A new opilioacarid species (Parasitiformes: Opilioacarida) from Crete (Greece) with notes on its karyotype
Vázquez, Maria Magdalena1 ; Ávila Herrera, Ivalú Macarena2 ; Just, Pavel3 ; Reyes Lerma, Azucena Claudia4 ; Chatzaki, Maria5 ; Heller, Tim Lukas6 and Král, Jiří7
1✉ Division de Ciencias e Ingenierias, Universidad de Quintana Roo, Chetumal, Quintana Roo, Mexico.
2Laboratory of Arachnid Cytogenetics, Department of Genetics and Microbiology, Faculty of Science, Charles University, Viničná 5, 128 44, Prague 2, Czech Republic.
3Laboratory of Arachnid Cytogenetics, Department of Genetics and Microbiology, Faculty of Science, Charles University, Viničná 5, 128 44, Prague 2, Czech Republic & Invertebrate Zoology Unit, Department of Zoology, Faculty of Science, Charles University, Viničná 7, 128 44, Prague 2, Czech Republic.
4Laboratory of Arachnid Cytogenetics, Department of Genetics and Microbiology, Faculty of Science, Charles University, Viničná 5, 128 44, Prague 2, Czech Republic.
5Department of Molecular Biology and Genetics, Democritus University of Thrace, GR - 68100, Alexandroupolis, Greece.
6Ludwig-Maximilians-University, Munich, Germany.
7Laboratory of Arachnid Cytogenetics, Department of Genetics and Microbiology, Faculty of Science, Charles University, Viničná 5, 128 44, Prague 2, Czech Republic.
2021 - Volume: 61 Issue: 3 pages: 548-563
https://doi.org/10.24349/acarologia/20214449ZooBank LSID: BD5ACF5D-93C2-4BC8-ABDF-4EB51A9044B1
Original research
Keywords
Abstract
Introduction
The suborder Opilioacarida is a small, a low-diversity acarine clade (Bernardi et al. 2018). Although these mites exhibit features of both acariform and parasitiform mites (Klompen et al. 2015), recent molecular analyses clearly support their placement among parasitiform mites (e.g. Klompen 2010). Opilioacarids are probably the most early-diverging lineage among the recent parasitiform mites (Walter and Harvey 2009, Pepato et al. 2010). They exhibit many morphological characters, which are considered ancestral within parasitiform mites and which are shared with other arachnid groups, e.g. two pairs of eyes and abdominal segmentation (Coineau and Legendre 1975; Vázquez and Palacios-Vargas 1988; Dunlop and Alberti 2008). Opilioacarids are the only parasitiform mites, which possess complete acarine ontogenetic sequence including a prelarva (Klompen 2000; Bernardi et al. 2013b). Due to the position of the opilioacarids as a basal group of parasitiform mites, their characters can be used for the reconstruction of parasitiform phylogeny (Grandjean 1936; van der Hammen 1970, Klompen et al. 2007, Pepato et al. 2010). Despite the considerable importance of opilioacarids for reconstruction of arachnid phylogeny, there are only few studies aimed at understanding their phylogeny, ethology, and ecology. Opilioacarids are relatively large, free-living scavengers of arthropods and pollen and possibly occasional predators (Walter and Proctor 1998). A recent study of Vázquez et al. (2018) suggests a complex behaviour of opilioacarids, which is, however, not sufficently understood and includes courtship, brood care and care for immature instars. Opilioacarids are usually found under stones or in leaf litter. Recent study from Belize also revealed opilioacarids under bark of fallen trees or under moss and lichens on tree bases (Vázquez et al. 2018). Despite their low diversity, the opilioacarids inhabit a wide range of elevations (from altitudes close to sea level up to 2,000m) and biotopes (from coastal plains and mangroves to uplands like altiplano and from semi-desert biotopes to wet tropical forests). They also occur in hypogean environments (Bernardi et al. 2012; Vázquez and Klompen 2015; Moraza et al. 2021).
The suborder Opilioacarida currently includes a single family Opilioacaridae comprising 13 genera, 52 species, and 2 subspecies (Araújo et al. 2020; Moraza et al. 2021). The first described opilioacarid was Opilioacarus segmentatus (the type species) from Algeria, which was described by Carl Johannes With in 1902 (With 1902). For the following 40 years, relatively little attention was paid to opilioacarids, the findings of which were limited to the Mediterranean region. During the last two decades, the number of studies dealing with opilioacarid taxonomy has considerably increased. Nowadays, opilioacarids are known from tropical and temperate regions of all continents except for Antarctica (Araújo et al. 2020). Remarkably, particular species are often endemic to relatively narrow regions. Opilioacarids were found in South America (Lehtinen 1980; Vázquez et al. 2014, 2015, 2018; Bernardi et al. 2012; 2013a, b; 2014; Araújo et al. 2018a), Mesoamerica and the Antilles (Juvara-Bals and Baltac 1977; Vázquez and Palacios-Vargas 1988; Vázquez and Klompen 2002, 2009, 2015), the southern part of the United States (Chamberlin and Mulaik 1942; van der Hammen 1966), sub-Saharan Africa including Madagascar (Naudo 1963; van der Hammen 1977; Vázquez and Klompen 2010), the Arabian peninsula (van der Hammen 1969), India (Das and Bastawade 2007), central (Krivolutsky 1965) and southeastern Asia (Leclerc, 1989), and Australia (Walter and Proctor 1998).
The type genus Opilioacarus is restricted to the Mediterranean region of Europe and North Africa (Vázquez and Klompen 2009). This genus includes six species, namely O. segmentatus With, 1902 (Algeria), O. italicus (With, 1904), O. brignolii Araújo and Di Palma, 2018 (Italy), O. baeticus Moraza, Prieto and Balanzategui, 2021 (Spain), and two fossil species from amber (Dunlop et al. 2010; Dunlop and Bernardi 2014). Previously, the genus Opilioacarus also included some New World species, which are currently placed into the genus Neocarus. The revision of various samples revealed that diagnostic characters of Neocarus are sufficiently robust to constitute a separate genus (Vázquez and Klompen 2002, 2009).
Specimens of Opilioacarus were also collected in Greece, namely Peloponnese peninsula and islands of Crete (Thaler and Knoflach 2002), Korfu (Silvestri 1905), Kassos, Karpathos, and Rhodes (Beron 1990). These specimens were classified as O. segmentatus. However, taxonomic revision of material from Greece is needed (Thaler and Knoflach 2002, Moraza et al. 2021). Our material collected on the island of Crete at the locality of Thaler and Knoflach (2002) allowed us to describe a new species of the genus Opilioacarus and to bring some information on its life history and karyotype. In contrast to the parasitiform suborders Ixodida and Mesostigmata, for opilioacarids the chromosome data are unknown.
Material and methods
Material
Specimens were collected during four expeditions in Greece, western Crete, Georgioupolis, hill to the west of the town (35.372193° N, 24.256336° E) (Figure 1). Three trips took place in early spring (25.III.–2.IV.2015, 22.IV.–1.V.2017, 1.–8.V.2019), another in early autumn (15.IX.–24.IX.2013). Opilioacarids were collected under large stones on moister spots of phrygana vegetation or inside stone accumulations (Figure 2), which were also inhabited by spider species, which normally occur in caves [pholcid Hoplopholcus labyrinthi (Kulczyński, 1903), leptonetid Sulcia cretica Fage, 1945]. One or two opilioacarid specimens occurred on stones. Howewer, in early May 2019 (beginning of the hot period) the distribution of specimens was different. During this period, opilioacarids were more abundant. Some stones were inhabited by more (up to eight) specimens.






After sampling, the specimens were reared in plastic containers with slightly moistened cellulose. Water as needed was provided in the form of droplets. Opilioacarids were fed by tiny granules of dry yeast, pollen, and crushed bodies of Drosophila and chironomids. They preferred the granules of dry yeast. Specimens were fed twice per week. Excess food was removed to prevent fungal growth.
Morphological studies
Some specimens used for study of morphology were slide-mounted. For this purpose, specimens were cleared in lactic acid for two days, washed in distilled water, dissected and mounted on slides in Hoyer's medium (Krantz and Walter 2009) with the aim to observe their structures under light microscopy. The main structures (idiosoma, gnathosoma, and legs) were mounted on separate slides and kept in the oven at 50 °C for a week. After that the slides were sealed.
Identification drawings were prepared using a Zeiss phase contrast microscope, connected to a drawing tube; measurements were taken using an ocular micrometer and are presented in micrometers (μm). Measurements of idiosomal length and width might be distorted, as they are based on slide-mounted specimens. The description of palps follows the nomenclature of Grandjean (1936) and Vázquez and Klompen (2002). Description of subcapitulum, chelicerae, and sternal area follows Klompen et al. (2015). The examined material is deposited in the collection of JK (Department of Genetics and Microbiology, Charles University, Prague, Czech Republic).
Chromosome preparations and their evaluation
Mitotic plates were obtained from the content of the idiosoma of five female specimens, using a spreading technique described by Dolejš et al. (2011). Briefly, the dissected tissues were hypotonized in 0.075M KCl for 20 min and then fixed in three rounds of freshly prepared ethanol:acetic acid (3:1) fixative (6, 10 and 20 min, RT). A piece of fixed tissue was suspended into a drop of 60% acetic acid using a pair of tungsten needles. Another tungsten needle was used to gradually spread the resulting cell suspension along the microscope slide placed on a heated histological plate (40˚C). Preparations were stained for 30 min in 5% Giemsa solution in Sörensen buffer (pH 6.8). They were inspected using the BX 50 microscope (Olympus). Images were taken under immersion objective 100x using a DP 71 CCD camera (Olympus) and optimized in Corel PHOTO-PAINT X4 programme.
Taxonomic section
Suborder Opilioacarida (following Krantz and Walter, 2009)
Family Opilioacaridae With, 1904
Opilioacarus With, 1902
Type species Opilioacarus segmentatus With, 1902: by original designation
The genus currently includes four extant species, namely O. segmentatus With, 1902 (Mediterranean region of Algeria), O. italicus (With, 1904) (Sicily), O. brignolii Araújo and Di Palma, 2018 (southern part of the Italian peninsula and Sardinia), and O. baeticus Moraza, Prieto and Balanzategui, 2021 (Spain). O. italicus is a nomen dubium (Araújo et al. 2018b). Types of this species are missing, the original description is insufficient, and the recollecting efforts at the type locality were unsuccessful (Araújo et al. 2018b). The main differences between the species of Opilioacarus described by With are in the size of their legs. Information on chaetotaxy, a standard feature nowadays, was provided only for species described recently (Araújo et al. 2018b, Moraza et al. 2021) and partially for O. segmentatus (Grandjean 1936). Recently, two fossil species have been placed putatively into the genus Opilioacarus, namely the Eocene O. aenigmus Dunlop et al., 2010 from Baltic amber (Dunlop et al. 2010) and the Upper Cretaceous O. groehni Dunlop and Bernardi, 2014 from Burmese amber (Dunlop and Bernardi 2014). These findings indicate a considerable antiquity of the clade containing Opilioacarus.
Diagnosis: 4–6 foliate d setae (d1) and one slightly pectinate d seta (d2) on palp. With´s organ membranous, discoid, hyaline. Two pairs of dorsolateral eyes, preanal region with 4–5 dorsal setae and 2 lateroventral setae. Dorsal segments between prodorsal shield and preanal region without setae. Leg I exhibits crown-like eupathidium (ζ1) close to main sensilla group.
Opilioacarus thaleri n. sp. Vázquez, Ávila and Just
ZOOBANK: 9E2813B3-3B51-424B-A57C-85DD2F0C95C4 ![]()
Figures 3–10



Material examined — Specimens examined were collected manually by J. Král, 1.–8.V.2019 in Greece, western Crete, Georgioupolis, hill to the west of the town, 35.372193° N, 24.256336° E. To evaluate characters, the slides were prepared from holotype female, 3 paratype females, and 4 paratype males. Specimens dissected for chromosomes were preserved in 96% ethanol. Some of them (6 females, 4 males) were also slide-mounted. These specimens are also designated as paratypes. The type material is deposited in the collection of JK (Department of Genetics and Microbiology, Charles University, Prague, Czech Republic).
Etymology — This species is dedicated to Konrad Thaler and his wife Barbara Thaler-Knoflach, outstanding Austrian arachnologists, who collected and reported opilioacarids from several places in Greece (Thaler and Knoflach 2002).
Diagnosis — Palp genu with ~6 p–type setae, palp femur with 13 p–type setae. Palp tarsus with foliate setae d1 with 3 rounded ending acute lobes each and 1 thin pectinate seta d2. Males and females differ by number of setae d1. While males exhibit 6 setae d1, females shows only 5 d1 setae. Pregenital region in males with 3 short and thick, ribbed and blunt pregenital setae. Pregenital region in females without pregenital setae. It has 6 eugenital setae. Genital region in males with 11 thin and lightly serrated and tapering setae. Ovipositor of the simple type, folded with 2 pairs of glands and two pairs of ducts on the basis. Internal basal structure of the ovispositor smooth, without any spikes, bifurcated on the top.
Description
Measurements
Measurements are summarized in Table 1.



Gnathosoma
Chelicera (Figure 4A). Basal segment with 1 seta, fixed digit with 3 setae. All setae are lightly barbed. Dorsal and antiaxial lyrifissures well developed. Fixed digit with one tooth, movable digit with 1–3 teeth and a well developed terminal hook. Movable digit with large denticle and two very small denticles. Without sexual dimorphism.



Subcapitulum (Figure 4B). All four pairs of paralabial setae are present: pl1 small, conical; With´s organ (pl2) membranous, hyaline, discoid and barbulated. Rutella (pl3) with 1 row of 5 teeth, inserted dorsolaterally; pl4 small but distintct, inserted dorsally on subcapitulum. In addition, 4 circumbuccal (cb) and 5–7 medium and subcapitular, latero-ventral (ventral medium in part), latero-ventral medium, latero-dorsal medium, ventral posterior, and latero-ventral posterior setae. Females with 5 subcapitular setae, males with 7 subcapitular setae. Lateral lips with distinct channels.
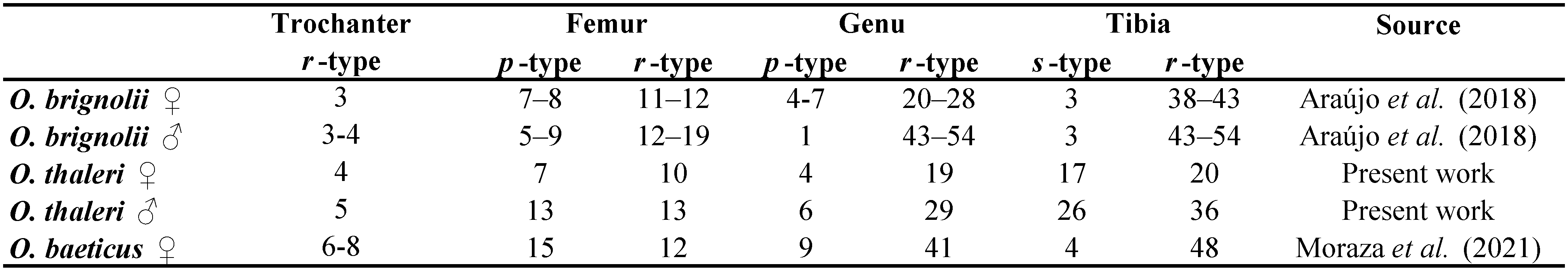
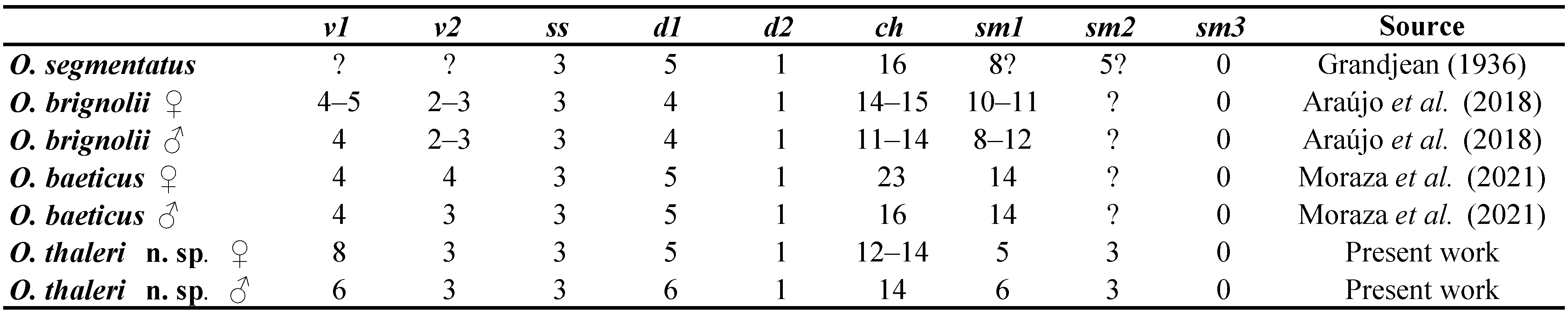
Palp (Figures 5–7, Tables 2, 3). Trochanter with 4 ribbed, tapering setae (= r–type) in females (Figure 5A), males with 5 r–type setae (Figure 6A); femur with 7 papilliform setae (= p–type) in females (Figure 5B), males with 13 p–type setae (Figure 6B); genu with 4 p–type setae and 19 r–type setae in females (Figure 5C), males with 6 p–type setae and 29 r–type setae (Figure 6C); tibia with 17 long lightly serrate and pointed setae (= s–type) and 20 r–type setae and two thin smoth sensilla (ss) in females (Figure 5D), males with 26 s–type setae and 36 r–type setae and 2 ss (Figure 6D). Tibia and tarsus partially fussed. Tarsus with 6 foliate (d1–type) and 1 pectinate (d2–type) setae in males (Figure 7A–B). Females with 5 foliate d1 setae and 1 pectinate d2 (Figure 7C–D). Females with 6 v1, 3 v2, 3 ss, 14 ch, 5 sm1, 3 sm2. Males with 8 v1, 3 v2, 3 ss, 12–14 ch, 6 sm1, 3 sm2 (Table 3). Lyrifisures iα and iπ present (Figure 7A–B). Pretarsus with a pair of well-developed, smoth, sessile claws (Figure 7).






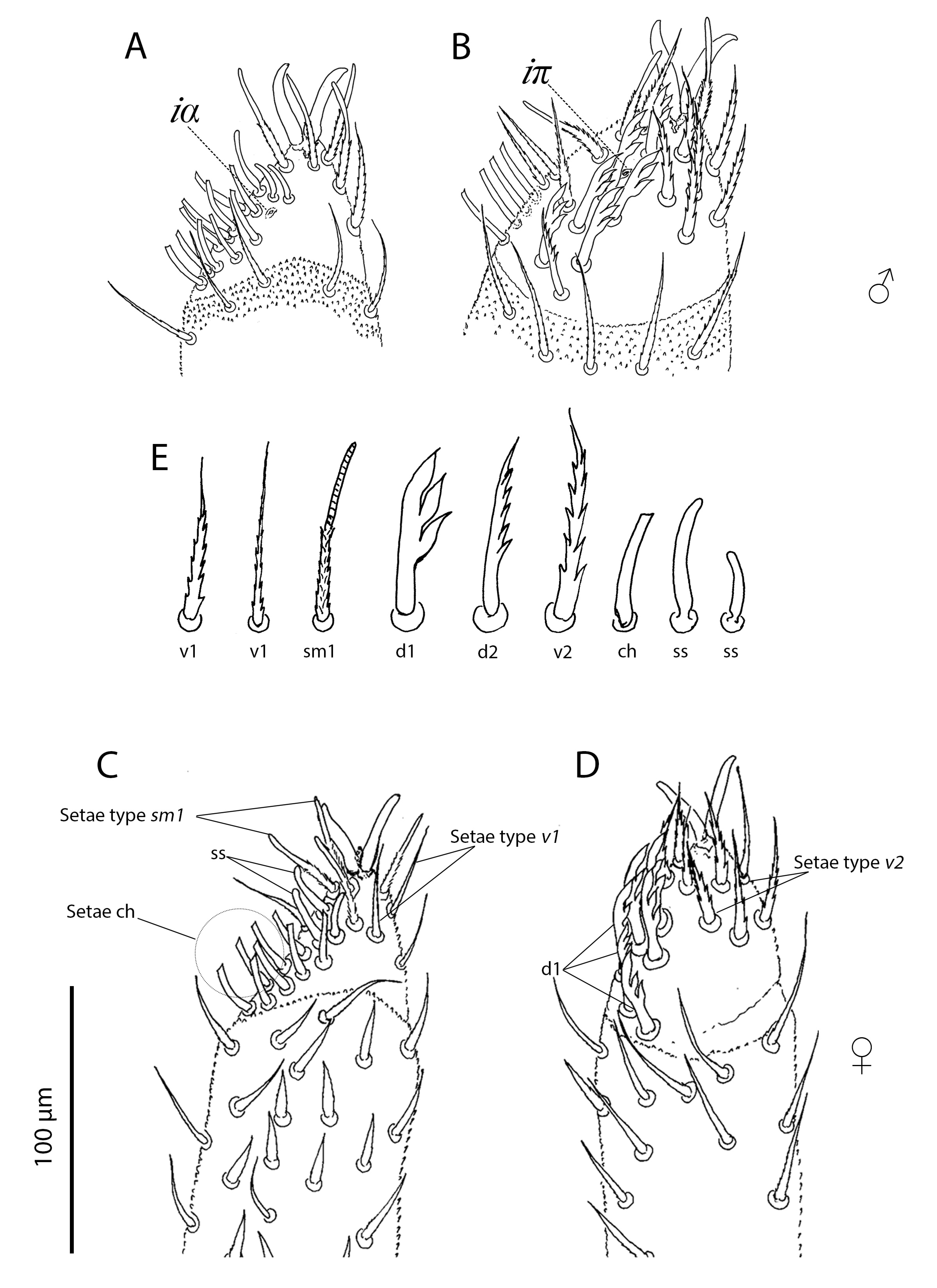






Idiosoma
Color. Light blue and violet body; combination of blue-purple and white stripes on the legs.
Sternogenital region (Figures 8–9, Table 4). Sternal verrucae in adults each with 3–4 large serrate and pointed and 1 composite (st1) setae. Remaining sternal region with 4–5 pairs of stout, ribbed, setae with blunt-tips, and 2 pairs of composite (st1) setae in both females and males. Pregenital capsules each with 1 long tapering seta (st5) and 4–5 stout, ribbed, blunt-tipped setae (Table 4).



Pregenital area in males with 3 short ribbed blunt-tips pregenital setae (Figure 8A). Pregenital area in females without pregenital setae (Figure 8B, Table 4). It has 6 thin lightly serrated eugenital setae (Figure 9A, Table 4). Genital region in males with 11 thin lightly serrated genital setae (Figure 8A). Ovipositor folded, with two pairs of gland-like structures and a pair of ducts. Internal basal structure smooth, without any spikes, bifurcated on the top (Figure 9B).
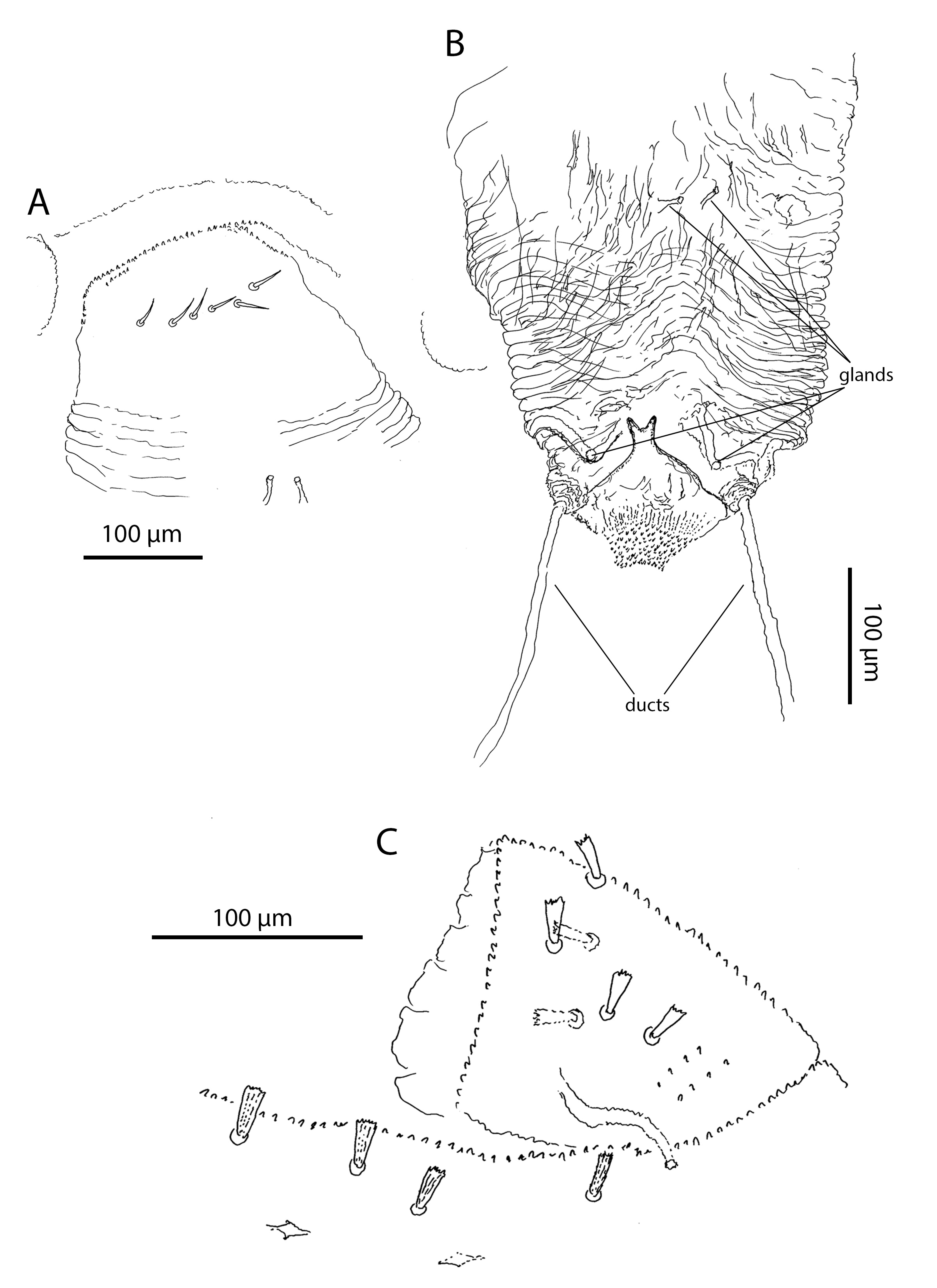


Dorsum. Prodorsal shield in adults with 60–70 stout ribbed setae and two pairs of lateral eyes. Dorsal idiosoma between the shield and the pre-anal segment without setae, but with numerous lyrifissures arranged in transverse rows. Pre-anal segment with 4–5 dorsal and two lateroventral short, stout and ribbed setae (Figure 9C). Anal plates with 12–13 papilliform setae. Dorsum without sexual dimorphism.



Taxonomic remarks
The material collected in Crete, Greece allowed us to describe a new species O. thaleri and to compare it with O. segmentatus, O. brignolii and O. baeticus. It should be underlined that the diversity of Opilioacarus could be higher than recognized. Specimens from other Greek localities [Peloponnese (Thaler and Knoflach 2002), island of Korfu (Silvestri 1905), islands of Kasos, Karpathos and Rhodes (Beron 1990)] were not revised yet. Moreover, data on Opilioacarus indicate that this genus occurs throughout all the coast of Mediterranean Sea forming many species, which inhabit a narrow geographic region.
The number of the setae on the penultimate segment is one of the main characteristics of the genus Opilioacarus. There are 4–5 dorsal and two lateroventral stout ribbed setae in both female and male. This characteristic is constant in the genus. The genus Neocarus has only one dorsal and two lateroventral stout ribbed setae on the penultimate segment.
There is some variation in the number of setae on the palp among Opilioacarus species. Opilioacarus segmentatus and O. baeticus have 5 foliated d1 type setae on the palp (Grandjean 1936; Moraza et al. 2021) while O. brignolii has 4 foliated d1 type setae (Araújo et al. 2018b). Males of O. thaleri present 6 foliated d1 setae while females of this species have 5 foliated d1 (this study). The lowest number of ch–type setae was found in O. brignolii (11–14) (Araújo et al. 2018b) and O. thaleri (12 in females and 14 in males) (this study). Opilioacarus segmentatus has 16 ch setae (Grandjean 1936) while O. baeticus exhibits 23 ch setae in females and 16 ch setae in males (Moraza et al. 2021).
In the genital area, the differences among Opilioacarus species are also significant. Pattern of eugenital setae in female pregenital area is often species-specific. Opilioacarus segmentatus and O. baeticus exhibits 5 eugenital setae (Grandjean 1936; Moraza et al. 2021). Opilioacarus brignolii presents 4–9 smooth eugenital setae (Araújo et al. 2018b) while O. thaleri shows 6 of these setae (this study). Pattern of setae in male pregenital and genital area is also species-specific. Males of O. brignolii have in trapezoidal area 2–7 acute ribbed genital setae (Araújo et al. 2018b). Males of O. segmentatus have 5 short tapering and smooth pregenital setae (Grandjean 1936). Males of O. baeticus have 5 pregenital and 5 genital setae (Moraza et al. 2021) while males of O. thaleri have 3 short ribbed blunt pregenital setae and 11 slightly serrated acute genital setae (this study), which is a very significant difference. Finally, there are significant interspecific differences in morphology of the ovipositor. In O. thaleri the ovipositor has 2 pairs of glands and 1 pair of ducts on the basis of the ovipositor. These structures were found neither in O. brignolii (Araújo et al. 2018b) or O. baeticus (Moraza et al. 2021).
Karyotype
In the present study, we reported for the first time chromosome data of opilioacarids. The female karyotype consists of 16 large monocentric chromosomes, predominantly with acrocentric morphology (Figure 10). The karyotype of O. thaleri n. sp. corresponds to the karyotypes of parasitiform suborders Ixodida and Mesostigmatida in terms of diploid number and chromosome morphology. While chromosomes of these groups were studied intensely due to their medical and economical importance, there are no cytogenetic data on Holothyrida, the fourth parasitiform suborder. Like in O. thaleri, 1) ixodids and mesostigmatids exhibit a low number of monocentric chromosomes (the diploid set of ixodids ranges from 12 to 32 chromosomes and diploid set of mesostigmatids from 6 to 26 chromosomes), and 2) karyotypes of most ixodids and mesostigmatids are predominated by acrocentric chromosomes (Oliver 1977, 1989; Norton et al. 1993). These features could be plesiomorphic for parasitiform mites.



Acknowledgements
We are very grateful to B. Thaler-Knoflach (University of Innsbruck, Austria) for specification of the opilioacarid locality in the neighbourhood of Georgioupolis, Crete. Furthermore, we are thankful to N. Kordatzakis and her family (Georgioupolis, Greece), P. Pousková (Charles University, Prague), H. Čermáková (Czech Ministry of Education, Youth, and Sports), and B. Lejtnarová (embassy of the Czech Republic in Greece), taking part in the organization of JK´s expeditions to Crete. These expeditions were supported by several scholarships, which were based on agreement between the Czech Ministry of Education, Youth, and Sports and the Greek Ministry of Education, Lifelong Learning, and Religious Affairs. Research stay of MMV at the Charles University (Prague) focused on opilioacarid taxonomy was supported by the Mobility Fund of this institution. Our study was also funded by the projects of the Czech Ministry of Education, Youth, and Sports (SVV 260314: ACRL, IMAH, JK; SVV 260571/2021: PJ; LTAUSA 19142: IMAH, JK) and Chilean National Agency for Research and Development (ANID) (IMAH).
References
- Araújo M.S., Bichuette M.E., Bauchan G.R., Ochoa R., Feres R.J.F. 2018a. A new species of cave dwelling Neocarus (Acari: Opilioacaridae) from Bahia state, Brazil, with remarks on taxonomic characters. Zootaxa, 4402(2): 303-322. doi:10.11646/zootaxa.4402.2.4
- Araújo M.S., Di Palma A., Feres R.J.F. 2018b. A new species of Opilioacarus With, 1902 (Acari: Opilioacaridae) from Italy, and a new diagnosis of the genus. Zootaxa, 4500(1): 135-145. doi:10.11646/zootaxa.4500.1.9
- Araújo, M.S., Di Palma, A., Feres, R.J.F. 2020. Catalog of the Opilioacarida (Acari: Parasitiformes). Zootaxa, 4895: 332-356. doi:10.11646/zootaxa.4895.3.2
- Bernardi L.F.O., Zacarias M.S., Ferreira R.L. 2012. A new species of Neocarus Chamberlin & Mulaik, 1942 (Acari: Opilioacarida) from Brazilian caves and karst areas. Zootaxa, 3416: 53-68. doi:10.11646/zootaxa.3416.1.5
- Bernardi L.F.O., Silva F.A.B., Zacarias M.S., Klompen H., Ferreira R.L. 2013a. Phylogenetic and biogeographic analysis of the genus Caribeacarus (Acari: Opilioacarida), with description of a new South American species. Invertebr. Syst., 27: 294-306. doi:10.1071/IS12041
- Bernardi L.F.O., Klompen H., Zacarias M.S., Ferreira R.L. 2013b. A new species of Neocarus Chamberlin & Mulaik, 1942 (Opilioacarida: Opilioacaridae) from Brazil, with remarks on postembryonic development. ZooKeys, 358: 69-89. doi:10.3897/zookeys.358.6384
- Bernardi L.F.O., Klompen H., Ferreira R.L. 2014. Neocarus caipora, a new mite species (Parasitiformes: Opilioacarida: Opilioacaridae) from brazilian Amazon caves. Acarologia, 54(1): 47-56. doi:10.1051/acarologia/20142113
- Bernardi L.F.O., Zacarias M.S., Borges-Filho E.L. 2018. Neocarus spelaion sp. n. (Parasitiformes, Opilioacaridae), a new species of cave dwelling Neocarus from Minas Gerais state, Brazil. Subterr. Biol., 27: 1-16. doi:10.3897/subtbiol.27.25777
- Beron P.K. 1990. On the occurence of Opilioacarus segmentatus With, 1903 (Arachnida, Opilioacarida) on the islands of Kassos, Karpathos and Rhodes (Greece). Acta Zool. Bulg., 39: 64-66.
- Chamberlin R.V., Mulaik S. 1942. On a new family of Notostigmata. Proc. Biol. Soc. Wash., 55: 125-132.
- Coineau Y., Legendre R. 1975. Sur un mode de régénération appendiculaire inédit chez les Arthropodes: la régénération des pattes marcheuses chez les Opilioacariens (Acari: Notostigmata). C. R. Hebd. Séanc. Acad. Sci. Paris, 280: 41-43.
- Das N.P.I., Bastawade D.B. 2007. The first report of the acarine suborder Opilioacarida from India, with description of new genus, Indiacarus, and a new species, Indiacarus pratyushi. Acarologia, 47(1-2): 3-11.
- Dolejš P., Kořínková T., Musilová J., Opatová V., Kubcová L., Buchar J., Král J. 2011. Karyotypes of central European spiders of the genera Arctosa, Tricca and Xerolycosa (Araneae: Lycosidae). Eur. J. Entomol., 108: 1-16. doi:10.14411/eje.2011.001
- Dunlop J.A., Alberti G. 2008. The affinities of mites and ticks: a review. J. Zool. Syst. Evol. Res., 46(1): 1-18.
- Dunlop J.A., Sempf C., Wunderlich J. 2010. A new opilioacarid mite in Baltic amber. In: Nentwig W., Entling M., Kropf C. (Eds). European Arachnology 2008. Bern: Natural History Museum. pp. 59-70.
- Dunlop J.A., Bernardi L.F.O. 2014. An opilioacarid mite in Cretaceous Burmese amber. Naturwissenschaften, 101(9): 759-763. doi:10.1007/s00114-014-1212-0
- Grandjean F. 1936. Un acarien synthétique: Opilioacarus segmentatus With. Bull. Soc. Hist. Nat. Afr. Nord, 27: 413-444.
- Juvara-Bals I., Baltac M. 1977. Deux nouvelles espèces d'Opilioacarus (Acarina: Opilioacarida) de Cuba. In: Orghidan T., Núñez Jiménez A., Decou V., Negrea S., Viña Bayés N. (Eds). Résultats des Expéditions Biospéleogiques Cubano-Roumaines á Cuba. Bucuresti: Academiei Republicii Socialiste Romania. pp. 169-184.
- Klompen H. 2000. Prelarva and larva of Opilioacarus (Neocarus) texanus (Chamberlin and Mulaik) (Acari: Opilioacarida) with notes on the patterns of setae and lyrissures. J. Nat. Hist., 34: 1977-1992. doi:10.1080/00222930050144819
- Klompen H. 2010. Holothyrids and ticks: new insights from larval morphology and DNA sequencing, with the description of a new species of Diplothyrus (Parasitiformes: Neothyridae). Acarologia, 50(2): 269-285. doi:10.1051/acarologia/20101970
- Klompen H., Lekveishvili M., Black W.C. 2007. Phylogeny of parasitiform mites (Acari) based on rRNA. Mol. Phylogenet. Evol. 43: 936-951. doi:10.1016/j.ympev.2006.10.024
- Klompen H., Vázquez M.M., Bernardi L.F.O. 2015. Post-embryonic development in the mite suborder Opilioacarida, with notes on segmental homology in Parasitiformes. Exp. Appl. Acarol., 67(2): 183-207. doi:10.1007/s10493-015-9939-7
- Krantz G.W., Walter D.E. 2009. A manual of acarology. 3rd Edition. Lubbock, TX: Texas Tech University Press.
- Krivolutsky D.A. 1965. A new finding of Opilioacarina in USSR. Russ. J. Zool., 44: 1413.
- Leclerc P. 1989. Considerations paléogéographique à propos de la découverte en Thaïlande d'Opilioacariens nouveaux (Acari-Notostigmata). C. R. Séances. Soc. Biogéogr., 65(4): 162-174.
- Lehtinen P.T. 1980. A new species of Opilioacarida (Arachnida) from Venezuela. Acta Biol. Venez., 10(2): 205-214.
- Moraza M.L., Prieto C.E., Balanzategui I. 2021. A new species of the genus Opilioacarus With, 1902 (Acari: Opilioacarida) for the Iberian Peninsula. Acarologia, 61: 128-¬147. doi:10.24349/acarologia/20214422
- Naudo M.H. 1963. Acariens Notostigmata de l'Angola. Publicaçoés Cult. Co. Diam. Angola, 63: 13-24.
- Norton R.A., Kethley J.B., Johnston D.E., O Connor B.M. 1993. Phylogenetic perspectives on genetic systems and reproductive modes of mites. In: Wrensch D.L., Ebbert, M.A. (Eds). Evolution and diversity of sex ratio in insects and mites. New York: Chapman & Hall. pp. 8-99. doi:10.1007/978-1-4684-1402-8_2
- Oliver J.H.jr. 1977. Cytogenetics of mites and ticks. Ann. Rev. Entomol., 22: 407-429. doi:10.1146/annurev.en.22.010177.002203
- Oliver J.H.jr. 1989. Biology and systematics of ticks (Acari: Ixodida). Ann. Rev. Ecol. Syst., 20: 397-430. doi:10.1146/annurev.es.20.110189.002145
- Pepato A.R., da Rocha C.E.F., Dunlop J.A. 2010. Phylogenetic position of the acariform mites: sensitivity to homology assessment under total evidence. BMC Evol. Biol., 10: 235. doi:10.1186/1471-2148-10-235
- Silvestri F. 1905. Note Aracnologiche III. Redia, 2: 239-261.
- Thaler K., Knoflach B. 2002. Neue Opilioacarus-Funde (Acari, Notostigmata) in Peloponnes und Ägäis (Griechenland). Entomolog. Nachr. Ber., 46: 271-277.
- Van der Hammen L. 1966. Studies on Opilioacarida (Arachnida) I. Description of Opilioacarus texanus (Chamberlin, Mulaik) and revised classification of the genera. Zool. Verh., 86: 1-80.
- Van der Hammen L. 1969. Studies on Opilioacarida (Arachnida) III. Opilioacarus platensis Silvestri, and Adenacarus arabicus (With). Zool. Meded., Leiden, 44(8): 113-131.
- Van der Hammen L. 1970. La Phylogenѐse des Opilioacarides, et leurs affinités avec les autres Acariens. Acarologia, 12(3): 465-473.
- Van der Hammen L. 1977. Studies on Opilioacarida (Arachnidea). IV The genera Panchaetes Naudo and Salfacarus gen. nov. Zool. Meded., Leiden, 51(4): 43-78.
- Vázquez M.M., Palacios-Vargas J.G. 1988: Algunas observaciones sobre el comportamiento de los acaros opilioacaridos (Acarida: Notostigmata). Rev. Nicar. Entomol., 6: 1-6.
- Vázquez M.M., Klompen H. 2002. The family Opilioacaridae (Acari: Parasitiformes) in North and Central America, with description of four new species. Acarologia, 42(4): 299-322.
- Vázquez M.M., Klompen H. 2009. New species of New World Opilioacaridae (Acari: Parasitiformes) with the description of a new genus from the Caribbean region. Zootaxa, 2061: 23-44. doi:10.11646/zootaxa.2061.1.2
- Vázquez M.M., Klompen H. 2010. The genus Salfacarus (Acari: Opilioacarida) in Madagascar. Zootaxa, 2482: 1-21. doi:10.11646/zootaxa.2482.1.1
- Vázquez M.M., Araújo M.S., Feres R.J.F. 2014. A new genus and two new species of Opilioacaridae (Acari: Parasitiformes) from Amazonia, Brazil with a key to the world genera. Zootaxa, 3814(2): 151-176. doi:10.11646/zootaxa.3814.2.1
- Vázquez M.M., Klompen H. 2015. The family Opilioacaridae (Parasitiformes: Opilioacarida) in Mexico, description of two new species and notes on biology and geographical distribution. Zootaxa, 3957(5): 535-552. doi:10.11646/zootaxa.3957.5.3
- Vázquez M.M., Araújo M.S., Feres R.J.F. 2015. Brasilacarus cocaris (Acari: Opilioacaridae), a new genus and species from Amazonia, Brazil. Zootaxa, 3915(3): 375-389. doi:10.11646/zootaxa.3915.3.3
- Vázquez M.M., May D., Alamilla E., Klompen H. 2018. A new species of Opilioacaridae (Parasitiformes: Opilioacarida) from Belize with some observations on life history and behavior. Syst. Appl. Acarol., 23(1): 132-144. doi:10.11158/saa.23.1.11
- Walter D.E., Proctor H.C. 1998. Feeding behaviour and phylogeny: Observations on early derivative Acari. Exp. Appl. Acarol., 22(1): 39-50. doi:10.1023/A:1006033407957
- Walter D.E., Harvey M.S. 2009. Order Opilioacarida. In: Krantz G.W., Walter D.E. (Eds.). A manual of acarology, Lubock, TX: Texas Tech University Press. pp. 104-106.
- With C.J. 1902. A new acaride Opilioacarus segmentatus. Forhandlingar vid Nordiska Naturforskareog Lakaremotet i Helsingfors den 7 till 12 Juli 1902 (Comptes Rendus du Congrès des Naturalistes et Médecins du Nord tenu à Helsingfors), Sektionen för Zoologi, 20: 4-5.
- With C.J. 1904. The Notostigmata, a new suborder of Acari. Vidensk. Meddel. Naturhist. Foren. Kjøbenhavn., 137-192.



2020-08-05
Date accepted:
2021-05-31
Date published:
2021-06-07
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2021 Vázquez, Maria Magdalena; Ávila Herrera, Ivalú Macarena; Just, Pavel; Reyes Lerma, Azucena Claudia; Chatzaki, Maria; Heller, Tim Lukas and Král, Jiří
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




