions of the genera Euseius, Typhloseiopsis, and the Typhlodromus occidentalis species group (Acari: Mesostigmata). Intern. J. Entomol. 25(4): 249-272.
McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Ann. Rev. Entomol. 42: 291-321. doi:10.1146/annurev.ento.42.1.291 ![]()
McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the life styles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol. 18: 297-320. doi:10.11158/saa.18.4.1 ![]()
McMurtry J.A., Sourassou N.F., Demite P.R. 2015. The Phytoseiidae (Acari: Mesostigmata) as Biological Control Agents. Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms : 133-149.
Mégevand B., Klay A., Gnanvossou D., Paraiso G. 1993. Maintenance and mass rearing of phytoseiid predators of the cassava green mite. Exp. Appl. Acarol. 17: 115-128.
Moraes G.J. de, Barbosa M.F. de C., Castro T.M.M.G. de 2013. Phytoseiidae (Acari: Mesostigmata) from natural ecosystems in the State of São Paulo, Brazil. Zootaxa 3700(3): 301-347. doi:10.11646/zootaxa.3700.3.1 ![]()
Moraes G.J. de, Kreiter S., Lofego A.C. 2000. Plant mites of the French Antilles. 3. Phytoseiidae. Acarologia 40: 237-264.
Moraes G.J. de, Lopes P.C., Fernando C.P. 2004a. Phytoseiid mite (Acari: Phytoseiidae) of coconut growing areas in Sri Lanka, with descriptions of three new species. J. Acarol. Soc. Jap. 13(2): 141-160. doi:10.2300/acari.13.141 ![]()
Moraes G.J. de, McMurtry J.A. 1983. Phytoseiid mites (Acarina) of northeastern Brazil with descriptions of four new species. Intern. J. Acarol. 9(3): 131-148. doi:10.1080/01647958308683326 ![]()
Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A catalog of the mite family Phytoseiidae. References to Taxonomy, Synonymy, Distribution and Habitat. Embrapa ed. and Pub., Brasilia, 353 pp + VII.
Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004b. A revised catalog of the mite family Phytoseiidae. Zootaxa 434: 1-494. doi:10.11646/zootaxa.434.1.1 ![]()
Moraes G.J. de, Mesa N.C. 1988. Mites of the family Phytoseiidae (Acari) in Colombia, with descriptions of three new species. Intern. J. Acarol. 14: 71-88. doi:10.1080/01647958808683790 ![]()
Moraes G.J. de, Mesa N.C., Braun A. 1991. Some phytoseiid mites of Latin America (Acari: Phytoseiidae). Intern. J. Acarol. 17: 117-139. doi:10.1080/01647959108683892 ![]()
Muma M.H. 1955. Phytoseiidae (Acarina) associated with citrus in Florida. Ann. Entomol. Soc. Am. 48: 262-272. doi:10.1093/aesa/48.4.262 ![]()
Muma M.H. 1961a. Subfamiles, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Fla. State Mus. Bull. 5(7): 267-302.
Muma M.H. 1962. New Phytoseiidae (Acarina: Mesostigmata) from Florida. Fla. Entomol. 45: 1-10. doi:10.2307/3492897 ![]()
Muma M.H. 1964. Annotated list and keys to Phytoseiidae (Acarina: Mesostigmata) associated with Florida citrus. Univ. Fla. Agric. Exp. Sta. Bull. 685: 1-42.
Muma M.H. 1967. Typhlodromalus peregrinus (Muma) (Acari: Phytoseiidae) on Florida citrus. Proceedings, 2nd International Congress of Acarology. Sutton Bonington, 19-25 July 1967, England. Akade´miai Kiado´, Budapest, Hungary: 135-148.
Muma M.H. 1969. Biological control of various insects and mites on Florida citrus. Proceedings, 1st International Citrus Symposium, 16-26 March 1969, Riverside, CA. University of California, Riverside: 863-870.
Muma M.H. 1971. Food habits of Phytoseiidae (Acarina: Mesostigmata) including common species on Florida citrus. Fla Entomol. 54(1): 21-34. doi:10.2307/3493786 ![]()
Muma M.H., Denmark H.A. 1962. Intraspecific variation in Phytoseiidae (Acarina: Mesostigmata). Fla. Entomol. 45: 57-65. doi:10.2307/3492217 ![]()
Muma M.H., Denmark H.A. 1969. The conspicua species-group of Typhlodromina Muma, 1961. Ann. Entomol. Soc. Am. 62: 406-413. doi:10.1093/aesa/62.2.406 ![]()
Muma M.H., Denmark H.A. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighboring land areas, 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, USA, 150 pp.
Myers N. 1988. Threatened biotas: hostspots in tropical forests. Environmentalist 8: 187-208. doi:10.1007/BF02240252 ![]()
Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature 403: 853-858. doi:10.1038/35002501 ![]()
Peña J.F. 1992. Predator-prey interactions between Typhlodromalus peregrinus and Polyphagotarsonemus latus: effects of alternative prey and other food resources. Fla. Entomol. 75: 241-248. doi:10.2307/3495626 ![]()
Prasad V. 2012. Checklist of Phytoseiidae of the world. Indira Publishing House, West Bloomfield, Michigan, USA, 1063 pp.
Quirós-McIntire E., Rodríguez H. 2010. Ácaros depredadores asociados a Steneotarsonemus spinki Smiley (Acari: Tarsonemidae) en Panamá. Revista de Protección Vegetal 25(2): 103-107.
Rezende J.M., Lofego A.C. 2011. Phytoseiidae (Acari: Mesostigmata) on plants of the central region of the Brazilian Cerrado. Acarologia 51(4): 449-463. doi:10.1051/acarologia/20112027 ![]()
Rezende J.M., Lofego A.C. 2012. Mites (Mesostigmata, Prostigmata, Astigmatina) associated with weeds among physic nut crops (Jatropha curcas L.: Euphorbiaceae) in Brazil. Syst. Appl. Acarol. 17(1): 15-26.
Rezende J.M., Lofego A.C., Navia D., Roggia S. 2012. Mites (Acari: Mesostigmata, Sarcoptiformes and Trombidiformes) associated to soybean in Brazil, including new records from the Cerrado areas. Fla. Entomol. 95(3): 683-693. doi:10.1653/024.095.0319 ![]()
Ribaga C. 1904 (1902). Gamasidi planticoli. Rivista di Patologia Vegetale 10: 175-178.
Rodriguez H., Miranda I., Louis J.L., Hernandez J. 2009. Comportamiento poblacional de Steneotarsonemus spinki (Acari: Tarsonemidae) en el cultivo del arroz (Oryza sativa L.). Tomas de Ciencia y Tecnologia 13(39): 55-66.
Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns of the dorsal shield in the family Phytoseiidae. Can. Entomol. 110: 859-876. doi:10.4039/Ent110859-8 ![]()
Schausberger P., Croft B.A. 1999a. Predation on and discrimination between con- and heterospecific eggs among specialist and generalist phytoseiid mites (Acari: Phytoseiidae). Environ. Entomol. 28: 523-528. doi:10.1093/ee/28.3.523 ![]()
Schausberger P., Croft B.A. 1999b. Activity, feeding, and development among larvae of specialist and generalist phytoseiid mite species (Acari: Phytoseiidae). Biological Control 28: 322-329. doi:10.1093/ee/28.2.322 ![]()
Schausberger P., Croft B.A. 2000a. Nutritional benefits of intraguild predation and cannibalism among generalist and specialist phytoseiid mites. Ecol. Entomol. 25: 1-8. doi:10.1046/j.1365-2311.2000.00284.x ![]()
Schausberger P., Croft B.A. 2000b. Cannibalism and intraguild predation among phytoseiid mites: are aggressiveness and prey preference related to diet specialization? Exp. Appl. Acarol. 24: 709-725. doi:10.1023/A:1010747208519 ![]()
Schicha E. 1975. Predacious mites (Acarina: Phytoseiidae) on sprayed apple trees at Bathurst (N.S.W.). J. Austral. Entomol. Soc. 14: 217-219. doi:10.1111/j.1440-6055.1975.tb02029.x ![]()
Schicha E. 1981. A new species of Amblyseius from Australia compared with ten closely related species from Asia, America and Africa. Intern. J. Acarol. 7: 203-216. doi:10.1080/01647958108683262 ![]()
Shicha E. 1987. Phytoseiidae of Australia and Neighboring Areas. Indira Publishing House, Oak Park, Michigan, USA. 187 p.
Tixier M.-S. 2012. Approaches to assess intraspecific variations of morphological continuous characters: the case study of the family Phytoseiidae (Acari: Mesostigmata). Cladistics 28(2012) 489-502. doi:10.1111/j.1096-0031.2012.00394.x ![]()
Tixier M.-S., Okassa M., Kreiter S. 2009. On the way to the molecular diagnostic of species of Phytoseiidae. Euraac Newsletter 1+2.
Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomol. Mem. Depart. Agric. Water Supply, RSA 73: 1-168.
Villanueva R.T., Childers C.C. 2004. Phytoseiidae increase with pollen deposition on citrus leaves. Fla. Entomol. 4: 609-611. doi:10.1653/0015-4040(2004)087[0609:PIWPDO]2.0.CO;2 ![]()
Villanueva R.T., Childers C.C. 2005. Diurnal and spatial patterns of Phytoseiidae in the citrus canopy. Exp. Appl. Acarol. 35(4): 269-280. doi:10.1007/s10493-004-5728-4 ![]()
Vitzthum H. von 1941. Acarina. In: Bronns, H.G. (Ed.), Klassen und Ordnungen des Tierreichs 5, Akademischer Verlag, Leipzig, Germany: 764-767.
Wainstein B.A. 1962. Révision du genre Typhlodromus Scheuten, 1857 et systématique de la famille des Phytoseiidae (Berlese 1916) (Acarina: Parasitiformes). Acarologia 4: 5-30.
Womersley H. 1954. Species of the subfamily Phytoseiinae (Acarina: Laelaptidae) from Australia-Austral. J. Zool. 2: 169-191. doi:10.1071/ZO9540169 ![]()
Zacarias M.S., Moraes G.J. de 2001. Phytoseiid mites (Acari) associated with rubber trees and other euphorbiaceous plants in southeastern Brazil. Neotrop. Entomol. 30: 579-586. doi:10.1590/S1519-566X2001000400011 ![]()



2017-08-31
Date accepted:
2017-11-21
Date published:
2018-03-15
Edited by:
Faraji, Farid

This work is licensed under a Creative Commons Attribution 4.0 International License
2018 Kreiter, Serge; Zriki, Ghais; Ryckewaert, Philippe; Pancarte, Clovel; Douin, Martial and Tixier, Marie-Stéphane
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



