Functional response of the predatory mite, Typhlodromus bagdasarjani (Acari: Phytoseiidae) to protonymphs of Eotetranychus frosti (Acari: Tetranychidae) on four apple cultivars
Jafarian, Fatemeh1
; Jafari, Shahriar  2
and Fathipour, Yaghoub
2
and Fathipour, Yaghoub  3
3
1Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khorramabad, Iran.
2✉ Department of Plant Protection, Faculty of Agriculture, Lorestan University, Khorramabad, Iran.
3Department of Entomology, Faculty of Agriculture, Tarbiat Modares University, Tehran, Iran.
2022 - Volume: 62 Issue: 2 pages: 454-464
https://doi.org/10.24349/7ejy-uk7sOriginal research
Keywords
Abstract
Introduction
Tetranychid mites are among the important pests of different agricultural crops, including field crops, fruit trees, vegetables and ornamental plants worldwide (Fathipour and Maleknia, 2016). Eotetranychus frosti (McGregor) (Tetranychidae) is considered to be one of the most serious pests of apple trees in Iran and maybe in other apple growing areas of the world (Darbemamieh et al., 2007; Khodayari et al., 2010; Jafarian and Jafari 2016 a, b; Jafarian et al., 2020). This species causes serious damages on fruit trees such as apple and citrus in different parts of Iran (Sadeghi-Namaghi, 1995; Darbemamieh et al., 2007; Khodayari et al., 2008, 2010; Darmiani et al., 2011; Jafari et al., 2014). The feeding of this pest with high population density on the lower surface of the leaves usually leads to decrease in the fruit quality, defoliation and losses in apple yield in the same and also following seasons (Jafari et al., 2014). The biological traits of this mite, including the irregular dense webs, depositing eggs on the leaf surface under the webs and the defecation on threads of complicated webs are close to CW (Complicated-web type) life type of tetranychid mites and subtype CW-r (Saito, 1983).
Control of spider mites relies heavily on the use of chemical acaricides (Badii et al., 2004). Due to the undesired effects of hazardous pesticides, the interest in biological control and resistant cultivars is growing. Host plant resistance is a main component of integrated pest management (IPM) programs that can potentially contribute to the efficacy of natural enemies (Lorenzen et al., 2001). The physical and the chemical properties of the host plant have different effects on phytophagous pests and their natural enemy's traits like developmental time, survival and fecundity (Madadi et al., 2007). The functional responses or other foraging behaviors of predators may be influenced by host plant in different ways such as the effect on prey size, shelter for prey, restricting the natural enemy movement by plant physical structures or reducing the searching efficiency of predator by sticky secretions (Price et al., 1980; Messina and Hanks, 1998; Sabelis et al., 1999). The physical properties of the plant such as shape and type of trichomes may influence the consumption rate of the predatory mites (Loughner et al., 2008). Since the population growth of a pest species is usually slower on resistant plants, host plant resistance is often assumed to increase the capacity of a biological control agent to keep the pest population under the economic damage threshold (Boethel and Eikenbary, 1986).
Typhlodromus bagdasarjani Wainstein and Arutunjan is a generalist predator (type III-a lifestyle) (McMurtry et al., 2013) that has relatively high population densities, especially on trees such as apple and it is a potential natural enemy of E. frosti in some regions of Iran (Kamali et al., 2001; Hajizadeh et al., 2002; Jafari, 2010). In addition, it can feed on mites belonging to the families Eriophyidae, Tenuipalpidae, Tetranychidae and Tydeidae as well as insect pests such as thrips and whiteflies (Hajizadeh et al., 2002; Jafari, 2010; Rahmani et al., 2010; Ganjisaffar et al., 2011). The preference of this predator to the protonymphal stage of E. frosti was reported (Bazgir et al., 2020b). Typhlodromus bagdasarjani is a widespread species in the Middle East and was reported from Armenia, Azerbaijan, Turkey, Turkmenistan and Lebanon (Demite et al., 2014).
The functional response parameters of natural enemies are considered to determine the efficiency and ability in regulating pest populations (Fantinou et al., 2012). Functional response in predators describes the relationship between consumption rate of a predator and different prey densities. Holling (1959) proposed three types of functional response, in type I the number of prey consumed linearly increased to reach a maximum, then remains constant with increasing the prey density, in type II the prey consumption increases at a decelerating rate towards an asymptote as prey density increases, and in type III the number of prey eaten approaches an asymptote as a sigmoid function. In functional response experiments searching ability and consumption potential of a predator are determined as two important factors in its efficiency (Holling, 1966). Understanding how various factors affect foraging behavior can provide better understanding of the selection of best conditions in pest control programs. The effect of host plant characters on functional response parameters of some natural enemies has been reported (Madadi et al., 2007; Ahn et al., 2010; Marafel et al., 2011; Rezaie et al., 2017). The significant effect of Lebanon Red, Kohanz Golab, Imperial Gala, Gala Royal, Fuji, Granny Smith and Golden cultivars of apple on life history performance of phytophagous mite E. frosti was detected in a previous study (Jafarian et al., 2020). In this regard, with the aim of completing the study of the tritrophic effects of some apple cultivars on the biological activities of E. frosti and its predator, the current study was designed and conducted. In this study, we evaluated the effect of four apple cultivars (Imperial Gala, Kohanz Golab, Granny Smith and Lebanon Red) on the functional response of adult females of the predatory mite, T. bagdasarjani to different densities of E. frosti protonymphs. Also, the leaf characteristics of different apple cultivars in terms of density and length of trichome effects on functional response of T. bagdasarjani was addressed.
Material and methods
Host plant and prey colony
The apple cultivars were provided from a complex of orchards in Tajareh region, Khorramabad, Western Iran. Four apple cultivars ten years old (planted in 2011) were evaluated, including Imperial Gala, Kohanz Golab, Granny Smith and Lebanon Red, with no pesticide applications conducted during and three years prior to the study. All cultivars were grown in outside with similar environmental conditions and had the same phenological stage. Fresh leaves of each apple cultivar were cut, transferred to the laboratory and used for the experiments. To prepare the colony of prey, the apple leaves infested by the tetranychid mite, E. frosti, were collected from apple trees at the vicinity of Khorramabad, Lorestan Province, Western Iran, during 2020. They were maintained at 27±1°C, 60±10% RH and a photoperiod of 16L: 8D h. The collected tetranychid mites were divided into four groups and reared separately on detached leaves of each apple cultivar for three generations before provisioning them as prey in the experiments described below.
Apple leaf characters
To investigate the role of leaf characters of four apple cultivars on the functional response of the predator, 20 leaves of each cultivar were randomly picked and the number of trichomes on the underside of the leaf in 1 cm2, as well as the length of each trichome, were accurately recorded and measured by means of a stereomicroscope (Olympus SZ-1145) equipped with a calibrated lens, respectively.
Predatory mite colony
The initial population of T. bagdasarjani was originally collected from leaves of apple trees located in Khorramabad, Iran during 2020. Laboratory colonies of T. bagdasarjani were reared on E. frosti on apple leaves in the laboratory. Before their use in the experiments, the predators were separately reared for two generations on the different apple cultivars, using immature stages of E. frosti as the sole food source.
Functional response experiments
According to our observations in apple orchards, around Khorramabad County, Iran, E. frosti were mostly found on the underside of apple leaves. The experimental arena for functional response experiments consisted of a piece of apple leaf from the above-mentioned cultivars (4 ×4 cm) placed upside down on a wet cotton layer in a Petri dish (6 cm diameter) with a 0.5 cm hole drilled in the center. This Petri dish was placed in the middle of a larger Petri dish (9 cm in diameter) containing water to keep the leaves fresh. To prevent escaping of the predator and prey, a water-saturated cotton strip was placed around the leaf margin.
To standardize the age of female T. bagdasarjani used in experiments, approximately 100 gravid females were randomly selected from the colony and transferred to apple leaf disc arenas supplied with immature stages of E. frosti. After 24 h, T. bagdasarjani females and all stages of the prey were removed, leaving only the newly laid T. bagdasarjani eggs, kept at 27±1°C, 60±10% RH and 16:8 (L: D)h and monitored at daily intervals. Subsequently, the emerging predator larvae were reared on immature stages of E. frosti to adulthood. A cohort of mated females (four days old) was used in the functional response experiments. According to results of our preliminarily test (Unpublished data) and those reported by Bazgir et al. (2020 b), T. bagdasarjani females preferred the protonymphal stage of E. frosti for feeding. To determine the functional response of the predator on the prey, a 24-h starved female was exposed to seven densities (2, 4, 8, 16, 32, 64 and 128) of newly emerged protonymphs of E. frosti. To obtain protonymph prey, approximately 50 adult female E. frosti were transferred from the stock colony to each leaf disc and allowed to oviposit for 24 h, following which the females were removed. The hatched eggs were monitored under a stereomicroscope until reaching the protonymph stage and subsequently provisioned with a fine paintbrush (number 0000) to the respective arenas to reach the designated prey density, along with the starved female predator. After 24 h, the predators were removed and the number of prey eaten was recorded. Ten replicates were prepared for each prey density. All experiments were conducted at a constant temperature of 27±1°C, 60±10% RH and 16:8 h L: D photoperiod in two incubators (Jal Tajhiz Company, Iran), which were able to control the RH, temperature and photoperiod.
Data analysis
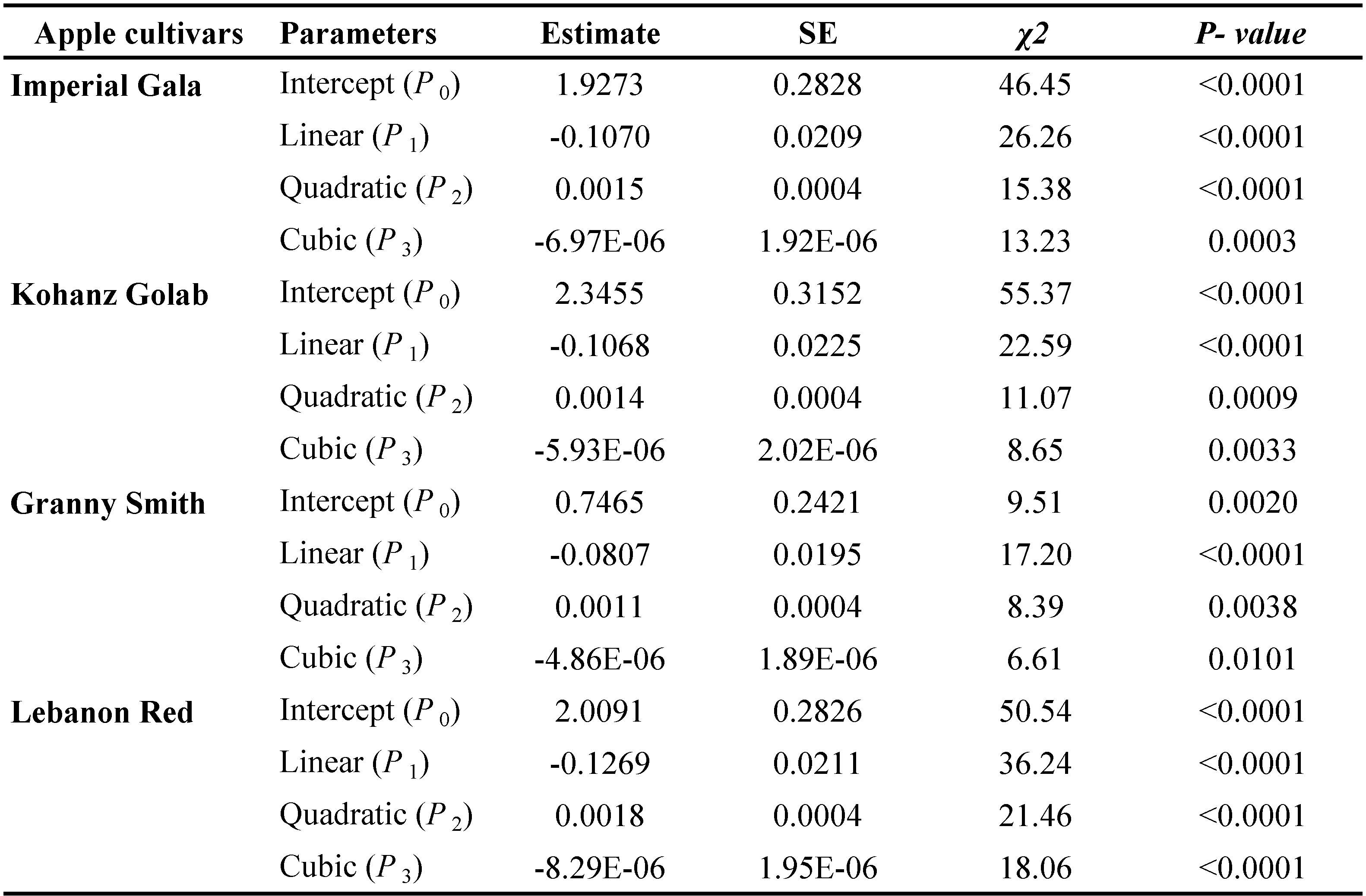
The data of functional response were analyzed in two steps (Juliano, 2001). First, to determine the type of functional response, the positive or negative sign of the linear coefficient was determined by logistic regression of the proportion of prey consumed (Na/Nt) as a function of prey density (Nt):
\[ \frac{N_a}{N_t} = \frac{exp(P_0 + P_1 N_t + P_2 N_t^2 + P_3 N_t^3)}{1 + exp(P_0 + P_1 N_t + P_2 N_t^2 + P_3 N_t^3)} \]
where Na is the number of prey consumed, Nt is the initial prey density, (Na/Nt) is the probability of being eaten and P0, P1, P2 and P3 are the intercept, linear, quadratic and cubic coefficients, respectively, estimated using the maximum likelihood method. The type of functional response was determined by the signs of P1 and P2. If the linear coefficient is negative (P1 < 0), it describes a type II functional response because the proportion of prey consumed declines gradually with increasing the initial prey density (Juliano, 2001). If P1< 0 and P2< 0, the proportion of prey consumed is positively density dependent describing a type III functional response (Juliano, 2001). In the next step, the handling time (Th ) and the attack rate (α) coefficients of a type II response were estimated using the Rogers' random predator equation (Royama, 1971; Rogers, 1972):
\[ N_a = N_t \{1 - exp [ -\alpha TP_t / (1 + \alpha T_h N_t ) ] \} \]
where Na is the number of prey consumed, Nt is the initial prey density, T is the total time available for predators during the experiment (in this study 24 hours), α is the attack rate (searching efficiency), and Th is the amount of time that a predator handles each prey individual (handling time) and Pt is the number of predators (Rogers, 1972). Nonlinear regression was used to estimate the attack rate and the handling time parameters (Proc NLIN, SAS Institute, 2003). The differences between numbers of prey consumed per day by each female of T. bagdasarjani on different densities of E. frosti on four apple cultivars and also the number and length of trichomes on each apple cultivars were analyzed using one way analysis of variance (ANOVA). When a significant difference was detected, the means were compared using Tukey's multiple range test (P\textless0.05). The ANOVA and mean comparison were carried out using the Minitab Version 18 (Minitab, 2017). The curves of the observed number and proportion of prey eaten by T. bagdasarjani females to the protonymphal stages of E. frosti at different densities on four apple cultivars were depicted by Excel software.
Results
Apple leaf characters
A significant difference was observed among the number of trichomes on 1 cm2 of lower surface of four apple cultivars. The highest and lowest number of trichomes were observed in Granny Smith and Kohanz Golab, respectively. Furthermore, the shortest trichomes was observed in Kohanz Golab (Table 1).



Functional response experiments
The logistic regression analysis of consumed prey by T. bagdasarjani at different densities of prey showed that P1< 0 for all cultivars (Table 2), indicating the percentage of prey consumed on each cultivar gradually declined as prey density increased. Therefore T. bagdasarjani showed a type II functional response on protonymphal stages of the E. frosti on all tested cultivars. Analysis of variance of the data obtained from the functional reaction test showed that at different prey densities there was a significant difference between the numbers of prey consumed by the predator in different cultivars (Table 3). As shown, with increasing the number of prey in all tested cultivars, the number of prey eaten increased, but on the contrary the ratio of prey eaten decreased. Only in Granny Smith the percentage of prey eaten firstly slightly increased, then similar to other cultivars decreased (Figures 1 and 2).
Rogers's type II model showed a good fit with the data obtained on the four tested cultivars. The highest and lowest values of attack rate were 0.0747 h- 1 and 0.0334 h-1, respectively, which were observed in Kohanz Golab and Granny Smith, respectively (Table 4). The handling time (Th ) of T. bagdasarjani was also affected by apple cultivars. The longest and shortest handling time were 1.1189 h on Granny Smith and 0.7005 h on Kohanz Golab, respectively. The theoretical maximum predation rate (T/Th ) is the upper asymptote of the functional response curve and represents the high potential of a predator to eat its prey during a period of 24 h (Cave and Gaylor, 1987; Jafari et al., 2012). Theoretical maximum predation rate (T/Th ) of the predatory mite was the highest and lowest on the Kohanz Golab (34.26 prey/day) and Granny Smith (21.45 prey/day) cultivars, respectively (Table 4).














Discussion
This is the first study that assessed host plant effects, using four apple cultivars, on the functional response of the predatory mite T. bagdasarjani to varying densities of E. frosti. In this study, the functional response of T. bagdasarjani females to different densities of E. frosti protonymphs in four apple cultivars was type II. The type II functional response has been reported for other phytoseiid mite species (Zhang et al., 1999; Skirvin and Felon, 2003; Badii et al., 2004; Gotoh et al., 2004; Shirdel et al., 2004; Sepulveda and Carrillo, 2008; Marafel et al., 2011; Ferla et al., 2011; Jafari et al., 2012; Bazgir et al. 2020a; Fu et al., 2021).
The surface properties of host plant including trichome density and shape may affect the functional response of predators by changing search efficiency or by affecting their prey (Price et al., 1980; Coll et al., 1997; Scott Brown et al., 1999; Madadi et al., 2009). It is expected that the host plant properties will affect the type of functional response and/or change in value of the functional response parameters. The results of the current study show that the physical traits of the plant surface can affect functional response parameters and predation rate of T. bagdasarjani feeding on E. frost protonymphs but not on the type of functional response. These findings are consistent with those of Rezaie et al., (2017) who found a type II functional response for N. californicus females on Frankliniella occidentalis (Pergande) larvae on different strawberry cultivars. Also, the functional response type of N. californicus to T. urticae on different strawberry cultivars was type II (Ahn et al., 2010). Unlike, Podisus nigrispinus (Dallas) (Het.: Pentatomidae) exhibited a type II functional response to beet armyworms on sweet pepper and cucumber and a type III on tomato (De Clercq et al., 2000).
Attack rate (a) and handling time (Th ) are two key parameters in evaluating the effectiveness of natural enemies that were estimated in functional response studies (Juliano, 2001) and determine the magnitude of the functional response (Pervez and Omkar, 2005). The predator shows the highest attack rate and the shortest handling time on Kohanz Golab in comparison to others. It is possible that the high rate of feeding on Kohanz Golab is related to the lower density and shortest length of trichome on the lower side leaves of this cultivar (table 1), facilitating access to prey. Similarly, Madadi et al. (2007) showed that the physical properties of host plants, especially difference in trichome density between the three plants including sweet pepper, eggplant and cucumber significantly affected the functional response parameters of the Neoseiulus cucumeris (Oudemans) to Thrips tabaci Lindeman. According to Krips et al. (1999) the attack rate of Phytoseiulus persimilis Athias-Henriot was lowest on gerbera cultivars that had higher trichome density. Koveos and Broufas (2000) reported that the searching efficiency of Euseius finlandicus (Oudemans) was lower on apple leaves in comparisons to peach, possibly due to the presence of trichomes on both surfaces of the apple leaf. A similar effect was reported for the predatory mite N. cucumeris on the leaves of Dombeya acutangula Cav., where fewer F. occidentalis were eaten due to the presence of trichomes on both leaf surfaces (Scott Brown et al., 1999). The presence of trichomes on leaf surfaces may physically disrupt the movement of either pest or predator physically by impeding or trapping them, or chemically by subjecting them to sticky, deterrent or toxic compounds in the exudates from glandular trichome (van Lenteren et al., 1995).
The handling time of a predator can be affected by time spent for pursuing and capturing, eating and digesting a prey (Hassell, 1978). The present study shows that the handling time of T. bagdasarjani to protonymphs of E. frosti on different apple cultivars was different. The shortest and longest handling times were obtained on Kohanz Golab and Granny Smith cultivars, respectively. These results show that on Granny Smith, the predator needed more time to manipulate and eat a prey, while on Kohanz Golab in a shorter time the predator can consume more prey. A possible reason for longer handling time of predators on Granny Smith leaves might be that Granny Smith leaf trichomes prevent the movement of predators, subsequently interfere with its searching efficiency or may provide shelter for prey and make them less vulnerable against their predators (Sabelis and van Rijn, 1997).
Also, the estimated a/Th in the functional reaction experiment showed that T. bagdasarjani has an effective predatory potential for the control of E. frosti on Kohanz Golab. In our previous study, Kohanz Golab was the least suitable for development and reproduction of E. frosti, whereas Granny Smith was the most suitable cultivar (Jafarian et al., 2020). Inadequacy of the host plant can affect the efficiency of its predator by affecting the size, weight and population growth of the prey. Also, according to estimate a/Th , T. bagdasarjani has less efficiency for the control of E. frosti on Granny Smith. The decrease in predation efficiency of T. bagdasarjani on Granny Smith may also affect its fecundity, resulting in population decrease and hampering the efficacy of T. bagdasarjani to control of the E. frosti.
Although the functional response is a relatively important and rapid way to determine the effects of the host plant's defense on the effectiveness of the natural enemy, it alone does not indicate the success or failure of a natural enemy in pest control. Many factors influence the efficiency of a predator that should be considered.
Acknowledgments
This research has been partially supported by Lorestan University, which is greatly appreciated. We also express our appreciation to the editor (Dr. Eric Palevsky) and two anonymous reviewers whose constructive comments helped us improve the manuscript.
References
- Ahn J., Kim K., Lee J.H. 2010. Functional response of Neoseiulus californicus (Acari: Phytoseiidae) to Tetranychus urticae (Acari: Tetranychidae) on strawberry leaves. J. Appl. Entomol., 134: 98-104. https://doi.org/10.1111/j.1439-0418.2009.01440.x
- Badii M.H., Hernández-Ortiz E., Flores A.E., Landeros J. 2004. Prey stage preference and functional response of Euseius hibisci to Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Exp. Appl. Acarol., 34: 263-273. https://doi.org/10.1023/B:APPA.0000049222.65883.77
- Bazgir F., Shakarami J., Jafari S. 2020a. Functional response of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) to Eotetranychus frosti (Tetranychidae) and Cenopalpus irani (Tenuipalpidae). Acarologia, 60(1): 30-39. https://doi.org/10.24349/acarologia/20204359
- Bazgir F., Shakarami J., Jafari S. 2020b. Prey-stage preferences, functional and numerical responses, and mutual interference of Typhlodromus bagdasarjani (Acari: Phytoseiidae) on Eotetranychus frosti (Tetranychidae). Int. J. Acarol., 46 (1). https://doi.org/10.1080/01647954.2020.1734657
- Boethel D.J., Eikenbary R.D. 1986. Interactions of plant resistance and parasitoids and predators of insects. Ellis Horwood, Chichester.
- Cave R.D., Gaylor M.J. 1987. Functional response of Telenomus reynoldsi (Hym., Scelionidae) at five constant temperatures and in an artificial plant area. Entomophaga, 34: 1-9. https://doi.org/10.1007/BF02372581
- Coll M., Smith L.A., Ridgway R.L. 1997. Effect of plants on the searching efficiency of a generalist predator: the importance of predator-prey spatial association. Entomol. Exp. Appl., 83: 1-10. https://doi.org/10.1046/j.1570-7458.1997.00151.x
- Darbemamieh M., Fathipour Y., Kamali K. 2007. Seasonal activity and spatial distribution pattern of Eotetranychus frosti (McGregor) (Acari: Tetranychidae) in an unsprayed apple orchard of Kermanshah, Western Iran. Persian J. Acarol., 1(2): 137-146.
- Darmiani F., Sadeghi-Namaghi H., Hatefi S. 2011. Part of mites (Acari) associated with fruit trees in Birjand region. 2th Iranian Pest Management Conference. Kerman, Iran, 61 pp.
- De Clercq P., Mohaghegh J., Tirry L. 2000. Effect of host plant on the functional response of the predator Podisus nigrispinus (Heteroptera: Pentatomidae). Biol. Control, 18: 65-70. https://doi.org/10.1006/bcon.1999.0808
- Demite P.R., McMurtry J.A., de Moraes G.J. 2014. Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa, 3795 (5): 571-577. https://doi.org/10.11646/zootaxa.3795.5.6
- Fathipour Y., Maleknia B. 2016. Mite Predators. In: Omkar (Ed.), Ecofriendly pest management for food security. Else¬vier, San Diego, USA, 329-366 pp. https://doi.org/10.1016/B978-0-12-803265-7.00011-7
- Fantinou A., Baxevani A., Drizou F., Labropoulos P., Perdikis D., Papadoulis G. 2012. Consumption rate, functional response and preference of the predaceous mite Iphiseius degenerans to Tetranychus urticae and Eutetranychus orientalis. Exp. Appl. Acarol., 58: 133-144. https://doi.org/10.1007/s10493-012-9557-6
- Ferla N.J., Marchetti M., Johann L., Haetinger C. 2011. Functional response of Phytoseiulus macropilis under different Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Zoologia, 28(1): 17-22. https://doi.org/10.1590/S1984-46702011000100003
- Fu X., Liu Q., Liu J., Meng R. 2021. Functional response of Amblyseius andersoni and Neoseiulus neoreticuloides (Acari: Phytoseiidae) to adults of the wolfberry gall mite Aceria pallida (Acari: Eriophyoidae). Syst. Appl. Acarol., 26(4): 809-817. https://doi.org/10.11158/saa.26.4.11
- Ganjisaffar F., Fathipour Y., Kamali K. 2011. Effect of temperature on prey consumption of Typhlodro¬mus bagdasarjani (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). Int. J. Acarol., 37 (6): 556-560. https://doi.org/10.1080/01647954.2010.528800
- Gotoh T., Yamaguchi K., Mori K. 2004. Effect of temperature on life history of Amblyseius californicus (Acari: Phytoseiidae). Exp. Appl. Acarol., 32: 15-30. https://doi.org/10.1023/B:APPA.0000018192.91930.49
- Hajizadeh J., Hosseini R., McMurtry J.A. 2002. Phytoseiid mites (Acari: Phytoseiidae) associated with eriophyid mites (Acari: Eriophyidae) in Guilan province of Iran. Int. J. Acarol., 28: 373-378. https://doi.org/10.1080/01647950208684313
- Hassell M.P. 1978. The dynamics of arthropod predator-prey systems.1st edn. Princeton University Press, Princeton, NJ.
- Holling C.S. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol., 91: 385-398. https://doi.org/10.4039/Ent91385-7
- Holling C.S. 1966. The functional response of invertebrate predators to prey density. Mem. Ent. Soc. Can., 48: 1-86. https://doi.org/10.4039/entm9848fv
- Jafari S. 2010. Phytoseiid mites of the Lorestan province and determining the predation efficiency of Neoseiulus barkeri (Phytoseiidae). Ph.D. thesis, Faculty of Agriculture, Tarbiat Modares University, 192 pp.
- Jafari S., Fathipour Y., Faraji F. 2012. The influence of temperature on the functional response and prey consumption of Neoseiulus barkeri (Acari: Phytoseiidae) on Tetranychus urticae (Acari: Tetranychidae). J. Entomol. Soc. Iran., 31: 39-52.
- Jafari S., Rahmati M., Bahirae F. 2014. Spatial and temporal distribution of Eotetranychus frosti and Cenopalpus irani and their predator Typhlodromus bagdasarjani in an unsprayed apple orchard at Khorramabad, Western Iran. Persian J. Acarol., 3: 51-61.
- Jafarian F., Jafari S. 2016a. The effect of temperature on life history and demographic parameters of Eotetranychus frosti (Acari: Tetranychidae). Syst. Appl. Acarol., 21: 957-967. https://doi.org/10.11158/saa.21.7.9
- Jafarian F., Jafari S. 2016b. Temperature-dependent life history of Eotetranychus frosti (Tetranychidae) fed on apple leaves. Int. J. Acarol., 42: 377-381. https://doi.org/10.1080/01647954.2016.1202319
- Jafarian F., Jafari S., Fathipour Y. 2020. Evaluation of antibiosis resistance in seven apple cultivars to Eotetranychus frosti (Tetranychidae). Syst. Appl. Acarol., 25(3): 525-537. https://doi.org/10.11158/saa.25.3.12
- Juliano S.A. 2001. Non-linear curve fitting: predation and functional response curve. Design and Analysis of Ecological Experiments. New York, Oxford University Press. p. 178-196.
- Kamali K., Ostovan H., Atamehr A. 2001. A catalogues of mites and ticks (Acari) of Iran. Islamic Azad University Scientific Publication Scientific Center, 192 pp.
- Khodayari S., Kamali K., Fathipour Y. 2008. Tetranychid mites and their natural enemies in Maragheh region and the first record of Neopronematus neglectus (Acari: Iolinidae) from Iran. J. Entomol. Soc. Iran., 28(2): 61-65 (In Persian with English abstract).
- Khodayari S., Fathipour Y., Kamali K., Naseri B. 2010. Seasonal activity of Zetzellia mali (Stigmaeidae) and its preys Eotetranychus frosti (Tetranychidae) and Tydeus longisetosus (Tydeidae) in unsprayed apple orchards of Maragheh, northwestern of Iran. J. Agri. Sci. Tech., 12: 549-558.
- Koveos D.S., Broufas G.D. 2000. Functional response of Euseius finlandicus and Amblyseius undersoni to Panonychus ulmi on apple and peach leaves in the laboratory. Exp. Appl. Acarol., 24: 247-256.
- Krips O.E., Kleijn P.W., Willems P.E.L., Gols G.J.Z., Dicke M. 1999. Leaf hairs influence searching efficiency and predation rate of the predatory mite Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol., 23: 119-131. https://doi.org/10.1007/978-94-017-1343-6_29
- Lorenzen J.H., Balbyshev, N.F., Lafta, A.M., Casper H., Tian X., Sagredo B. 2001. Resistant potato selections contain leptine and inhibit development of the colorado potato beetle (Coleoptera: Chrysomelidae). J. Econ. Entomol., 94: 1260-1267. https://doi.org/10.1603/0022-0493-94.5.1260
- Loughner R., Goldman K., Loeb G., Nyrop J. 2008. Influence of leaf trichomes on predatory mite sp. (Typhlodromus pyri) abundance in grape varieties. Exp. Appl. Acarol., 45: 111-122. https://doi.org/10.1007/s10493-008-9183-5
- Madadi H., Enkegaard A., Brodsgaard H.F., Kharrazi -Pakdel A., Mohaghegh J., Ashouri A. 2007. Host plant effects on the functional response of Neoseiulus cucumeris to onion thrips larvae. J. Appl. Entomol., 131: 728-733. https://doi.org/10.1111/j.1439-0418.2007.01206.x
- Madadi H., Enkegaard A., Brodsgaard H.F., Kharrazi-Pakdel A., Ashouri A., Mohaghegh-Neishabouri J. 2009. Interactions between Orius albidipennis (Heteroptera: Anthocoridae) and Neoseiulus cucumeris (Acari: Phytoseiidae): effects of host plants under microcosm condition. Bio. Control, 50: 137-142. https://doi.org/10.1016/j.biocontrol.2009.03.015
- Marafel P.P., Reis P.R., Silveira E.C., Toledo M.A., Souza-Pimentel G.C. 2011. Neoseiulus californicus preying in different life stages Tetranychus urticae (Acari: Phytoseiidae, Tetranychidae). Acarologia, 51: 499-506. https://doi.org/10.1051/acarologia/20102031
- Messina F.J., Hanks, J.B. 1998. Host plant alters the shape of the functional response of an aphid predator (Coleoptera: Coccinellidae). Environ. Entomol., 27: 1196-1202. https://doi.org/10.1093/ee/27.5.1196
- McMurtry J.A., Moraes G.J., Famah Sourassou N. 2013. Revision of the lifestyles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Minitab. 2017. Minitab 18 statistical software. State College (PN): Minitab Inc.
- Pervez A., Omkar. 2005. Functional responses of coccinellid predators: an illustration of a logistic approach. J. Insect Sci., 5: 1-6. https://doi.org/10.1673/031.005.0501
- Price P.W., Bouton C.E., Gross P., Mcpheron B.A., Thompson J.N., Weis A.E. 1980. Interaction among three trophic levels: Influence of plants on interaction between insect herbivores and natural enemies. Ann. Rev. Ecol. Syst., 11: 41-65. https://doi.org/10.1146/annurev.es.11.110180.000353
- Rahmani H., Kamali K., Faraji F. 2010. Predatory mite fauna of Phytoseiidae of northwest Iran (Acari: Mesostigma). Turk. J. Zool., 34: 497-508.
- Rezaie M., Baniamerie V., Saboori A. 2017. Functional response and predation interference of Neoseiulus californicus (Acari: Phytoseiidae) feeding on the western flower thrips larvae on several commercial strawberry cultivars. Plant Pest Res., 6(4): 1-15.
- Rogers D. 1972. Random search and insect population models. J. Anim. Ecol., 41: 353-360. https://doi.org/10.2307/3474
- Royama T. 1971. A comparative study of models for predation and parasitism. Res. Pop. Ecol., 13(1): 1-91. https://doi.org/10.1007/BF02511547
- Sabelis M.W., van Baalen M., Bakker F.M., Bruin J., Drukker B., Eggas M., Janssen A.R.M., Lesna I.K., Pels B., van Rijn P., Scutareanu P. 1999. The evolution of direct and indirect plant defence against herbivorous arthropods. in: Brown OH, Drent rH (eds), Herbivores: Between Plants and Predators, pp.109-166. Blackwell, Oxford.
- Sabelis M.W., van Rijn P.C.J. 1997. Predation by insect and mites. In: Lewis T. (Ed.), Thrips as a crop pest, CABI publishing, London. pp. 259-354.
- Sadeghi-Namaghi H. 1995. Mites associated with pomefruit trees in Mashhad and vicinity. J. Agri. Sci. Tech., 9(1): 110-120 (In Persian).
- Saito Y. 1983. The concept of life types in Tetranychinae. An attempt to classify the spinning behaviour of Tetranychinae. Acarologia, 24: 377-391.
- SAS Institute. 2003. GLM: a guide to statistical and data analysis, version 9.1. Cary (NC): SAS Institute.
- Scott Brown A.S., Simmonds M.S.J., Blaney W.M. 1999. Influence of species of host plants on the predation of thrips by Neoseiulus cucumeris, Iphiseius degenerans and Orius laevigatus. Ent. Exp. Appl., 92: 283-288. https://doi.org/10.1046/j.1570-7458.1999.00548.x
- Sepulveda F., Carrillo R. 2008. Functional response of the predatory mite Chileseius camposi (Acarina: Phytoseiidae) on densities of its prey, Panonychus ulmi (Acarina: Tetranychidae). Rev. Biol. Trop., 56: 1255-1260. https://doi.org/10.15517/rbt.v56i3.5707
- Shirdel D., Kamali K., Ostovan H., Arbabi M. 2004. Functional response of the predatory mite Typhlodromus kettanehi Dosse (Acari: Phytoseiidae) on two-spotted spider mite. In 16th Iranian Plant Protection Congress, p. 264.
- Skirvin D., Felon J.S. 2003. The effect of temperature on the functional response of Phytoseiulus persimilis (Acari: Phytoseiidae). Exp. Appl. Acarol., 31: 37-49. https://doi.org/10.1023/B:APPA.0000005107.97373.87
- van Lenteren J.C., Hua L.Z., Kamerman J.W., Rumei X. 1995. The parasite-host relationship between Encarsia formosa (Hym., Aphelinidae) and Trialeurodes vaporariorum (Hom., Aleyrodidae): XXVI. Leaf hairs reduce the capacity of Encarsia to control greenhouse whitefly on cucumber. J. Appl. Entomol., 119: 553-559. https://doi.org/10.1111/j.1439-0418.1995.tb01335.x
- Zhang Y., Zhang Z-Q, J.J., Lin J. 1999. Predation of Amblyseius longispinosus (Acari: Phytoseiidae) on Schizotetranychus nanjingensis (Acari: Tetranychidae) a spider mite injurious to preferences bamboo in Fujian, China. Syst. Appl. Acarol., 4: 63-68. https://doi.org/10.11158/saa.4.1.9



2021-10-24
Date accepted:
2022-04-10
Date published:
2022-05-05
Edited by:
Palevsky, Eric

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Jafarian, Fatemeh; Jafari, Shahriar and Fathipour, Yaghoub
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




