Occurrence of the introduced snake mite, Ophionyssus natricis (Gervais, 1844), in the wild in Australia
Norval, Gerrut1 ; Halliday, Bruce2 ; Sih, Andrew3 ; Sharrad, Robert D.4 and Gardner, Michael G.5
1✉ College of Science and Engineering, Flinders University, Sturt Rd., Bedford Park SA 5042, Australia.
2Australian National Insect Collection, CSIRO, Canberra, Australian Capital Territory, Australia.
3Department of Environmental Science and Policy, University of California, Davis, CA 95616, USA.
4College of Science and Engineering, Flinders University, Sturt Rd., Bedford Park SA 5042, Australia.
5College of Science and Engineering, Flinders University, Sturt Rd., Bedford Park SA 5042, Australia & Evolutionary Biology Unit, South Australian Museum, North Terrace Adelaide 5000, South Australia, Australia.
2020 - Volume: 60 Issue: 3 pages: 559-565
https://doi.org/10.24349/acarologia/20204385Original research
Keywords
Abstract
Introduction
Of all the mite species that are known to infest reptiles, one of the most problematic is the snake mite, Ophionyssus natricis (Gervais, 1844) because it is of animal and human health significance. Snakes and lizards infested by this mite may suffer from abnormal shedding (i.e. dysecdysis), and heavy infestation can cause anemia and even result in mortality (Frank, 1981; Lane and Mader, 1996; Šlapeta et al., 2018). This mite has also been identified as a possible vector of several blood-borne bacterial, filariid and viral pathogens of snakes (Wozniak and DeNardo, 2000), and has also been implicated in instances of dermatitis in humans (Schultz, 1975; Beck, 1996; Amanatfard et al., 2014).
The native range of Op. natricis is uncertain. Ophionyssus natricis was originally described from specimens taken from snakes in a zoo in Europe and was subsequently also collected from areas around Paris and Rome (Gervais, 1844). However, it has subsequently been reported in many parts of the world from captive snakes and lizards in zoo collections and the pet trade, and even a few instances from reptiles in the wild (Yunker, 1956; Goldberg and Bursey, 1991; Miranda et al., 2017), which indicates that it is easily spread via the movement of animals. In Australia Op. natricis is considered an introduced species and has been recorded in parts of coastal eastern and southern Australia (Domrow, 1988). Herein we report infestation by Op. natricis of three wild skinks (family: Scincidae) from three localities in South Australia, with comments on the distribution of this parasite in Australia based on museum specimens and published records.
Methods and results
Observations
Case 1 — On 19 September 2017, seven (four female and three male) sleepy lizards (Tiliqua rugosa) were collected from a study site surrounding the Bundey Lutheran Church ruins, ca. 130 km north-northeast of Adelaide City, in a pastoral district in the mid north region of South Australia. The habitat of this study site is a chenopod shrubland, dominated by the shrubs Atriplex vesicaria, Maireana pyramidata and Maireana sedifolia, with scattered stands of the tree Casuarina pauper. The lizards were collected as part of an ongoing study into the roles of personality and sociality in the spread of parasites in these lizards (Sih et al., 2018), and were kept individually overnight in sterilised calico bags. The following morning (20 September 2019) after the lizards had been released back into the wild, 14 tiny (ca. 1 mm in length) arthropods were collected from inside one of the fabric bags. The arthropods were preserved in 95% ethanol, and were later determined to be one larva of the ixodid tick Bothriocroton hydrosauri, four larvae of the argasid tick Ornithodoros gurneyi, and nine females of Op. natricis. In a subsequent follow-up investigation between 17 August 2019 and 18 November 2019, 26 T. rugosa were caught in the vicinity of the abovementioned study site and four were caught in the vicinity of an earth dam located ca. 3.5 km southwest of the study site. An additional three T. rugosa were caught in the outskirts of Eudunda, the nearest town, ca. 35 km southwest of the study site. The lizards were held within a plastic container and brushed with a fine paintbrush (Figure 1) prior to being released at the point of capture. The container was then rinsed out with 70% ethanol, that was poured through a fine (0.25 x 0.25 mm mesh) polyester fabric. The fabric was subsequently examined under a dissection microscope for the presence of Op. natricis. None of the examined lizards were found to be infested by snake mites except for one that was collected on 17 August 2019 inside a dried-up earth dam (Figure 2; S33.88845 E139.31065; datum WGS84) at the study site surrounding the Bundey Lutheran Church ruins. Four females of Op. natricis (Figure 3) were collected from the lizard, but when it was subsequently recaptured on 18 November 2019 less than 50 m from where it was initially captured (Figure 2), no Op. natricis were found. None of the other lizards were captured more than once. The Op. natricis specimens were deposited in the Australian National Insect Collection (ANIC), CSIRO, Canberra (voucher numbers ANIC 51-006454 to ANIC 51-006462).









Case 2 — On 12 June 2018, a juvenile eastern bluetongue lizard (Tiliqua scincoides) that had been injured by a domestic cat (Felis catus), was collected by a wildlife rescuer from the garden of a private residence on Beach Road in Hackham, a suburban neighborhood of Adelaide, and taken to a veterinarian. Several suspected mites were collected, preserved in 95% ethanol, and donated to us to determine the species. These mites were determined to be five females and two males of Op. natricis, which were also subsequently deposited in ANIC (females - ANIC 51-0006463 to ANIC 51-006467; males - ANIC 51-006468 and ANIC 51-006469)
Case 3 — On 04 August 2019, another T. scincoides that had been injured by a domestic cat, was collected by a wildlife rescuer from the garden of a private residence in Morphett Vale, a suburban neighborhood of Adelaide, and taken to a veterinarian. The lizard was suspected to be infested by Op. natricis and was treated accordingly. The kitchen tissue towels that were used as a substrate in the container the lizard was transported in were examined under a dissection microscope and one female of Op. natricis was found and subsequently deposited in ANIC (ANIC 51-006513)
Museum records and literature review
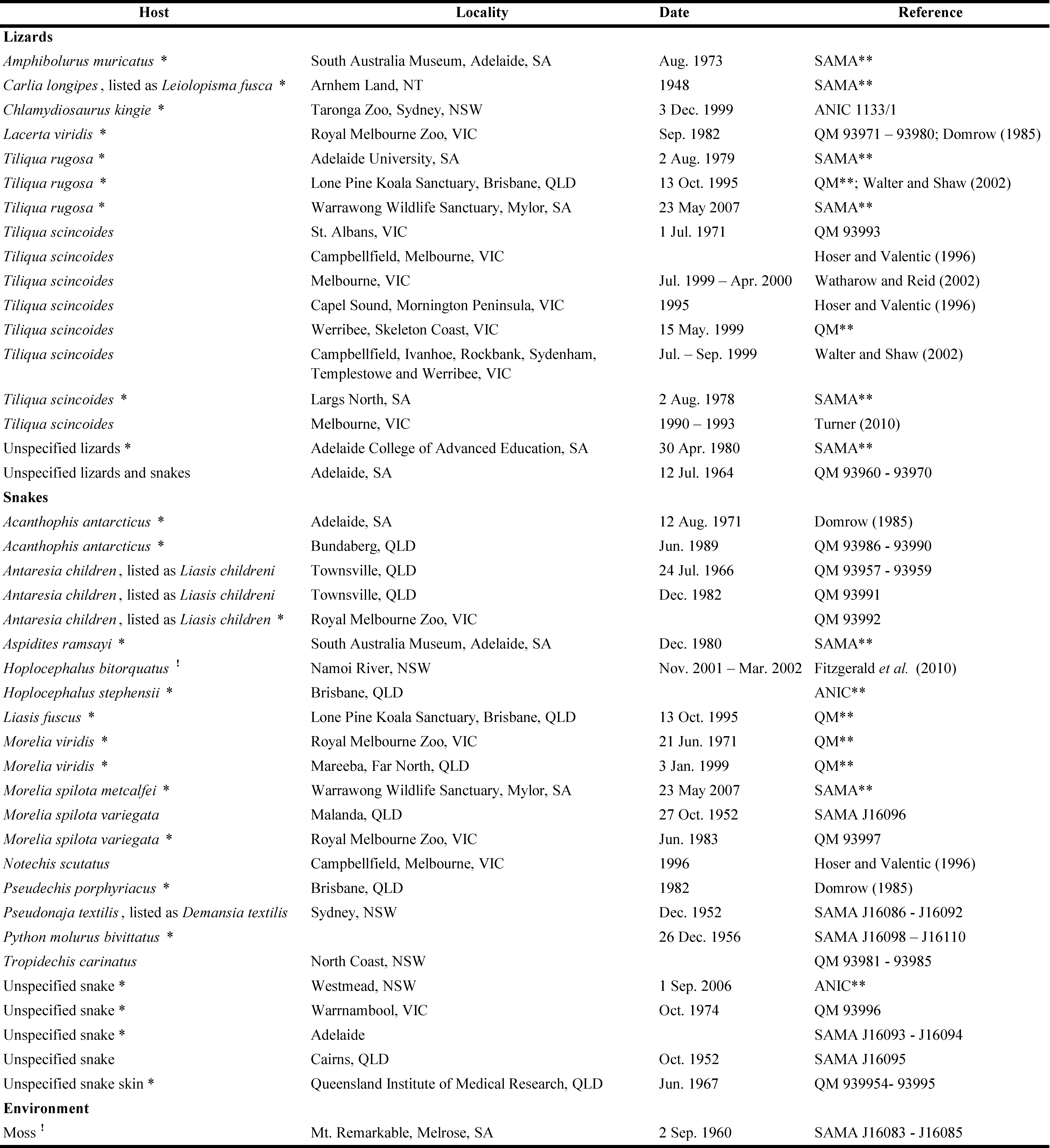
We found 42 records of Op. natricis specimens collected in Australia from the Northern Territory, Queensland, Victoria and South Australia (Table 1). Six lizard and 13 snake species have been reported as hosts of Op. natricis in Australia. Of the 17 records of lizards as hosts of this parasite, 52.9% were animals in captivity and of the 24 records of snakes as hosts, 62.5% were animals in captivity. One record was of mite that were collected from the environment.


Discussion
Our record of Op. natricis from the mid-north of South Australia shows that this parasite is present on wild populations of T. rugosa and that it has become naturalized in the wild in Australia. Ophionyssus natricis was first reported from Australia by Womersley (1956), on the basis of specimens from snakes at Sydney, New South Wales, and Malanda, Queensland. Domrow (1985) recorded it on the European green lizard, Lacerta viridis, in Melbourne, which suggests a possible pathway for the introduction of the mite into Australia. Domrow (1988) summarised previous records of Op. natricis on several species of snakes in Australia and pointed out that the records often came from hosts in captivity. Our summary of known hosts of this mite in Australia supports this conclusion (Table 1). In addition, most records of these mites on free-living reptiles involve animals that were found in the proximity of suburban areas, so the likelihood of these reptiles being escaped pets or that the mite was spread by escaped pets cannot be excluded. Wildlife Health Australia (2018) stated that ''there are no confirmed reports of snake mite in free-ranging reptiles in Australia''. The nearest human settlement to our study site in the mid north of South Australia is a small village (Robertstown, 2006 population 318), ca. 17 km away and the nearest town (Eudunda, 2006 population 640) is ca. 35 km away, so the presence of the mite in the study site is unlikely due to a close proximity to pet reptiles. In addition, the likelihood that the infested lizard was a released pet can be ruled out since the lizards we collected are marked with unique toe-clips and have been captured in consecutive years. Our records of Op. natricis from the mid-north of South Australia therefore shows that the parasite is present on wild populations of hosts. In addition, the specimens that were collected from moss on Mt. Remarkable near Melrose in South Australia (Table 1), and the instances of infestation of the endangered pale-headed snake (Hoplocephalus bitorquatus) in a pastoral area near Pilliga in New South Wales (Fitzgerald et al., 2010) indicates that these mite are present in the wild in other parts of southeastern and southern Australia, leading us to the conclusion the species has become naturalised.
Ophionyssus natricis can readily be accidentally introduced into new localities along with their hosts. The larvae of Op. natricis are fairly sedentary, while the protonymph, deutonymph and adult (male and female) life stages are more active (Camin, 1953). Only the protonymph and adult life stages are parasitic and, as they are photophobic, tend to attach under scales of the host (Camin, 1953; Wozniak and DeNardo, 2000). They can be easily overlooked, particularly if they are un-engorged. Case in point, the first record of Op. natricis in New Zealand was from dead juvenile eastern blue-tongued lizards that were obtained from Melbourne Zoo by Wellington Zoo in New Zealand (Heath, 1986). It is therefore imperative that wildlife rescuers in areas where this mite is present should take this into consideration when reptiles are relocated, and ideally, such reptiles should be treated for possible mite infestation prior to release.
Interestingly, our observations in the mid-north of South Australia were from a xeric environment, which indicates that the distribution of Op. natricis can extend beyond high rainfall parts of Australia. Ophionyssus natricis is generally considered sensitive to desiccation and eggs and larvae suffer high mortality in conditions with a relative humidity lower than 75% (Camin, 1953; Wozniak and DeNardo, 2000). The distribution in drier areas is of concern regarding the potential for spread of this invasive species.
Our findings also highlight the importance of continued monitoring of Op. natricis in the wild. During the 2017 field season it was noted that what appeared to be snake mite were quite common on sleepy lizards at the study site surrounding the Bundey Lutheran Church ruins (Sih 2017, personal observation). The study site typically experiences rainfall of less than 300 mm during late winter to early summer (August to December) (Kerr and Bull, 2004) and the only record of Op. natricis at the study site in 2019 was in mid-August when the study site was experiencing a severe drought, which lasted throughout the time the study was conducted. It is therefore possible that no other instances of mite infestation were found at the study site because the mite population in this area may have been drastically reduced or may even have died out due to desiccation stress. It is therefore recommended that follow up investigations be done during non-drought periods to verify the status and distribution of this snake mite population.
Herein we describe instances of Op. natricis parasitising skinks from South Australia and summarised the known hosts and distribution records of this mite in Australia. Our observation from the mid-north of South Australia is a new distribution record for Op. natricis in Australia and appears to be the first confirmed instance of these mite infesting a sleepy lizard in the wild. Additional surveys are needed to confirm the extent of the distribution of this mite in Australia, and we encourage the submittal of mite specimens for species verification and the reporting of the host species and localities where Op. natricis are found in Australia.
Disclosures
This study was partly funded by a National Science Foundation grant (DEB 1456730) to Andrew Sih and a grant from the Royal Society of South Australia to Gerrut Norval. Clearance for the research was granted by the Animal Welfare Committee of Flinders University (No.: E454/17) and the Department of Environment, Water and Natural Resources of the Government of South Australia (Permit No.: A23436-25 and A23436-27). The opinions expressed herein are those of the authors and are not to be construed as official or representing the views of the institutions they are affiliated to.
Acknowledgments
We thank Simon Adamczyk of Animal Relocation & Education for the mite specimens collected from the eastern blue-tongued lizards. We also express our gratitude to Dr. Matthew Shaw (South Australian Museum) and Dr. Owen Seeman (Queensland Museum) for information and access to the specimens in the collections under their management, and Grant Gully (College of Science and Engineering, Flinders University) for his assistance with the preparation of the micrograph of the snake mite.
References
Amanatfard E., Youssefi M.R., Barimani A. 2014. Human dermatitis caused by Ophionyssus natricis, a snake mite. Iranian Journal of Parasitology, 9: 594-596. http://ijpa.tums.ac.ir/index.php/ijpa/article/view/360 ![]()
Beck W. 1996. Tierische Milben als Epizoonoseerreger und ihre Bedeutung in der Dermatologie. Der Hautarzt, 47: 744-748. doi:10.1007/s001050050501 ![]()
Camin J.H. 1953. Observations on the life history and sensory behavior of the snake mite Ophionyssus natricis (Gervais) (Acarina: Macronyssidae). Chicago Academy of Sciences Special Publication, 10: 1-75. doi:10.1086/400476 ![]()
Domrow R. 1985. Species of Ophionyssus Mégnin from Australian lizards and snakes (Acari: Dermanyssidae). Australian Journal of Entomology, 24: 149-153. doi:10.1111/j.1440-6055.1985.tb00213.x ![]()
Domrow R. 1988. Acari Mesostigmata parasitic on Australian vertebrates: an annotated checklist, keys and bibliography. Invertebrate Taxonomy, 1: 817-948. doi:10.1071/IT9870817 ![]()
Fitzgerald M., Brian L., Shine R. 2010. Ecology and conservation of the pale-headed snake (Hoplocephalus bitorquatus, Elapidae). Australian Zoologist, 35: 283-290. doi:10.7882/AZ.2010.017 ![]()
Frank W. 1981. Ectoparasites. In: Cooper J.E., Jackson O.F., (Eds). Diseases of the Reptilia. London, UK: Academic Press Inc. (london) Ltd. p. 359-383.
Gervais P. 1844. Histoire Naturelle des Insectes. Aptères. Paris, France: Roret.
Goldberg S.R., Bursey C.R. 1991. Duration of attachment by mites and ticks on the iguanid lizards Sceloporus graciosus and Uta stansburiana. Journal of Wildlife Diseases, 27: 719-722. doi:10.7589/0090-3558-27.4.719 ![]()
Heath A.C.G. 1986. First occurrence of the reptile mite, Ophionyssus natricis (Acari: Dermanyssidae) in New Zealand. New Zealand Veterinary Journal, 34: 78. doi:10.1080/00480169.1986.35304 ![]()
Hoser R., Valentic R. 1996. Notes on a field trip to the Lower Mornington Peninsula with comments on the wider significance of some observations. Monitor, 8: 24-34.
Kerr G.D., Bull C.M. 2004. Field observations of extended locomotor activity at sub-optimal body temperatures in a diurnal heliothermic lizard (Tiliqua rugosa). Journal of Zoology, London, 264: 179-188. doi:10.1017/S0952836904005734 ![]()
Lane T.J., Mader D.R. 1996. Parasitology. In: Mader D.R., (Ed). Reptile Medicine and Surgery. Philadelphia, U.S.A.: W.B. Saunders Company. p. 185- 203.
Miranda R.J., Cleghorn J.E., Bermudez S.E., Perotti M.A. 2017. Occurrence of the mite Ophionyssus natricis (Acari: Macronyssidae) on captive snakes from Panama. Acarologia, 57: 365-368. doi:10.1051/acarologia/20164161 ![]()
Schultz H. 1975. Human infestation by Ophionyssus natricis snake mite. British Journal of Dermatology, 93: 695-697. doi:10.1111/j.1365-2133.1975.tb05120.x ![]()
Sih A., Spiegel O., Godfrey S., Leu S., Bull C.M. 2018. Integrating social networks, animal personalities, movement ecology and parasites: a framework with examples from a lizard. Animal Behaviour, 136: 195-205. doi:10.1016/j.anbehav.2017.09.008 ![]()
Šlapeta J., Modrý D., Johnson R. 2018. Reptile parasitology in health and disease. In: Doneley B., Monks D., Johnson R., Carmel B., (Eds). Reptile Medicine and Surgery in Clinical Practice. West Sussex, UK: John Wiley & Sons Ltd. p. 425-439. doi:10.1002/9781118977705.ch31 ![]()
Turner G.S. 2010. Natural history notes on the eastern blue-tongued skink Tiliqua scincoides scincoides from the basalt plains around Melbourne. The Victorian Naturalist, 127: 70-78. https://search.informit.com.au/documentSummary;dn=282048267549394;res=IELHSS;subject=Arts ![]()
Walter D.E., Shaw M. 2002. First record of the mite Hirstiella diolii Baker (Prostigmata: Pterygosomatidae) from Australia, with a review of mites found on Australian lizards. Australian Journal of Entomology, 41: 30-34. doi:10.1046/j.1440-6055.2002.00272.x ![]()
Watharow S., Reid A. 2002. The introduced snake mite Ophionyssus natricis on wild populations of eastern blue tongue lizards (Tiliqua scincoides). Herpetofauna, 32: 26-29.
Wildlife Health Australia. Snake mite (Ophionyssus natricis) [Internet]. Wildlife Health Australia; [cited]. Available from: https://wildlifehealthaustralia.com.au/FactSheets.aspx ![]()
Womersley H. 1956. On some new Acarina - Mesostigmata from Australia, New Zealand and New Guinea. Journal of the Linnean Society of London, Zoology, 42: 505-599. doi:10.1111/j.1096-3642.1956.tb02218.x ![]()
Wozniak E.J., DeNardo D.F. 2000. The biology, clinical significance and control of the common snake mite, Ophionyssus natricis, in captive reptiles. Journal of Herpetological Medicine and Surgery, 10: 4-10. doi:10.5818/1529-9651-10.3.4 ![]()
Yunker C.E. 1956. Studies on the snake mite, Ophionyssus natricis, in nature. Science, 124: 979-980. doi:10.1126/science.124.3229.979 ![]()



2020-02-28
Date accepted:
2020-07-21
Date published:
2020-07-23
Edited by:
Roy, Lise

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Norval, Gerrut; Halliday, Bruce; Sih, Andrew; Sharrad, Robert D. and Gardner, Michael G.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




