New records of phytoseiid mites from Mauritius Island (Acari: Mesostigmata)
Kreiter, Serge  1
and Abo-Shnaf, Reham I. A.
1
and Abo-Shnaf, Reham I. A.  2
2
1✉ Montpellier SupAgro, UMR CBGP INRA/ IRD/ CIRAD/ SupAgro, 755 Avenue du Campus Agropolis (Baillarguet), CS 30016, 34988 Montferrier-sur-Lez cedex, France.
2Plant Protection Research Institute (PPRI), Agricultural Research Centre (ARC), 7 Nadi El-Seid Street, Dokii, 12611 Giza, Egypt.
2020 - Volume: 60 Issue: 3 pages: 520-545
https://doi.org/10.24349/acarologia/20204382Original research
Keywords
Abstract
Introduction
Mites of the family Phytoseiidae are known for their predatory behaviors on phytophagous mites and small insects on cultivated plants and wild vegetation. Several species are used for the control of pest organisms in agricultural open fields and above all protected crops all around the world (McMurtry and Croft 1997; McMurtry et al. 2013). This family is widespread around the world, present on all inhabited continents and consists presently of 2,521 valid species of 94 genera belonging to three sub-families (Demite et al. 2020).
Biodiversity surveys in poorly investigated areas is still an urgent need and might resulted in the discovery of additional species potentially useful for biological control as well as having more information on the biodiversity of these areas (Kreiter et al. 2018a, b, c, 2020a, b, c).
Most of the Indian Ocean constitutes one of the world's biodiversity hotspots, a concept defined by Myers (1988) in order to identify the most immediately important areas for biodiversity conservation. The characteristics of these hotspots are to hold high endemism levels and have lost at least 70% of their original natural vegetation (Myers et al. 2000). Knowledge of the phytoseiid diversity in these areas may contribute to future establishment of conservation programs.
Located in the Indian Ocean at 1,000 km from the eastern coast of Madagascar, Mauritius is one of the three main islands constituting Mascareignes Archipelago, together with La Réunion and Rodrigues.
Only three phytoseiid species had been reported from this island for a long time (Moutia 1958, Schicha 1984, Demite et al. 2020), namely Amblyseius caudatus Berlese, Euseius ovalis (Evans) and Phytoseius coheni Swirski and Shechter. Kreiter et al. (2018a) had reported the occurrence of four additional species, namely Paraphytoseius orientalis (Narayanan, Kaur and Ghai), Phytoseiulus persimilis Athias-Henriot, Scapulaseius reptans (Blommers) and Typhlodromips culmulus (van der Merwe). Ferragut and Baumann (2019) had added eight additional species to these seven previous ones: Scapulaseius asiaticus (Evans), Amblyseius largoensis (Muma), A. passiflorae Blommers, A. tamatavensis Blommers, Transeius pungi Ferragut, Phytoseius haroldi Ueckermann and Kreiter, P. crinitus Swirski and Shechter and Typhlodromus (Anthoseius) recurvitremus Ferragut, bringing the number of recorded species for the Mauritius' fauna to 15.
The objective of this paper is to present the phytoseiid mites species reported in a new survey conducted in October and November 2018 in Mauritius.
Materials and methods
The survey took place in Mauritius at the end of October and beginning of November 2018. Plant inhabiting mites were collected from cultivated and wild plants in several locations of the island. Mites were directly collected on leaves with a fine brush or by beating the plants and collecting the mites in a black plastic rectangular saucer 45 x 30 cm (Ref. STR 45, BHR, 71370 Saint-Germain-du-Plain, France), depending on the plant investigated:
• large leaves of shrubs and trees with the direct collection method or by beating;
• very small leaves or spiny shrubs and trees with the beating method;
• and herbaceous plants with a brush.
Collected mites were then transferred with a brush into small plastic vials containing 1.5 ml of 70% ethanol. Mites were then all mounted on glass slides using Hoyer's medium and all identified using a phase and interferential contrast microscope (DMLB, Leica Microsystèmes SAS, Nanterre, France). Characters of specimens were measured using a graded ocular micrometre (Leica, see above).
Chant and McMurtry's (1994, 2007) concepts of the taxonomy of the family Phytoseiidae and the world catalogue database of Demite et al. (2014, 2020) were used for identification and for distribution and information on descriptions and re-descriptions, respectively. The setal nomenclature system adopted was that of Lindquist and Evans (1965) and Lindquist (1994) as adapted by Rowell et al. (1978) and Chant and Yoshida-Shaul (1992) for the dorsum and by Chant and Yoshida-Shaul (1991) for the venter. The notation for solenostomes and poroids is based on Athias-Henriot (1975). Numbers of teeth on the fixed and movable cheliceral digits do not include the respective apical teeth. Setae not referred to in the results section should be considered as absent. All measurements are given in micrometres (µm) and presented with the mean in bold followed by the range in parenthesis.
Specimens of each species are deposited in the mite collections of Montpellier SupAgro conserved in UMR CBGP INRA/IRD/CIRAD/SupAgro/University of Montpellier.
Specimens collected in fields in Mauritius within these surveys were all identified and only very few single males or immatures collected during this study are not taken into account.
The following abbreviations are used in this paper for morphological characters: dsl = dorsal shield length just under j1 to just below J5; dsw = dorsal shield width at the level of s4; Z4 ser., Z5 ser. = Z4, Z5 serrated (if Z4 and Z5 without ser. = not serrated); gensl = genital shield length; gensw post. cor. = genital shield width posteriorly; lisl = Largest inguinal sigilla (= ''metapodal plate'') length; lisw = Largest inguinal sigilla (= ''metapodal plate'') width; sisl = smallest inguinal sigilla (= ''metapodal plate'') length; sisw = smallest inguinal sigilla (= ''metapodal plate'') width; vsl = ventrianal shield length; gv3 - gv3 = distance between solenostomes gv3 on the ventrianal shield; vsw ZV2 & vsw anus = ventrianal shield width at ZV2 level and at paranal setae level; scl.: calyx length; scw = calyx widest width; Fdl = fixed digit length; Mdl = movable digit length; Nb teeth Fd = number of teeth on the fixed digit; Nb teeth Md = number of teeth on the movable digit; Shaft = length of the shaft of spermatodactyl; toe = length of the toe; BCA = Biological control agent; aasl = altitude above sea level.
The following abbreviations are used in this paper for institutions: CBGP = Centre de Biologie pour la Gestion des Populations; CIRAD = Centre International de Recherche Agronomique pour le Développement; INRA = Institut National de la Recherche Agronomique; IRD = Institut de Recherche pour le Développement; MSA = Montpellier SupAgro, France; UMR = Unité Mixte de Recherche.
Results and discussion
We have collected a total of 22 species, 21 being presented thereafter (a new species will be described in a following paper).
Subfamily Amblyseiinae Muma
Amblyseiinae Muma 1961: 273.
Tribe Neoseiulini Chant & McMurtry
Neoseiulini Chant & McMurtry 2003a: 6
Genus Neoseiulus Hughes
Neoseiulus Hughes 1948: 141.
Neoseiulus houstoni Schicha
Neoseiulus houstoni, Schicha 1987: 111; Chant & McMurtry 2003a: 23; Moraes et al. 2004: 123.
Neoseiulus recifensis Gondim Jr. & Moraes 2001: 77 (synonymy according to Kreiter et al. 2020c).
Neoseiulus barreti Kreiter in Furtado et al. 2005: 135 (synonymy according to Kreiter et al. 2020c).
This species belongs to the cucumeris species group of Neoseiulus. It was collected on Vigna unguiculata (L.) Walpers in Queensland, Australia and described by Schicha (1987). Then described a long time after under two different species names, N. recifensis Gondim Jr and Moraes and N. barreti Kreiter. Kreiter et al. (2020c) had put those two species as junior synonyms of N. houstoni and described for the first time the male of N. houstoni. Biology of this species remains totally unknown and it is the first record from Mauritius.
World distribution: Australia, Brazil, Reunion Island.
Specimens examined: 2 ♀♀ in total. Pamplemousse, Botanical Garden (altitude above sea level = aasl 41 m, lat. 20°06'24'' S, long. 57°34'49'' E), 1 ♀ on Acacia glauca Willdenow (Fabaceae), 3/XI/2018; Bel Ombre, Resorts (aasl 11 m, lat. 20°06'24'' S, long. 57°34'49'' E), 1 ♀ on Thespesia populneoides (L.) Solander ex Corrêa (Malvaceae), 5/XI/2018.
Remarks: morphological and morphometric characters and all measurements fit well measurements in Kreiter et al. (2020c). This species was described from Australia, but recorded also in Brazil and was first mentioned in the Indian Ocean from La Réunion Island, an Island distant of only 225 km from Mauritius. Several species were shared by the two Islands and probably by many others.
Tribe Kampimodromini Kolodochka
Kampimodromini Kolodochka 1998: 59; Chant & McMurtry 2003b: 189, 2006: 137, 2007: 33.
Subtribe Paraphytoseiina Chant & McMurtry
Paraphytoseiina Chant & McMurtry 2003b: 211.
Genus Paraphytoseius Swirskii & Shechter
Paraphytoseius Swirski & Shechter 1961: 113; Moraes et al. 1986: 104, 2004: 160; Chant & McMurtry 2003b: 216, 2007: 49.
Paraphytoseius horrifer (Pritchard & Baker)
Amblyseius (Ptenoseius) horrifer Pritchard & Baker 1962: 295.
Amblyseius horrifer, Meyer & Rodrigues 1966: 30.
Amblyseius (Paraphytoseius)* horrifer, van der Merwe 1968: 169.
Proprioseius (Paraphytoseius) horrifer, Karg 1983: 302.
Paraphytoseius horrifer, Moraes et al. 1986: 105, 2004: 152; Beard 2001: 84; Chant & McMurtry 2003a: 37, 2007: 53.
In our specimens of this genus, setae S5 are absent. So accordingly, with Chant and McMurtry (2003b) all specimens belong to the orientalis species group. Accordingly, with these previous authors, and with Moraes et al. (2007), we consider that P. horrifer and P. orientalis are different valid species. Our specimens with longer setae s4, Z4, Z5, and lacking a distinctly short, thick, spatulate macroseta on genu I belongs to the former species. Paraphytoseius horrifer is widely distributed in Sub-Saharan Africa and Madagascar. Its biology remains totally unknown and it is the first record from Mauritius.
World distribution: Benin, DR Congo, Ghana, India, Kenya, La Réunion Island, Madagascar Island, Malawi, Mozambique, Senegal, South Africa, Uganda.
Specimens examined: 10 ♀♀ in total. Curepipe, Anderson street (aasl 540 m, lat. 20°19'11'' S, long. 57°31'52'' E), 1 ♀ on Litsea monopetala (Roxburgh) Person and 1 ♀ on Litsea glutinosa (Loureiro) C.B. Robinson (Lauraceae), 27/X/2018; Côte d'Or, Bridge (aasl 443 m, lat. 20°15'26'' S, long. 57°32'21'' E), 1 ♀ on Tristemma mauritianum J.F. Gmelin (Melastomataceae), 28/X/2018; Curepipe, Botanical Garden (aasl 540 m, lat. 20°19'28'' S, long. 57°30'50'' E), 6 ♀♀ on Rubus alceifolius Poiret (Rosaceae) and 1 ♀ on Clidemia hirta (L.) D. Don (Melastomataceae), 29/X/2018.
Remarks: morphological and morphometric characters and all measurements fit well measurements in Kreiter et al. (2020b, c). This species was described from Africa (Pritchard and Baker 1962), but recorded also in Vietnam (Kreiter et al. 2020b) and was first mentioned in the Indian Ocean from La Réunion Island by Kreiter et al. (2020c), an Island distant of only 225 km from Mauritius with several species shared by the two Islands (this study and Kreiter et al. 2020c).
Paraphytoseius orientalis (Narayanan, Kaur & Ghai)
Typhlodromus (Amblyseius) orientalis Narayanan, Kaur & Ghai 1960: 394.
Paraphytoseius orientalis, Moraes et al. 1986: 105, 2004: 162; Chant & McMurtry 2003b: 220, 2007: 53.
Amblyseius ipomeai Narayanan, Kaur & Ghai 1960: 394 (synonymy according to El-Banhawy 1984).
Paraphytoseius multidentatus Swirski & Shechter 1961: 114 (synonymy according to Matthysse & Denmark 1981 in Denmark et al. 1999).
Paraphytoseius narayanani Ehara 1967: 67 (synonymy according to Ehara & Ghai, in Ehara 1967).
This species belongs to the genus Paraphytoseius and to the orientalis species group (Chant and McMurtry 2003b). Our specimens with relatively shorter setae s4, Z4 and Z5, having a distinctly short, thick, spatulate macroseta on genu I is belonging to the species P. orientalis. It is widely distributed in tropical and subtropical areas in South America, Africa and Asia. It belongs to a genus included in the large polyphagous generalist group named type III phytoseiid mites (McMurtry and Croft 1997; McMurtry et al. 2013). Navasero and Navasero (2016) had studied the life history of P. orientalis on the broad mite (Polyphagotarsonemus latus) (Banks) as prey and reported high predation rates on the eggs of P. latus, suggesting good potential for the control of this pest. Paraphytoseius orientalis had been collected before in Mauritius (Kreiter et al. 2018a; Ferragut and Baumann 2019).
World distribution: Argentina, Brazil, Burundi, India, Japan, Kenya, La Réunion Island, Madagascar Island, Martinique Island, Mauritius Island, Mozambique, Rwanda.
Specimens examined: 20 ♀♀ and 3 ♂♂ in total. Rivière des Anguilles, Bridge (aasl 158 m, lat. 20°28'49'' S, long. 57°33'37'' E), 1 ♀ on Solanum mauritianum Scopoli (Solanaceae), 31/X/2018; Réserve Nationale de Pétrins, Chemin de Maccabée (aasl 676 m, lat. 20°29'26'' S, long. 57°33'31'' E), 19 ♀♀ and 3 ♂♂ on Urena lobata L. (Malvaceae), 6/XI/2018.
Remarks: this species was reported before by Kreiter et al. (2018a), but with only one female collected. Ferragut and Baumann (2019) had also reported P. orientalis, but with a lot of specimens. Morphological and morphometric characters and all measurements fit well measurements in Ferragut and Baumann (2019) and Kreiter et al. (2020b, c). This species was described from Asia (Narayanan et al. 1960) and recently recorded from Vietnam (Kreiter et al. 2020b), and also in La Réunion Island (Kreiter et al. 2020c). It seems more common than P. horrifer in Mauritius Island.
Tribe Typhlodromipsini Chant & McMurtry
Typhlodromipsini Chant & McMurtry 2005c: 318.
Genus Scapulaseius Karg & Oomen-Kalsbeek
Scapulaseius Karg & Oomen-Kalsbeek 1987: 132; Chant & McMurtry 2005c: 331, 2007: 65.
Scapulaseius asiaticus (Evans)
Typhlodromus asiaticus Evans 1953: 461.
Typhlodromus (Amblyseius) asiaticus, Chant 1959: 80.
Amblyseius (Typhlodromopsis) asiaticus, Muma 1961: 289.
Amblyseius (Amblyseius) asiaticus, Ehara 1966: 20; Ehara & Bhandhufalck 1977: 58.
Amblyseius asiaticus, Carmona 1968: 280; Gupta, 1975: 32.
Amblyseius (Neoseiulus) asiaticus, Ehara 2002: 127.
Typhlodromips asiaticus, Moraes et al. 2004: 207.
Scapulaseius asiaticus, Chant & McMurtry 2005b: 335, 2007: 67.
Scapulaseius linearis Corpuz & Rimando 1966: 125 (synonymy according to Schicha & Corpuz-Raros 1992).
Scapulaseius siaki Ehara & Lee 1971: 64 (synonymy according to Ehara & Bhandhufalck 1977).
Species of this genus are supposed to be of type III (McMurtry and Croft 1997; McMurtry et al. 2013), i.e., a polyphagous generalist predator. However, the biology of S. asiaticus remains totally unknown. This species had been collected before in Mauritius (Kreiter et al. 2018a; Ferragut and Baumann 2019).
World distribution: Angola, China, Cyprus, Hong Kong, India, Indonesia, Malaysia, Philippines, Singapore, Sri Lanka, Thailand, Vietnam.
Specimens examined: 27 ♀♀ and 5 ♂♂ in total. Côte d'Or, Village (aasl 443 m, lat. 20°15'26'' S, long. 57°32'21'' E), 2 ♀♀ on Clibadium surinamense L. (Asteraceae), 28/X/2018; Curepipe, Trou aux cerfs (aasl 593 m, lat. 20°19'04'' S, long. 57°30'47'' E), 1 ♀ on Rubus apetalus Poiret (Rosaceae), 29/X/2018; Mare aux Vacoas (aasl 572 m, lat. 20°21'40'' S, long. 57°29'59'' E), 11 ♀♀ on Tibouchina heteromalla Cogniaux (Melastomataceae) and 2 ♀♀ on Litsea monopetala (Roxburgh) Person (Lauraceae), 30/X/2018; Quartier Militaire (aasl 472 m, lat. 20°19'11'' S, long. 57°36'05'' E), 1 ♀ on Clidemia hirta (L.) D. Don (Melastomataceae), 1/XI/2018; Curepipe, Bld Pasteur (aasl 510 m, lat. 20°19'21'' S, long. 57°31'45'' E), 1 ♀ on Ageratum conyzoides L. (Asteraceae), 4/XI/2018; Curepipe, Anderson street (aasl 560 m, lat. 20°19'11'' S, long. 57°31'52'' E), 5 ♀♀ and 3 ♂♂ on Erigeron canadensis (L.) Cronquist (Asteraceae) and 3 ♀♀ and 2 ♂♂ on Sonchus oleraceus L. (Asteraceae), 4/XI/2018; Mare aux Vacoas (aasl 581 m, lat. 20°22'05'' S, long. 57°29'31'' E), 1 ♀♀ on Ludwigia octovalvis (Jacquemin) P.H.Raven (Onagraceae), 5/XI/2018.
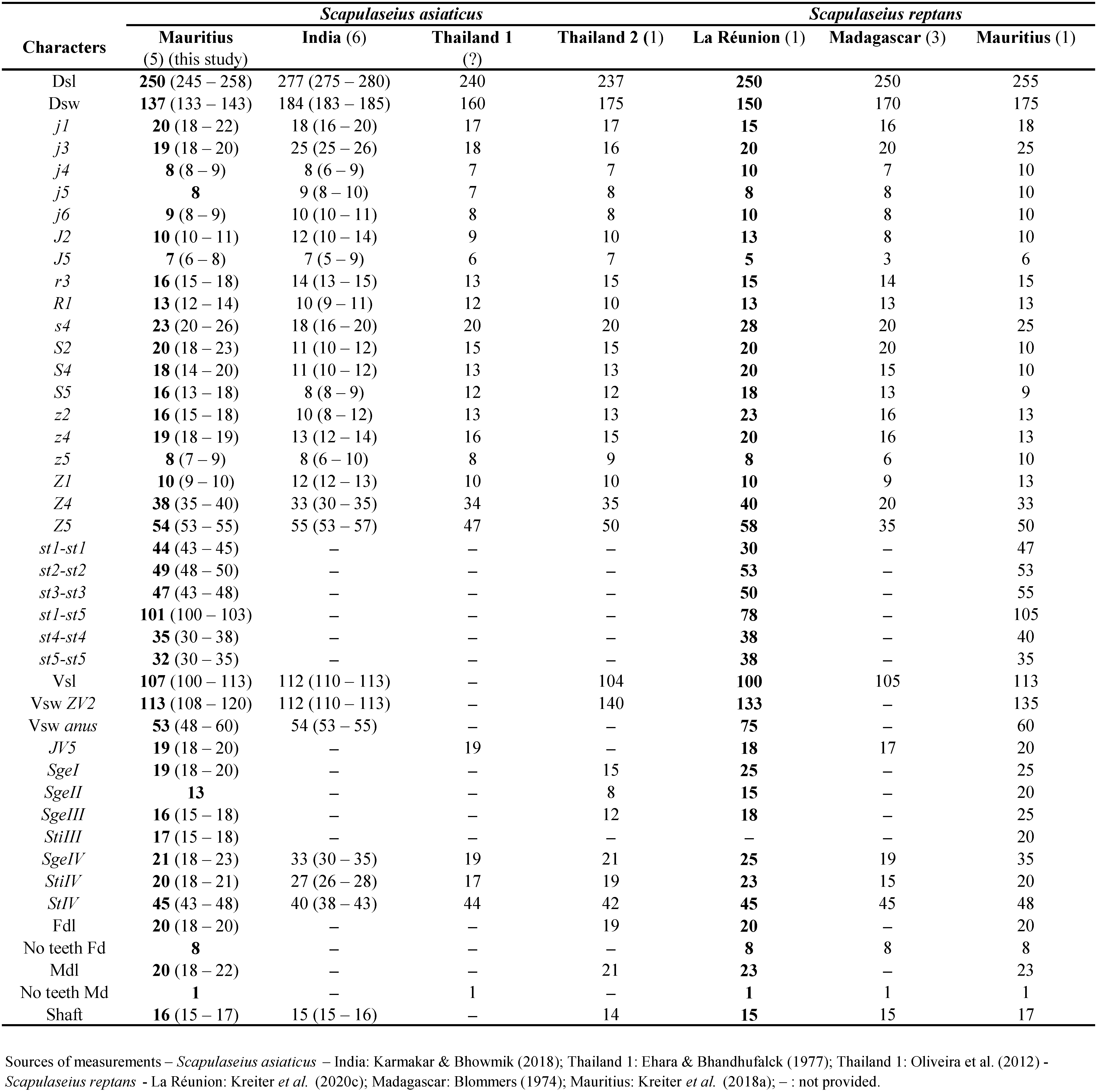
Remarks: Scapulaseius asiaticus was described by Evans (1953) under the name Typhlodromus asiaticus from specimens collected in Java island, Indonesia. The closely related Scapulaseius reptans (Blommers) was described by Blommers in 1974 from Madagascar in Tamatave from specimens collected on Psidium guajava L.
Character measurements of 12 of the 27 females (Table 1) and of the five males (Table 2) collected in Mauritius agree very well with those obtained from specimens of S. asiaticus or S. reptans. We consider so far that our specimens can be anyone of the two species and that examination of the specimens collected in this study can lead to anyone of the two species. Consequently, the morphometrics strongly suggest synonymy.
There are however some discrepancies between our measurements and observations and previous descriptions of the two species. In the two descriptions:
• dorsal shield is reticulated in the description of S. reptans in the anterior lateral margins and on all the posterior part of the dorsal shield except the centre. This was not illustrated in the original description of S. asiaticus by Evans (1953), but was illustrated by Ehara and Bhandhulfalck (1977) and by Ferragut and Baumann (2019);
• Ehara and Bhandhulfalck (1977) pointed out that the seta R1 is inserted on a lateral projection of the dorsal shield, trait taken back also by Ferragut and Baumann (2019). Scapulaseius reptans is morphologically very close to S. asiaticus, but with setae R1 located off the dorsal shield in the description of Blommers (1974). Taking this trait into consideration as an apomorphic character, Chant and McMurtry (2005c) placed the two species within different groups, S. asiaticus in the asiaticus species group characterized by having R1 on the dorsal shield and S. reptans in the ficilocus species group with species bearing R1 on the lateral integument. However, in S. asiaticus the position of this seta is variable even among individuals of the same population. Ehara and Bhandhufalck (1977) were the first to mention this variability. Ferragut and Baumann (2019) had examined 19 females from Mauritius: eleven females (58%) have both R1 setae on the dorsal shield, four females (26%) have one setae of the pair on a lateral projection of the shield and the other on the soft integument, and three females (16%) have both setae R1 on the lateral integument. If the majority of specimens have both or one seta on the dorsal shield, 16% is not a negligible proportion;
• a peculiar trait in S. asiaticus not mentioned by previous authors and especially by Blommers (1974) for S. reptans is the position of the dorsal solenostome gd3. Ferragut and Baumann (2019) stated that while in females of the family Phytoseiidae, this pore-like structure is usually placed on the peritremal plate, in S. asiaticus, it is on the lateral integument, between the peritremal and dorsal shields, posterior to setae r3 and close to the margin of dorsal shield.
Our examination of the material collected in Mauritius (this study) and Vietnam (Kreiter et al 2020b) and identified as S. asiaticus and of the material collected in Mauritius (Kreiter et al. 2018a) and in La Réunion (Kreiter et al. 2020c) and identified as S. reptans along with the original descriptions of S. asiaticus and S. reptans shows:
• Re-examination of our specimens from La Réunion (Kreiter et al. 2020c) and of Mauritius (Kreiter et al. 2018a) show that the dorsal shields of the two species present exactly the same reticulation as drawn by Bommers (1974) for the description of S. reptans and in Ehara and Bhandhulfalck (1977) for the redescription of S. asiaticus;
• In our 27 specimens females of Mauritius (this study), we have 21 females out of 27 (77.8%) with R1 on the dorsal shield, four females / 27 (14.8%) with one of these setae on and the other one off shield and two females / 27 (7.4%) with setae R1 both off shield, compared to 58, 26 and 16% for Ferragut and Baumann (2019), respectively. In specimens from Vietnam (Kreiter et al. 2020b), we had four females out of seven (57.1%) with R1 on the dorsal shield, only one female / seven (14.3%) with one of these setae on and the other one off shield and two females / seven (28.6%) with setae R1 both off shield. In the two specimens from Maurice (Kreiter et al. 2018a), both specimens have R1 on the dorsal shield. And in the two female specimens from La Réunion (Kreiter et al. 2020c), one specimen has both R1 on the dorsal shield, but the other specimens have one seta on and the other one off the dorsal shield.
• solenostomes gv3 are on integument in all our specimens from Mauritius (this study), from Vietnam (Kreiter et al. 2020b), but also on those from Mauritius (Kreiter et al. 2018a) and La Réunion (Kreiter et al. 2020c) previously identified as S. reptans.
Given this variability in S. asiaticus, we agree with Ferragut and Baumann (2019) that the taxonomic status of S. reptans is uncertain, because the holotype used to describe the latter species could represent, in fact, a female of S. asiaticus with setae R1 outside their most common position. Setal measurements and other morphological features of the specimens collected in Mauritius agree well with both, with those of the original description and subsequent redescriptions of S. asiaticus by Ehara and Bhandhufalck (1977), Moraes et al. (2004), Oliveira et al. (2012), Karmakar and Bhowmik (2018) and Ferragut and Baumann (2019); as well as with the morphological data provided in the original description of S. reptans, and the redescriptions given by Kreiter et al. (2018a, 2020c).
Considering all this information, we can conclude that our specimens from Mauritius (this study and Kreiter et al. 2018a) and from La Réunion (Kreiter et al. 2020c) must be all identified as S. asiaticus. Previous specimens collected in La Réunion Island (Kreiter et al. 2020c) and in Mauritius Island (Kreiter et al. 2018a) and previously identified as S. reptans are consequently belonging all to S. asiaticus. Consequently, this is the third report of that species in Mauritius Island after Kreiter et al. (2018a) and Ferragut and Baumann (2019).
Unfortunately, the confinement during the Covid-19 pandemic has not given us the possibility to borrow type materials of both species in order to compare specimens of the two species. Despite this fact, we strongly suspect just like Ferragut and Baumann (2019) that S. reptans is a junior synonym of S. asiaticus.





Tribe Amblyseiini Muma
Amblyseiinae Muma 1961: 273..
Amblyseiini Muma, Wainstein 1962: 26.
Subtribe Amblyseiina Muma
Amblyseiina Muma, Chant & McMurtry 2004: 179.
Genus Amblyseius Berlese
Amblyseius Berlese 1914: 143.
Amblyseius haleakalus Prasad
Amblyseius haleakalus Prasad 1968: 1516; Moraes et al. 1986: 14, 2004: 27; Denmark & Muma 1989: 97; Chant & McMurtry 2004: 199, 2007: 78; Denmark & Evans 2011: 75.
This species belongs to the obtusus species group as seta z4 is minute and female ventral shield is not vase-shaped or divided. It belongs to the andersoni species subgroup as the spermatheca has a differentiated atrium, a calyx not dotted or annulated, not swollen basally and calyx dish-, cup-, bell- or V-shaped. The biology is totally unknown.
World distribution: Hawaii.
Specimens examined: 8 ♀♀ and 1 ♂ in total. Curepipe, Anderson street (aasl 560 m, lat. 20°19'11'' S, long. 57°31'52'' E), 8 ♀♀ and 1 ♂ on Araucaria columnaris (Forster) Hook (Araucariaceae), 4/XI/2018.
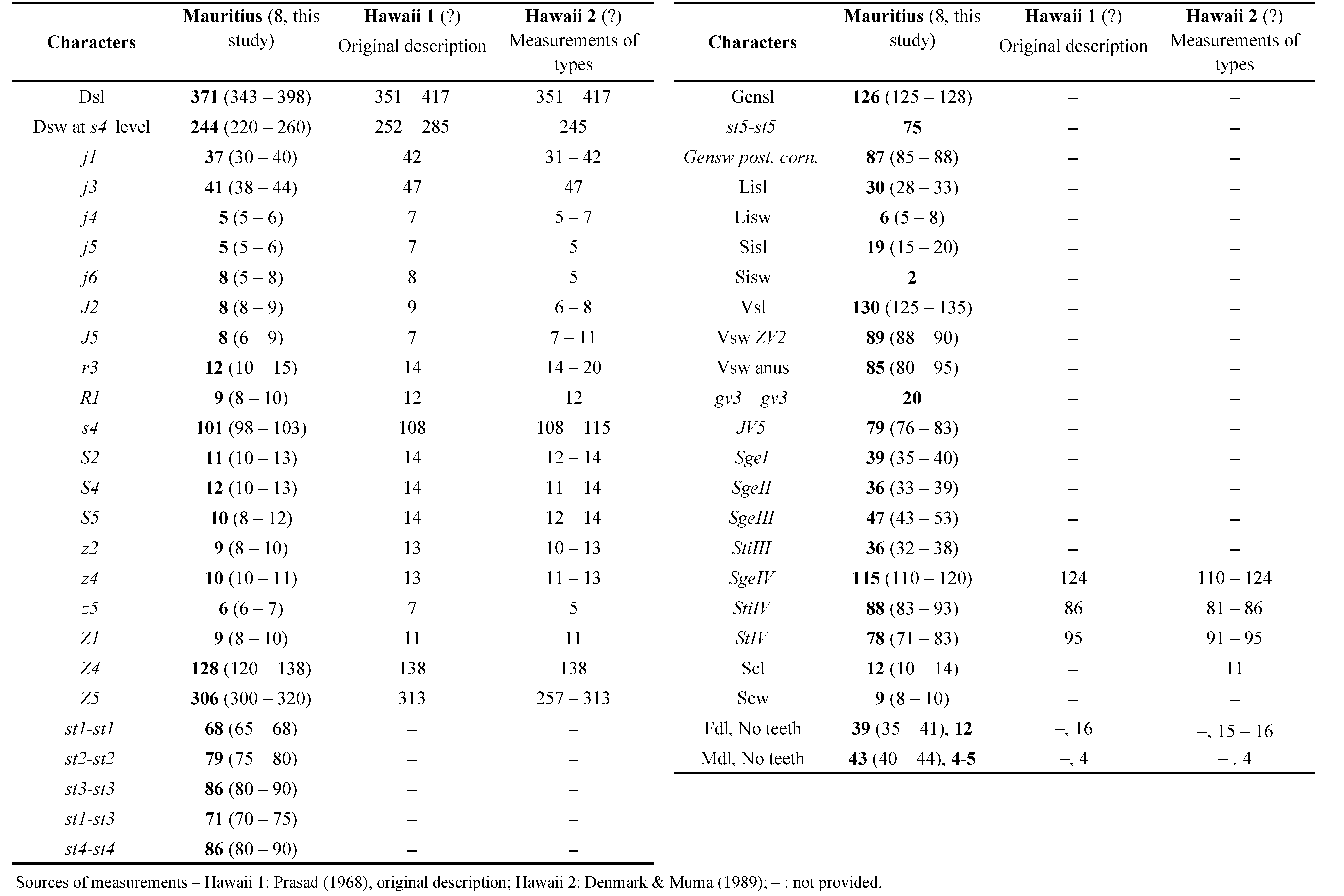
Remarks: this is the first mention of this species from Indian Ocean and in an area outside the land from which the species was described, Hawaii. The measurements of specimens collected in Mauritius Island (Table 3) are very close to those obtained from specimens of Hawaii by Prasad (1968) in the original description and by Denmark and Muma (1989) in the only available redescription of the type material, except for general slightly shorter dimensions of characters and especially of setae j3 and s4 and of the macroseta StIV and lower number of teeth in both movable and fixed digits of the chelicera.
The male of A. haleakalus is unknown and the description of the single male specimen collected will be provided in another article (Kreiter and Abo-Shnaf, in progress).


Amblyseius herbicolus (Chant)
Typhlodromus (Amblyseius) herbicolus Chant 1959: 84.
Amblyseius (Amblyseius) herbicolus, Muma 1961: 287.
Typhlodromus herbicolus, Hirschmann 1962: 23.
Amblyseius herbicolus, Moraes et al. 1986: 14, 1989: 79, 2004: 27; Chant & McMurtry 2004: 208, 2007: 78.
Amblyseius impactus Chaudhri 1968: 553 (synonymy according to Daneshvar & Denmark 1982; Denmark & Muma 1989).
Typhlodromus (Amblyseius) amitae Bhattacharyya 1968: 677 (synonymy according to Denmark & Muma 1989).
Amblyseius deleoni Muma & Denmark 1970: 68 (synonymy according to Daneshvar & Denmark 1982; Denmark & Muma 1989).
Amblyseius giganticus Gupta 1981: 33 (synonymy according to Gupta 1986).
Amblyseius (Amblyseialus) thermophilus Karg 1991: 12 (synonymy according to El-Banhawy & Knapp 2011).
This species belongs to the largoensis species group as setae J2 and Z1 are present, setae s4 are minute and the ventrianal shield of the female is vase-shaped. It belongs to the largoensis species subgroup as setae Z4 are long, spermatheca has the calyx elongate and the female ventrianal shield is entire (Chant and McMurtry 2004). Amblyseius herbicolus is widespread in all tropical and subtropical regions of the world. It is the second most abundant phytoseiid mites on Coffea arabica L. in Brazil, associated with Brevipalpus phoenicis (Geijskes), vector of the coffee ring spot virus and it was found to be an efficient predator (Reis et al. 2007). Amblyseius herbicolus is also found associated with the broad mite, P. latus in crops such as chili pepper (Capsicum annuum L.) in Brazil and has also a good potential for controlling the pest. Rodriguez-Cruz et al. (2013) had studied biological, reproductive and life table parameters of A. herbicolus on three different diets: broad mites, castor bean pollen (Ricinus communis L.) and sun hemp pollen (Crotalaria juncea L.). The predator was able to develop and reproduce on all these three diets. However, its intrinsic growth rate was higher on broad mites and castor bean pollen. Feeding on alternative food such as pollen can facilitate the predator's mass rearing and maintain its population on crops when prey is absent or scarce. Many polyphagous generalist phytoseiid mites are important natural enemies because they can feed on plant provided pollen and various prey species, and thus persist in crops even in the absence of target pests (McMurtry et al. 2013). Hence, populations of these predators can be established in a crop by providing alternative food, thus increasing biological control. Alternative food affects P. latus control on chilli pepper plants by predatory mites (Duarte et al. 2015). Amblyseius herbicolus had high oviposition and population growth rates when fed with cattail pollen (Typha latifolia L.), chilli pepper pollen and bee-collected pollen, and a low rate on the alternative prey (Tetranychus urticae Koch). Supplementing pepper plants with pollen resulted in better control of broad mite populations (Duarte et al. 2015). Release of A. herbicolus on young plants with weekly addition of honeybee pollen or cattail pollen until plants produce flowers seems a viable strategy to sustain populations of this predator (Duarte et al. 2015). Amblyseius herbicolus was previously recorded in Comoros Archipelago (Kreiter et al. 2018b) and in La Réunion (Quilici et al. 1997, 2000; Kreiter et al. 2020c), but this is the first mention from Mauritius despite the fact that with 115 female specimens collected, this is one of the more common species.
World distribution: Argentina, Australia, Azores, Benin, Brazil, Burundi, Canary Islands, China, Colombia, Grande Comore Island, Costa Rica, Dominican Republic, Dr Congo, El Salvador, Ghana, Guadeloupe Island, Guatemala, Hawaii, Honduras, India, Iran, Kenya, Les Saintes Island, La Réunion Island, Madagascar Island, Malawi, Malaysia, Martinique Island, New Caledonia Island, Papua New Guinea, Peru, Philippines, Portugal, Puerto Rico, Rwanda, Senegal, Singapore, South Africa, Spain, Taiwan, Thailand, Turkey, USA, Venezuela, West Indies.
Specimens examined: 116 ♀♀ and 3 im. in total. Curepipe, Anderson street (aasl 560 m, lat. 20°19'11'' S, long. 57°31'52'' E), 1 ♀ on Codiaeum variegatum (L.) Jussieu (Euphorbiaceae) and 8 ♀♀ on Cleome viscosa L. (Cleomaceae), 27/X/2018; Curepipe, La Marie (aasl 600 m, lat. 20°19'02'' S, long. 57°31'36'' E), 4 ♀♀ on Clidemia hirta (L.) D. Don (Melastomataceae), 27/X/2020; Curepipe, Usine à thé (aasl 557 m, lat. 20°18'54'' S, long. 57°31'29'' E), 1 ♀ on Antigonon leptopus Hooker and Arnott (Polygonaceae) and 2 ♀♀ on Hibiscus genevii Bojer ex Hook. (Malvaceae), 27/X/2020; Curepipe, Botanical Garden (aasl 540 m, lat. 20°19'28'' S, long. 57°30'50'' E), 11 ♀♀ on C. hirta, 6 ♀♀ on Syzygium jambos (L.) Alston (Myrtaceae), 2 ♀♀ on Impatiens flaccida Arnott (Balsaminaceae), 1 ♀ on Vernicia montana Loureiro (Euphorbiaceae) and 1 ♀ on Pachira glabra Aublet (Malvaceae), 29/X/2018; Curepipe, Trou aux cerfs (aasl 593 m, lat. 20°19'04'' S, long. 57°30'47'' E), 1 ♀ and 2 im. on Pinus massoniana D. Don in Lambert (Pinaceae), 29/X/2018; Mare aux Vacoas (aasl 572 m, lat. 20°21'40'' S, long. 57°29'59'' E), 1 ♀ on Litsea monopetala (Roxburgh) Person (Lauraceae), 30/X/2018; Mare aux Vacoas (aasl 589 m, lat. 20°22'36'' S, long. 57°29'10'' E), 4 ♀♀ on Cyathea excelsa Swartz (Cyatheaceae), 30/X/2018; Piton Grand Bassin, Carrefour Chamarel (aasl 665 m, lat. 20°24'30'' S, long. 57°28'24'' E), 1 ♀ on Callistemon citrinus (William Curtis) Homer Collar Skeels (Myrtaceae), 1 ♀ on Boehmeria penduliflora Weddell ex D.G.Long (Urticaceae) and 1 ♀ on Pinus eliottii Engelmann (Pinaceae), 30/X/2018; Bassin Blanc (aasl 665 m, lat. 20°24'30'' S, long. 57°28'24'' E), 16 ♀♀ on C. hirta, 30/X/2018; Nouvelle-France (aasl 442 m, lat. 20°22'34'' S, long. 57°35'58'' E), 7 ♀♀ on Camelia sinensis (L.) Kuntze (Theaceae), 31/X/2018; Quartier Militaire (aasl 472 m, lat. 20°19'11'' S, long. 57°36'05'' E), 1 ♀ on C. hirta, 1/XI/2018; Curepipe, Morcellement de Séneville (aasl 534 m, lat. 20°19'05'' S, long. 57°32'17'' E), 6 ♀♀ on Ocimum gratissimum L. (Lamiaceae), 2 ♀♀ on Brillantaisia owariensis Beauvois (Acanthaceae) and 1 ♀ on Persea americana Miller (Lauraceae), 2/XI/2018; Curepipe, Bld Pasteur (aasl 510 m, lat. 20°19'21'' S, long. 57°31'45'' E), 2 ♀♀ on Acalypha hispida Burman (Euphobiaceae), 8 ♀♀ on Ageratum conozoides L. (Asteraceae), 2 ♀♀ on Strobilanthes hamiltonianus (Steudel) Bosser et Heine (Acanthaceae), 8 ♀♀ on Duranta erecta L. (Verbenaceae) and 1 ♀ on Terminalia mantaly Perrier (Combretaceae), 4/XI/2018; Curepipe, Domaine Les Aubineaux (aasl 560 m, lat. 20°19'21'' S, long. 57°31'45'' E), 1 ♀ on S. jambos, 4 ♀♀ and 1 im. on Thumbergia sp. (Acanthaceae) and 10 ♀♀ on Cupressus sempervirens L. (Cupressaceae), 4/XI/2018; Mare aux Vacoas (aasl 581 m, lat. 20°21'05'' S, long. 57°29'31'' E), 1 ♀ on Ludwigia octovalvis (Jacquemin) P.H.Raven (Onagraceae), 30/X/2018.
Remarks: this species was reported before by Kreiter et al. (2018b) in the Grande Comore Island of the Comoros Archipelago in the Indian Ocean with two females collected. Curiously, Ferragut and Baumann (2019) had not reported it from Mauritius in their study despite the fact that it seems to be the more common species in Mauritius Island and at least the more numerous species after our study. It is interesting to notice that, despite the large number of female specimens, no male was collected just like in La Réunion Island (Kreiter et al. 2020c).
Amblyseius herbicolus was also recently reported from Vietnam (Kreiter et al. 2020b) and La Réunion Island (Kreiter et al. 2020c). Morphological and morphometric characters and all measurements fit well previous measurements in Kreiter et al. (2018b, 2020b, c).
Amblyseius largoensis (Muma)
Amblyseiopsis largoensis Muma 1955: 266.
Typhlodromus (Amblyseius) largoensis, Chant 1959: 96.
Amblyseius (Amblyseialus) largoensis, Muma 1961: 287.
Typhlodromus largoensis, Hirschmann 1962: 2.
Amblyseius (Amblyseius) largoensis, Ehara 1966: 22.
Amblyseius largoensis, Swirski & Golan 1967: 225; Moraes et al. 1986: 17, 2004: 33; Chant & McMurtry 2004: 208, 2007: 78.
Amblyseius magnolia Muma 1961: 289 (synonymy according to Denmark & Evans 2011).
Amblyseius sakalava Blommers 1976: 96 (synonymy according to Ueckermann & Loots 1988).
Amblyseius amtalaensis Gupta 1977: 53 (synonymy according to Gupta 1986).
This species belongs also to the largoensis species group and the largoensis species subgroup (see for the previous species).
It is widespread in all tropical and subtropical regions of the world and was the most abundant species collected by Moraes et al. (2000) in French Caribbean Islands and a potential BCA of Raoiella indica Hirst in La Réunion Island (Moraes et al. 2012).
Using morphometric analyses of 36 characters, molecular analyses and crossing tests, Navia et al. (2014) studied specimens collected in Brazil, La Réunion Island and Trinidad and Tobago to determine whether A. largoensis populations from different geographic origins belong to the same taxonomic entity. Though differences in the lengths of some setae were observed, molecular analyses and crossing experiments indicated that populations from Indian Ocean and America were conspecific. Amblyseius largoensis was previously recorded from Mauritius by Ferragut and Baumann (2019) and so this is the second record of that species.
World distribution: this species is widely distributed in the tropical and subtropical regions of Africa, America, Asia and the Pacific Islands.
Specimens examined: 7 ♀♀ in total. Pamplemousse, Botanical Garden (aasl 41 m, lat. 20°06'24'' S, long. 57°34'49'' E), 3 ♀♀ on Mangifera indica L. (Anacardiaceae), 3 ♀♀ on Psiadia viscosa (Asteraceae) and 1 ♀ on Hyophorbe lagenicaulis (L.H. Bailey) H.E. Moore (Arecaceae), 3/XI/2018.
Remarks: morphological and morphometric characters and all measurements fit well values given by Zannou et al. (2007) for specimens from Africa, Navia et al. (2014) for specimens from Brazil, La Réunion and Trinidad and Tobago, and Ferragut and Baumann (2019) for specimens from Mauritius.
Amblyseius neolargoensis van der Merwe
Amblyseius (Amblyseius) neolargoensis van der Merwe 1965: 59.
Amblyseius neolargoensis, Moraes et al. 1986: 23, 2004: 41; Chant & McMurty 2004: 208, 2007: 80; Zannou et al. 2007: 20.
Amblyseius vazimba Blommers & Chazeau 1974: 312 (synonymy according to Ueckermann & Loots 1988).
This species also belongs to the largoensis species group and the largoensis species subgroup (see above for A. largoensis). Its biology is totally unknown. It is the first mention of Amblyseius neolargoensis from Mauritius.
World distribution: Cape Verde, Hawaii, Mozambique, La Réunion Island, South Africa, Zimbabwe.
Specimens examined: a single ♀ collected in Mauritius during the survey. Nouvelle-France (aasl 442 m, lat. 20°22'34'' S, long. 57°35'58'' E), 1 ♀ on Camelia sinensis (L.) Kuntze (Theaceae), 31/X/2018.
Remarks: morphological and morphometric characters and all measurements fit well measurement values given by van der Merwe (1965) for specimens from South Africa and Zannou et al. (2007) for specimens from South Africa and Mozambique.
Amblyseius passiflorae Blommers
Amblyseius passiflorae Blommers 1974: 145; Moraes et al. 1986: 27, 2004: 46; Denmark and Muma 1989: 49; Chant & McMurty 2004: 210, 2007: 80.
This species belongs to the largoensis species group and the arcus species subgroup as the spermatheca is dish-, cup- or bell-shaped and to the vasiformis species complex as seta Z5 is very long. Its biology is totally unknown.
It was previously only known from the type series (five females and one male) (Blommers 1974). The original description was rather complete, providing comprehensive information on female and male morphology. Ferragut and Baumann (2019) had added information on dorsal adenotaxy and poroidotaxy. Amblyseius passiflorae was collected by the latter authors and was thus already recorded from Mauritius.
World distribution: Madagascar, Mauritius.
Specimens examined: 63 ♀♀ and 1 ♂ in total. Brittania (aasl 442 m, lat. 20°27'01'' S, long. 57°33'42'' E), 17 ♀♀ on Cryptomeria japonica (L. f.) D. Don (Taxodiaceae) and 3 ♀♀ on Allamanda cathartica L. (Apocynaceae), 31/X/2018; Rivière des Anguilles, Bridge (aasl 158 m, lat. 20°28'49'' S, long. 57°33'37'' E), 6 ♀♀ on Mangifera indica L. (Anacardiaceae) and 4 ♀♀ on Asplenium nitens Swartz (Aspleniaceae), 31/X/2018; Le Val, close to the river (aasl 153 m, lat. 20°22'29'' S, long. 57°35'12'' E), 5 ♀♀ on Euphorbia pulcherima Willdenow ex Klotzsch (Euphorbiaceae) and 3 ♀♀ on Callistemon citrinus (William Curtis) Homer Collar Skeels (Myrtaceae), 1/XI/2018; Quartier militaire (aasl 472 m, lat. 20°15'11'' S, long. 57°34'32'' E), 15 ♀♀ on C. japonica, 1/XI/2018; Pamplemousse, Botanical Garden (aasl 41 m, lat. 20°06'24'' S, long. 57°34'49'' E), 1 ♀ on Artocarpus heterophyllus Lamarck (Moraceae), 1 ♀ on Hyophorbe lagenicaulis (L.H. Bailey) H.E. Moore (Arecaceae), 5 ♀♀ and 1 ♂ on Acalypha hispida Burman (Euphorbiaceae) and 1 ♀ on Saraca indica L. (Fabaceae), 3/XI/2018; Curepipe, Bld Pasteur (aasl 510 m, lat. 20°19'21'' S, long. 57°31'45'' E), 1 ♀ on Sphaeropteris cooperi (Hooker ex F. Mueller) R.M. Tryon (Cyatheaceae) and 1 ♀ on Ixora coccinea L. (Rubiaceae), 4/XI/2018.
Remarks: morphological and morphometric characters and all measurements of our specimens fit well values in Blommers (1974) and Ferragut and Baumann (2019). This species is the third most collected species in our study and probably one of the three more common species with A. herbicolus and E. ovaloides.
Amblyseius tamatavensis Blommers
Amblyseius tamatavensis Blommers 1974: 144; Moraes et al. 1986: 31, 2004: 52; Denmark & Muma 1989: 13; Chant & McMurtry 2004: 203, 2007: 81; Ehara & Amano 2004: 17.
Amblyseius (Amblyseius) tamatavensis, Ehara 2002: 33; Ehara & Amano 2002: 322.
Amblyseius maai Tseng 1976: 123 (synonymy according to Denmark & Muma 1989).
Amblyseius aegyptiacus Denmark & Matthysse in Matthysse & Denmark 1981: 343 (synonymy according to Denmark & Muma 1989)
This species belongs to the obtusus species group as setae J2 and Z1 are present, setae z4 are minute and the female ventrianal shield is not vase-shaped or divided. It belongs to the aerialis species subgroup (46 species) as the calyx of the spermatheca is tubular (Chant and McMurtry 2004).
It seems to fit the functional type III-b (generalist predators living on glabrous leaves) group defined by McMurtry et al. (2013). Cavalcante et al. (2017) reported this species as a promising natural enemy of Bemisia tabaci (Gennadius). Experimental releases of this predator on caged plants in a screenhouse caused the reduction of the density of B. tabaci on pepper plants by up to 60-80% (Massaro and Moraes 2019). It can be easily produced in large numbers (Massaro et al. 2018) when fed with astigmatine mites, which could allow the mass production for augmentative biological control. This species is reported in tropical areas from over 20 countries around the world (Africa, Asia, America and Oceania). It was recently recorded from La Réunion since previous studies (Quilici et al. 2000).
World distribution: this species was described from Madagascar, but is actually widely distributed in the tropical and subtropical regions of Africa, America, Asia and the Pacific Islands.
Specimens examined: 7 ♀♀ in total. Nouvelle-France (aasl 442 m, lat. 20°22'34'' S, long. 57°35'58'' E), 4 ♀♀ on Camelia sinensis (L.) Kuntze (Theaceae), 31/X/2018; Riambel, Sea Front (aasl 11m, lat. 20°31'15'' S, long. 57°30'45'' E), 2 ♀♀ on Musa paradisiaca L. (Musaceae), 31/X/2018; Curepipe, Anderson street (aasl 560 m, lat. 20°19'11'' S, long. 57°31'52'' E), 1 ♀ on Sonchus oleraceus L. (Asteraceae), 4/XI/2018.
Remarks: this species was described from Madagascar (Blommers 1974), then mentioned in the Indian Ocean from La Réunion Island (Quilici et al. 2000) and recently from Mauritius (Ferragut and Baumann 2019). Morphological and morphometric characters and all measurements of our specimens fit well measurements in Blommers (1974), Ferragut and Baumann (2019) and Kreiter et al. (2020c).
Tribe Euseiini Chant & McMurtry
Euseiini Chant & McMurtry 2005b: 191.
Subtribe Typhlodromalina Chant & McMurtry
Typhlodromalina Chant & McMurtry 2005b: 195.
Genus Typhlodromalus Muma
Amblyseius (Typhlodromalus) Muma 1961: 288; Typhlodromalus De Leon 1966: 87.
Typhlodromalus spinosus (Meyer & Rodrigues)
Amblyseius spinosus Meyer & Rodrigues 1966: 30; Moraes et al. 1986: 31.
Kampimodromus spinosus, Quilici et al. 2000: 100.
Typhlodromalus spinosus, Moraes et al. 2004: 204; Chant & McMurtry 2005a: 199, 2007: 111.
This species belongs to the athiasae species group as setae J1 and S5 are absent. This species group contains six species (Chant and McMurtry 2005b, Moraes et al. 2006).
Typhlodromalus spinosus was collected from Eastern, Western, but mainly Southern Africa and La Réunion (Demite et al. 2020). The rapid multiplication of this species on the western flower thrips (WFT), Frankliniella occidentalis Pergande, was confirmed and clear evidence that T. spinosus predates on WFT under laboratory and field conditions, but not on T. urticae (Mwangi et al. 2015). This species seems abundant in low vegetation as it was found in high populations in a study of companion plants in citrus orchard (Le Bellec et al. unpub. data).
This species has never been record from Guadeloupe or Martinique in similar studies, but it is interesting to notice that in those islands, another Typhlodromalus was collected, T. peregrinus (Muma) (Mailloux et al. 2010; Kreiter et al. 2013, 2018c). Typhlodromalus spinosus was recorded in low numbers from La Réunion by Quilici et al. (2000) and in high numbers by Kreiter et al. (2020c). This is the first mention of this species from Mauritius Island.
World distribution: Benin, Burundi Dr Congo, Kenya, Malawi, Mozambique, La Réunion Island.
Specimens examined: 3 ♀♀ in total. Côte d'Or, Village (aasl 443 m, lat. 20°15'26'' S, long. 57°32'21'' E), 1 ♀ on Clibadium surinamense L. (Asteraceae), 28/X/2018; Le Val, close to the river (aasl 157 m, lat. 20°22'47'' S, long. 55°36'18'' E), 1 ♀ on Callistemon citrinus (William Curtis) Homer Collar Skeels (Myrtaceae), 1/XI/2018; Curepipe, Bld Pasteur (aasl 510 m, lat. 20°19'21'' S, long. 57°31'45'' E), 1 ♀ on C. citrinus, 4/XI/2018.
Remarks: this species was described from Mozambique (Meyer and Rodrigues 1966), then mentioned in the Indian Ocean from La Réunion Island (Quilici et al. 2000; Kreiter et al. 2020c). Morphological and morphometric characters and all measurements of our specimens fit well measurements in Kreiter et al. (2020c).
Subtribe Euseiina Chant & McMurtry
Euseiina Chant & McMurtry 2005b: 209.
Genus Euseius Wainstein
Amblyseius (Amblyseius) section Euseius Wainstein 1962: 15; Euseius De Leon 1966: 86.
Euseius gallicus Kreiter & Tixier
Euseius gallicus Kreiter and Tixier in Tixier et al. 2010: 242.
This species was described from Southern France (Tixier et al. 2010). It had also been recorded from Tunisia, Belgium, Germany, the Netherlands, and Turkey (Kreiter et al. 2010; Döker et al. 2014) and recently from Slovenia (Kreiter et al. 2020a).
Unlike most phytoseiid species, which are classified as generalist predators of small insects and mites (type III), Euseius species are pollen-feeding generalist predators (type IV) (McMurtry and Croft 1997; McMurtry et al. 2013). Type III phytoseiids also feed on pollen, but prefer or show better performance on insect or mite prey. Type IV predatory mites have their highest reproductive capacity when feeding on pollen, and populations in the field often increase significantly when the crop or the surrounding vegetation is flowering (McMurtry et al. 2013).
Recently, E. gallicus had shown potential as a biocontrol agent for thrips and whiteflies in roses when Typha sp. (cattail) pollen was supplied as an additional food source (Biobest 2013; Wackers 2013). Provision of pollen as a supplementary food source can improve biological control of whiteflies and thrips by type III phytoseiids (van Rijn and Sabelis 1993; Nomikou et al. 2010), and control works excellently in crops where pollen is naturally available (Calvo et al. 2012). The populations of Euseius species can grow faster than the populations of type III phytoseiids when pollen is provided as a food source.
This is the first report of this species from Mauritius, but also the first mention from the Indian Ocean, very far from the European area where the species was originally described and recorded.
World distribution: Belgium, France, Germany, Italy, The Netherlands, Slovenia, Tunisia, Turkey.
Specimens examined: a single ♀ collected in Mauritius during the survey. Mare aux Vacoas (aasl 572 m, lat. 20°21'40'' S, long. 57°29'59'' E), 1 ♀ on Tibouchina heteromalla Cogniaux (Melastomataceae), 30/X/2018.
Remarks: morphological and morphometric characters and all measurements of our specimens fit well measurements in Tixier et al. (2010) and Döker et al. (2014).
Euseius hima (Pritchard & Baker)
Amblyseius (Amblyseius) hima Pritchard & Baker 1962: 257; Blommers 1976: 89.
Euseius hima, Moraes et al. 1986: 46, 2004: 71; Quilici et al. 2000: 99; Chant & McMurtry 2005b: 215, 2007: 121.
This species was recorded from several countries of Sub-Saharan Africa, but also from Madagascar, India (Demite et al. 2020) and La Réunion (Quilici et al. 2000; Kreiter et al. 2020c). All details of collections were provided in those papers. Its biology remains totally unknown. This is the first report of E. hima from Mauritius.
World distribution: Cameroon, Equatorial Guinea, La Réunion Island, Madagascar Island.
Specimens examined: 4 ♀♀ and 1 ♂ in total. Morne-Brabant (aasl 249 m, lat. 20°22'05'' S, long. 57°29'31'' E), 4 ♀♀ and 1 ♂ on Lantana camara L. (Verbenaceae), 5/XI/2018.
Remarks: measurements of specimens collected and identified were published only in Kreiter et al. (2020c). Morphological and morphometric characters and all measurements of our specimens fit well measurements in Kreiter et al. (2020c).
Euseius ovaloides (Blommers)
Amblyseius (Amblyseius) ovaloides Blommers 1974: 147.
Euseius ovaloides Moraes et al. 1986: 51, 2004: 78; Chant & McMurtry 2005a: 215, 2007: 121.
This species was described by Blommers (1974) from specimens collected on Citrus hystrix and Persea americana in Madagascar. Like all Euseius species, it belongs to the type IV (pollinophagous generalist predators) of McMurtry and Croft (1997) and McMurtry et al. (2013). The species had been occasionally recorded from Madagascar (Blommers 1974), Papua-New Guinea (Schicha and Gutierrez 1985), Seychelles (Schicha 1987), La Réunion Island, (Quilici et al. 1997, 2000), Guadeloupe, Martinique and Marie-Galante (Moraes et al. 2000; Kreiter et al. 2006) on various plants, though its biology remains unknown. It is suspected to be a poor predator of tetranychid mites (Gutierrez and Etienne 1986), but can be considered as a potentially good predator of thrips and of whiteflies. This is one of the more common species on La Réunion Island. This is the first mention of E. ovaloides from Mauritius.
World distribution: Guadeloupe, Madagascar Island, Marie-Galante, Martinique, Papua New Guinea, La Réunion Island, Seychelles Archipelago.
Specimens examined: 83 ♀♀, 7 ♂♂ and 1 im. in total. Curepipe, Anderson street (aasl 560 m, lat. 20°19'11'' S, long. 57°31'52'' E), 21 ♀♀ on Codiaeum variegatum (L.) Jussieu (Euphorbiacae), 27/X/2018; Curepipe, Botanical Garden (aasl 540 m, lat. 20°19'28'' S, long. 57°30'50'' E), 1 ♀ + 1 ♂ on Cinnamomum camphora (L.) Presl (Lauraceae), and on Vernicia montana Loureiro (Euphorbiaceae), 29/X/2018; Curepipe, Trou aux cerfs (aasl 593 m, lat. 20°19'04'' S, long. 57°30'47'' E), 1 ♀ on Solanum mauritianum Scopoli (Rosaceae), 29/X/2018; Baie du Cap (aasl 55 m, lat. 20°29'11'' S, long. 57°22'43'' E), 8 ♀♀ + 1 ♂ on Terminalia cattapa L. (Combretaceae), 31/X/2018; Mahébourg, Pointe des Régates (aasl 2 m, lat. 20°24'15'' S, long. 57°42'35'' E), 1 ♀ on T. cattapa, 31/X/2018; Riambel, Sea Front (aasl 11m, lat. 20°31'15'' S, long. 57°30'45'' E), 5 ♀♀ on Carica papaya L. (Caricaceae), 2 ♀♀ on Musa paradisiaca L. (Musaceae) and 2 ♀♀ on Pithecellobium dulce (Roxburgh) Bentham (Mimosaceae), 31/X/2018; Mare d'Albert (aasl 160 m, lat. 20°25'13'' S, long. 57°38'16'' E), 7 ♀♀ on C. papaya and 3 ♀♀ on Litchi sinensis Sonnerat (Sapindaceae), 1/XI/2018; Anse aux petits sables (aasl 2 m, lat. 20°24'15'' S, long. 57°42'35'' E), 2 ♀♀ on Ricinus communis L. (Euphorbiaceae), 1/XI/2018; Belle Rive (aasl 158 m, lat. 20°19'24'' S, long. 57°42'08'' E), 4 ♀♀ on Acalypha hispida Burman (Euphorbiaceae), 1/XI/2018; Pamplemousse, Botanical Garden (aasl 41 m, lat. 20°06'24'' S, long. 57°34'49'' E), 1 ♀ + 1 ♂ on Terminalia arjuna (Roxburgh) Wight et Arnott (Combretaceae), 1 ♂ on Acacia glauca (L.) Moench (Fabaceae) and 2 ♀♀ on Saraca indica (Roxburgh) Willdenow (Fabaceae), 3/XI/2018; Curepipe, Bld Pasteur (aasl 510 m, lat. 20°19'21'' S, long. 57°31'45'' E), 4 ♀♀ on Sphaeropteris cooperi (Hooker ex F. Mueller) R.M. Tryon (Cyatheaceae), 1 ♀ on Psidium guajava L. (Myrtaceae), 7 ♀♀ + 1 ♂ on Hibiscus rosa-sinensis L. (Malvaceae), 3 ♀♀ on Ageratum conyzoides L. (Asteraceae), 1 ♀ on Duranta erecta L. (Verbenaceae) and 5 ♀♀ on Terminalia mantaly Perrier (Combretaceae), 4/XI/2018; Réduit, Sugar reaearch institute (aasl 273 m, lat. 20°14'48'' S, long. 57°21'25'' E), 2 ♀♀ + 2 ♂♂ + 1 im. on Acalypha wilkesiana Müller Argoviensis (Euphorbiaceae), 6/XI/2018.
Remarks: this species was recently reported from Vietnam (Kreiter et al. 2020b). Morphological and morphometric characters and all measurements of our specimens fit well measurements in Kreiter et al. (2020b). Euseius ovaloides is the second most collected species in our study after A. herbicolus and probably one of the three more common species with A. herbicolus and A. passiflorae.
Subfamily Phytoseiinae Berlese
Phytoseiini Berlese 1913: 3; Phytoseiinae Vitzthum 1941: 767.
Genus Phytoseius Ribaga
Phytoseius Ribaga 1904: 177.
Phytoseius coheni Swirski & Shechter
Phytoseius (Dubininellus) macropilis coheni Swirski & Shechter 1961: 104.
Phytoseius (Phytoseius) macropilis coheni, Ehara 1966: 26.
Phytoseius (Dubininellus) coheni, Swirski & Golan 1967: 226; Wu 1997: 153.
Phytoseius (Phytoseius) coheni, Moraes et al. 1986: 219.
Phytoseius coheni, Moraes et al. 2004: 235; Chant & McMurtry 2007: 129.
Phytoseius hawaiiensis Prasad 1968: 1460 (synonymy according to Denmark & Evans 2011).
Phytoseius huangi Ehara 1970: 62 (synonymy according to Ehara 2002).
Phytoseius jianfengensis Chen, Chu & Zhou 1980: 15 (synonymy according to Wu 1997).
This species belongs to the horridus species group as setae J2 and R1 are absent (Chant and McMurtry 1994). It was described from Hong-Kong by Swirski and Shechter (1961) collected on a wide range of plants and very common on citrus. Although species of the genus Phytoseius are considered to belong to the type III (polyphagous generalist predators) of McMurtry and Croft (1997) and McMurtry et al. (2013), its specific biology is totally unknown.
World distribution: Australia, China, Hawaii, Hong-Kong, India, Indonesia, Japan, Malaysia, Mauritius, Papua New Guinea, Philippines, Singapore, Tahiti, Taiwan, Thailand, USA.
Specimens examined: 4 ♀♀ and 1 ♂ in total. Curepipe, Bld Pasteur (aasl 510 m, lat. 20°19'21'' S, long. 57°31'45'' E), 4 ♀♀ and 1 ♂ on Sphaeropteris cooperi (Hooker ex F. Mueller) R.M. Tryon (Cyatheaceae), 4/XI/2018.
Remarks: This species was first reported from Mauritius by Schicha (1984) under the junior synonym name P. hawaiensis. Ferragut and Baumann (2019) recovered the species. Kreiter et al. (2020b) had recently reported this species from Vietnam. Morphological and morphometric characters and all measurements of our specimens fit well measurements in Kreiter et al. (2020b).
Phytoseius crinitus Swirski & Shechter
Phytoseius (Dubininellus) crinitus Swirski & Shechter 1961: 102; Amitai & Swirski 1966: 21; Denmark 1966: 66; Swirski & Amitai 1966: 11; Moraes et al. 1986: 220.
Phytoseius crinitus, Moraes et al. 2004: 236; Chant & McMurtry 2007: 129.
This species belongs to the horridus species group (Chant and McMurtry 1994). It was recorded in several countries of Asia, in Burundi, Madagascar (Demite et al. 2020) and La Réunion (Quilici et al. 2000). The biology of P. crinitus remains totally unknown.
World distribution: Burundi, China, Hong Kong, India, Indonesia, Japan, Madagascar Island, Philippines, La Réunion Island, Singapore, Taiwan.
Specimens examined: 21 ♀♀ in total. Curepipe, Anderson street (aasl 560 m, lat. 20°19'11'' S, long. 57°31'52'' E), 13 ♀♀ on Litsea glutinosa (Loureiro) Robinson (Lauraceae), 27/X/2018; Curepipe, Botanical Garden (aasl 540 m, lat. 20°19'28'' S, long. 57°30'50'' E), 1 ♀ on Vernicia montana Loureiro (Euphorbiaceae), 29/X/2018; Nouvelle-France (aasl 442 m, lat. 20°22'34'' S, long. 57°35'58'' E), 1 ♀ on Camelia sinensis (L.) Kuntze (Theaceae), 31/X/2018; Belle Rive (aasl 158 m, lat. 20°19'24'' S, long. 57°42'08'' E), 1 ♀ on Acalypha hispida Burman (Euphorbiaceae), 1/XI/2018; Baie du Cap, sea front (aasl 158 m, lat. 20°30'14'' S, long. 57°23'02'' E), 3 ♀♀ on Casuarina equisetifolia L. (Casuarinaceae), 5/XI//2018; Morne-Brabant (aasl 249 m, lat. 20°22'05'' S, long. 57°29'31'' E), 2 ♀♀ on Chromolaena odorata (L.) R.M. King and H. Robinson (Asteraceae), 5/XI/2018.
Remarks: this species is the more numerous species of Phytoseius collected in the present study. This was also the case with Ferragut and Baumann (2019) along with Phytoseius haroldi. It was reported for the first time by these authors from Mauritius, but was already reported by Quilici et al. (2000) from Mascareignes Archipelago in La Réunion Island where Kreiter et al. (2020c) had recovered high numbers of the species. Morphological and morphometric characters and all measurements of our specimens fit well measurements in Kreiter et al. (2020c).
Phytoseius duplus Ueckermann & Loots
Phytoseius (Phytoseius) duplus Ueckermann & Loots 1985: 37.
Phytoseius duplus, Moraes et al. 2004: 237; Chant & McMurtry 2007: 129.
This species belongs to the horridus species group. Its biology is unknown, this is the single report of that species since the original description and is the first report of that species from Mauritius Island.
World distribution: South Africa.
Specimens examined: 4 ♀♀ in total. Plaisance (aasl 55 m, lat. 20°25'59'' S, long. 57°40'44'' E), 4 ♀♀ on Litsea monopetala (Roxburgh) Person (Lauraceae), 31/X/2018.
Remarks: this is the first mention of this species from Indian Ocean. The measurements of specimens collected in Mauritius Island (Table 4) are very close to those given by Ueckermann and Loots (1985) for two specimens in the original description, except for the shorter dimensions of the ventral shields (Table 4).



Phytoseius haroldi Ueckermann & Kreiter
Phytoseius haroldi Ueckermann & Kreiter in Kreiter et al. 2002: 339; Chant & McMurtry 2007: 129.
This species belongs to the horridus species group as setae J2 and R1 are absent (Chant and McMurtry 1994). It was described by Ueckermann and Kreiter in Kreiter et al. (2002). It was abundant on lower vegetation in a study of companion plants in citrus orchards in La Réunion Island (Kreiter et al. 2020c). It seems that this species prefers low plants, but despite this observation has to be confirmed, its biology remains totally unknown. This is the second report in Mauritius after recent report by Ferragut and Baumann (2019).
World distribution: La Réunion Island, Mauritius Island.
Specimens examined: 2 ♀♀ and 1 ♂ in total. Plaisance (aasl 55 m, lat. 20°25'59'' S, long. 57°40'44'' E), 2 ♀♀ on Litsea monopetala (Roxburgh) Person (Lauraceae), 31/X/2018; Morne-Brabant (aasl 249 m, lat. 20°22'05'' S, long. 57°29'31'' E), 1 ♂ on Lantana camara L. (Verbenaceae), 5/XI/2018.
Remarks: this species was described by Ueckermann and Kreiter in Kreiter et al. (2002) from La Réunion Island. Morphological and morphometric characters and all measurements of our specimens fit well measurements of the original description in Kreiter et al. (2002) concerning specimens from La Réunion Island, Ferragut and Baumann (2019) for specimens from Mauritius, and Kreiter et al. (2020c) for additional specimens from La Réunion Island.
Subfamily Typhlodrominae Wainstein
Typhlodromini Wainstein 1962: 26; Typhlodrominae Chant and McMurtry 1994: 235.
Genus Typhlodromus (Anthoseius) Scheuten
Typhlodromus (Anthoseius) De Leon 1959: 258; van der Merwe 1968: 20; Karg 1982: 194; Chant and McMurtry 1994: 250, 2007: 149.
Typhlodromus (Anthoseius) lobatus Zannou, Moraes & Oliveira
Typhlodromus (Anthoseius) lobatus Zannou, Moraes & Oliveira in Ueckermann et al. 2008: 59.
This species belongs to the large rhenanus species group (Chant and McMurtry 1994). Its biology is totally unknown and this is the first report of T. (A.) lobatus from Mauritius.
World distribution: Ghana.
Specimens examined: 2 ♀♀ and 1 ♂ in total. Chamouny (aasl 192 m, lat. 20°28'42'' S, long. 57°29'11'' E), 1 ♀ on Cupressus sempervirens L. (Cupressaceae), 31/X/2018; Morne-Brabant (aasl 249 m, lat. 20°22'05'' S, long. 57°29'31'' E), 1 ♀ and 1 ♂ on Chromolaena odorata (L.) R.M. King and H. Robinson (Asteraceae), 5/XI/2018.
Remarks: morphological and morphometric characters and all measurements of our specimens fit well measurements of the original description by Zannou, Moraes and Oliveira in Ueckermann et al. (2008) concerning specimens from Ghana, in Western Africa.
Typhlodromus (Anthoseius) muliebris van der Merwe
Typhlodromus (Anthoseius) muliebris van der Merwe 1968: 28; Moraes et al. 2004: 337; Chant & McMurtry 2007: 155; Ueckermann et al. 2008: 69.
Amblydromella muliebris, Moraes et al. 1986: 168.
Amblydromella (Amblydromella) muliebris, Denmark & Welbourn 2002: 308.
This species belongs to the large rhenanus species group (Chant and McMurtry 1994). It was described by van der Merwe (1968) from South Africa and then reported by El-Banhawy and Knapp (2011) from Kenya. The biology of this species is totally unknown. This is the first report of this species from Mauritius and from a country outside the Africa continent.
World distribution: Kenya, South Africa.
Specimens examined: 3 ♀♀ in total. Chamouny (aasl 192 m, lat. 20°28'42'' S, long. 57°29'11'' E), 3 ♀♀ on Solanum torvum Swartz (Solanaceae), 31/X/2018.
Remarks: morphological and morphometric characters and all measurements of our specimens fit well measurements of the original description by van der Merwe (1968) and new descriptions by Ueckermann et al. (2008) and El-Banhawy and Knapp (2011) concerning specimens from South Africa and Kenya, respectively.
Typhlodromus (Anthoseius) recurvitremus Ferragut
Typhlodromus (Anthoseius) recurvitremus Ferragut in Ferragut & Baumann 2019: 847-851.
As underlined by Ferragut and Baumann (2019), this species can hardly be accommodated in any of the species groups proposed by Chant and McMurtry (1994) for the subgenus Typhlodromus (Anthoseius). It seems most closely related to Typhlodromus (Anthoseius) elaeis Zannou, Moraes and Oliveira described from Cameroon (Ueckermann et al. 2008) which can be probably raised as a new species group for the subgenus Typhlodromus (Anthoseius) including the two species (to be called elaeis species group). The biology of this species is totally unknown. This is the second report of T. (A.) recurvitremus from Mauritius.
World distribution: Mauritius.
Specimens examined: 4 ♀♀ and 2 ♂♂ in total. Chamouny (aasl 192 m, lat. 20°28'42'' S, long. 57°29'11'' E), 4 ♀♀ and 2 ♂♂ on Agarista salicifolia (Commerson ex Lam) G. Don (Ericaceae), 31/X/2018.
Remarks: morphological and morphometric characters and all measurements of our female and male specimens fit well measurements of the original description of Ferragut and Baumann (2019).
Conclusion
The results of an additional survey made in 2018 in Mauritius Island is presented in this paper. A total of 12 new records: nine Amblyseiinae, one Phytoseiinae and two Typhlodrominae, have been obtained, namely Neoseiulus houstoni, Paraphytoseius horrifer, Amblyseius haleakalus, A. herbicolus, A. neolargoensis, Typhlodromalus spinosus, Euseius gallicus, E. hima, E. ovaloides, Phytoseius duplus, Typhlodromus (Anthoseius) lobatus and T. (A.) muliebris.
The fauna of Mauritius after our study is composed of 27 species: 20 Amblyseiinae, four Phytoseiinae and three Typhlodrominae.
Among the 12 newly recorded species, at least 3 species (A. herbicolus, E. gallicus and E. ovaloides) are known as BCAs. In addition to the intrinsic value of phytoseiid mite biodiversity in tropical environments, demonstration of the natural occurrence of efficient BCAs in a developing country such as Mauritius is of great agricultural, commercial and strategic interests for the country.
Acknowledgements
Acknowledgements are first due to the Department to which the senior author belongs for his research activities that have funded this work for travels and accommodations in Mauritius: UMR CBGP (Internal call for proposals 2018). Many thanks are also due to Mrs Marie Anne Edouard for hosting the senior author and for his son for helping with car problems. Great thanks to Mrs Claudia Baider, responsible for the Mauritius Herbarium, who has identified some plants and advice on Island locations and Mauritius biodiversity. Thanks also to National Authorities of Mauritius for the signature of a Memorandum of agreement for the supply of biological material by the Government of Mauritius and a Phytosanitary certificate. Also thanks to Le Vélo Vert Association, especially Mrs Géraldine d'Unienville for e-mail exchanges and advices. Thanks also to the I-SITE Montpellier Université d'Excellence (MUSE) for the international mobility support to the junior author (Explore \#2, the MUSE International Mobility program, 2019).
References
Amitai S., Swirski E. 1966. Illustrations of spermathecae in several previously described phytoseiid mites (Acarina) from Hong Kong and Israel. Isr. J. Agric. Res., 16: 19-24.
Athias-Henriot C. 1975. Nouvelles notes sur les Amblyseiini. II. Le relevé organotaxique de la face dorsale adulte (Gamasides protoadéniques, Phytoseiidae). Acarologia, 17(1): 20-29.
Beard J.J. 2001. A review of Australian Neoseiulus Hughes and Typhlodromips De Leon (Acari: Phytoseiidae: Amblyseiinae). Invert. Taxon., 15: 73-158. doi:10.1071/IT99017 ![]()
Berlese A. 1913. Systema Acarorum genera in familiis suis disposita. Acaroteca Italica, 1-2: 3-19.
Berlese A. 1914. Acari nuovi. Manipulus IX. Redia, 10: 113-150.
Bhattacharyya S.K. 1968. Two new phytoseiid mites from eastern India (Acarina: Phytoseiidae). J. Bombay Nat. Hist. Soc., 65(3): 677-680.
Biobest. 2013. Biobest introduces Dyna-Mite®: a new predatory mite strategy in rose. Biobest Belgium N. V. http://www.biobest.be/nieuws/289/3/0/ ![]()
Blommers L. 1974. Species of the genus Amblyseius Berlese, 1914, from Tamatave, east Madagascar (Acarina: Phytoseiidae). Bulletin Zoologisch Museum Universiteit van Amsterdam, 3: 143-155.
Blommers L. 1976. Some Phytoseiidae (Acarina: Mesostigmata) from Madagascar, with descriptions of eight new species and notes on their biology. Bijdragen tot Dierkunde, 46(1): 80-106. doi:10.1163/26660644-04601005 ![]()
Blommers L., Chazeau J. 1974. Two new species of predatory mites of the genus Amblyseius Berlese (Acarina: Phytoseiidae) from Madagascar. Zeitschrift fur Angewandte Entomologie, 75: 308-315. doi:10.1111/j.1439-0418.1974.tb01856.x ![]()
Calvo F.J., Bolckmans K., Belda J.E. 2012. Biological control-based IPM in sweet pepper greenhouses using Amblyseius swirskii (Acari: Phytoseiidae). Biocontrol Sci Technol., 22(12): 1398-1416. doi:10.1080/09583157.2012.731494 ![]()
Carmona M.M. 1968. Contribuicao para o estudo de alguns acaros fitofagos e depredadores, de Angola. Agron. Lusit., 29: 267-288 + 12 plates.
Cavalcante A.C.C., Demite P.R, Amaral F.S.R., Lofego A.C., Moraes G.J. de 2017. Complementary description of Neoseiulus tunus (De Leon) (Acari: Mesostigmata: Phytoseiidae) and observation on its reproductive strategy. Acarologia, 57(3): 591-599. doi:10.24349/acarologia/20174178 ![]()
Chant D.A. 1959. Phytoseiid mites (Acarina: Phytoseiidae). Part I. Bionomics of seven species in southeastern England. Part II. A taxonomic review of the family Phytoseiidae, with descriptions of thirty-eight new species. Can. Entomol., 61(12): 1-166. doi:10.4039/entm9112fv ![]()
Chant D.A., McMurtry J.A. 1994. A review of the subfamilies Phytoseiinae and Typhlodrominae (Acari: Phytoseiidae). Intern. J. Acarol., 20(4): 223-310. doi:10.1080/01647959408684022 ![]()
Chant D.A., McMurtry J.A. 2003a. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part I. Neoseiulini new tribe. Intern. J. Acarol., 29(1): 3-46. doi:10.1080/01647950308684319 ![]()
Chant D.A., McMurtry J.A. 2003b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part II. The tribe Kampimodromini Kolodochka. Intern. J. Acarol., 29(3): 179-224. doi:10.1080/01647950308684331 ![]()
Chant D.A., McMurtry J.A. 2004. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part III. The tribe Amblyseiini Wainstein, subtribe Amblyseiina n. subtribe. Intern. J. Acarol., 30(3): 171-228. doi:10.1080/01647950408684388 ![]()
Chant D.A., McMurtry J.A. 2005a. A review of the subfamily Amblyseiina Muma (Acari: Phytoseiidae): Part V. Tribe Amblyseiini, subtribe Proprioseiopsina Chant and McMurtry. Intern. J. Acarol., 31(1): 3-22. doi:10.1080/01647950508684412 ![]()
Chant D.A., McMurtry J.A. 2005b. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VI. The tribe Euseiini n. tribe, subtribes Typhlodromalina n. subtribe, Euseiina n. subtribe, and Ricoseiina n. subtribe. Intern. J. Acarol., 31(3): 187-224. doi:10.1080/01647950508684424 ![]()
Chant D.A., McMurtry J.A. 2005c. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae) Part VII. Typhlodromipsini n. tribe. Intern. J. Acarol., 31(4): 315-340. doi:10.1080/01647950508683673 ![]()
Chant D.A., McMurtry J.A. 2006. A review of the subfamily Amblyseiinae Muma (Acari: Phytoseiidae): Part IX. An overview. Intern. J. Acarol., 32(2): 1-27. doi:10.1080/01647950608684453 ![]()
Chant D.A., McMurtry J.A. 2007. Illustrated keys and diognoses for the genera and subgenera of the Phytoseiidae of the world (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, 219 pp.
Chant D.A., Yoshida-Shaul E. 1991. Adult ventral setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol., 17(3): 187-199. doi:10.1080/01647959108683906 ![]()
Chant D.A., Yoshida-Shaul E. 1992. Adult idiosomal setal patterns in the family Phytoseiidae (Acari: Gamasina). Intern. J. Acarol., 18(3): 177-193. doi:10.1080/01647959208683949 ![]()
Chaudhri W.M. 1968. Six new species of mites of the genus Amblyseius (Phytoseiidae) from Pakistan. Acarologia, 10: 550-562.
Chen S.-W., Chu C.-M., Zhou F.-W. 1980. On the phytoseiid mites of Guangdong (Acarina: Phytoseiidae). J. Jiagxi Univ., 4(1), 15-20 [in Chinese with English abstract].
Corpuz L.A., Rimando L. 1966. Some Philippine Amblyseiinae (Phytoseiidae: Acarina). Philip. Agric., 50: 114-136.
Daneshvar H., Denmark H.A. 1982. Phytoseiids of Iran (Acarina: Phytoseiidae). Intern. J. Acarol., 8(1): 3-14. doi:10.1080/01647958208683272 ![]()
De Leon, D. 1959. Two new genera of phytoseiid mites with a note on Proprioseius meridionalis Chant (Acarina: Phytoseiidae). Entomol. News, 70(10): 257-262.
De Leon D. 1966. Phytoseiidae of British Guyana with keys to species (Acarina: Mesostigmata). Stud. Fauna Suriname and other Guyanas, 8: 81-102.
Demite P.R., Moraes G.J. de, McMurtry J.A., Denmark H.A., Castilho R.C. 2020. Phytoseiidae Database. Available from: www.lea.esalq.usp.br/phytoseiidae ![]() (last access 30/III/2020).
(last access 30/III/2020).
Demite P.R., McMurtry J.A., Moraes G.J. de. 2014. Phytoseiidae Database: a website for taxonomic and distributional information on phytoseiid mites (Acari). Zootaxa, 3795(5): 571-577. doi:10.11646/zootaxa.3795.5.6 ![]()
Denmark H.A. 1966. Revision of the genus Phytoseius Ribaga, 1904 (Acarina: Phytoseiidae). Fla Dep. Agric. Bul., 6: 1-105.
Denmark H.A., Evans G.A. 2011. Phytoseiidae of North America and Hawaii (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, USA, 451 pp.
Denmark H.A., Evans G.A., Aguilar H., Vargas C., Ochoa R. 1999. Phytoseiidae of Central America (Acari: Mesostigmata). Indira Publishing House, West Bloomfield, Michigan, USA, 125 pp.
Denmark H.A., Muma M.H. 1989. A revision of the genus Amblyseius Berlese, 1914 (Acari: Phytoseiidae). Occas. Pap. Fla State Coll. Arthropods, USA, 4, 149 pp.
Denmark H.A., Welbourn W.C. 2002. Revision of the genera Amblydromella Muma and Anthoseius De Leon (Acari: Phytoseiidae). Intern. J. Acarol., 28(4): 291-316. doi:10.1080/01647950208684308 ![]()
Döker I., Witters J., Pijnakker J., Kazak C., Tixier M.-S., Kreiter S. 2014. Euseius gallicus Kreiter and Tixier (Acari: Phytoseiidae) is present in four more countries in Europe: Belgium, Germany, The Netherlands and Turkey. Acarologia, 54(3), 245-248. doi:10.1051/acarologia/20142132 ![]()
Duarte M.V.A, Venzon M., Bittencourt M.C.de S., Rodriguez-Cruz F.A., Pallini A., Janssen A. 2015. Alternative food promotes broad mite control on chilli pepper plants. BioControl, 60: 817-825. doi:10.1007/s10526-015-9688-x ![]()
Ehara S. 1966. A tentative catalogue of predatory mites of Phytoseiidae known from Asia, with descriptions of five new species from Japan. Mushi, 39: 9-30.
Ehara S. 1967. Phytoseiid mites from Okinawa Island (Acarina: Mesostigmata). Mushi, 40(6): 67-82.
Ehara S. 1970. Phytoseiid mites from Taiwan (Acarina: Mesostigmata). Mushi, 43(6), 55- 63.
Ehara S. 2002. Some phytoseiid mites (Arachnida: Acari: Phytoseiidae) from west Malaysia. Species Div., 7: 29-46. doi:10.12782/specdiv.7.29 ![]()
Ehara S., Amano H. 2002. Some Japanese phytoseiid mites (Acari: Phytoseiidae) mostly from Ishigaki and Taketomi Islands. Entomol. Sc., 5(3): 321-329.
Ehara S., Amano H. 2004. Checklist and keys to Japanese Amblyseiinae (Acari: Gamasina: Phytoseiidae). J. Acarol. Soc. Japan, 13(1): 1-30. doi:10.2300/acari.13.1 ![]()
Ehara S., Bhandhufalck A. 1977. Phytoseiid mites of Thailand (Acarina: Mesotigmata). J. Fac. Educ. Tottori University, Nat. Sc., 27(2): 43-82.
Ehara S., Lee L.H.Y. 1971. Mites associated with plants in Hong Kong. J. Fac. Educ., Tottori Univ., Natur. Sci., 22(2): 61-78.
El-Banhawy E.M. 1984. Description of some phytoseiid mites from Brazil (Acarina: Phytoseiidae). Acarologia, 25(2): 125-144.
El-Banhawy E.M., Knapp M. 2011. Mites of the family Phytoseiidae Berlese from Kenya (Acari: Mesostigmata). Zootaxa, 2945: 1-176. doi:10.11646/zootaxa.2945.1.1 ![]()
Evans G.O. 1953. On some mites of the genus Typhlodromus Scheuten, 1857, from S. E. Asia. Ann. Mag. Nat. Hist., 6: 449-467. doi:10.1080/00222935308654444 ![]()
Ferragut F., Baumann J. 2019. New phytoeiid mites (Mesostigmata: Phytoseiidae) of Mauritius, with the description of two new species. Syst. Appl. Acarol., 24(5): 825-856. doi:10.11158/saa.24.5.8 ![]()
Furtado I.P., Kreiter S., Moraes G.J. de, Tixier M.-S., Flechtmann C.H.W., Knapp M. 2005. Plant mites (Acari) from Northeastern Brazil, with description of two new species of the family Phytoseiidae (Mesostigmata). Acarologia, 45(2-3): 131-143.
Gondim Jr. M.G.C., Moraes G.J. de 2001. Phytoseiid mites (Acari: Phytoseiidae) associated with palm trees (Arecaceae) in Brazil. Syst. Appl. Acarol., 6: 65-94. doi:10.11158/saa.6.1.11 ![]()
Gupta S.K. 1975. Mites of the genus Amblyseius (Acarina: Phytoseiidae) from India with descriptions of eight new species. Intern. J. Acarol., 1(2): 26-45. doi:10.1080/01647957508683746 ![]()
Gupta S.K. 1977. Some undescribed and little-known species of Amblyseius (Acarina: Phytoseiidae) from western and northern India. Ind. J. Acarol., 1: 28-37.
Gupta S.K. 1981. Phytoseiidae (Acari: Mesostigmata) from Jammu and Kashmir, India, with descriptions of five new species. Ind. J. Acarol., 5: 37-49.
Gupta S.K. 1986. Fauna of India (Acari: Mesostigmata) Family Phytoseiidae. Zoological Survey of India, Calcutta, India, 350 pp.
Gutierrez J., Etienne J. 1986. Les Tetranychidae de l'île de la Réunion et quelques-uns de leurs prédateurs. L'Agronomie Tropicale, 41(1): 84-91.
Hirschmann W. 1962. Gangystematik der Parasitiformes. Acarologie Schriftenreihe fur Vergleichende Milbenkunde, Hirschmann-Verlag, Furth/Bay, 5(5-6), 80 pp.+ 32 plates.
Hughes A.M. 1948. The mites associated with stored food products. Ministry of Agriculture and Fisheries, H. M. Stationary Office, London, 168 pp.
Karg W. 1982. Diagnostic and systematics of predatory mites of the family Phytoseiidae Berlese in orchards. Zool. Jahrb. Syst., 109: 188-210.
Karg W. 1983. Systematische untersuchung der Gattungen und Untergattungen der Raubmilbenfamilie Phytoseiidae Berlese, 1916, mit der beschreibung von 8 neuen Arten. Mitt. Zool. Mus. Berlin, 59(2): 293-328. doi:10.1002/mmnz.4830590203 ![]()
Karg W. 1991. Die Raubmilbenarten der Phytoseiidae Berlese (Acarina) Mitteleuropas sowie angrenzender Gebiete. Zool. Jahrb. Syst., 118(1): 1-64.
Karg W., Oomen-Kalsbeek F. 1987. Neue Raubmilbenarten der Gattung Amblyseius Berlese (Acarina, Parasitiformes, Phytoseiidae) Antagonisten der unechten Spinnmilbe Brevipalpus phoenicis Geijskes. Zool. Jahrb. Syst., 114(1): 131-140.
Karmakar K., Bhowmik S. 2018. Description of eight new species and of four species belonging to the family Phytoseiidae (Acari: Mesostigmata) from West Bengal, India. Zootaxa, 4422(1), 41-77. doi:10.11646/zootaxa.4422.1.3 ![]()
Kolodochka L.A. 1998. Two new tribes and the main results of a revision of Paleartic phytoseiid mites (Parasitiformes, Phytoseiidae) with the family system concept. Vest. Zool., 32(1-2): 51-63 [in Russian].
Kreiter S., Amiri K., Douin M., Bohinc T., Trdan S., Tixier M.-S. 2020a. Phytoseiid mites of Slovenia (Acari: Mesostigmata): new records and first description of the male of Amblyseius microorientalis. Acarologia 60(2): 203-242. doi:10.24349/acarologia/20204364 ![]()
Kreiter S., Bopp M.-C., Douin M., Nguyen D.T., Wyckhuys K. 2020b. Phytoseiidae of Vietnam (Acari: Mesostigmata) with description of a new species. Acarologia 60(1): 75-110. doi:10.24349/acarologia/20204362 ![]()
Kreiter S., Fontaine O., Payet R.-M. 2018a. New records of Phytoseiidae (Acari: Mesostigmata) from Mauritius. Acarologia, 58(4): 773-785. doi:10.24349/acarologia/20184273 ![]()
Kreiter S., Mailloux J., Tixier M.-S., Le Bellec F., Douin M., Guichou S., Etienne J. 2013. New phytoseiid mites of the French West Indies, with description of a new species, and new records (Acari: Mesostigmata). Acarologia, 53(3): 285-303. doi:10.1051/acarologia/20132095 ![]()
Kreiter S., Payet R.-M., Fillâtre J., Abdou Azali H. 2018b. First records of Phytoseiidae from one island of the Comoros Archipelago. Acarologia, 58(3): 529-545. doi:10.24349/acarologia/20184256 ![]()
Kreiter S., Payet R.-M., Douin M., Fontaine O., Fillâtre J., Le Bellec F. 2020c. Phytoseiidae of La Réunion Island (Acari: Mesostigmata): three new species and two males described, new synonymies, and new records. Acarologia 60(1): 111-195. doi:10.24349/acarologia/20204361 ![]()
Kreiter S., Tixier M.-S., Etienne J. 2006. New records of phytoseiid mites (Acari: Mesostigmata) from the French Antilles, with description of Neoseiulus cecileae sp. nov. Zootaxa, 1294: 1-27. doi:10.11646/zootaxa.1294.1.1 ![]()
Kreiter S., Tixier M.-S., Sahraoui H., Lebdi-Grissa K. Chabaan S.B., Chatti A., Chermiti B., Khoualdia O., Ksantini M. 2010. Phytoseiid mites (Acari: Mesostigmata) from Tunisia: catalogue, biogeography, and key for identification. Tunis. J. Plant Protec., 5(2): 151-178.
Kreiter S., Ueckermann E.A., Quilici S. 2002. Seven new phytoseiid species, with a new generic assignement and a key to the species of La Reunion Island (Acari: Mesostigmata). Acarologia, 42(4): 335-350.
Kreiter S., Zriki Z., Ryckewaert P., Pancarte C., Douin M., Tixier M.-S. 2018c. New phytoseiid mites of Martinique, with redescription of four species and new records. Acarologia, 58 (2): 366-407. doi:10.24349/acarologia/20184248 ![]()
Lindquist E.E. 1994. Some observations on the chaetotaxy of the caudal body region of gamasine mites (Acari: Mesostigmata), with a modified notation for some ventrolateral body setae. Acarologia, 35: 323-326.
Lindquist E.E., Evans G.W. 1965. Taxonomic concepts in the Ascidae, with a modified setal nomenclature for the idiosoma of the Gamasina (Acarina: Mesostigmata). Mem. Entomol. Soc. Canada, 47: 1-64. doi:10.4039/entm9747fv ![]()
Mailloux J., Le Bellec F., Kreiter S., Tixier M.-S., Dubois P. 2010. Influence of ground cover management on diversity and density of phytoseiid mites (Acari: Phytoseiidae) in Guadeloupean citrus orchards. Exp. Appl. Acarol., 52: 275-290. doi:10.1007/s10493-010-9367-7 ![]()
Massaro M., Montrazi M., Melo J.W.S., Moraes G.J. 2018. Production of Amblyseius tamatavensis with Thyreophagus crasentiseta (Acari: Phytoseiidae, Acaridae). Intern. J. Pest Manag. (in press).
Massaro M., Moraes G.J. de. 2019. Predation and oviposition potential of Brazilian populations of the predatory mite Amblyseius tamatavensis (Acari: Phytoseiidae) on eggs of Bemisia tabaci (Insecta: Hemiptera). Acarologia, 59(1): 120-128. doi:10.24349/acarologia/20194314 ![]()
Matthysse J.G., Denmark H.A. 1981. Some phytoseiids of Nigeria (Acarina: Mesostigmata). Fla Entomol., 64: 340-357. doi:10.2307/3494585 ![]()
McMurtry J.A., Croft B.A. 1997. Life-styles of phytoseiid mites and their roles in biological control. Ann. Rev. Entomol., 42: 291-321. doi:10.1146/annurev.ento.42.1.291 ![]()
McMurtry J.A., Moraes G.J. de, Sourassou N.F. 2013. Revision of the life styles of phytoseiid mites (Acari: Phytoseiidae) and implications for biological control strategies. Syst. Appl. Acarol., 18: 297-320. doi:10.11158/saa.18.4.1 ![]()
Meyer M.K.P., Rodrigues M. da C. 1966. Acari associated with Cotton in Southern Africa. References to other plants. Garcia de Orta, Rev. Junta Investig., 13: 27-31.
Moraes G.J. de, Castro T.M.G. de, Kreiter S., Quilici S., Gondim Jr. M.G.C., Sá L.A. 2012. Search for natural enemies of Raoiella indica Hirst in Réunion Island (Indian Ocean). Acarologia, 52(2): 129-134. doi:10.1051/acarologia/20122043 ![]()
Moraes G.J. de, Zannou I.D., Ueckermann E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2007. Phytoseiid mites of the tribes Afroseiulini, Kampimodromini and Phytoseiulini, and complementary notes on mites of the tribes Euseiini and Neoseiulini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1628: 1-22. doi:10.11646/zootaxa.1628.1.1 ![]()
Moraes G.J. de, Kreiter S., Lofego A.C. 2000. Plant mites (Acari) of the French Antilles. 3. Phytoseiidae (Gamasida). Acarologia, 40(3): 237-264.
Moraes G.J. de, McMurtry J.A., Denmark H.A. 1986. A catalog of the mite family Phytoseiidae. References to taxonomy, synonymy, distribution and habitat. EMBRAPA - DDT, Brasilia, Brazil, 353 pp.
Moraes G.J. de, McMurtry J.A., Denmark H.A., Campos C.B. 2004. A revised catalog of the mite family Phytoseiidae. Zootaxa, 434: 1-494. doi:10.11646/zootaxa.434.1.1 ![]()
Moraes G.J. de, McMurtry J.A., van den Berg H., Yaninek J.S. 1989. Phytoseiid mites (Acari: Phytoseiidae) of Kenya, with descriptions of five new species and complementary descriptions of eight species. Intern. J. Acarol., 15(2): 79-93. doi:10.1080/01647958908683829 ![]()
Moraes G.J. de, Zannou I.D., Oliveira A.R., Yaninek J.S., Hanna R. 2006. Phytoseiid mites of the subtribes Typhlodromalina and Euseiina (Acari: Phytoseiidae: Euseiini) from sub-Saharan Africa. Zootaxa, 1114: 1-52. doi:10.11646/zootaxa.1114.1.1 ![]()
Moutia L.A. 1958. Contribution to the study of some phytophagous Acarina and their predators in Mauritius. Bull. Entomol. Res., 49: 59-75. doi:10.1017/S0007485300053438 ![]()
Muma M.H. 1955. Phytoseiidae (Acarina) associated with citrus in Florida. Ann. Entomol. Soc. Amer., 48: 262-272. doi:10.1093/aesa/48.4.262 ![]()
Muma M.H. 1961. Subfamiles, genera, and species of Phytoseiidae (Acarina: Mesostigmata). Fla St. Mus. Bul., 5(7): 267-302
Muma M.H., Denmark H.A. 1970. Phytoseiidae of Florida. Arthropods of Florida and neighboring land areas, 6. Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, USA, 150 pp.
Mwangi E., Kiarie A., Wainwright H. 2015. Typhlodromalus spinosus as a potentially new biological control agent for Western Flower Thrips. Glob. Adv. Res. J. Agric. Sc., 4 (3): 162-165.
Myers N. 1988. Threatened biotas: hostspots in tropical forests. Environmentalist, 8: 187-208. doi:10.1007/BF02240252 ![]()
Myers N., Mittermeier R.A., Mittermeier C.G., Da Fonseca G.A., Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature, 403: 853-858. doi:10.1038/35002501 ![]()
Narayanan E.S., Kaur R.B.N., Ghai S. 1960. Importance of some taxonomic characters in the family Phytoseiidae., with new records and descriptions of species. Proceed. Nat. Inst. Sc. India, 26B: 384-394.
Navasero M.M., Navasero M.V. 2016. Biology of Paraphytoseius orientalis (Narayanan et al.) reared on the broad mite, Polyphagotarsonemus latus (Banks) (Acari: Phytoseiidae, Tarsonemidae) in the Philippines. Philip. Entomol., 30 (1): 21-28.
Navia D., Domingos C.A., Mendonça R.S., Ferragut F., Rodrigues M.A.N., de Morais E.G.F., Tixier M.-S., Gondim Jr. M.G.C. 2014. Reproductive compatibility and genetic and morphometric variability among populations of the predatory mite, Amblyseius largoensis (Acari: Phytoseiidae), from Indian Ocean Islands and the Americas. Biol. Cont., 72: 17-29. doi:10.1016/j.biocontrol.2014.01.011 ![]()
Nomikou M., Sabelis M.W., Janssen A. 2010. Pollen subsidies promote whitefly control through the numerical response of predatory mites. Biocontrol, 55: 253-260. doi:10.1007/s10526-009-9233-x ![]()
Oliveira D.C., Charanasri V., Kongchuensin M., Konvipasruang P., Chandrapatya A., Moraes G.J. de. 2012. Phytoseiidae of Thailand (Acari: Mesostigmata), with a key for their identification. Zootaxa, 3453: 1-24. doi:10.11646/zootaxa.3453.1.1 ![]()
Prasad V. 1968. Amblyseius mites from Hawaii. Ann. Entomol. Soc. Amer., 61(6): 1514-1521. doi:10.1093/aesa/61.6.1514 ![]()
Pritchard A.E., Baker E.W. 1962. Mites of the family Phytoseiidae from Central Africa, with remarks on the genera of the world. Hilgardia, 33(7): 205-309. doi:10.3733/hilg.v33n07p205 ![]()
Quilici S., Kreiter S., Ueckermann E. A., Vincenot D. 1997. Predatory mites (Acari) from various crops on Réunion Island. Intern. J. Acarol., 23(4): 283-291. doi:10.1080/01647959708683578 ![]()
Quilici S., Ueckermann E. A., Kreiter S., Vayssières J.-F. 2000. Phytoseiidae (Acari) of La Réunion Island. Acarologia, 41(1-2): 97-108.
Reis P.R., Teodoro A.V., Pedro Neto M., Da Silva E.A. 2007. Life history of Amblyseius herbicolus (Chant) (Acari: Phytoseiidae) on coffee plants. Neotrop. Entomol., 36(2): 282-287. doi:10.1590/S1519-566X2007000200016 ![]()
Ribaga C. 1904. Gamasidi planticoli. Riv. Patol. Veget., 10: 175-178.
Rodriguez-Cruz F.A., Venzon M., Pinto C.M.F. 2013. Performance of Amblyseius herbicolus on broad mites and on castor bean and sunnhemp pollen. Exp. Appl. Acarol., 60: 497-507. doi:10.1007/s10493-013-9665-y ![]()
Rowell H.J., Chant D.A., Hansell R.I.C. 1978. The determination of setal homologies and setal patterns on the dorsal shield in the family Phytoseiidae (Acarina: Mesostigmata). Can. Entomol., 110: 859-876. doi:10.4039/Ent110859-8 ![]()
Schicha E. 1984. Contribution to the knowledge of the genus Phytoseius Ribaga in Australia, the South Pacific and Indian Ocean regions with four new species and records of known species (Acarina: Phytoseiidae). Intern. J. Acarol., 10(2): 117-128. doi:10.1080/01647958408683361 ![]()
Schicha E. 1987. Phytoseiidae of Australia and neighboring areas. Indira Publishing House, West Bloomfield, Michigan, USA, 187 pp.
Schicha E., Gutierrez J. 1985. Phytoseiidae of Papua New Guinea, with three new species, and new records of Tetranychidae (Acari). Intern. J. Acarol., 11(3): 173-181. doi:10.1080/01647958508683412 ![]()
Schicha E., Corpuz-Raros L.A. 1992. Phytoseiidae of the Philippines. Indira Publishing House, West Bloomfield, Michigan, USA, 190 pp.
Swirski E., Amitai S. 1966. Descriptions of the males of four phytoseiid mites (Acarina) from Hong Kong. Isr. J. Agric. Res., 16: 11-18.
Swirski E., Golan Y. 1967. On some phytoseiid mites (Acarina) from Luzon Island (Phillipines). Israel J. Agric. Res., 17: 225-227.
Swirski E., Shechter R. 1961. Some phytoseiid mites (Acarina: Phytoseiidae) of Hong-Kong, with a description of a new genus and seven new species. Isr. J. Agric. Res., 11: 97-117.
Tixier M.-S., Kreiter S., Okassa M., Cheval B. 2010. A new species of the genus Euseius Wainstein (Acari: Phytoseiidae) from France. J. Nat. Hist., 44(3-4): 241-254. doi:10.1080/00222930903383529 ![]()
Tseng Y.H. 1976. Systematics of the mite family Phytoseiidae from Taiwan, with a revised key to genera of the world (II). J. Agric. Ass. China New Series, 94: 85-128.
Ueckermann E.A., Loots G.C. 1985. A review of the Afrotropical species of the genus Phytoseius Ribaga, 1902 (Acari: Phytoseiidae) Phytophylactica, 17: 31-39.
Ueckermann E.A., Loots G.C. 1988. The African species of the subgenera Anthoseius De Leon and Amblyseius Berlese (Acari: Phytoseiidae). Entomol. Mem., Dep. Agric. Water Supply, Rep. South Africa 73, 168 pp.
Ueckermann, E.A., Zannou, I.D., Moraes, G.J. de, Oliveira, A.R. de, Hanna, R., Yaninek J.S. 2008. Phytoseiid mites of the tribe Typhlodromini (Acari: Phytoseiidae) from sub-Saharan Africa. Zootaxa, 1901: 1-122. doi:10.11646/zootaxa.1901.1.1 ![]()
van der Merwe G.G. 1965. South African Phytoseiidae (Acarina). I. Nine new species of the genus Amblyseius Berlese. J. Entomol. Soc. South Afr., 28: 57-76.
van der Merwe G.G. 1968. A taxonomic study of the family Phytoseiidae (Acari) in South Africa with contributions to the biology of two species. Entomol. Mem. South Africa Dep. Agric. Techn. Serv., 18: 1-198.
van Rijn P.C.J., Sabelis M.W. 1993. Does alternative food always enhance biological control? The effect of pollen on the interaction between western flower thrips and its predators. IOBC/WPRS Bull., 16(8): 123-125.
Vitzthum H. von 1941. Acarina. In: Bronns, H.G. (Ed.), Klassen und Ordnungen des Tierreichs 5, Akademischer Verlag, Leipzig, Germany, pp. 764-767.
Wackers F. 2013. Food for thought: Nutritional supplements to boost biocontrol. Presentation at the Annual Biocontrol Industry Meeting (ABIM), October 21-23, Congress Centre Basel, Switzerland. http://www.abim.ch/fileadmin/documents-abim/Presentations_2013/ABIM_2013_3_2_Wackers_01.pdf ![]()
Wainstein B.A. 1962. Révision du genre Typhlodromus Scheuten, 1857 et systématique de la famille des Phytoseiidae (Berlese 1916) (Acarina: Parasitiformes). Acarologia, 4: 5-30.
Wu W.N. 1997. A review of taxonomic studies of the genus Phytoseius (Acari: Phytoseiidae) from China. Syst. Appl. Acarol., 2: 149-160. doi:10.11158/saa.2.1.21 ![]()
Zannou I.D., Moraes G.J. de, Ueckermann E.A., Oliveira A.R., Yaninek J.S., Hanna R. 2007. Phytoseiid mites of the subtribe Amblyseiina (Acari: Phytoseiidae: Amblyseiini) from sub-Saharan Africa. Zootaxa, 1550: 1-47. doi:10.11646/zootaxa.1550.1.1 ![]()



2020-05-05
Date accepted:
2020-06-16
Date published:
2020-06-24
Edited by:
Tsolakis, Haralabos

This work is licensed under a Creative Commons Attribution 4.0 International License
2020 Kreiter, Serge and Abo-Shnaf, Reham I. A.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




