First record of the genus Tanytydeus (Acari: Paratydeidae) from South America with description of a new species from the Patagonian forests of Argentina
Kun, Marcelo E.  1
1
1✉ Laboratorio de Zoología, Centro Regional Universitario Bariloche, Universidad Nacional del Comahue, 8400 San Carlos de Bariloche, Provincia of Río Negro, Argentina.
2022 - Volume: 62 Issue: 4 pages: 1084-1097
https://doi.org/10.24349/piy2-100qZooBank LSID: 09DBD3CE-867E-49BF-8EF4-A11A30598340
Original research
Keywords
Abstract
Introduction
The Paratydeidae comprises three genera, Scolotydaeus Berlese 1910, Tanytydeus Theron et al. 1969 and Neotydeus Baker 1950, and 20 extant and 2 fossil species, distributed worldwide (Klimov et al. 2020). Previous reports of paratydeid mites indicate this group lives in edaphic and arboreal habitats such as litter, moss, rotten wood and under tree bark, but also taking shelter in bird nests (Theron et al. 1969; Price 1973; Delfinado & Baker 1974; Seeman & Walter 1999; Dönel et al. 2012) or termite nests (Khaustov et al. 2019). Species of Paratydeidae with eyes such as those belonging to Scolotydaeus apparently thrive on exposed habitats such as bark, moss, or litter while the blind genus Tanytydeus is generally found in soil excepting T. lamington.
The sickle-shaped chelicera of Paratydeidae could advocate for a predatory way of life, but some mites with a similar shape of chelicerae are suspected to be moss feeders such as the genus Eustigmaeus (Stigmaeidae) (Flechtmann 1985).
Only one species, Scolotydaeus corticicola Flechtmann, 1992, was previously reported from the Neotropical region (in Brazil). A recent revision of the family Paratydeidae included three new species: Scolotydaeus uralensis, Tanytydeus cubanus and T. kethleyi (Khaustov 2017). More recent work added T. theroni from South Africa (Khaustov et al. 2019) and two fossil Paratydeidae: Scolotydaeus vlaskini and Tanytydeus pogrebnyaki from amber of Ukraine (Klimov et al. 2020).
This study presents the description of a new species of Tanytydeus representing the first record of this genus in South America and the first record of the Paratydeidae from Argentina.
Materials and methods
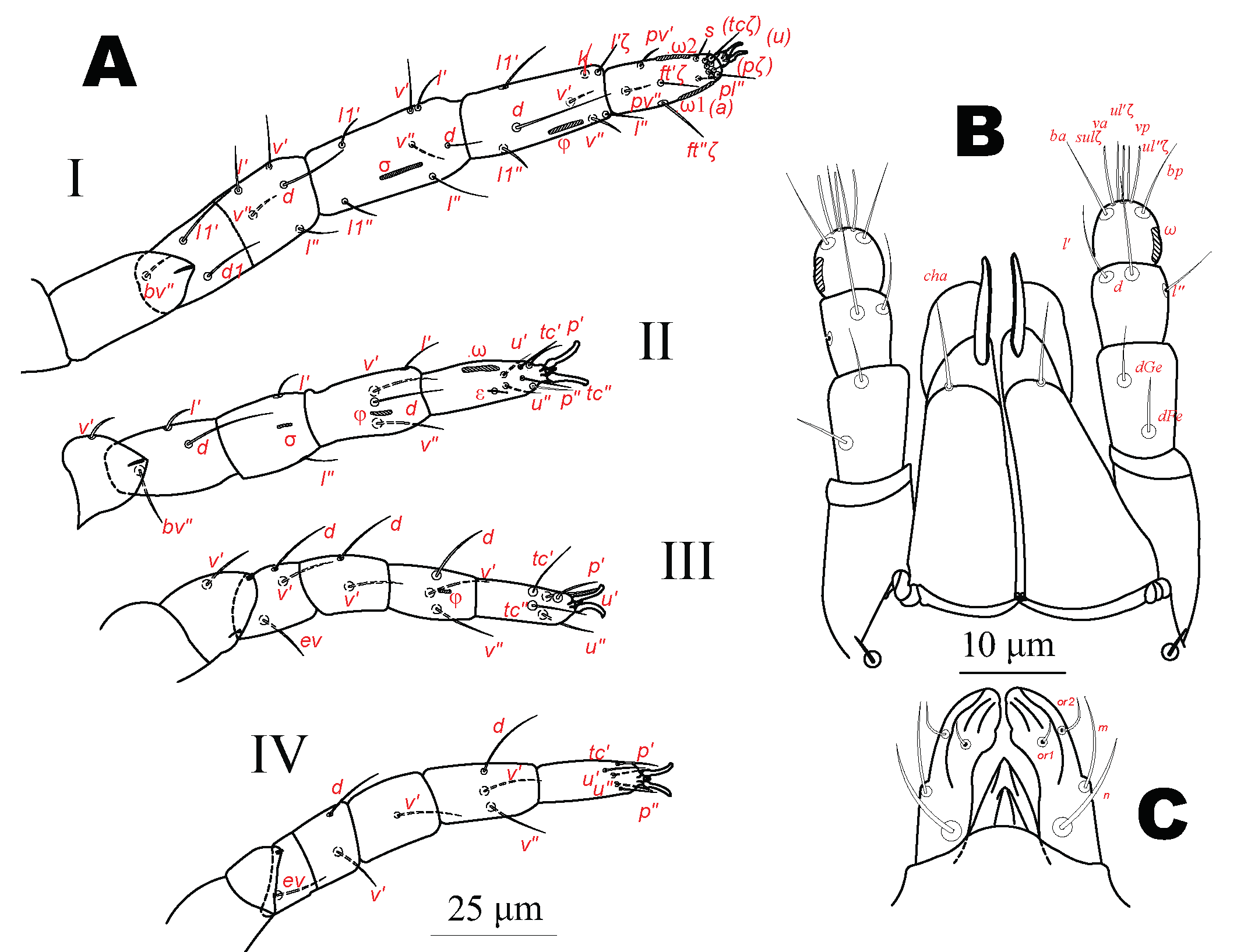
Bark fragments were obtained from coihue trees, Nothofagus dombeyi, from Parque Municipal Llao Llao in three locations, (1) path to the Cerro Llao Llao, (2) path to Lago Escondido, (3) path to Puente Romano and from Parque Nacional Nahuel Huapi, Villa Los Coihues near the path to Cascada Los Duendes. Mites were extracted during ten days in Tullgren funnels, at 20 °C, cleared one day in Nesbitt's fluid, mounted in Hoyer's medium, dried, measured, identified and drawn with Olympus CH5-260 and Zeiss BX40 microscopes. The latter was equipped with a drawing tube and phase contrast (PH) and Differential Interference Contrast objectives and was used for detailed analysis and PH micrographs. Measurements were made with an ocular eyepiece provided with a grid and calibrated with a stage micrometer. Photos were made with a 12-megapixel Sony Camera model SS adapted to the microscope tube. Drawings were made with nanoCAD 5.0 with micrographs as a background. The notation applied to the body and leg setae follow that of Grandjean's system, overviewed by Kethley (1990) and Norton (1977), respectively, and the palpal setation follows Grandjean (1946).
Ventral idiosomal setal designation follows the epimeral setation of Grandjean (1934). All measurements are given in micrometers (µm). Legs were measured from the base of trochanters to the tip of the tarsi without considering the claws.
The female holotype and 4 paratypes (1 male, 1 tritonymph, 1 deutonymph, and 1 protonymph) are deposited in the Museo Nacional de La Plata and remaining paratypes in the Laboratorio de Zoología del Centro Regional Universitario Bariloche de la Universidad Nacional del Comahue.
Results
Tanytydeus nothofagi n. sp.
ZOOBANK: FA829149-49AE-4839-AEC1-51DEF6314972 ![]()
Figures 1–10
Description






Female — (Figs. 1-3)
Length of idiosoma 495 (495-525), width 111 (111-157). Body elongated, mauve to grayish purple, with violet pigmented granules sparsely distributed all over striated body (Fig. 1A). Gnathosoma (Fig. 3B) with palptarsus with three eupathidia (sul, ul´ and ul″). Subcapitular setae n 10 (9-11), slightly longer than m 8 (6-8), and m longer than or1-2 6 (5-6). Triangular ventral lip (labium) present between lateral lips of subcapitulum (Fig. 3C). Chelicerae pyriform and movable digit curved and blade-like with cheliceral setae 9 (9- 12). Peritremes linear, widened laterally and hidden medially under anterior margin of prodorsum. Gnathosomal blunt ended setae ep 4 (3-4) and epl 4 (4-4).
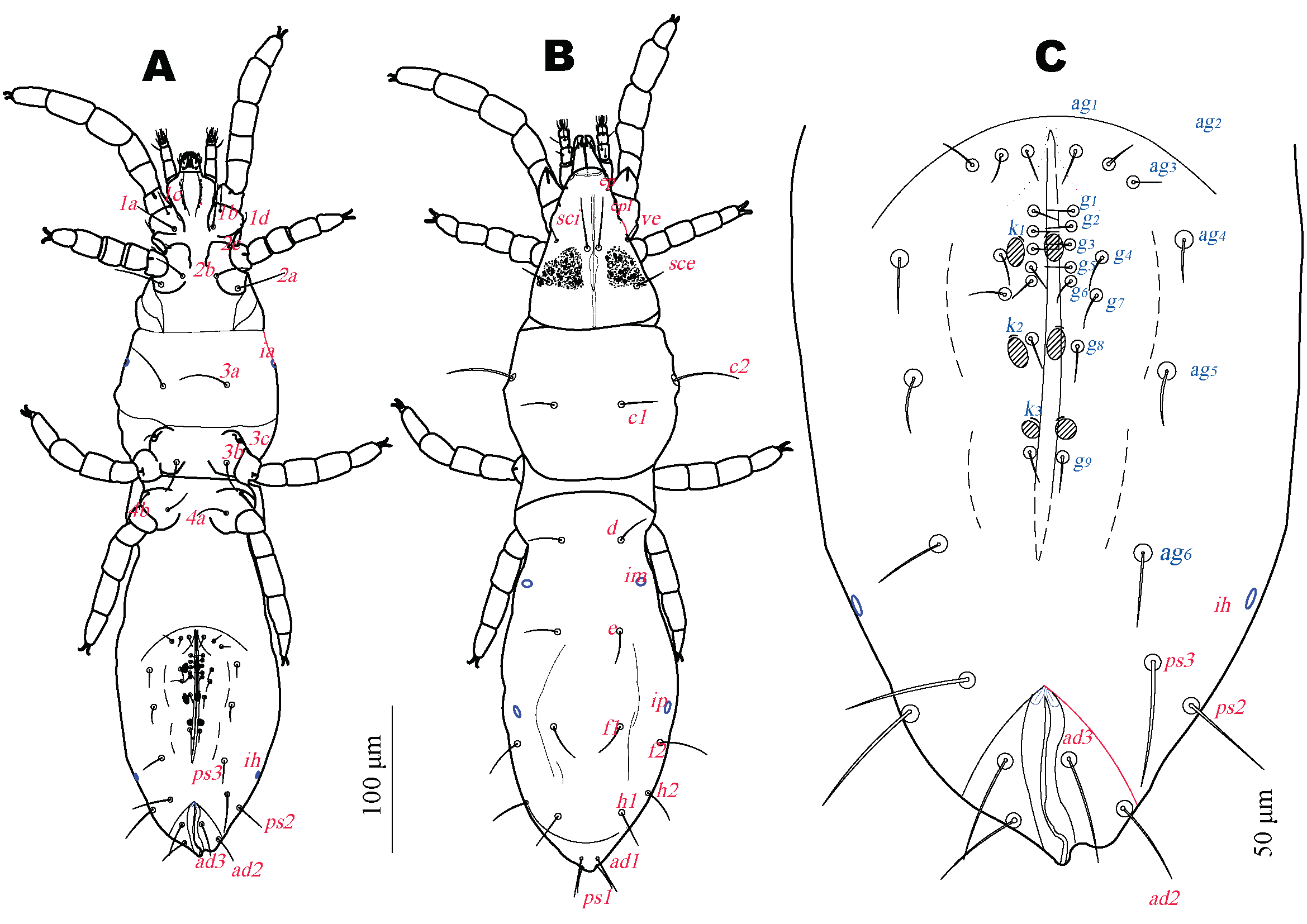
Idiosomal dorsum (Fig. 2B). Prodorsum with linear crista-like shield and 3 pairs of setae: long simple trichobothria sci, located on shield margin and simple setae ve and sce, on striated cuticle, supracoxal peg-like setae, ep and pl on dorsal palpcoxae and coxae I. Prodorsum lacking eyes but with 1 pair of subcuticular eyespots (Fig. 1A) located between second legs and trichobothrial setae sci represented by subdermal dark violet pigmented granules, each granule rounded to elongated 2 to 5 microns diameter. Hysterosoma with 4 transverse dorsal furrows between setal rows C and D, D and E, E and F and H and PS; furrows between E and F and H and PS incomplete not reaching hysterosomal margin. Furrow between setal rows C-D divides hysterosoma into anterior and posterior hysterosomal regions, anterior margin of latter overlaps caudal margin of anterior hysterosomal region. All dorsal setae smooth, pointed. Cupules im situated anterolaterally to setae e near lateral margin, cupules ip situated posteriad setae f1, anteromedially to setae f2.
Idiosomal venter (Fig. 2A). Ventral setae smooth and pointed. With 4 pairs of aggenital setae and 5 pairs of genital setae, 3 pairs of genital acetabulae, each associated with a pair of acetabular setae k1, k2 and k3 (Fig. 2C). Cupules ia situated laterally to3a.
Cupules ih situated posterolaterally to setae ag4 near lateral margin of body and anterior to setae ps3 . Anal region anteromedially with 1 pair of oval pits. Length of idiosomal setae: ve 8 (8-13), sci 34 (34-38), sce 26 (22-29), c1 16 (15-22), c2 42 (47-53), d 21 (15-21), e 14 (13-14), f1 16 (15-17), f2 42 (30-42), h1 23 (22-25), h2 34 (33-44), ps1 18 (18-23), ps2 32 (21-32), ps3 32 (28-33), ad1 22 (21-23), ad2 17 (17-24), ad3 20 (16-22), 1a 23 (23-32), 1c 6 (6-11), 1b 15 (11-19), 1d 8 (7-12), 2c 7 (5-9), 2b 18 (17-21), 2a 27 (27-29), 3a 44 (37-45), 3c 11 (9-12), 3b 23 (21-24), 4a 20 (16-20), 4b 11 (10-13), ag1 13 (13-17), ag2 17 (14-17), ag3 16 (12-18), ag4 13 (13-21), g1 6 (5-6), g2 7 (6-8), g3 7 (6-9), g4 7 (5-7), g5 5 (5-7).
Legs (Fig. 3A) Length of legs: leg I 139 (124-129), leg II 87 (87-97), leg III 97 (97-106), leg IV 104 (97-116). Solenidia sunken elongate-oval pits (Figs. 1B, 3A). Claws of legs I small and nearly one half as long as claws of legs II-IV. All empodia small, about five times shorter than tarsal claws.





Male — (Figs 4-6)





Length of idiosoma 505, width 149. Gnathosoma, prodorsum, and anal region as in female. Subcapitular setae n 8, slightly longer than m 8, and m longer than or1-2, 4, cheliceral setae 10, all slightly shorter than in female (Fig. 6B). Gnathosomal blunt ended setae ep 3 and epl 3.
Idiosomal dorsum (Fig. 5B). Hysterosoma with transverse furrow between setal rows H and PS present. Dorsal setae pointed. Cupules im situated anterolaterally to setae e near lateral margin, cupules ip situated anteriad to setae f1and f2. Setae f2 shorter than in female.
Idiosomal venter (Fig. 5A). Setae ps2 shorter than in female. Genital area with 6 pairs of aggenital setae and 9 pairs of genital setae, 3 pairs of genital acetabulae with, near each, 1 pair of acetabular setae, k1, k2 and k3 (Fig. 5C) and 10 pairs of eugenital setae, eu7 and eu8 with alveoli coalescent (Fig. 6A).



Length of idiosomal setae: ve10, sci 36, sce 27, c1 15, c2 44, d 14, e 15, f1 16, f2 27, h1 25, h2 32, ps1 23, ps2 21 , ps3 35, ad1 19, ad2 20, ad3 17, 1a 30, 1c 5, 1b 16, 1d 9, 2c 7, 2b 21, 2a 31, 3a 42, 3c 12, 3b 26, 4a 15, 4b 18, ag1 6, ag2 8, ag3 9, ag4 10, ag5 11, ag6 15, g1 5, g2 6, g3 8, g4 5, g5 4, g6 6, g7 7, g8 4, g9 5.
Legs (Length of legs: leg I 149, leg II 101, leg III 106, leg IV 116. Leg setation as in female (Table 1).
Larva — (Fig. 7)



Length of idiosoma 313, width 86. Body color and pigmentation as in female. Subcapitular setae n (3), m (4), and or1 (3), or2 absent. Cheliceral setae 7. Gnathosomal blunt ended setae ep 2 and epl 2.
Idiosomal dorsum. Prodorsum as in female. Hysterosoma with furrow between setal rows C and D present. Cupules ia situated anteriad to setae c2.
Idiosomal venter. Claparède organs between coxae I and II. Genital area without setae only represented by a sinuate middle slit between two flanking parallel linear folds.
Length of idiosomal setae: ve 7, sci 29, sce 28, c1 14, c2 39, d 13, e 13, f1 22, f2 30, h1 22, h2 25, ps1 9, ps2 7, ps3 6, 1a 24, 1c 1, 1b 3, 2a 17, 3a 35, 3b 19.
Legs (Fig. 8). Length of legs: leg I 120, leg II 75, leg III 73. Legs with empodia thinner, about one fifth longer than tarsal claws (Fig.7C).



Protonymph — (Fig. 8)



Length of idiosoma 404, width 114. Body color and pigmentation, gnathosoma and prodorsum as in female. Subcapitular setae n 8, m 7, and or1-2 5, cheliceral setae 12. Gnathosomal blunt ended setae ep 3, epl 3.
Idiosomal dorsum (Fig. 8B). Hysterosoma with incomplete furrows not reaching hysterosomal margin between the E and F and F and PS setal rows present.
Idiosomal venter (Fig. 8A) Genital area with 1 pair of aggenital setae and 1 pair of genital acetabulae with near each a pair of acetabular setae k. Length of idiosomal setae: ve 13, sci 28, sce 21, c1 13, c2 40, d 12, e 11, f1 17, f2 31, h1 20, h2 26, ps1 18, ps2 11, ps3 15, ad1 4, ad2 6, ad3 6, 1a 24, 1c 4, 1b 12, 1d 8, 2b 15, 2a 19, 3a 35, 3b 18, g1 10.
Legs (Fig. 8C) Length of legs: leg I 121, leg II 82, leg III 94, leg IV 104. Claws of legs I small, almost one-half length of claws of legs II - IV. Empodia of leg IV longer and slender than tarsal claws, remaining empodia shorter than tarsal claws.
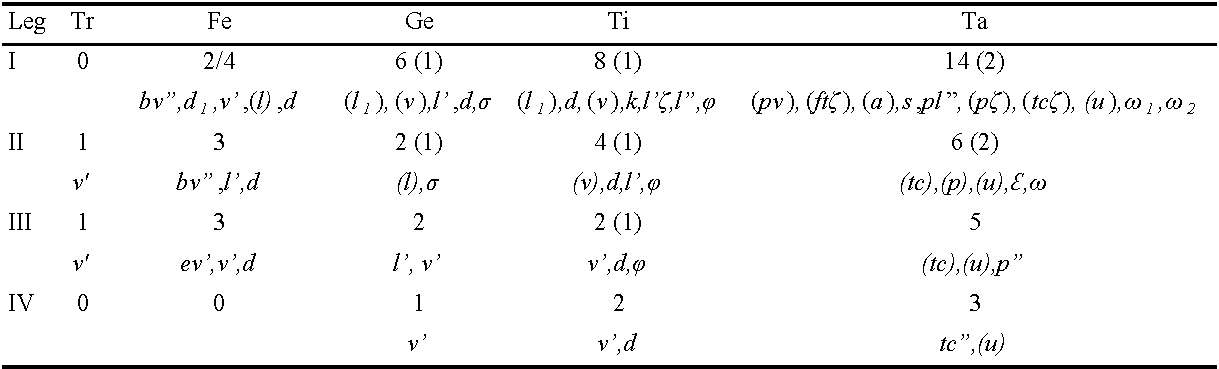


Deutonymph — (Fig. 9)



Length of idiosoma 425, width 121. Body color and pigmentation, gnathosoma and prodorsum as in female. Subcapitular setae n 9, m 6, and or1-2 6-7. Cheliceral setae 11. Peritremes as in female. Gnathosomal blunt ended setae ep 3, epl 3.
Idiosomal dorsum (Fig. 9B). Hysterosoma with furrows between rows of setae C and D and H and PS. The latter incomplete not reaching hysterosomal margin. Cupules im situated anterolaterally to setae e near lateral margin, cupules ip situated as in female.
Idiosomal venter (Fig. 9A). Ventral setae smooth and pointed. Genital area with 2 pairs of aggenital setae, 2 pairs of genital setae and 2 pairs of genital acetabulae, near each a pair of acetabular setae k. Cupules ia and ih situated as in female. Anal region with two anterior oval pits. Length of idiosomal setae: ve 9, sci 33, sce 22, c1 14, c2 50, d 13, e 12, f1 17, f2 31, h1 25, h2 33, ps1 23, ps2 18, ps3 20, ad1 16, ad2 14, ad3 14, 1a 25, 1b 6, 1c 12, 1d 7, 2b 18, 2c 7, 2a 22, 3a 43, 3b 21, 3c 7, 4a 14, ag1 15, ag2 16, g1 8, g2 8.
Legs (Fig. 9C). Length of legs: leg I 125, leg II 87, leg III 97, leg IV 109. Solenidia as in other stages. Claws of legs I small almost one half the length of claws of legs II - IV. Empodia shorter than tarsal claws.



Tritonymph — (Fig. 10)



Length of idiosoma 455, width 121. Body color and pigmentation, gnathosoma and prodorsum as in female. Subcapitular setae n 9, m 7, and or1-2 6-7. Cheliceral setae 11. Peritremes as in female. Gnathosomal blunt ended setae ep 3 and epl 3.
Idiosomal dorsum (Fig. 10B). As in female. Idiosomal venter (Fig. 10A) ventral setae smooth and pointed. Hysterosoma with transverse dorsal furrows between C and D and and H and PS. Genital and anal areas widely separated. Genital area with 4 pairs of aggenital setae and 4 pairs of genital setae, 3 pairs of genital acetabulae , near each a pair of acetabular setae k1, k2 and k3 (Fig. 10A). Cupules ia and ih and anal region as in female. Length of idiosomal setae: ve 12, sci 33, sce 22, c1 15, c2 42, d 13, e 12, f1 14, f2 37, h1 24, h2 34, ps1 18, ps2 23, ps3 30, ad1 23, ad2 18, ad3 19, 1a 25, 1c 5, 1b 16, 1d 9, 2b 16, 2c 6, 2a 21, 3a 31, 3b 7, 3c 20, 4a 19, 4b 7, ag1 13, ag2 13, ag3 14, ag4 12, g1 5, g2 7, g3 5, g4 8.
Legs (Fig.10C) Length of legs: leg I 139, leg II 92, leg III 94, leg IV 106. Femur I with only two setae present on basifemur (Table 5).
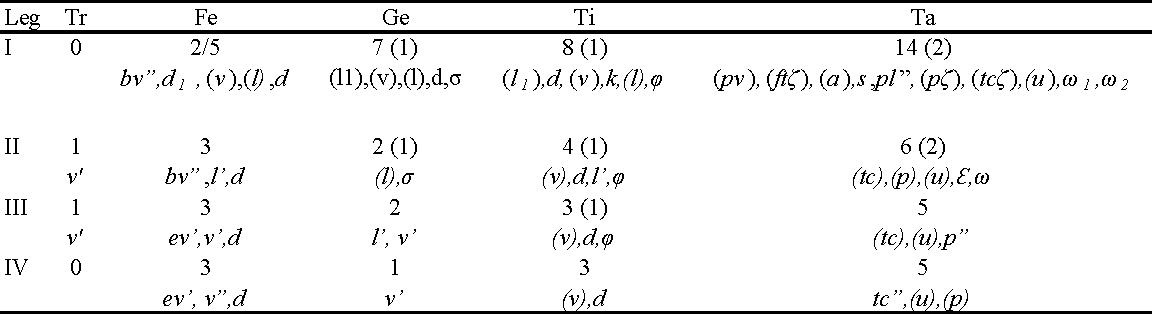


In Fig. 10B a symmetrical structure appears under the cuticle. The origin of it cannot be attributed at this point to either the beginning of the formation of male genitalia or an internal organ.
Material examined
Four males, 1 female, 1 tritonymph, 3 deutonymphs, 1 protonymph and 1 larva on 11 microscopic preparations path to Cerro LLao LLao, Parque Municipal LLao LLao (Río Negro) 41°02′52″S, 071°33′09″W, 972 m a.s.l., 14 April 2007 leg. M.E. Kun; 2 females, 1 protonymph on 3 microscopic preparations path to Cerro LLao Llao, Parque Municipal LLao LLao (Río Negro), 21 January 2007 Leg. M.E. Kun; 3 females, 1 tritonymph, 3 deutonymphs on 7 microscopic preparations path to Cascada Los Duendes, Villa Los Coihues, Parque Nacional Nahuel Huapi (Río Negro) 41°10′42″S, 71°25′01″W, 862 m a.s.l., 14 April 2007 leg. M.E. Kun; 2 males, 1 female, 1 tritonymph, 1 deutonymph, 1 protonymph on 6 microscopic preparations, Puente romano, Parque Municipal LLao LLao (Río Negro) 41°03′01″S, 71°33′57″W, 817 m a.s.l., 25 November 2014 leg. M.P. Salaberry; 3 males, 2 females, 1 tritonymph, 2 deutonymphs, 2 protonymphs on 10 microscopic preparations path to Lago Escondido, Parque Municipal LLao LLao (Río Negro) 41°03′28″S, 71°33′55″W, 801 m a.s.l., May 2015 leg. M.P. Salaberry. All specimens on bark of Nothofagus dombeyi.
Differential diagnosis
Tanytydeus nothofagi n. sp. may be unique in Tanytydeus by the presence of subdermal eyespots which are not reported in the genus. The presence of 3 pairs of genital acetabulae (instead of 2 in the adult) places this species in a group comprising T. beyzavii, T. kakadu, T. lamington , T. simplex and T. theroni (Delfinado and Baker 1974, Seeman and Walter 1999, Khanjani et al. 2014, Khaustov et al. 2019). T. nothofagi n. sp. differs from T. beyzavii, T. kakadu and T. theroni by the presence of 8 setae on femur I, from T. simplex by the presence 3 setae on Fe II and 6 setae on Ta II, from T. lammingtoni by the presence of 7 setae on Ge I, 2 setae on Ge II, 4 setae on Ti II, 5 setae on Ta III.
Remarks
Species of Paratydeidae with eyes as those belonging to genus Scolotydaeus apparently live on exposed habitats such as bark, moss, or litter while the blind genus Tanytydeus is generally found in soil excepting T. lamington. The finding of T. nothofagi n. sp. living on bark instead of soil poses the question if the presence of eyespots could be a mild adaptation for the perception of shifts in light intensity which is an advantageous ability for surviving in a light exposed habitat.
Etymology
The species is named after Nothofagus dombeyi, the tree on which the mites were collected.
Acknowledgments
This work was realized under the project 04/B215 of the Universidad Nacional del Comahue, named ''Diversidad de los artrópodos en la Patagonia argentina y sus relaciones con los animales, las plantas y el hombre'' and project 04/B243 of the Universidad Nacional del Comahue, named ''Biodiversidad, biología e interacciones ecológicas y antrópicas de artrópodos y vertebrados nativos y exóticos en el NO de Patagonia'' in the laboratory de Zoología of the Centro Regional Universitario Bariloche (Universidad Nacional del Comahue). The author wishes to thank Doctor Alexander Khaustov and Doctor Owen Seeman for their suggestions, helpful comments and for sharing their knowledge about Paratydeidae. The author thanks also Dra Maria Inés Messuti and Dr Gernot Vobis for their support and encouragement for carrying out research on arthropods and their relations with lichens and Lic. María Paula Salaberry for lending collected material on bark of Nothofagus dombeyi.
References
- Berlese, A. 1910 Acari nuovi. Redia, 6, 199-234.
- Baker, E.W. (1950) Further notes on the family Paratydeidae (Acarina), with a description of another new genus and species. Journal of The Washington Academy of Sciences, 40, 289-291.
- Delfinado D.M., Baker E.W. 1974. Terrestrial mites of New York (Acarina: Prostigmata), I - Tarsocheylidae, Paratydeidae and Pseudocheylidae. J. N.Y. Entom. Soc. 82(3):202-211.
- Dönel G., Seeman O.D., Doğan S. 2012. The first Paratydeidae (Trombidiformes: Paratydeoidea) in Turkey: Scolotydaeus anatolicus sp. nov. Int. J. of Acarol., 38(5):436- 444. https://doi.org/10.1080/01647954.2012.669527
- Flechtmann C.H.W. 1985. Eustigmaeus bryonemus sp. n., a moss feeding mite from Brasil (Acari, Prostigmata : Stigmaeidae). Rev. bras. Zool., S Paulo 2(6): 387-391. https://doi.org/10.1590/S0101-81751984000200010
- Flechtmann C.H.W. 1992. First record of a Paratydeidae (Acari, Prostigmata) in South America with description of Scolotydaeus corticicola sp. n. Rev. Bras. Zool. 9(3- 4):299-304. https://doi.org/10.1590/S0101-81751992000200017
- Grandjean, F. 1934. Les poils des épimères chez les Oribates (Acariens). Bull. Mus. Nat. Hist. Natur., Sér. 2(6):504-512.
- Grandjean, F. 1946. Au sujet de l′organe de Claparède, des eupathides multiples et des taenidies mandibulaires chez les Acariens actinochitineux. Archives des Sciences physiques et naturelles, 28: 63-87.
- Kethley, J. 1990. Acarina: Prostigmata (Actinedida). In: Dindal, D.L. (Ed.), Soil Biology Guide. John Wiley & Sons, New York, pp. 667-756.
- Khanjani, M., Nadri, A.R., Khanjani, M. & Seeman, O.D. 2014. Post larval stages of Tanytydeusbeyzavii sp. nov. (Acari: Paratydeidae) from Iran. Zootaxa, 3895 (2): 170- 182. https://doi.org/10.11646/zootaxa.3895.2.2
- Khaustov A. 2017. Review of the Paratydeidae (Acari: Prostigmata), with description of three new species. Zootaxa 4303(2):151-212. https://doi.org/10.11646/zootaxa.4303.2.1
- Khaustov, A.A., Hugo-Coetzee E.A. & Ermilov S.G. 2019. A new species of Tanytydeus (Acari: Paratydeidae) from termite nests in South Africa. Syst. Appl. Acarol. 24(9): 1604-1619. https://doi.org/10.11158/saa.24.9.3
- Klimov, P.B., Khaustov, A.A., Vorontsov, D.D., Perkovsky, E.E., Pepato A.R. &Sidorchuk, E.A. 2020. Two new species of fossil Paratydeidae (Acari: Trombidiformes) from the late Eocene amber highlight ultraslow morphological evolution in a soil-inhabiting arthropod lineage. J. Systematic. Paleontology, 18(7):607-629. https://doi.org/10.1080/14772019.2019.1655496
- Norton, R.A. 1977. A review of F. Grandjean's system of leg chaetotaxy in the Oribatei and its application to the Damaeidae. In: Dindal, D.L. (Ed.), Biology of Oribatid Mites. SUNY College of Environmental Science and Forestry, Syracuse, pp. 33-62.
- Price, D.W. 1973. Abundance and vertical distribution of microarthropods in the surface layers of a California pine forest soil. Hilgardia 42:121-147. https://doi.org/10.3733/hilg.v42n04p121
- Seeman O.D., Walter D.E. 1999. A review of the Paratydeidae (Acari: Prostigmata) with description of the first Australian Representatives Tanytydeus lamington sp. nov. and T. Kakadu sp. nov. Acarologia. 40(4):393-400.
- Theron P.D., Meyer M.K.P, Ryke P.A.J. 1969. Two new genera of the family Paratydeidae (Acari: Prostigmata) from South African soils. Acarologia. 11(4):697-710.



2022-07-01
Date accepted:
2022-09-29
Date published:
2022-10-04
Edited by:
Auger, Philippe

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Kun, Marcelo E.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




