Agistemus collyerae (Acari: Trombidiformes: Stigmaeidae) in South Africa: first record, introduction pathways and a re-description including additional life stages
Saccaggi, Davina L.1 and Ueckermann, Edward A.2
1✉ Plant Health Diagnostic Services, Department of Agriculture, Forestry and Fisheries (DAFF), Private Bag X5015, Stellenbosch, 7599 South Africa.
2Unit for Environmental Sciences and Management, Potchefstroom Campus, North-West University, Private Bag X6001, Potchefstroom, 2520 South Africa.
2018 - Volume: 58 Issue: 1 pages: 116-130
https://doi.org/10.24349/acarologia/20184235ZooBank LSID: 96B9114B-6665-400A-9D00-465FF77F3974
Keywords
Abstract
Many mite species have been transported and introduced beyond their natural ranges, due in part to the ease with which they can accidentally be transported on internationally traded commodities (Navajas & Ochoa 2013). Many agricultural pest species have been introduced this way, but less is known about the introduction of non-native predators in agricultural systems (Palevsky et al. 2013; Saccaggi et al. 2016).
In 2015 South African authorities detected a newly introduced pest of quarantine status on grapevine, Brevipalpus lewisi (Trombidiformes: Tenuipalpidae) (Saccaggi et al. 2017). During the delimiting survey that followed this detection, a non-native predator, Agistemus collyerae (Trombidiformes: Stigmaeidae) was found on some samples around the same location where B. lewisi was first detected. The new discovery of two non-native mite species raised questions as to the introduction pathways of both mites. We investigated the presence and identity of mites on agricultural imports to South Africa. During this investigation, we noted differences in the specimens examined and found that the male and deutonymph of A. collyerae had not been described.
Agistemus collyerae falls within the family Stigmaeidae, which consists of 577 species in 34 genera, with Agistemus the third largest genus with 87 species, after Stigmaeus (133 species) and Eustigmaeus (124 species) (Fan et al. 2016). Stigmaeidae are present in all geographical regions, with most species described from the Palaearctic (289 species). Africa has only 45 Stigmaeidae species described, with most of those stemming from South Africa (Fan et al. 2016). Members of this family are considered economically important predators of mites and small insects (Santos & Laing 1985; Gerson et al. 2003; Fan and Zhang 2005; Fan & Flechtmann 2015).
Agistemus collyerae González-Rodriguez 1963 was described from New Zealand, where it is assumed to be native (González-Rodrigues 1963). It is also recorded from Australia, Iran, Portugal, Turkey and Italy (Fan et al. 2016). It has been recorded from various cultivated and wild plants, and is known to feed on Tetranychus lambi (Tetranychidae) and Aculus fockeui [syn. Aculus cornutus] (Eriophyidae) (Fan and Zhang 2005).
Here we report Agistemus collyerae (Acari: Trombidiformes: Stigmaeidae) for the first time from South Africa. Possible introduction pathways of A. collyerae into South Africa are investigated and discussed. We re-describe the species based on South African specimens, including for the first time a description of the male and deutonymph.
Plant samples were collected from grapevine (Vitis vinifera, various cultivars) throughout South Africa as part of a nation-wide survey from March 2015 – November 2016 (for more details see Saccaggi et al. 2017). Shoots and leaves from each plant were collected, sealed in plastic bags, and transported to the laboratory for microscopic inspection. Plant parts were examined under a stereomicroscope for the presence of mites. Any mites found were preserved in 70% ethanol until clearing in lactic acid and mounting on glass slides in PVA mounting medium, following the general protocols outlined in Krantz & Walter (2009).
Slide-mounted specimens were identified by EU using a compound microscope with phase contrast and measured with a Zeiss Image Analysing System. All measurements are in micrometers (μm). Specimens were identified as Agistemus collyerae using the keys to Raphignathoidea and Stigmaeidae provided in Fan and Zhang (2005) and Fan et al. (2016). Specimens were compared to the original description of Agistemus collyerae by González-Rodrigues (1963) and re-descriptions by Fan and Zhang (2005) and Yeşilayer and Çobanoğlu (2013). Specimens from South Africa, New Zealand, Australia, Italy, USA, Chile, Yemen, Spain and France were examined and compared to each other.
The introduction pathways of Agistemus collyerae to South Africa were investigated by searching for records of mites detected on imported plant products. We examined the interception databases maintained by the Plant Health Diagnostic Division of South Africa’s Department of Agriculture, Forestry and Fisheries (DAFF). These databases contain records of all organisms (mites, insects, nematodes, fungi, bacteria, viruses and phytoplasms) detected on imported plant material by DAFF since 2004 (Saccaggi and Pieterse 2013). Additional A. collyerae specimens intercepted from other countries before 2004 were found in the South African National Collection of Arachnida (NCA), housed at the Agricultural Research Council (ARC) at Roodeplaat, Pretoria, Gauteng Province.
Specimens of A. collyerae detected on imported material were compared to published descriptions as described above, to specimens from New Zealand, and to the specimens collected in this study.
New species record: Agistemus collyerae
This is the first report of Agistemus collyerae (Trombidiformes: Stigmaeidae) from grapevine in South Africa. It was detected on three samples collected in March and April 2015 in the Western Cape Province. It was found in association with Brevipalpus lewisi, B. obovatus (Trombidiformes: Tenuipalpidae) (Saccaggi et al. 2017) and Colomerus cf. vitis (Trombidiformes: Eriophyidae), on which it is assumed to be feeding.
Family Stigmaeidae Oudemans
Genus Agistemus Summer
Agistemus collyerae González-Rodrigues, 1963: 349; 1965: 34; Fan & Zhang, 2005: 41; Ferreira et al., 2007: 56; Rahmani et al., 2012: 105; Beyzavi et al., 2013: 397; Yeşilayer and Çobanoğlu, 2013: 96; Fan et al., 2016: 31.Zetzellia collyerae (González-Rodrigues) Wood, 1967: 131.
Material examined — Eight females, two males and one deutonymph from grapevine, Wellington, 16 April 2015; one female from Malmesbury, 16 March 2015; and one female from Riebeek West, 17 April 2015. All these locations are within the Western Cape Province, South Africa. Specimens are deposited in the National Collection of Arachnida (NCA) in Pretoria, South Africa. Measurements are given in Table 1. Other features are described below.
Diagnosis (Female) — Dorsal shields reticulated, setae c1 and d1 shorter than distances to setae d1 and e1, respectively; setal formulae of genua 3-0-0-0, femora 4-4-2-2 and tibiae 6-6-6-4, coxa IV with one seta.
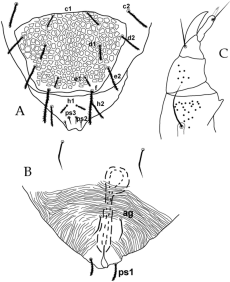
Female (Figures 1-5, Table 1) (n=10 for qualitative characters) — Idiosoma oval.
Dorsum (Figures 1-2) — Dorsal shields (fig 1-2A) covered with thick-walled reticulations, each cell with about 11-14 vacuoles; setae short, barbed (fig 2B) and gradually becoming longer caudally; prodorsum with one pair of eyes and one pair of postocular bodies. Setae c2 are situated on small spindle-shaped platelets.






Gnathosoma (Figures 3B-C) — Subcapitulum (fig 3C) with 2 pairs of setae (m and n) and two pairs of oral setae (or1 and or2). Chelicerae free, with movable digits. Palp six-segmented (fig 3B); palp tarsus with four simple setae, one solenidion, one tridentate and one simple eupathidium; palp tibia with two smooth setae, one stout claw and one seta-like accessory claw; palp genu with one long simple seta; palp femur with two serrate setae; palp trochanter without setae and palp coxa with a small spine.
Venter (Figure 3A) — Covered with striae with three ventral setae (1a, 3a and 4a). Anogenital area (fig 3A) with two pairs of aggenital setae (ag1-2), on a smooth horse-shoe like area, one pair of very long genital setae (g) stretching past setae ps3 and three pairs of anal setae (ps1-3), latter all smooth except for ps3.



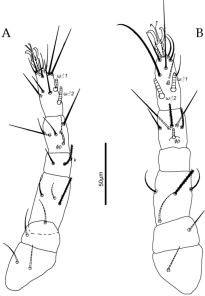
Legs (Figures 4-5) — Setal formulae of leg segments: coxae 2+elcp-1-2-1, trochanters 1-1-1-1, femora 4-4-2-2, genua 3 (including k)-0-0-0, tibiae 5+1φ – 5+1φ – 5+1φ – 4, tarsi 11+1ω – 9+1ω – 7+1ω – 7.






Male (Figures 6-8, Table 1) (n=2) — Idiosoma oval, similar to female.
Dorsum (Figure 6A) — All dorsal shields reticulated with setae short and barbed with setae f longest. Median shield with six pairs of setae, f included.
Gnathosoma (Figure 6C) — Like that of female but more robust. Palp femur with a spur on inner margin.
Venter (Figure 6B) — Like that of the female except that setae ag2 and g are absent.


Legs (Figures 7-8) — Leg chaetotaxy similar to that of the female, except for tarsi: 11+1ω+1ω♂ – 9+1ω+1ω♂ – 7+1ω – 7+1ω.


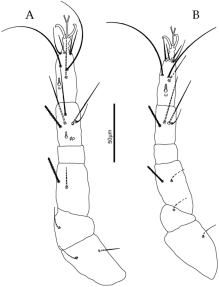


Dorsum — Dorsal shields weakly reticulate, prodorsum with eyes and postocular bodies. Dorsal setae also barbed and gradually become longer caudally.
Gnathosoma — Like that of female.
Legs — Leg chaetotaxy like that of the female, except for trochanters 1 – 1 – 1 – 0.
Remarks — The South African specimens resemble the original descriptions and re-descriptions in most respects, except for the lengths of the dorsal setae and the leg solenidia (see Table 1). The lengths of dorsal setae in the published descriptions are 32 – 46 μm in González-Rodrigues (1963), 28 – 50 μm in Fan and Zhang (2005) and 30 – 46 μm in Yeşilayer & Çobanoglu (2013). Specimens from the native range in New Zealand, measured in this study (Table 1), have dorsal setal lengths from 24 to 46 μm. Except for h2, the lengths of the dorsal setae in the South African specimens are markedly shorter than the same setae in the published descriptions or the New Zealand specimens, with ranges usually not overlapping. The dorsal setae of the South African specimens range from 15 to 38 μm. However, the leg chaetotaxy, which is considered unique for this species (González-Rodrigues 1963, 1965), is identical to the descriptions. Additionally, the setal ratios, dorsal shields ornamentation and anogenital area are similar, and we do not consider the difference in setal and solenidia lengths enough evidence to justify a new species. This is also the first time that a male and deutonymph are described. The male is unique in having a spur on the inner margin of the palp femur and apparently having only one pair of ag setae instead of two pairs as in the female.



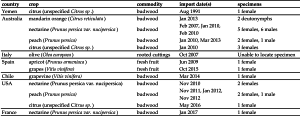
From analysis of the records kept by DAFF and the NCA, eighteen detections of A. collyerae on imported agricultural goods have been recorded, with the first recorded detection in 1991. These have not been reported previously, as interception of non-quarantine species (such as predatory mites) is usually not considered to be of economic concern (Saccaggi et al. 2016). Table 2 shows a list of these detections. In addition to detections on material from Australia and Italy, A. collyerae was also detected from the USA, Chile, Yemen, Spain and France, countries where it has previously not been known to occur. Thus this paper also serves to document the presence of A. collyerae in these countries. The unreported presence of A. collyerae in these countries is not unusual, as new introductions of small or cryptic organisms (such as mites) are difficult to detect and therefore often go unnoticed. Usually such introductions are noticed either because of damage to a crop (Navia et al. 2010) or during a routine survey, as was the case here. The introduction data and pathway of entry of A. collyerae to South Africa is unknown, but it is likely that it entered as a contaminant on agricultural goods, as is common for terrestrial invertebrates (Faulkner et al. 2015).


When these intercepted A. collyerae specimens were compared to published descriptions and specimens from South Africa and New Zealand, the female dorsal setae lengths were more similar to those of the South African specimens, 15 – 40 μm in length, than to the New Zealand specimens or published descriptions. The males and deutonymphs intercepted were also comparable to those newly described from the South African samples.
We initially suspected that this discrepancy in female dorsal setal lengths may have been due to differences in methods of measurements used in the published descriptions and the current study. However, after obtaining specimens from the native range in New Zealand and measuring them ourselves, it seems that this may reflect a true difference between populations. If this is the case, it suggests that at least two lineages of A. collyerae exist, one restricted to New Zealand and the other spread globally to other countries. It is plausible that a strain or race of A. collyerae with these shorter setal lengths was transported out of New Zealand to a secondary country, which then acted as a “distribution hub” for the species to spread to other countries. It is increasing recognised that this type of global spread of a species, sometimes termed the “bridgehead effect”, is extremely common, and that many exotic species populations are a mixture of introductions and back-introductions from different origins (Garnas et al. 2016). Further investigation into possible morphological similarities, possible transport routes and population genetic linkages would be necessary to investigate this possibility.
The frequency with which non-quarantine organisms (such as predatory mites) are present on imported goods is unknown. It is highly likely that live specimens of non-quarantine mites are able to enter a country relatively easily, increasing the chances of establishment. The ecological and economic effects of introducing predatory arthropods into a novel region are poorly studied. In many cases they are seen as benign or even beneficial introductions, enhancing biological control of pest species (Palevsky et al. 2013; Hajek et al. 2016). However, the ecological effects are largely unknown. There are some notable cases in which introduction of an arthropod predator to enhance biocontrol has generated significant negative impacts – the case of the Harlequin ladybug (Harmonia axyridis (Coccinellidae)) being a prime example (Stals & Prinsloo, 2007; Kenis et al. 2016). Mite biology and ecology have not been studied extensively enough to understand nor predict the impact an introduced predatory species may have.
The authors would like to express their thanks to DAFF Inspection Services and personnel for use of their resources and time. In particular, we thank the Western Cape DAFF inspectors for collection of field material; H. Ramukhesa and S. Rosenberg for laboratory collection of mites; N. Ngubane-Ndhlovu and I. Dhanani for slide-mounting; and N. Africander, M. Arendse and T. Pongolo (DAFF – Diagnostic and Quarantine Services) for permission to use the interception databases. A special thanks to Dr Qing-Hai Fan of the Plant Health & Environmental Laboratory, Ministry for Primary Industries, Auckland, New Zealand for the loan of A. collyerae specimens.
This work is based on research supported in part by the National Research Foundation of South Africa (UID) 85288. Any opinion, findings and conclusions or recommendations expressed in the material are those of the authors and therefore the NRF does not accept any liability in regard thereto.
Beyzavi G., Ueckermann E.A., Faraji F., Ostovan H. 2013 — A catalog of Iranian prostigmatic mites of superfamilies Raphignathoidea & Tetranychoidea (Acari) — Persian Journal of Acarology 2(3): 389-474.
Fan Q.-H., Flechtman C.H.W. 2015 — Chapter 7. Stigmaeidae — In: Carrillo D., de Moraes G.J., Peña J. (Eds). Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms. New York, USA: Springer, pp. 185-206. doi:10.1007/978-3-319-15042-0_7 ![]()
Fan Q.-H., Flechtmann C.H.W., De Moraes G.J. 2016 — Annotated catalogue of Stigmaeidae (Acari: Prostigmata), with a pictorial key to genera — Zootaxa 4176: 1-199.
Fan Q.-H., Zhang Z.-Q. 2005 — Raphyignathoidea (Acari: Prostigmata) — Lincoln, Canterbury, New Zealand: Fauna of New Zealand 52. 400 pp.
Faulkner K.T., Robertson M.P., Rouget M., Wilson J.R.U. 2015 — Understanding and managing the introduction pathways of alien taxa: South Africa as a case study — Biol. Invasions 18: 73-87. doi:10.1007/s10530-015-0990-4 ![]()
Ferreira M.A.S., Ramos N., Soares C., Fernandes J.E. 2007 — Ácaro do abacateiro no Algarve — Frutas, Legumes e Flores 15(97): 56-57.
Garnas J.R., Auger-Rozenberg M.A., Roques A., Bretelsmeier C., Wingfield M.J., Saccaggi D.L., Roy H.E., Slippers B. 2016 — Complex patterns of global spread in invasive insects: eco-evolutionary and management consequences — Biol. Invasions 18(4): 935-952. doi:10.1007/s10530-016-1082-9 ![]()
Gerson U., Smiley R.L., Ochoa R. 2003 — Mites (Acari) for Pest Control — Oxford, UK: Blackwell Science, 539 pp. doi:10.1002/9780470750995 ![]()
González-Rodrigues R.H. 1963 — Four new mites of the genus Agistemus Summers, 1960 (Acarina: Stigmaeidae) — Acarologia 5: 342-350.
González-Rodrigues R.H. 1965 — A taxonomic study of the genera Mediolata, Zetzallia and Agistemus (Acarina: Stigmaeidae) — University of Californica Publications in Entomolgy, 41: 64pp.
Hajek A.E., Hurley B.P., Kenis M., Garnas J.R., Bush S.J., Wingfield M.J., van Lenteren J.C., Cock M.J.W. 2016 — Exotic biological control agents: A solution or contribution to arthropod invasions? — Biol. Invasions 18: 953-969. doi:10.1007/s10530-016-1075-8 ![]()
Kenis M., Adriaens T., Brown P.M.J., Katsanis A., San Martin G., Branquart E., Maes D., Eschen R., Zindel R., Van Vlaenderen J., Babendreier D., Roy H.E., Hautier L., Poland R.L. 2016 — Assessing the ecological risk posed by a recently established invasive alien predator: Harmonia axyridis as a case study — BioControl 1-14.
Krantz G.W., Walter D.E. 2009 — A manual of Acarology, 3rd edition — Lubbock, USA: Texas Tech University Press, 807 pp.
Navajas M., Ochoa R. 2013 — Integrating ecology and genetics to address Acari invasions — Exp. Appl. Acarol. 59: 1-10. doi:10.1007/s10493-012-9636-8 ![]()
Navia D., Ochoa R., Welbourn C., Ferragut F. 2010 — Adventive eriophyoid mites: A global review of their impact, pathways, prevention and challenges — Exp. Appl. Acarol. 51: 225-255. doi:10.1007/s10493-009-9327-2 ![]()
Palevsky E., Gerson U., Zhang Z.-Q. 2013 — Can exotic phytoseiids be considered ``benevolent invaders" in perennial cropping systems? — Exp. Appl. Acarol. 59: 11-26. doi:10.1007/s10493-012-9575-4 ![]()
Rahmani H., Fathipour Y., Kamali K. 2012 — First record of Agistemus collyerae (Acarina: Stigmaeidae) from Iran — Journal of Entomological Society of Iran 31(2): 105-107.
Saccaggi D.L., Karsten M., Robertson M.P., Kumschick S., Somers M.J., Wilson J.R.U., Terblanche J.S. 2016 — Methods and approaches for the management of arthropod border incursions — Biol. Invasions. 18(4): 1057-1075. doi:10.1007/s10530-016-1085-6 ![]()
Saccaggi D.L., Pieterse W. 2013 — Intercepting aliens: Insects and mites on budwood imported to South Africa — J. Econ. Entomol. 106: 1179-1189. doi:10.1603/EC12465 ![]()
Saccaggi D.L., Ueckermann E.A., du Toit I., Ngubane-Ndhlovu N.P. 2017 — First records of Brevipalpus lewisi McGregor (Acari: Trombidiformes: Tenuipalpidae) in South Africa, with notes on distribution and field ecology — Afr. Entomol. 25(2): 523-528. doi:10.4001/003.025.0523 ![]()
Santos M.A., Laing J.E. 1985 — Stigmaeid predators — In: Helle W., Sabelis M.W. (Eds). Spider mites, their Biology, natural Enemies and control. Vol. 1B. Amsterdam, The Netherlands: Elsevier, pp. 197-203.
Stals R., Prinsloo G. 2007 — Discovery of an alien invasive, predatory insect in South Africa: the multi-coloured Asian ladybird beetle, Harmonia axyridis (Pallas) (Coleoptera: Coccinellidae) — South African Journal of Science 103(3-4): 123-126.
Wood T.G. 1967 — New Zealand mites of the family Stigmaeidae (Acari, Prostigmata) — Transactions of the Royal Society of New Zealand 9(9): 93-139.
Yeşilayer A., Çobanoğlu S. 2013 — Determination of Raphignathoid mites (Acari: Prostigmata: Raphignathoidea) — Turk. Entomol. Derg. 37(1): 93-103.



2017-06-07
Date accepted:
2017-08-12
Date published:
2018-01-30
Edited by:
Tixier, Marie-Stéphane

This work is licensed under a Creative Commons Attribution 4.0 International License
2018 Saccaggi, Davina L. and Ueckermann, Edward A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)



