Comparison of natural prey Tetranychus turkestani, date palm pollen, and bee pollen diets on development, reproduction, and life table parameters of the predator Amblyseius swirskii
Rahmani Piyani, Atefeh1 ; Shishehbor, Parviz2 ; Kocheili, Farhan3 and Riddick, Eric W.4
1Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz 6135783151, Iran.
2Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz 6135783151, Iran.
3Department of Plant Protection, Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz 6135783151, Iran.
4✉ National Biological Control Laboratory, Agricultural Research Service, USDA, Stoneville, MS 38776, USA.
2021 - Volume: 61 Issue: 4 pages: 890-900
https://doi.org/10.24349/G9ed-QB9hOriginal research
Keywords
Abstract
Introduction
Amblyseius swirskii Athias-Henriot (Acari: Phytoseiidae) is a type III generalist predatory mite, which has a wide diet range including insect and mite prey, pollen, plant exudates, and honeydew (McMurtry et al. 2013). More specifically, laboratory studies indicate that A. swirskii develops and reproduces on a diet of tetranychid mites (Riahi et al. 2017b), eriophyid mites (Park et al. 2010), tenuipalpid mites (Peña et al. 2009), tarsonemid mites (Abou-Awad et al. 2014), psyllids (Juan-Blasco et al. 2012), whiteflies (Medd and Greatrex 2014), thrips (Messelink et al. 2006), and plant pollen (Nemati et al. 2019). Amblyseius swirskii is used in augmentative biological control of pests on greenhouse-grown crops such as cucumber Cucumis sativus L., and sweet pepper Capsicum anuum L. in more than 50 countries (Knapp et al. 2018).
Amblyseius swirskii was first discovered along the eastern Mediterranean coast but has expanded its natural range throughout the Middle East and neighboring regions (Calvo et al. 2014). It is a candidate for augmentative biological control of the strawberry spider mite Tetranychus turkestani Ugarov and Nikolskii (Acari: Tetranychidae) on crop plants in greenhouses and fields in Khuzestan province, southwestern Iran, and elsewhere. Tetranychus turkestani is an important pest of crop plants in multiple families in the southwestern region of Iran (Mossadegh and Kocheili 2003; Sohrabi and Shishehbor 2008) and other countries including Russia (Popov 1981), USA (Jeppson et al. 1975), China (Li et al. 2014), India and some European countries (Migeon and Dorkled 2021). Characterized by frequent outbreaks coupled with acaricide resistance, this mite poses a threat to field and glasshouse crop producers (Sohrabi and Shishehbor 2008). Tetranychus turkestani has several generations during the growing season, tolerates high temperatures and low humidity, and has a short generation time of approximately 6 to 7 days (Sohrabi and Shishehbor 2008). Different developmental stages initially feed on the lower leaf surface but can cover an entire plant as populations increase. Early damage symptoms appear as chlorotic stipples on leaves, but large areas will turn yellow as feeding damage builds-up. Leaves may also become bronzed, and the plant can defoliate. Webbing is often very evident, giving a bright appearance to the plant (Jeppson et al. 1975).
Developing or improving techniques to mass produce A. swirskii for augmentative releases against T. turkestani are needed. In efforts to reduce rearing costs, factitious prey, e.g., Carpoglyphus lactis (L.) (Acari: Carpoglyphidae), has been used to rear A. swirskii (San et al. 2020). Yet, additional time, labor, and space will be required to maintain C. lactis colonies. Hence, the incorporation of a non-prey diet would be most cost-effective. Research has tested the utility of non-prey diets, e.g., plant pollen, in lieu of natural prey. For example, pollen from cattail Typha latifolia L., almond Prunus amygdalus Batsch, date palm Phoenix dactylifera L., castor bean Ricinus communis L., maize Zea mays L., and apricot Prunus armeniaca L. are potential plant pollen diets for mass rearing A. swirskii (Riahi et al. 2017a). Bee-collected pollen (from unidentified plant sources) is less suitable for A. swirskii development and reproduction (Goleva and Zebitz 2013; Calvo et al. 2014), but this is not always the case if the nutritional content of bee pollen exceeds that of date palm pollen (Khanamani et al. 2017). If plant pollen is readily obtained from plants grown locally or purchased from a supplier in the region, A. swirskii mass production could become more time and cost efficient. Culturing of tetranychids requires more time and labor because host plants must also be cultured to provide food for the tetranychids. Currently, there are no suitable artificial diets for continuous mass rearing of tetranychids (Van der Geest et al. 1983; He et al. 2019).
Although biological characteristics of A. swirskii fed tetranychids such as Tetranychus urticae Koch (Fadaei et al. 2018), Panonychus citri (McGregor) (Ji et al. 2013), Eotetranychus frosti (McGregor) (Bazgir et al. 2018), and Eutetranychus orientalis (Klein) (All-Azzazy and Alhewairini 2020) have been studied previously, no information is available on A. swirskii attacking or feeding on T. turkestani in Iran or elsewhere. Therefore, the objectives of this study were to compare the effects of natural prey T. turkestani, date palm pollen, and bee pollen diets on A. swirskii development, reproduction, and life table parameters.
Material and methods
Spider mite and predatory mite colonies
The T. turkestani used in this study originated from field bindweed Convolvulus arvensis L. (Solanales: Convolvulaceae) growing on the premises of the Faculty of Agriculture, Shahid Chamran University of Ahvaz, Ahvaz, Iran. A stock colony of T. turkestani was maintained on seedlings of cowpea Vigna unguiculata (L.) Walp. (Fabales: Fabaceae) grown from seeds and transplanted into compost in plastic pots (20 cm diam.) in a laboratory of the Department of Plant Protection. Infested plants were held in wooden framed rearing cages (120 × 60 × 60 cm) covered with nylon mesh, 210 µm aperture. The cages were maintained in a laboratory at 25 ± 2 °C, 50 ± 5% RH and a 16: 8 (L: D) h with illumination (4000 lux) provided by fluorescent lamps. New plants were introduced as required.
The A. swirskii population was obtained from laboratory colonies at Bu-Ali Sina University, Hamedan, Iran, which had been purchased from Koppert Biological Systems (Berkel en Rodenrijs, The Netherlands) in 2012. It was reared on T. urticae at Bu-Ali Sina University. At Shahid Chamran University, Department of Plant Protection, the A. swirskii colony was maintained in a laboratory in rearing units comprised of a plastic sheet (11 x 7 x 0.1 cm) on a foam mat maintained in humid conditions (approximately 70% RH) with distilled water in a Plexiglas box (as described by Wazler and Schausberger 1999). To provide food, cowpea leaves infested with T. turkestani were added to the units twice a week. Date palm pollen was also made available to immature and adult A. swirskii in the rearing units for several generations when T. turkestani immatures and adults were in short supply. The A. swirskii colony was reared for several generations prior to commencing the experiments.
Pollen
Date palm production is a major industry in Ahaz city, Iran. Very fresh date palm pollen of the Stamaran cultivar was harvested directly from a tree growing in Ahvaz city in April 2019. Even if date palm pollen was not harvested from the field, it could have been purchased at a low price. In a previous study, date palm pollen sheaths were obtained from a rural supplier in Ahvaz at a cost of 3.0-3.5 USD per kg (equivalent to four sheaths); approximately 6 g of pollen grains were within each kg of sheath (Ebrahimifar et al. 2021). Commercial bee-collected pollen (of unknown plant origin) was purchased from a rural supplier in Ahvaz city. The average price of bee pollen was 12.0 USD per kg in Ahvaz (PS, personal observations). Bee pollen was stored in a freezer at -18 °C for up to four months, before experimentation.
Experimental setup
Experimental units were prepared according to the method described by Riahi et al. (2017a) with some modifications. Each experimental unit was comprised of a green plastic sheet (3 x 3 cm) located on a thick foam pad, cut to a similar size (3 x 3 cm), in a plastic Petri dish (9 cm diam., 2 cm high) that was half-filled with distilled water (Figure 1). The edges of the plastic sheet were covered with moist tissue paper to prevent A. swirskii from escaping. In addition, a few cotton threads were placed on each plastic sheet to serve as shelter and oviposition substrates for A. swirskii. Eggs of A. swirskii (less than 24 h old) were transferred individually to each experimental unit using a fine squirrel-hair brush. After emergence of the larvae, mites were fed according to treatments, i.e., T. turkestani prey, date palm pollen, or bee pollen. The experimental units were monitored daily, and developmental duration and survival of immature stages were recorded. Molting was confirmed by the presence of exuviae in the experimental units. After adult emergence, each newly emerged female and male (less than 24 h old) was coupled and transferred to a new experimental unit provided with the same food they were fed in immature stages. The experimental units were checked daily and pre-oviposition period, oviposition period, post-oviposition period, adult longevity, and total fecundity were recorded. The experiments were carried-out in a completely randomized design to test the effects of diet treatment type on A. swirskii life history parameters. All experiments were conducted in a growth chamber at 25 ± 1 °C, 65 ± 5% RH and a 16L: 8D h photoperiod.
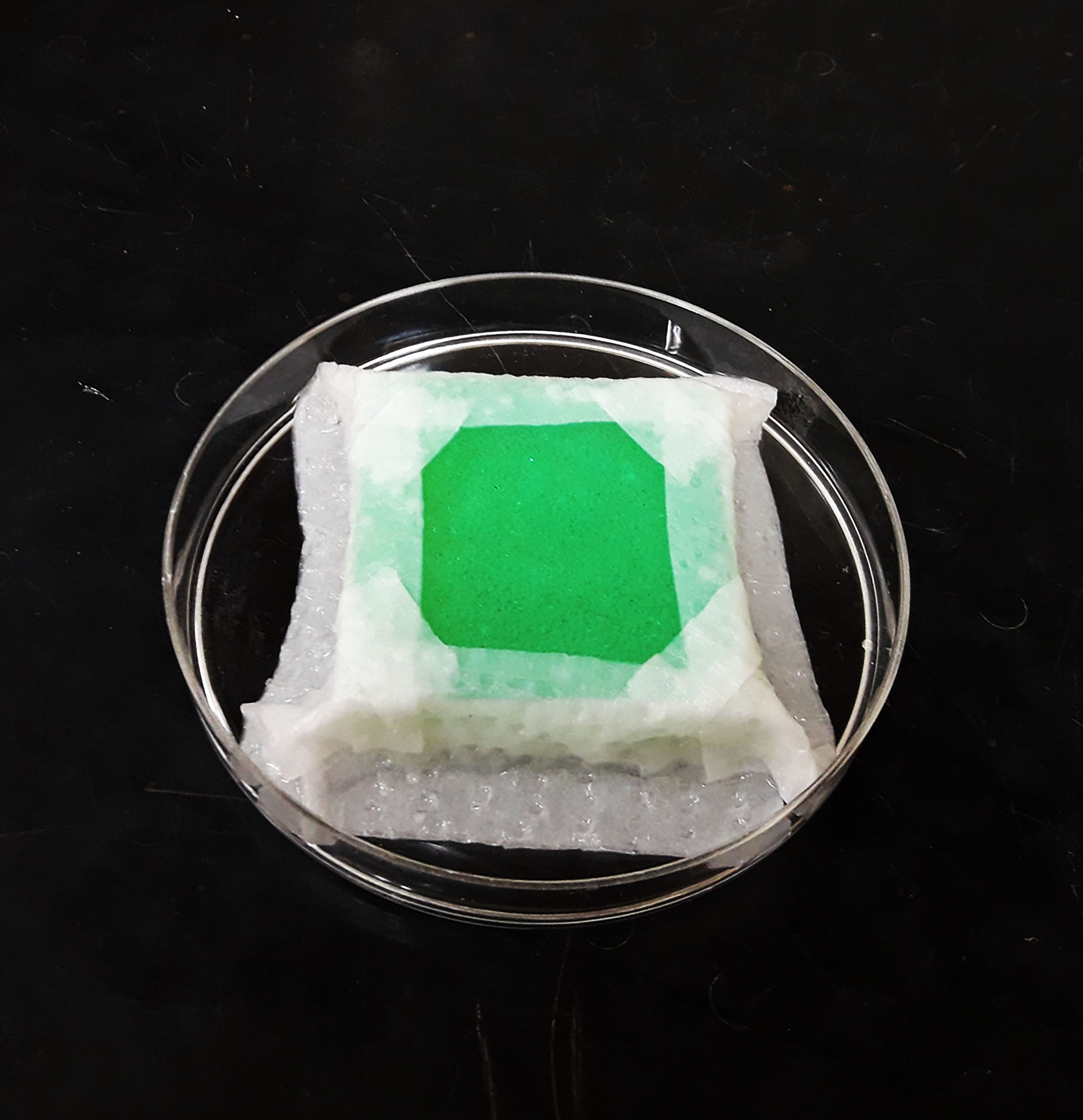


Approximately 5 mg of date palm pollen or bee pollen was added to respective treatment dishes with a single A. swirskii male or female. Every other day, old pollen was replaced with new pollen. In the prey treatment, live T. turkestani immatures and adults were added to dishes with a single A. swirskii male or female. Every other day, dead and uneaten T. turkestani were replaced with live T. turkestani from the stock colony. Sample sizes for female development and immature survival tests were 25, 35, and 33 individuals in T. turkestani, date palm pollen, and bee pollen treatments, respectively. Sample sizes for male development and immature survival tests included 20, 9, and 11 individuals in T. turkestani, date palm pollen, and bee pollen treatments, respectively. Sample sizes for reproduction and longevity tests included 25, 35, and 33 individuals in T. turkestani, date palm pollen, and bee pollen treatments, respectively. Number of replications (n) for immature development (female and male), reproduction and longevity (female and male) of diet treatments are listed in Tables 1, 2, and 3.
Statistical analysis
The datasets were first verified for normal distribution by the Kolmogorov-Smirnov test. Developmental time, immature survival, preoviposition period, oviposition period, postoviposition period, adult longevity, and total fecundity of A. swirskii fed different diets were analyzed using a one-way analysis of variance (one-way ANOVA). Immature survival data were arcsine transformed to homogenize variances prior to the one-way ANOVA. Means were separated using Tukey's Honestly Significant Difference (HSD) test at α = 0.05. SPSS version 22 statistical software (SPSS for windows, SPSS Institute Inc., Chicago, IL, USA.) was used for data analysis.
Life table parameters are important in defining the effects of external factors on populations (Birch 1948). Life table parameters can confirm which diets are most suitable for predator life history parameters (Grenier and De Clercq 2003). In the context of this study, they were used to confirm results obtained from experiments determining the effects of three diets on A. swirskii development and reproduction. An age-stage, two-sex life-table procedure (Chi and Liu 1985) was selected for data analysis to account for variable development rates among individuals and stages of development of A. swirskii fed different diets. Life table, i.e., population growth parameters including net reproductive rate (R0), gross reproductive rate (GRR), intrinsic rate of natural increase (rm), finite rate of increase (λ), and mean generation time (T) were calculated with the TWOSEX-MSChart program (Chi 2018). Population doubling time (DT), the number of days required for the A. swirskii population to double in numbers, was also calculated DT = ln (2)/ rm. Standard error (SE) of the population growth parameters was obtained using the bootstrap technique and multiple comparisons were made by the paired bootstrap test with 100,000 samples (Maia et al. 2000).
Results
Immature development and survival rate
The time in the egg stage was shortest for A. swirskii in the date palm pollen treatment than the T. turkestani or bee pollen treatments. Parental females and males in the stock colony had exposure to date palm pollen, T. turkestani, but not bee pollen. The protonymph stage was shorter for females and males in the date palm pollen treatment than the T. turkestani treatment. Total developmental time of A. swirskii females and males was affected significantly by diet type (females: F = 40.83; df = 2, 90; P < 0.001; males: F = 6.64; df = 2, 37; P = 0.003) (Tables 1 and 2). Total development time was significantly shorter for females fed date palm pollen than T. turkestani or bee pollen (Table 1). It was significantly shorter for males fed date palm pollen than T. turkestani or bee pollen (Table 2). Diet type had no significant effect on percent survival rate of males and females, combined (F = 1.50; df = 2, 6; P = 0.29); it was 100 ± 0.00a, 98.22 ± 1.82a, and 97.78 ± 1.81a for A. swirskii fed T. turkestani, date palm pollen, and bee pollen, respectively.
Diet type had a significant effect on pre-oviposition (F = 31.64; df = 2, 90; P < 0.001), oviposition (F = 43.12; df = 2, 90; P < 0.001) and post-oviposition (F = 12.66; df = 2, 90; P < 0.001) periods of A. swirskii females (Table 3). Pre-oviposition period was longest for females fed bee pollen rather than date palm pollen or T. turkestani. Oviposition and post-oviposition periods were longest for females fed T. turkestani than date palm pollen or bee pollen. Diet type had a significant effect on total fecundity (F = 31.64; df = 2, 90; P < 0.001; Table 3); females fed T. turkestani laid 1.7 and 5.7 times more eggs than females fed date palm pollen and bee pollen, respectively.









Significant differences in longevity were observed amongst the diets (F = 65.57; df = 2, 90; P < 0.001 for females; F = 13.91; df = 2, 37; P < 0.001 for males; Table 3). Both A. swirskii females and males lived longer when fed the T. turkestani diet rather than the date palm pollen or bee pollen diet. Longevity of T. turkestani-fed females and males was 34.57 d and 34.15 d, respectively (Table 3). Longevity of date palm pollen-fed and bee pollen-fed females (or males) did not differ significantly.
Life table analysis
Life table analysis confirmed that diet type had significant effects on A. swirskii life history (Table 4). The highest intrinsic rate of natural increase (rm) was observed for A. swirskii fed date palm pollen rather than T. turkestani or bee pollen. The finite rate of increase (λ), net reproductive rate (R0), and gross reproductive rate (GRR) did not differ significantly between A. swirskii fed date palm pollen or T. turkestani. But A. swirskii fed bee pollen had the lowest intrinsic and finite rates of increase and the lowest net and gross reproductive rates. Mean generation time (T) and population doubling time (DT) were significantly shorter for A. swirskii fed date palm pollen rather than T. turkestani or bee pollen (Table 4). Note that A. swirskii fed bee pollen had the longest mean generation and population doubling times.



Discussion
Diet type had a distinct influence on immature development of A. swirskii in this study. Total development time was shortest when A. swirskii immatures were fed the date palm pollen diet. Perhaps, nutrients in date palm pollen were easier to digest or assimilate than those in bee pollen or T. turkestani. A comparison of macronutrients, i.e., amino acids, lipids, carbohydrates, minerals, and phytochemicals, amongst the three diets would be necessary to help explain these results. In addition, geographic origin, climatic conditions, and soil type of individual plants from which date palm pollen and bee pollen originated could have influenced nutritional quality. The plant origin of bee pollen in this study was unknown. The plant species represented in the bee-collected pollen can have important consequences on its nutritional quality, i.e., amino acid profile and content (Taha et al. 2019).
The developmental time of 4.20 d for A. swirskii females fed date palm pollen in this study was short when compared to 9.09 d reported by Riahi et al. (2017a) and 8.3 d reported by Nemati et al. (2019). The developmental time of 5.79 d for females fed bee pollen in this study was also short in comparison to 10.38 d reported by Riahi et al. (2017a). Note that almond pollen, castor bean pollen, and date palm pollen were superior to bee-collected pollen as a diet to support the development of the phytoseiid Neoseiulus cucumeris (Oudemans) (Yazdanpanah et al. 2021). The authors indicated that bee pollen had the lowest nutritional value for N. cucumeris. In contrast, Khanamani et al. (2017) indicated that date palm pollen was inferior to bee pollen (collected from unidentified plant species), probably because the proportion of protein, lipids, and sugars were significantly lower in date palm pollen.
Prior research has not tested the effects of a diet of T. turkestani on development time of A. swirskii. Total development time of A. swirskii fed T. turkestani (at 25 °C, 5.97 d for females and 5.50 d for males) in this study was shorter than for A. swirskii fed T. urticae (7.60 d, Riahi et al. 2017a), T. urticae (6.89 d, Fadaei et al. 2018), E. frosti (6.94 d, Bazgir et al. 2018), and E. orientalis (11.17 d, All-Azzazy and Alhewairini 2020). Also, T. turkestani-fed A. swirskii developed faster than their prey. For example, development time of T. turkestani was approximately 10 d at 25°C (Bazazzadeh et al. 2020). Development time of A. swirskii fed T. turkestani in this study was also shorter than the development time reported for potential insect prey such as Bemisia tabaci (Gennadius) Bet-Dagen strain (Homoptera: Aleyrodidae) (6.3 d, after modification, Nomokou et al. 2001), and two thripids Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae) and Thrips tabaci Lindeman (7.8 d at 25°C, Wimmer et al. 2008). The ability of A. swirskii to develop faster than prey species has profound implications for augmentative biological control. It suggests that augmentative releases of A. swirskii could eliminate pest populations in the greenhouse or open field.
Diet type had a distinct effect on reproduction (fecundity) in this study. The observation that A. swirskii produced more progeny, i.e., more eggs, when fed the T. turkestani diet (rather than the date palm pollen or bee pollen diet) could suggest that the quantity or quality of macronutrients necessary for ovary (gonad) development and egg maturation are greater in T. turkestani. Unfortunately, the macronutrient profile in T. turkestani is unknown and specific nutrients that could stimulate A. swirskii oviposition have not been reported. Note that A. swirskii reproduction (fecundity) was greatest on a diet of T. turkestani (this study) than on a diet of T. urticae (Abou-Awad et al. 1992; Riahi et al. 2017a, b; Fadaei et al. 2018), E. frosti (Bazgir et al. 2018), E. orientalis (All-Azzazy and Alhewairini 2020), B. tabaci (Nomokou et al. 2001), F. occidentalis, or T. tabaci (Wimmer et al. 2008). These observations suggest that a diet of T. turkestani is suitable for A. swirskii reproduction.
Although the T. turkestani diet was best for A. swirskii reproduction, this observation does not diminish the value of the date palm pollen diet. In prior research, A. swirskii fecundity was greatest on a diet of date palm pollen rather than eggs of two scale insects Insulaspis pallidula (Green) and Phoenicococcus marlatti Cockerell (Homoptera: Diaspididae) (Abou-Elella et al. 2013). Similarly, A. swirskii fecundity was greater on a diet of date palm pollen than a diet of T. urticae eggs or immatures, but less than on a diet of E. orientalis immatures or adults (Ali and Zaher 2007). Date palm pollen contains sterols, saponins, flavonoids, fatty acids, amino acids, and other components that purportedly improve fertility in humans (Tahvilzadeh et al. 2016). The presence of these nutrients at suitable concentrations could affect the fecundity of A. swirskii. More research is necessary to identify the nutrients in date palm pollen and to manipulate their concentrations to maximize reproduction in A. swirskii and other phytoseiids in mass rearing systems.
Diet type also affected longevity (adult lifespan) of A. swirskii in this study; A. swirskii males and females lived longer when fed T. turkestani than date palm pollen or bee pollen. Many highly fecund arthropod species are not long-lived because energy and resources are diverted to oviposition rather than body maintenance (Hosking et al. 2019). The observation that the most fecund A. swirskii females (fed the T. turkestani diet) lived longer in this study does not follow this pattern. More research on the potential association between fecundity and longevity in phytoseiids is warranted. If phytoseiid females emerge as adults with only one or two mature eggs in their ovaries, it is possible that long-lived females generate more eggs than short-lived ones over time. The rate at which eggs mature in the ovaries of phytoseiids would also provide clues to whether fecundity and longevity are positively or negatively related. Research on egg maturation or ovarian dynamics in phytoseiids is scarce (Di Palma and Alberti 2001).
The benefits of date palm pollen and T. turkestani diets on A. swirskii development and reproduction was confirmed by a life table analysis. Although intrinsic rate of natural increase (rm) was greatest for A. swirskii fed the date palm pollen rather than T. turkestani, the finite rate of increase (λ), net reproductive rate (R0), and gross reproductive rate (GRR) did not differ significantly between the two diets. The observation that mean generation time (T) and doubling time (DT) were least for A. swirskii fed date palm pollen (rather than T. turkestani) suggests that A. swirskii will require less time to complete a generation and double in population size when fed date palm pollen.
The highest intrinsic rate of natural increase (rm) in this study was recorded when A. swirskii was fed date palm pollen (0.396 d-1). This value is higher than those reported by Riahi et al. (2017a) (0.080 d-1), Fadaei et al. (2019) (0.050 d-1) and Nemati et al. (2019) (0.031 d-1) for A. swirskii fed date palm pollen. These dissimilarities might be explained by disparities in freshness of date pam pollen or differences in date palm cultivar. A follow-up study to examine date palm pollen quality in relation to freshness, cultivar, and geographic location would be informative.
In addition, the rm value calculated for A. swirskii fed date palm pollen in this study was higher than the rm value reported for A. swirskii fed cattail pollen (0.158 d-1), a commonly used and recommended food to maintain A. swirskii laboratory colonies (Lee and Gillespie 2011). Furthermore, the rm found in this study was higher than those reported for A. swirskii fed T. urticae (0.167 d-1) (El-Leithy and Fouly 1992), the western flower thrip F. occidentalis (0.056 d-1), and onion thrip T. tabaci (0.024 d-1) (Wimmer et al. 2008), and the eriophyoid fig mites Aceria ficus (Cotte) (0.155 d-1) and Rhyncaphytoptus ficifoliae Keifer (0.122 d-1) (Abou-Awad et al. 2000), tomato russet mite Aculops lycopersici (Massee) (0.201 d-1) (Park et al. 2011), and cotton whitefly B. tabaci (0.213 d-1) (Nomikou et al. 2001).
In conclusion, date palm pollen has great potential as a diet to support A. swirskii in a cost-effective mass rearing system. Date palm pollen is readily available in Iran, easily harvested, amenable to cold storage, and very inexpensive to purchase. In contrast, additional labor costs and rearing space would be required to maintain a T. turkestani colony and host plants as food. Host plants are necessary because a satisfactory artificial diet for T. turkestani is not available. Although factitious prey, e.g., C. lactis, is available for A. swirskii (San et al. 2020), additional time, labor, and space will be required to maintain C. lactis colonies. Therefore, the incorporation of a non-prey diet would be most cost-effective. Although the date palm pollen diet would be more advantageous for mass rearing of A. swirskii, future research must determine if long-term rearing on this diet would cause any deterioration of the colony. Research is also necessary to determine if long-term rearing on date palm pollen reduces the capacity of A. swirskii to locate, capture, and consume natural prey, such as T. turkestani, on plants in greenhouses or open fields.
Acknowledgements
The authors thank the research deputy of Shahid Chamran University of Ahvaz for supporting this research. Two anonymous peer reviewers commented on a previous version of this manuscript. The U.S. Government has the right to retain a non-exclusive, royalty-free license in and to any copyright of this article. This article reports the results of research only. Mention of a commercial or proprietary product does not constitute an endorsement of the product by the U.S. Department of Agriculture or Shahid Chamran University of Ahvaz.
References
- Abou-Awad B.A., Reda A., Elsawi S. 1992. Effects of artificial and natural diets on the development and reproduction of two phytoseiid mites Amblyseius gossipi and Amblyseius swirskii (Acari: Phytoseiidae). Int. J. Trop. Insect Sci., 13. 441-445. https://doi.org/10.1017/S1742758400013746
- Abou-Awad B.A., El-Sawaf B.M., Abdel-Khalek A.A. 2000. Impact of two eriophyoid fig mites, Aceria ficus and Rhyncaphytoptus ficifoliae, as prey on postembryonic development and oviposition rate of the predacious mite Amblyseius swirskii. Acarologia, 40: 367-371.
- Abou-Awad B.A., Hafez S.M., Farhat B.M. 2014. Biological studies of the predacious mite Amblyseius swirskii, a predator of the broad mite Polyphagotarsonemus latus on pepper plants (Acari: Phytoseiidae: Tarsonemidae). Arch. Phytopathol. Plant Prot., 47: 349-354. https://doi.org/10.1080/03235408.2013.809908
- Abou-Elella G.M., Saber S.A., El-Sawi S.A. 2013. Biological aspects and life tables of the predacious mites, Typhlodromips swirskii (Athias-Henriot) and Euseius scutalis (Athias-Henriot) feeding on two scale insect species and plant pollen. Arch. Phytopathol. Plant Prot., 46: 1717-1725. https://doi.org/10.1080/03235408.2013.774715
- Ali F.S., Zaher M.A. 2007. Effect of food and temperature on the biology of Typhlodrompis swirskii (Athias-Henriot) (Acari: Phytoseiidae). ACARINES: J. Egypt. Soc. Acarol., 1: 17-21. https://doi.org/10.21608/ajesa.2007.4986
- All-Azzazy M.M., Alhewairini S.S. 2020. Effect of temperature and humidity on development, reproduction, and predation rate of Amblyseius swirskii (Phytoseiidae) fed on Phyllocoptruta oleivore (Eriophyiidae) and Eutetranychus orientalis (Tetranychidae). Int. J. Acarol., 46: 304-312. https://doi.org/10.1080/01647954.2020.1773922
- Bazgir F., Shakarami J., Jafari SH. 2018. Life table and predation rate of Amblyseius swirskii (Acari: Phytoseiidae) fed on Eotetranychus frosti (Tetranychidae) and Cenopalpus irani (Tenuipalpidae). Syst. Appl. Acarol., 23: 1614-1626. https://doi.org/10.11158/saa.23.8.11
- Bazazzadeh F., Shishehbor P., Esfandiari M., Farahi S. 2020. Development, reproduction and life table parameters of Tetranychus turkestani (Acari: Tetranychidae) on three different host plants. Acarologia, 60: 643-655. https://doi.org/10.24349/acarologia/20204393
- Birch L.C. 1948. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol., 17: 15- 26. https://doi.org/10.2307/1605
- Calvo F.J., Knapp M., Yvonne M., Houten V., Hoogerbrugge H., Belda J.E. 2014. Amblyseius swirskii: What made this predatory mite such a successful biocontrol agent? Exp. Appl. Acarol., 65: 419-433. https://doi.org/10.1007/s10493-014-9873-0
- Chi H., Liu H. 1985. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sinica, 24: 225-240.
- Chi H. 2018. TWOSEX-MSChart: a computer program for the age-stage, two-sex life-table analysis. http://140.120.197.173/Ecology/prod02.htm

- Di Palma A., Alberti G. 2001. Fine structure of the female genital system in phytoseiid mites with remarks on egg nutrimentary development, sperm-access system, sperm transfer, and capacitation (Acari, Gamasida, Phytoseiidae). Exp. Appl. Acarol., 25, 525-591.
- Ebrahimifar J., Shishehbor P., Rasekh A., Hemmati S.A., Riddick E.W. 2021. Evaluation of Artemia franciscana cysts to improve diets for mass rearing Stethorus gilvifrons, a predator of Tetranychus turkestani. Insects, 12: 632. https://doi.org/10.3390/insects12070632
- El-Laithy A.Y.M., Fouly H. 1992. Life table parameters of the two phytoseiid predators Amblyseius scutalis (Athias-Henriot) and A. swirskii A.-H. (Acari: Phytoseiidae) in Egypt. J. Appl. Entomol., 113: 8-12. https://doi.org/10.1111/j.1439-0418.1992.tb00631.x
- Fadaei E., Hakimitabar M., Seiedy M., Sarraf Moaieri H.R. 2018. Effects of different diets on biological parameters of the predatory mite Amblyseius swirskii (Acari: Phytoseiidae). Int. J. Acarol., 44: 341-346. https://doi.org/10.1080/01647954.2018.1525428
- Goleva I., Zebitz C.P.W. 2013. Suitability of different pollen as alternative food for the predatory mite Amblyseius swirskii (Acari, Phytoseiidae). Exp. Appl. Acarol., 61: 259-283. https://doi.org/10.1007/s10493-013-9700-z
- Grenier S., De Clercq P. 2003. Comparison of artificially vs. naturally reared natural enemies and their potential for use in biological control. In: van Lenteren J.C. (Ed.) Quality control and production of biological control agents: theory and testing procedures. CABI Publishing, Wallingford, UK, pp. 115- 131. https://doi.org/10.1079/9780851996882.0115
- He Y., Lv J., Wang E., Xu X. 2019. Reproductive performance and transcriptome analyses of Tetranychus urticae when feeding on bean leaves and an artificial diet. Syst. Appl. Acarol., 24: 1272-1283. https://doi.org/10.11158/saa.24.7.11
- Hosking C.J., Raubenheimer D., Charleston M.A., Simpson S.J., Senior A.M. 2019. Macronutrient intakes and the lifespan-fecundity trade-off: a geometric framework agent-based model. J. Royal Soc. Interface, 16:20180733. https://doi.org/10.1098/rsif.2018.0733
- Jeppson L.R., Keifer H.H., Buker E.W. 1975. Mites injurious to economic plants. Univ. Calif. Press. Berkeley, pp. 614. https://doi.org/10.1525/9780520335431
- Ji J., Lin T., Zhang Y., Lin J., Sun L., Chen X. 2013. A comparison between Amblyseius (Typhlodromips) swirskii and Amblyseius eharai with Panonychus citri (Acari: Tetranychidae) as prey: developmental duration, life table and predation. Syst. Appl. Acarol., 18: 123-129. https://doi.org/10.11158/saa.18.2.4
- Juan-Blasco M., Oureshi J.A., Urbaneja A., Stansly P.A. 2012. Predatory mite, Amblyseius swirskii (Acari: Phytoseiidae), for biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Fla. Entomol., 95: 543-551. https://doi.org/10.1653/024.095.0302
- Khanamani M., Fathipour Y., Talebi A.A., Mehrabadi M. 2017. Linking pollen quality and performance of Neoseiulus californicus (Acari: Phytoseiidae) in two-spotted spider mite management programmes. Pest Manag. Sci., 73: 452-461. https://doi.org/10.1002/ps.4305
- Knapp M., van Houten Y., van Ball E., Groot T. 2018. Use of the predatory mites in commercial biocontrol: current status and future prospects. Acarologia, 58 (Suppl): 72-82. https://doi.org/10.24349/acarologia/20184275
- Lee H., Gillespie D. 2011. Life table and development of Amblyseius swirskii (Acari: Phytoseiidae) at different temperatures. Exp. Appl. Acarol., 53: 17-27. https://doi.org/10.1007/s10493-010-9385-5
- Li G.Y., Li J.J., Xia W., Qu H.L., Yang S., Zhang J.P. 2014. Effects of Bt+ Cp TI transgenic cotton on the performance of Tetranychus turkestani (Acari: Tetranychidae). Syst. Appl. Acarol., 19: 236-247. https://doi.org/10.11158/saa.19.2.14
- Maia A.H.N., Alfredo J.B., Campanhola C. 2000. Statistical influence on associated fertility life table parameters using Jackknife technique: computational aspects. J. Econ. Entomol., 93: 511-518. https://doi.org/10.1603/0022-0493-93.2.511
- McMurtry J.A., Moraes G.J., Famah Sourasson, N.F. 2013. Revision of the lifestyle of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. https://doi.org/10.11158/saa.18.4.1
- Medd N.C., Greatrex R.M. 2014. An evaluation of three predatory mite species for the control of greenhouse whitefly (Trialeurodes vaporariorum). Pest Manag. Sci., 70. 1492-1496. https://doi.org/10.1002/ps.3794
- Messelink G.J., Van Steenpaal E.F., Ramakers P.M.J. 2006. Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. BioControl, 51: 753-768. https://doi.org/10.1007/s10526-006-9013-9
- Migeon A., Dorkled F. 2021. Spider Mites Web: a comprehensive database for the Tetranychidae. Available from: http://www1.montpellier.inra.fr/CBGP/spmweb
 (Accessed 14/08/2021)
(Accessed 14/08/2021) - Mossadegh M.S., Kocheili F. 2003. A semi-descriptive checklist of identified species of arthropods (agricultural, medical) and other pests from Khuzestan, Iran. Shahid Chamran University of Ahvaz Press, Ahvaz, Iran.
- Nemati A., Riahi E., Khalili Moghadam A., Gwiazdowicz D.J., Bahari M.R., Amini P. 2019. Comparison of different pollen grains and a factitious prey as food sources for Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol., 24: 2427-2438. https://doi.org/10.11158/saa.24.12.10
- Nomikou M., Janssen A., Schraag R., Sabelis M.W. 2001. Phytoseiid predators as potential biological control agents for Bemisia tabaci. Exp. Appl. Acarol., 25: 271-291. https://doi.org/10.1023/A:1017976725685
- Overmeer W.P.J. 1985. Alternative prey and other food resources. In Helle W., Sabelis M.W. (Eds.), World crop pests. Spider mites. Their biology, natural enemies and control. Vol. 1B. Elsevier, Amsterdam, pp: 131- 139.
- Park H., Shipp L., Buitenhuis R. 2010. Predation, development, and oviposition by the predatory mite Amblyseius swirskii (Acari: Phytoseiidae) on tomato russet mite (Acari: Eriophyidae). J. Econ. Entomol., 103: 563-569. https://doi.org/10.1603/EC09161
- Park H.-H., Shipp L., Buitenhuis R., Ahn J.J. 2011. Life history parameters of a commercially available Amblyseius swirskii (Acari: Phytoseiidae) fed on cattail (Typha latifolia) pollen and tomato russet mite (Aculops lycopersici). J. Asia-Pac. Entomol., 14: 497-501. https://doi.org/10.1016/j.aspen.2011.07.010
- Peña J.E., Rodrigues J.C.V., Roda A., Carrillo D., Osborne L.S. 2009. Predator-prey dynamics and strategies for control of the red palm mite (Raoiella indica) (Acari: Tenuipalpidae) in areas of invasion in the Neotropics. IOBC WPRS Bull, 50: 69-79.
- Popov S. J. 1981. Survival tables and biological parameters of the spider mite Tetranychus turkestani. Izvestia Timiryazeveskoi Kakhazyaistvennoi Akademii, 1: 124- 133.
- Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017a. Linking life table and consumption rate of Amblyseius swirskii (Acari: Phytoseiidae) in presence and absence of different pollens. Ann. Entomol. Soc. Am., 110: 244-253. https://doi.org/10.1093/aesa/saw091
- Riahi E., Fathipour Y., Talebi A.A., Mehrabadi M. 2017b. Natural diets versus factitious prey: Comparative effects on development, fecundity and life table of Amblyseius swirskii (Acari: Phytoseiidae). Syst. Appl. Acarol., 22: 711-723. https://doi.org/10.11158/saa.22.5.10
- San P.P., Tuda M., Nakahira K., Takagi M. 2020. Optimal rearing medium for the population growth of the predatory mite, Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae). Egypt. J. Biol. Pest Control 30: 130. https://doi.org/10.1186/s41938-020-00332-y
- Schausberger, P., Croft, B.A. 1999. Activity, feeding and development among larvae of specialist and generalist phytoseiid mite species (Acari: Phytoseiidae). Environ. Entomol., 28: 322-329. https://doi.org/10.1093/ee/28.2.322
- Sohrabi F., Shishehbor P. 2008. Effects of host plant and temperature on growth and reproduction of the strawberry spider mite Tetranychus turkestani Ugarov & Nikolskii (Acari: Tetranychidae). Syst. Appl. Acarol., 13: 26-32. https://doi.org/10.11158/saa.13.1.2
- Taha E.-K.A., Al-Kahtani S., Taha, R. 2019. Protein content and amino acids composition of bee-pollens from major floral sources in Al-Ahsa, eastern Saudi Arabia. Saudi J. Biol. Sci., 19: 232-237. https://doi.org/10.1016/j.sjbs.2017.06.003
- Tahvilzadeh M., Hajimahmoodi M., Rahimi R. 2016. The role of date palm (Phoenix dactylifera L.) pollen in fertility: A comprehensive review of current evidence. J. Evidence-Based Comp. Alter. Med., 21: 320-324. https://doi.org/10.1177/2156587215609851
- Van der Geest L.P.S., Bosse Th.C., Veerman A. 1983. Development of an artificial diet for the two-spotted spider mite Tetranychus urticae. Entomol. Exp. Appl., 33: 297-302. https://doi.org/10.1111/j.1570-7458.1983.tb03272.x
- Wazler A., Schausberger P. 1999. Predation preferences and discrimination between con- and heterospecific prey by phytoseiid mites Phytoseiulus persimilis and Neoseiulus californicus. BioControl, 43: 469- 478. https://doi.org/10.1023/A:1009974918500
- Wimmer D., Hoffmann D., Schausberger P. 2008. Prey suitability of western flower thrips, Frankliniella occidentalis, and onion thrips, Thrips tabaci, for the predatory mite Amblyseius swirskii. Biocontrol Sci. Technol., 18: 533-542. https://doi.org/10.1080/09583150802029784
- Yazdanpanah S., Fathipour Y., Riahi E. 2021. Pollen grains are suitable alternative food for rearing the commercially used predatory mite Neoseiulus cucumeris (Acari: Phytoseiidae). Sys. Appl. Acarol., 26: 109-1020. https://doi.org/10.11158/saa.26.5.14



2021-05-05
Date accepted:
2021-10-12
Date published:
2021-10-21
Edited by:
Palevsky, Eric

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Rahmani Piyani, Atefeh; Shishehbor, Parviz; Kocheili, Farhan and Riddick, Eric W.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




