Notes on the embryological development of the Brevipalpus yothersi (Acari: Tenuipalpidae)
Sinico, Thaís Elise  1
; Nunes, Maria Andréia
1
; Nunes, Maria Andréia  2
; Kitajima, Elliot Watanabe
2
; Kitajima, Elliot Watanabe  3
; Cunha, Bruna Aparecida
3
; Cunha, Bruna Aparecida  4
and Novelli, Valdenice Moreira
4
and Novelli, Valdenice Moreira  5
5
1Agronomic Institute, Sylvio Moreira Citrus Center, (IAC-CCSM), Cordeirópolis, SP, Brazil & Biological Institute (IB), São Paulo, SP, Brazil.
2Agronomic Institute, Sylvio Moreira Citrus Center, (IAC-CCSM), Cordeirópolis, SP, Brazil & University of Piauí (UESPI), Corrente, PI, Brazil.
3University of São Paulo, Luiz de Queiroz College of Agriculture (USP-ESALQ), Piracicaba, SP, Brazil.
4Agronomic Institute, Sylvio Moreira Citrus Center, (IAC-CCSM), Cordeirópolis, SP, Brazil.
5✉ Agronomic Institute, Sylvio Moreira Citrus Center, (IAC-CCSM), Cordeirópolis, SP, Brazil.
2022 - Volume: 62 Issue: 1 pages: 113-119
https://doi.org/10.24349/0gr3-3a6bOriginal research
Keywords
Abstract
Introduction
Flat mites in the genus Brevipalpus Donnadieu (Tenuipalpidae) have a worldwide distribution with more than 300 plant species described as their host (Beard et al. 2015). Some Brevipalpus species are notorious by harboring Cardinium symbiont and to be only organism within the Metazoa that exists as female haploid state (Weeks et al. 2001) and there are ability to transmit plant viruses to important crops such as orchids and others ornamental plants, coffee, passion fruit and citrus (Childers and Rodrigues 2011; Dietzgen et al. 2018).
Among these species, there is Brevipalpus yothersi Baker that is the main vector of citrus leprosis (CL) disease (Bassanezi et al. 2019). CL severely affects the citrus industry production and has a multi-etiological viral cause (Freitas-Astúa et al. 2018). Nevertheless, citrus leprosis virus C (CiLV-C; family Kitaviridae; genus Cilevirus) and B. yothersi are the prevalent complex (virus-vector) present in citrus orchards of Latin America (Andrade et al. 2018; Bassanezi et al. 2019; Chabi-Jesus et al. 2021). Despite the importance of B. yothersi and the improvement in microscopy in the last years, studies on biology and embryological development are scarce.
Actually, embryology of the mites is difficult due to adhesion of the eggs to the substrate, and removal causes rupture due to the diminutive size, besides the impermeability of eggshell to fixatives (Thomas and Telford 1999; Laumann et al. 2010a). However, embryological development studies were successfully performed with mites from Tetranychidae and Eriophyoidea family/superfamily (Gotoh et al. 1994; Dearden et al. 2002; Chetverikov and Desnitskiy 2016) suggesting that it is possible to advance with information to the peculiar flat mites. Tetranychus urticae studies have focused embryology to phylogeny, through knowledge of genes responsible to embryo segmentation and germ-cell specification, and gene silencing studies (Dearden et al. 2002, 2003; Khila and Grbic 2007). Here, due to the current importance of the B. yothersi as biological system and viral vector, the main goal of this study was to provide information about embryonic development using light microscopy and scanning electron microscopy.
Adult females of B. yothersi isoline were transferred from orange fruits to arenas mounted on square Petri dishes (120 x 120 mm) containing jack bean leaves [Canavalia ensiformis (L.) DC] surrounded by moistened cotton. These arenas were observed hourly using the stereoscopic microscope, looking for the oviposition to collect the eggs and maintained under temperature 25 °C ± 5 °C and relative humidity 60% ± 10%. To observe the early stages of the development, freshly laid eggs were transferred to microscope slides containing one drop of mineral oil (liquid paraffin) and observed for seven hours. Embryos older than 20 hours after oviposition were immersed in Hoyer's medium on the microscope slide. These samples were examined in a Leica DM750 light microscope (LM) (Leica Microsystems, Co., Wetzlar, Germany).
Three hundred eggs of B. yothersi were fixed in Carnoy's solution for 30 minutes, washed twice in 90% ethanol for 10 minutes and transferred to 0.1M pH 7.4 phosphate buffer solution. The eggs were dehydrated in a graded series of acetone (30, 50, 70, 80, and 100% for 10 minutes), then critical point dried with liquid CO2 (Baltec CPD 030), and transferred to aluminum stubs and sputter-coated with gold (Baltec SCD 050). Examinations were made using a tabletop Phenom scanning electron microscope (SEM).
Results and discussion
Brevipalpus yothersi embryological development



The B. yothersi embryological development was observed for seven days under experimental conditions and the eggs were easily visible by LM (Figure 1). Eggs collected soon after oviposition were slightly elongated and elliptical in shape (average of 108 μm length x 70 μm width) (Haramoto 1969), with light orange coloration, and nuclei that appeared red.
At the earliest cleavage stages, about one-hour post-laying, the nucleus occupied the center of the egg (Figure 1b). Two hours after oviposition, the first division began with the separation of the nucleus into two blastomeres of the same size and shape (Figure 1c) divided by a thin membrane. At three hours post-oviposition, an equal cleavage resulted in the total division of blastomeres into four cells (Figure 1d). Five hours after, two new divisions occurred with the formation of the eight and 16-cells stages (Figure 1e, f). After, a large number of cells were observed, suggesting the occurrence of three more cleavages, reaching a total of about 128 blastomeres (Figure 1g), following the formation of a cluster of cells, the morula.
In a period of 20 to 24 hours after egg-laying, the cells migrated and agglomerated in the peripheral region of the egg forming the blastula (Figure 1h). After 48 hours, the formation of the germinal disc (periblastula) in the ventral region was observed (Figure 1i). The periblastula started to stretch and then becomes flattened (Figure 1j). After three days, the embryo was laterally inside the egg, initiating the formation of the primitive body (Figure 1k), where it was visible the formation of metamers (Figure 1l). From the fourth to the fifth day, the formation of primordial appendages was evident (Figure 1m). On the sixth post-oviposition day, the dark-colored ocelli formation was initiated (Figure 1n), being more visible on the seventh day before the larvae hatched (Figure 1o), and ending with hatching of a hexapod larvae (Figure 1p). This pattern is according to other arachnids that only acquire the fourth pair of legs after their larvae undergo moults for the subsequent developmental stage (Dearden et al. 2002; Sharma 2018).
The analysis of the B. yothersi eggs in LM allowed the description of the different embryological stages. To our best knowledge, this report is the first embryological description of false spider mites and demonstrated that the use of the liquid paraffin was essential to observe the first hours of the false spider mite development, since it enables us to monitor the sequence of embryonic divisions and to establish the exact period it occurred.
The eggs structure of the B. yothersi is a centrolecithal type, where the nucleus occupies a central position (Figure 1b), as described in Ornithodoros moubata Murray, an Argasidae tick (Aeschlimann and Hess 1984). Here, the cleavage observed was a superficial type, similar to descriptions made on Prostigmata mites (Gotoh et al. 1994; Dearden et al. 2002), but differs from previous proposals that holoblastic cleavage was a standard feature of Acari (Laumann et al. 2010b). However, embryological studies of the Eriophyoidea superfamily have reported that their cleavage is meroblastic type, with the nuclei founded in a single region, whereas the other regions of the egg contains only the yolk (Chetverikov and Desnitskiy 2016). These authors observed that nucleus division occurs at the egg periphery, a peculiarity of the embryological development of the Eriophyoidea family and differing from the other organisms of the Acari group. It particularly raises a possible hypothesis that a lineage of eriophyid mites could have diverged from the primitive Acari and transitioned from a superficial cleavage to a total cleavage and thereby acquiring it unusual meroblastic pattern.
The first cleavages of B. yothersi were similar to the mite species of the Panonychus genus (Tetranychidae) as reported by Gotoh et al. (1994). As illustrated and described in the 18 stages, noting that the interval between the first cell divisions was about one hour each and that from 100 blastomeres it was difficult to distinguish them. After observation of the cellular stage composed of 128-cells, the formation of the morula occurred, as visualized in B. yothersi.
The development of the B. yothersi embryos was slow compared to the tenuipalpid Dolichotetranychus coco Flechtmann & Fernando, maintained at a temperature of 25 °C ± 1 °C, where the formation of the first pair of legs and morphogenesis occurred after 35 and 47 hours, respectively (Santhosh et al. 2009). Here, the initial formation of the primitive body occurred on the third day after oviposition. Studies have reported that temperature variations and host plants affect the life cycle and embryonic development of different species of the mites (Haramoto 1969; Chiavegato 1986; Teodoro and Reis 2006; Santhosh et al. 2009). Thus, to avoid any possible influence, during egg analysis, the environmental conditions were controlled.
The chelicerates have some conserved characteristics during embryonic development. First, the prosoma is emerged followed by the development of the appendices, which appear as small buds of tissue (Sharma 2018) observed between the third and fifth days of the development of B. yothersi embryos. Other conserved characteristics were the migration from the mouth to a terminal region below the chelicerae and the formation of the ocelli (Sharma 2018). The total development time observed in B. yothersi was seven days (from the first cleavage to larvae hatching), differing from that observed in the T. urticae model mite, which has a complete embryonic development in 39 hours (25 °C ± 1 °C) and completing the life cycle in less than seven days (Rao et al. 1996; Dearden et al. 2002; Grbic et al. 2007; Khila and Grbic 2007).
Brevipalpus yothersi egg morphology



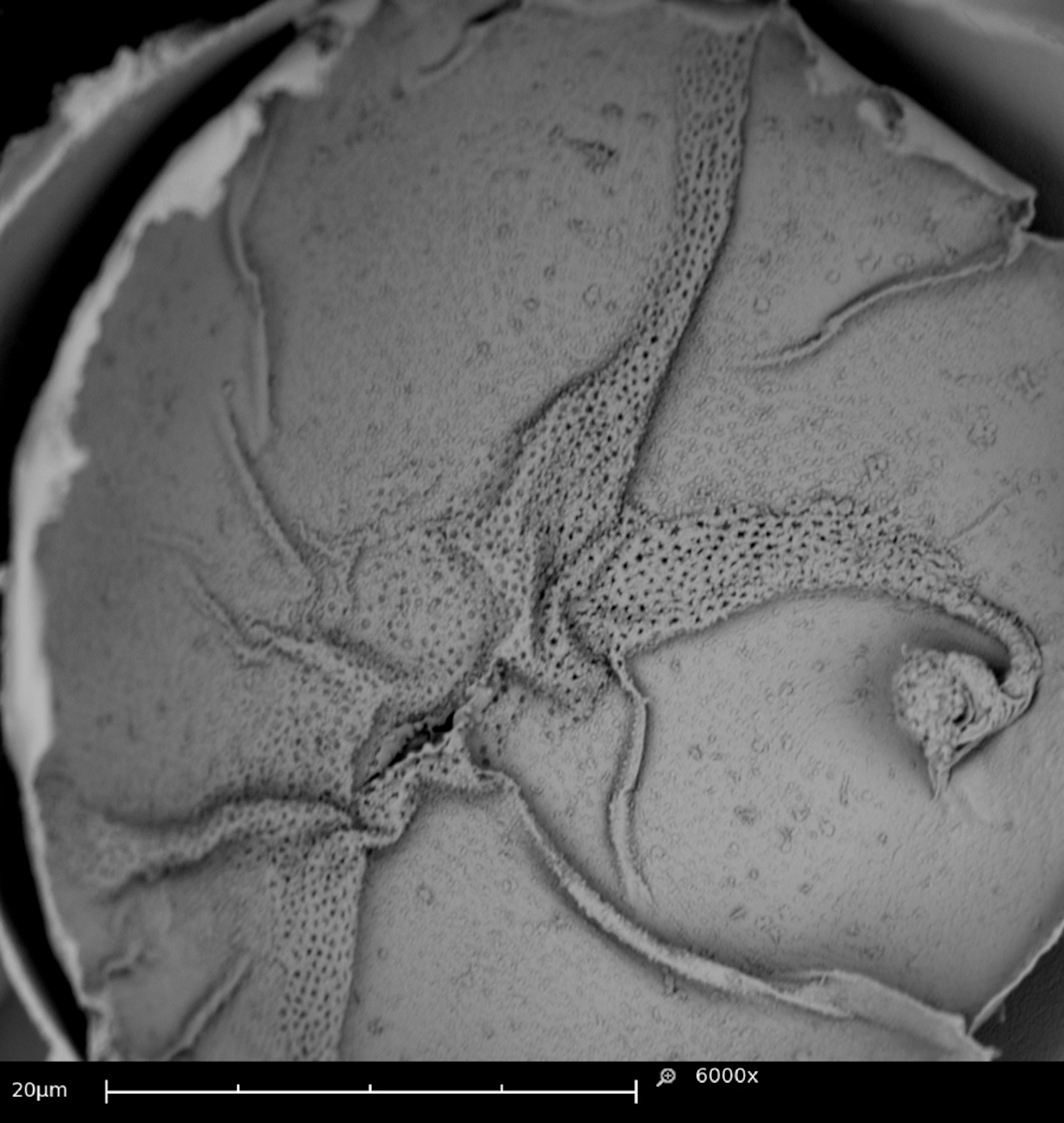


Details of external eggs morphology could be followed under SEM. Transverse streaks in the outer layer of the eggs called carenas (Rodrigues and Machado 1999) (Figure 2a) and a fold-like structure in the equatorial region of the egg (Figure 2b). An external globular structure were observed in studies by Rodrigues and Machado (1999). However, it was observed only in eggs with a more advanced development time but morphologically different from the one found in our study.
Eggshells frequently broke during fixation in Carnoy's solution, allowing for observation of the internal structure of eggs. Additionally, channels similar to small perforations were seen extending between the shell and intermediate lamella (Figure 3). These channels may be associated with the respiratory tract of the embryo. Such structures were also observed by Dittrich (1971) in studies with T. urticae, and they have been shown to give mites protection against desiccation and gas exchange.
The B. yothersi mite has a peculiar form of reproduction in the animal kingdom, harboring Cardinium symbiont and resulting in an entirely haploid species (Weeks et al. 2001). These peculiarities making it an extremely interesting organism for genetic and biological studies. Its anatomy and post-embryonic biology have been explored, but the present work pioneered to show of the embryo formation and development stages. This information is essential for B. yothersi as biology preliminary knowledge and it also provides us with the visualization of the possible respiratory apparatus in embryos, which was associated to specific morphological adaptation of Tetranychidae. Further studies can be complemented by other robust techniques such as immunolabeling and confocal microscopy.
Conclusion
This study its first to provides timeline data of the B. yothersi embryological development. Also, it contributed to the establishment of appropriate protocols to B. yothersi embryological investigations, using light and scanning electron microscopy.
Acknowledgments
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (2009/13959-9; 2011/13869-0; 2013/07369-0; 2014/08458-9; 2016/21749-8). We are grateful to Acarology laboratory members for helpful discussions.
Authors' contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by TES, MAN, BAC, EWK. The first draft of the manuscript was written by TES and VMN, and all authors commented on previous versions of the manuscript. Funding acquisition: VMN, TES, EWK. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no conflicts of interest.
References
- Aeschlimann A., Hess E. 1984. What is our current knowledge of acarine embryology? Acarology, 6: 90-99.
- Andrade D.J.; Lorençon J.R.; Siqueira D.S.; Novelli V.M.; Bassanezi R.B. 2018. Space-time variability of citrus leprosis as strategic planning for crop management. Pest. Manag. Sci., 74: 1798-1803. https://doi.org/10.1002/ps.4877
- Bassanezi R.B.; Czermainski A.B.C.; Laranjeira F.F.; Moreira A.S.; Ribeiro Junior. P.J.; Krainski E.T.; Amorim L. 2019. Spatial patterns of the Citrus leprosis virus and its associated mite vector in systems without intervention. Plant Pathol.; 68: 85-93. https://doi.org/10.1111/ppa.12930
- Beard J.J., Ochoa R., Braswell W.E., Bauchan G.R. 2015. Brevipalpus phoenicis (Geijskes) species complex (Acari: Tenuipalpidae) - a closer look. Zootaxa, 3944. https://doi.org/10.11646/zootaxa.3944.1.1
- Chabi-Jesus C.; Ramos-González P.L.; Postclam-Barro M.; Fontenele R.S.; Harakava R.; Bassanezi R.B.; Moreira A.S.; Kitajima E.W.; Varsani A.; Freitas-Astúa J. 2021. Molecular epidemiology of citrus leprosis virus C: a new viral lineage and phylodynamic of the main viral subpopulations in the Americas. Front. Microbiol., 12: 641252. https://doi.org/10.3389/fmicb.2021.641252
- Chetverikov P.E., Desnitskiy A.G. 2016. A study of embryonic development in eriophyoid mites (Acariformes, Eriophyoidea) with the use of the fluorochrome DAPI and confocal microscopy. Exp. Appl. Acarol., 68: 97-111. https://doi.org/10.1007/s10493-015-9982-4
- Chiavegato L.G. 1986. Biologia do ácaro Brevipalpus phoenicis em citros. Brasília: Pesqui. Agropecu. Brasil., 21: 813-816.
- Childers C.C., Rodrigues J.C.V. 2011. An overview of Brevipalpus mites (Acari: Tenuipalpidae) and the plant viruses they transmit. Zoosymposia, 6: 180-192. https://doi.org/10.11646/zoosymposia.6.1.28
- Dearden P.K., Donly C., Grbic M. 2002. Expression of pair-rule gene homologues in a chelicerate: early patterning of the tow-spotted spider mite Tetranychus urticae. Development, 129: 5461-5472. https://doi.org/10.1242/dev.00099
- Dearden P., Grbic M., Donly C. 2003. Vasa expression and germ-cell specification in the spider mite Tetranychus urticae. Dev. Genes Evol., 212: 599-603. https://doi.org/10.1007/s00427-002-0280-x
- Dietzgen R.G., Freitas-Astúa J., Chabi-Jesus C., Ramos-González P.L., Goodin M.M., Kondo H., Tassi A.D., Kitajima E.W. 2018. Dichorhaviruses in their host plants and mite vectors. Adv. Virus Res., 102: 119-148. https://doi.org/10.1016/bs.aivir.2018.06.001
- Dittrich V. 1971. Electron-microscopic studies of the respiratory mechanism of spider mite eggs. Ann. Entomol. Soc. Am., 64: 1134-1143. https://doi.org/10.1093/aesa/64.5.1134
- Freitas-Astúa J., Ramos-González P.L., Arena G.D., Tassi A.D., Kitajima E.W. 2018. Brevipalpus-transmitted viruses: parallelism beyond a common vector or convergent evolution of distantly related pathogens? Curr. Opin. Virol., 33: 66-73. https://doi.org/10.1016/j.coviro.2018.07.010
- Gotoh T., Kamoto T., Hatakeyama M., Gomi K. 1994. Embryonic development and diapause stage in Panonychus mites (Acari: Tetranychidae). Appl. Entomol. Zool., 29: 507-515. https://doi.org/10.1303/aez.29.507
- Grbic M., Khila A., Lee K.-Z., Bjelica A., Grbic V., Whistlecraft J., Verdon L., Navajas M., Nagy L. 2007. Mity model: Tetranychus urticae, a candidate for chelicerate model organism. BioEssays, 29: 489-496. https://doi.org/10.1002/bies.20564
- Haramoto F.H. 1969. Biology and control of Brevipalpus phoenicis (Geijskes) (Acari: Tenuipalpidae). 68th ed. Technical Bulletin. Honolulu: University of Hawaii, pp. 63.
- Khila A., Grbic M. 2007. Gene silencing in the spider mite Tetranychus urticae: dsRNA and siRNA parental silencing of the Distal-less gene. Dev. Genes Evol., 217: 241-251. https://doi.org/10.1007/s00427-007-0132-9
- Laumann M., Norton R.A., Heethoff M. 2010a. Acarine embryology: inconsistencies, artificial results and misinterpretations. Soil Organisms, 82: 217-235.
- Laumann M., Bergmann P., Norton R.A., Heethoff M. 2010b. First cleavages, preblastula and blastula in the parthenogenetic mite Archegozetes longisetosus (Acari, Oribatida) indicate holoblastic rather than superficial cleavage. Arthropod Struct. Dev., 39: 276-286. https://doi.org/10.1016/j.asd.2010.02.003
- Rao P.P., Praslicka J., Sutakova G. 1996. Effect of temperature and rearing method on development and fecundity of Tetranychus urticae (Acarina, Tetranychidae). Biologia, 51: 509-516.
- Rodrigues J.C.V., Machado M.A. 1999. Notes on a probable respiratory apparatus in eggs of Brevipalpus phoenicis (Acari: Tenuipalpidae). Int. J. Acarol., 25: 231-234. https://doi.org/10.1080/01647959908684157
- Santhosh P.P., Haq M.A., Ramani N. 2009. Biological studies of coconut infesting mite - Dolichotetranychus cocos. Advances in Environmental Biology, 3: 263-268.
- Sharma P.P. 2018. Chelicerates. Curr. Biol., 28: R774-R778. https://doi.org/10.1016/j.cub.2018.05.036
- Teodoro A.V., Reis P.R. 2006. Reproductive performance of the mite Brevipalpus phoenicis (Geijskes, 1939) on citrus and coffee, using life table parameters. Braz. J. Biol., 66: 899-905. https://doi.org/10.1590/S1519-69842006000500016
- Thomas R.H., Telford M.J. 1999. Appendage development in embryos of the oribatid mite Archegozeles longisetosus (Acari, Oribatei, Trhypochthoniidae). Acta Zool., 80: 193-200. https://doi.org/10.1046/j.1463-6395.1999.00016.x
- Weeks A.R., Marec F., Breeuwer J.A.J. 2001. A mite species that consists entirely of haploid females. Science, 292: 2479-2483. https://doi.org/10.1126/science.1060411



2021-08-25
Date accepted:
2022-01-23
Date published:
2022-01-26
Edited by:
Navia, Denise

This work is licensed under a Creative Commons Attribution 4.0 International License
2022 Sinico, Thaís Elise; Nunes, Maria Andréia; Kitajima, Elliot Watanabe; Cunha, Bruna Aparecida and Novelli, Valdenice Moreira
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




