A new mite species of the genus Neottialges (Acariformes: Hypoderatidae) from the black stork Ciconia nigra (Ciconiiformes: Ciconiidae) in Portugal
Mironov, Sergey V.1 and Ramilo, David W.R.2
1✉ Zoological Institute of the Russian Academy of Sciences, Universitetskaya Embankment 1, 199034, Saint Petersburg, Russia & Tumen State University, 10 Semakova Str., 625003, Tumen, Russia.
2CIISA – Centre for Interdisciplinary Research in Animal Health, Faculty of Veterinary Medicine, University of Lisbon, Avenida da Universidade Técnica, 1300-477 Lisbon, Portugal.
2019 - Volume: 59 Issue: 2 pages: 279-287
https://doi.org/10.24349/acarologia/20194332ZooBank LSID: 9E696F61-CB06-42F0-8BAD-1964ABBECF57
Original research
Keywords
Abstract
Introduction
Astigmatan mites of the family Hypoderatidae are mainly associated with birds and occasionally with some rodents. At the stage of deutonymph, also referred in astigmatan mites to as hypopus, these mites are subcutaneous or visceral tissue parasites, while remaining stages of their life cycle are commensals inhabiting nests of the corresponding vertebrate hosts (Fain and Bafort 1967; OConnor 1982, 1985; Wurst and Havelka 1997; Krantz and Walter 2009; Mironov and Kivganov 2010; Mironov and OConnor 2013). The most surprising feature of these mites is that the deutonymph, which is the main (or in some genera the only) feeding stage in the life cycle, lacks any mouthparts and even a mouth opening. The feeding process of hypoderatid deutonymphs was for a long time an enigma. It was suggested that they probably uptake tissue fluids of their hosts taking them directly though the body cuticle (Mironov and Kivganov 2010). In the course of feeding, which can take a year until the next reproductive period of hosts, deutonymphs grow greatly by the way of neosomy that causes strong changes in their general appearance, particularly in the size and form of the idiosoma, the pattern of cuticlar sclerotization, and relative position of legs and idiosomal setae. Recent investigation has shown that genital papillae are apparently nutrient-intake organs adsorbing liquid materials from host to mite (Alberti et al. 2016).
Other active stages of hypoderatids living in bird's nests are saprophages or aphages. The full life-cycle has been described only for the four species, Apodidectes verrucosus Mironov and OConnor, 2013, Hypodectoides propus (Nitzsch, 1861), Suladectes hughesae antipodus Fain and Clark, 1994, and Tytodectes strigis (Gené, 1845) (Fain and Bafort 1966, 1967, Fain and Clark 1994, Wurst and Havelka 1997; Mironov and OConnor 2013). In the genera Apodidectes Mironov and OConnor, 2013 and Tytodectes Fain, 1966, the larva, protonymph, tritonymph, and adults have the appearance of typical free-living astigmatans such as Acaridae, with well-developed legs and normally developed mouthparts. Moreover, it was shown that Tytodectes mites can omit the deutonymphal stage and complete a full generation in the nest (Wurst and Havelka 1997), indicating that these stages do feed in the nest of their hosts. In contrast to that, in the genus Hypodectoides, the tritonymph, protonymph, and larva are strongly reduced (calyptostases) and represented by sack-like cuticle shells lacking legs and mouthparts and are obviously unable to feed. Adults of H. propus have normally developed legs but very odd mouthparts — in females, chelicerae are vestigial and in males, they are strongly hypertrophied (Fain and Bafort 1967). Therefore it is still unknown, whether adult hypoderatids with modified chelicerae are capable of feeding.
The family Hypoderatidae currently includes over 80 species arranged in 24 genera and two subfamilies. The subfamily Hypoderatinae (about 75 species in 22 genera) includes mites associated with birds (Fain 1967; OConnor 1985; Fain and Lukoschus 1986; Mironov and Kivganov 2010; Mironov and OConnor 2013). A comprehensive taxonomic revision of most species known at that time from deutonymphs associated with birds was given by Fain (1967). To date, representatives of this subfamily have been recorded from avian hosts of 14 orders as classified by Gill and Donsker (2018). Five species in two genera, restricted to certain desert and borrowing rodents, constitute the second subfamily, Muridectinae, known from deutonymphs only (Fain 1968; Fain and Lukoschus 1977, 1978). Most hypoderatid species are known only from deutonymphs, while adult and/or tritonymphal stages have been described for a few species from 11 genera. Mironov and Kivganov (2010) provided a list of species for which non-deutonymphal stages are known. Because of the large gap in the knowledge of adult morphology, the taxonomic system of hypoderatids is generally based on the morphological structures of deutonymphs.
In the present paper we describe a new species of the genus Neottialges Fain, 1966 based on deutonymphs collected from the black stork, Ciconia nigra, in Portugal.
Material and methods
The individual black stork, from which hypoderatid mites were collected, was found near the Alqueva Dam (at the border of Beja and Évora Districts, Portugal). It was transported to Lisbon Recovery Center of Sylvatic Animals (LxCRAS) by the Instituto de Conservação da Natureza e Florestas, I.P. (ICNF) personnel, Lisbon, Portugal, where it was treated for three days. After that, the bird died and was delivered by LxCRAS personnel to the Faculty of Veterinary Medicine, University of Lisbon. Several mite specimens were found under the skin of this bird during the process of necropsy and parasitological examination (at the Pathology Laboratory of the Faculty of Veterinary Medicine, University of Lisbon, Portugal), fixed in 96 % ethanol and then mounted on microslides in Hoyer's medium (Krantz and Walter 2009). Mites were studied and figured using a Leica DM2500 microscope with DIC and equipped with a camera lucida.
The description of new species follows the modern format used for hypoderatid mites of the genus Neottialges and related genera (Fain and Clark 1994; Mironov and Kivganov 2010; Mironov and OConnor 2013). The idiosomal chaetotaxy is that of Grandjean (1939a) as modified by Griffiths et al. (1990) and Norton (1998); the leg chaetotaxy is that of Grandjean (1939b). All measurements in descriptions are in micrometers. Classification and Latin names of birds follow Gill and Donsker (2018). Abbreviations used in access number and depositories of type specimens: FMV — Faculty of Veterinary Medicine, University of Lisbon, Lisbon, Portugal; UMMZ — Museum of Zoology, University of Michigan, Ann Arbor, USA; ZISP — Zoological Institute of the Russian Academy of Sciences, Saint Petersburg, Russia.
Systematics
Family Hypoderatidae Murray, 1877
Subfamily Hypoderatinae Murray, 1877
Genus Neottialges Fain, 1966
Type species: Neottialges (Neottialges) geopeliae Fain, 1966, by original designation.
The genus Neottialges was established by Fain (1966) and originally included six species; at present, including the new species described below, it incorporates 35 species and is the most speciose within the family (Fain 1966, 1967, 1969; Fain and Amerson 1968; Pence 1971, 1973; Pence and Courtney 1973; Fain and Kigaye 1976; Fain and Domrow 1978; Fain and Lawrence 1979, 1986; Young and Pence 1979; OConnor 1985; Fain and Lukoschus 1986; Pence and Duncan 1995; Pence and Newman 1997; Pence et al. 1997; Mironov and Kivganov 2010; De Liberato et al. 2017). Fain (1966, 1967) recognized originally three subgenera within this genus: Caloenectes Fain, 1966, Neottialges s. str., and Pelecanectes Fain, 1966. Later, Fain and Lukoschus (1986) established two more subgenera, Ardeidectes Fain and Lukoschus, 1986 and Heronidectes Fain and Lukoschus, 1986. Adult mites are known only in a few species from each subgenus, except Caloenectes, whose adults are not yet known. The latest key to deutonymphs of most known species was given by Fain and Lawrence (1986); for major references for previously described Neottialges species see Mironov and Kivganov (2010).
Representatives of the genus Neottialges are presently known from birds of the orders Accipitriformes, Ciconiiformes, Charadriiformes, Columbiformes, Falconiformes, Musophagiformes, and Pelecaniformes (Fain 1967; Fain and Lawrence 1986; Fain and Lukoschus 1986; Mironov and Kivganov 2010). Of previously known species of this genus, three species of the subgenus Caloenectes have been described from storks (Ciconiiformes: Ciconiidae): Neottialges (Caloenectes) distinctus Fain and Lawrence, 1986 from the Maguari stork, Ciconia maguari (Gmelin, JF), N. ( C.) ellipticus (Nitzsch in: Giebel (1861)) n. comb. from the white stork C. ciconia Linnaeus, and N. ( C.) mycteriae Pence, 1973 from the wood stork, Mycteria americana Linnaeus, and painted stork, M. leucocephala (Pennant) (Giebel 1861; Fain 1967, 1973; Pence 1973; Fain and Lawrence 1986). Neottialges ellipticus was originally described in the genus Hypoderas Nitzsch, 1861 (now Hypodectes). Since the illustration of this species was rather schematic, Fain (1967) in his revision of hypoderatids parasitizing birds treated this mite as a species inquirenda; however the size and general shape of idiosoma, proportion of legs and their segments allow this mite to be referred to the genus Neottialges.
Neottialges (Caloenectes) ciconiae n. sp.
ZOOBANK: 99A9608D-C8F3-478A-8138-85EB5EAE0E88 ![]()
(Figures 1–3)
Type material — Holotype deutonymph (FMV 2001) and 10 deutonymph paratypes from Ciconia nigra (Linnaeus) (Ciconiiformes: Ciconiidae), Portugal, border of Beja and Évora Districts, Alqueva Dam, 38°11'51''N, 7°29'47''W, 23 March 2018, collected by ICNF (Instituto de Conservação da Natureza e das Florestas, I.P.) personnel.
Depositories — Holotype and 3 paratypes — FMV, remaining paratypes in UMMZ and ZISP.
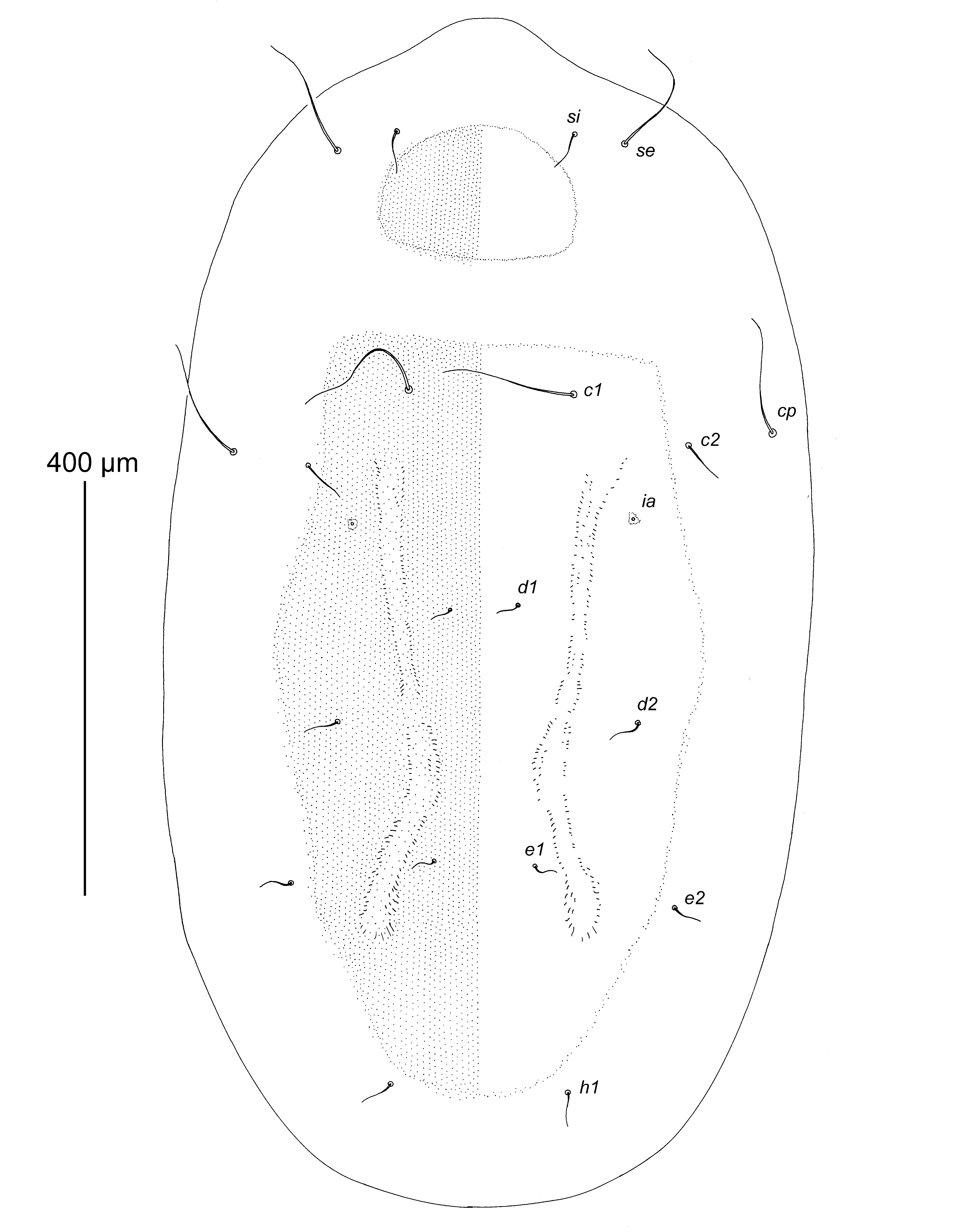


Description — Deutonymph (holotype, range for 10 paratypes in parentheses). Idiosoma ovate, with very short and wide rostral extension, length 1040 (950–1050), greatest width 500 (600–450). Median area of dorsal side of idiosoma occupied by roughly punctate prodorsal and hysteronotal shields with blurred and indistinct borders (Figure 1). Sejugal furrow not expressed dorsally. Prodorsal shield transversely ovate in form, occupying central part of propodosoma, not encompassing bases of scapular setae si and se. Setae si situated slightly anterior to level of setae se; distances between scapular setae: se:se 260 (215–260), si:si 170 (125–170); length of setae: se 115 (110–120), si 30 (20–30). Hysteronotal shield occupying only median area of hysterosoma, anterior margin straight, posterior margin rounded, surface with a pair of poorly sclerotized longitudinal bands. Setae cp situated dorsally. Setae c2 posterior to level of setae cp. Setae c1, d1, d2 and e1 situated on hysteronotal shield, setae cp, c2, e2, and h1 situated off hysteronotal shield. Cupules ia situated posteromesal to setae c2; cupules im, ip and ih indistinct. Four pairs of hysterosomal setae (cp, c1, c3, and h3) elongate and comparable in length to scapular setae se; remaining setae of dorsal side of hysterosoma and ventral setae of posterior end of opisthosoma very thin and not exceeding 50 long; length of long hysterosomal setae: cp 120 (100–140), c1 130 (90–130), c3 110 (100–120), h3 140 (130–150).
Gnathosoma situated ventrally, reduced to small trapezoidal sclerite 15 (15–17) long and 28 (25–28) wide at base, with two pairs of rudimentary setae represented by alveoli (Figures 2 and 3A). Vertical setae vi filiform, about 15 long, situated ventrally near anterior end of gnathosomal plate. Supracoxal setae scx situated near lateral margins of gnathosomal plate in small invaginations. Sternum 20 (12–20) long, much shorter than free parts of epimerites I. Coxal fields I, II and IV open; coxal fields III closed. Inner tips of epimerites III, IV with irregular pennate striation. Bases of trochanters I–IV flanked by narrow sclerotized bands. Coxal setae 1a and 3a rudimentary, represented by alveoli. Genital field ovate, situated at level of trochanters IV, well outlined. Genital apodeme rudimentary, represented by a pair of small inverted L-shaped sclerites anterior to genital papillae; surface between papillae poorly sclerotized (Figure 3B). Genital papillae ovate, small, anterior and posterior pairs similar in size, length 15 (13–15). Anal opening rudimentary, distant from genital papillae. Coxal setae 4b 45 (30–45) long, situated at midlevel of coxal fields III, genital setae g 40 (35–50) long. Postanal shield absent, setae h2 and h3 situated on soft tegument.



Leg segments normally developed, tarsi I, II approximately 1.5 times longer than corresponding tibiae and genua, tarsi III, IV 2.3–2.5 times longer than corresponding tibiae and genua. Length of legs excluding trochanters: I 87 (85–88), II 92 (90–92), III 125 (120–128), IV 110 (105–115); length of tarsi: I 37 (35–40), II 42 (37–42), III 68 (62–70), IV 65 (58–68). Tarsus I with setae aa, d, wa long filiform with simple tips; setae la, ra, p, q long filiform with foliate distal tips, seta e short filiform, seta f spine-like, solenidion ω1 distinctly punctate and slightly tapering apically; solenidion ω3 elongate, situated apically; famulus ε spine-like, situated at midlevel of tarsus, at same level as setae aa and wa ; solenidion ω2 vestigial, situated anterior to base of seta aa (Figure 3C). Tarsus II similar in structure to tarsus I (except for absence of seta aa and famulus and presence of ba), solenidion ω1 punctate and slightly thickened in basal third (Figure 3D). Tarsus III strongly elongate and straight, with small apical spine, with 8 setae: seta d long filiform, exceeding the length of segment; setae w, r filiform, nearly half as long as segment; setae e, f, p, q, filiform with foliate apices; seta s short spiculiform (Figure 3E). Tarsus IV with small apical spine and with 4 setae of uncertain homology: apical seta (possibly d) represented by macroseta, nearly 3 times longer than leg IV and with small sparse barbs in basal part; two spine-like setae (possibly w, r) situated basally, and thin spine-like seta (possibly s) situated subapically (Figure 3F). Empodial claws of tarsi I, II shorter than half-length of corresponding tarsi, acute and slightly curved apically, both 12 (11–13) long; empodial claw of tarsus III bidentate apically and slightly curved, 10 (8–10) long. Tibia I with longitudinal dorsal crest, seta gT long filiform, seta hT thick spine-like, solenidion φ subequal in length to solenidion ω1 of tarsus I. Tibia II similar to tibia I except both setae gT and hT spine-like, solenidion φ twice as long as solenidion ω1 of tarsus II. Tibia III with seta kT thick, spine-like, solenidion φ short, about 2/3 the length of this segment. Tibia IV with seta kT thick, spine-like and solenidion φ about 1/3 the length of this segment. Genu I with setae mG, cG filiform, solenidion σ2 a very short and blunt spine. Genu II with seta mG filiform, seta cG as on tarsus I, and solenidion σ spiculiform. Genu III with seta nG thick spine-like, with solenidion σ small, spiniform. Femoral setae vFI, vFII, and wFIV filiform, shorter than corresponding legs. Setae pR of trochanters I, II subequal in length to corresponding tarsi, seta sR of trochanter III shorter than corresponding tarsus. Leg setation (solenidia in parentheses): tarsi 10(3)-9(1)-8-4, tibiae 2(1)-2(1)-1(1)-1(1), genua 2(1)-2(1)-1(1)-0, femora 1-1-0-1, trochanters 1-1-1-0.



Differential diagnosis — Among previously known species, Neottialges (Caloenectes) ciconiae n. sp. is most similar to N. ( C.) distinctus Fain and Lawrence, 1986 in having the median dorsal sclerotization of the idiosoma represented by separate prodorsal and hysteronotal shields, the median sclerotized bridge connecting the ventral propodosomal sclerotized area (fused coxal fields I, II) and ventral hysterosomal sclerotized area (coxal fields III, IV), and only two long pairs of hysteronotal setae (c1 and cp). Neottialges (Caloenectes) ciconiae differs from the latter species by the following features: the bases of setae si, se are not encompassed by the prodorsal shield, hysteronotal setae cp, c2, e2 and h1 are situated off the hysteronotal shield, the area between the genital papillae is completely sclerotized, and its anterior end is fused with the sclerotized area of coxal fields III, IV, and the postanal shield on the ventral side of opisthosoma is absent. In deutonymphs of N. ( C.) distinctus, the bases of setae si, se are encompassed by the prodorsal shield, hysteronotal setae cp, c2, e2 and h1 are situated on the hysteronotal shield, the area between the genital papillae is sclerotized only medially, and its anterior end is separated from the sclerotized area of coxal fields III, IV, and the ventral side of the opisthosoma bears a rhomboid postanal shield encompassing the bases of setae h2 and h3.
Acknowledgements
The authors thank to the ICNF personnel, Dr. Inês Caetano and Dr. Érica Brizio (LxCRAS) for delivering the Ciconia nigra specimen at FMV, Leonor Antunes (Student of Integrated Master in Veterinary Medicine) who found the mites, to the supervisors of DR, Prof. Isabel Fonseca and Prof. Luís Cardoso, and to the Pathology Laboratory and the Laboratory of Parasitology and Parasitological Diseases of the Faculty of Veterinary Medicine, University of Lisbon, Lisbon, Portugal. The study was supported by The Russian Academy of Science (State theme No АААА-А19-119020790133-6) for SM, and by the Centre for Interdisciplinary Research in Animal Health, project UID/CVT/00276/2019, and the post-doctoral grant SFRH/BPD/115202/2016 for DR.
References
Alberti G., Kanarek G., Dabert J. 2016. Unusual way of feeding by the deutonymph of Neottialges evansi (Actinotrichida, Astigmata, Hypoderatidae), a subcutaneous parasite of cormorants, revealed by fine structural analyses. J. Morphol., 277: 1368-1389. doi:10.1002/jmor.20584 ![]()
De Liberato C., Magliano A., Tancredi F., Eleni C., Posillico M., Mironov S. 2018. Neottialges (Caloenectes) vulturis (Dubinin, 1956) (Acari: Hypoderatidae) from the Eurasian griffon vulture (Gyps fulvus) in Italy: first record in Europe, redescription and pathological changes in the host. Acarologia, 58: 255-264. doi:10.24349/acarologia/20184239 ![]()
Fain A. 1966. Note sur les Acariens nidicoles à deutonymphe parasite tissulaire des oiseaux (Hypodectidae: Sarcoptiformes) (Note préliminaire). Rev. Zool. Bot. Afr., 74: 324-330.
Fain A. 1967. Les hypopes parasites des tissus cellulaires des oiseaux (Hypodectidae : Sarcoptiformes). Bull. Inst. Roy. Sci. Nat. France, 43: 1-139.
Fain A. 1968. Un hypope de la famille Hypoderidae Murray 1877 vivant sous la peau d'un ronger (Hypoderidae: Sarcoptiformes). Acarologia, 10: 111-115.
Fain A. 1969. Noveaux hypopes parasites des tissues cellulares d'oiseaux. Bull. Ann. Soc. Roy. Entomol. France, 105: 91-102.
Fain A. 1973. Hyperparasitism of storks by hypopi of Hypoderidae with description of a new species of the genus Neottialges (Acarina: Sarcoptiformes). Bull. Ann. Soc. Roy. Belge Entomol., 109: 191-194.
Fain A., Amerson Jr. A.B. 1968. Two new heteromorphic deutonymphs (hypopi) (Acarina: Hypoderidae) from the great frigatebird (Fregata minor). J. Nat. Hist., 5: 320-324. doi:10.1093/jmedent/5.3.320 ![]()
Fain A., Bafort J. 1966. Les hypopes parasitant les tissus cellulaires des pigeons sont les deutonymphes d'un acarien libre et pas celles d'un acarien plumicole (Note préliminaire). Rev. Zool. Bot. Afr., 74: 313-316.
Fain A., Bafort J. 1967. Cycle évolutif et morphologie de Hypodectes (Hypodectoides) propus (Nitzsch) acarien nidicole à deutonymphe parasite tissulaire des pigeons. Acad. Roy France, Bull. Classe Sci., 5e Série, 53: 501-533
Fain A., Clark J.M. 1994. Description and life cycle of Suladectes hughesae antipodus subsp. n. (Acari: Hypoderatidae) associated with Sula bassana serrator Gray (Aves: Pelecaniformes) in New Zealand. Acarologia, 35: 361-371.
Fain A., Domrow R. 1978. The family Hypoderidae (Acari) in Australia. Proc. Linnaean Soc. New South Wales, 103: 43-46.
Fain A., Kigaye M. 1976. Neottialges (Pelecanectes) leptoptilus sp. n. from the marabou (Acarina, Astigmata, Hypoderidae). Rev. Zool. Afr., 90: 30-32.
Fain A., Lawrence B.R. 1979. Neottialges (Pelecanectes) platalea sp. nov. and other hypoderid mites (Acarina, Astigmata, Hypoderidae) from the spoonbill, Platalea leucorodia L. J. Nat. Hist., 13: 333-336. doi:10.1080/00222937900770271 ![]()
Fain A., Lawrence B.R. 1986. Two new species of Neottialges Fain (Acari, Hypoderatidae) under the skin of birds, with a key to the hypopi of this genus. J. Nat. Hist., 20: 849-856. doi:10.1080/00222938600770621 ![]()
Fain A., Lukoschus F. 1977. New endofollicular or subcutaneous hypopi from mammals (Acarina: Astigmata). Acarologia, 19: 484-493.
Fain A, Lukoschus F. 1978. Dipodomydectes americanus gen. et sp. n. (Acari: Hypoderidae) from the kangaroo rat. J. Parasitol., 64: 137-138. doi:10.2307/3279625 ![]()
Fain A., Lukoschus F. 1986. Observation on the life cycle of Neottialges (Pelecanectes) evansi Fain, 1966 and Phalacrodectes whartoni Fain, 1967 with description of new taxa (Acari, Hypoderatidae). Syst. Parasitol., 84: 291316. doi:10.1007/BF00009738 ![]()
Giebel C. 1861. Die Milbenarten der Gattung Hypoderas Nitzsch. Zeitschrift für die Gesammten Naturwissenschaften, 18: 438-444.
Gill F., Donsker D. (Eds.) 2018. IOC World Bird List (v 8.1). doi: 10.14344/IOC.ML.8.1. / Accessed 20 April 2018. doi:10.14344/IOC.ML.8.1 ![]()
Grandjean F. 1939a. Quelques genres d'Acariens appartenant au groupe des Endeostigmata. Ann. Sci. Nat., Zool., 2: 1-122.
Grandgean F. 1939b. La chaetotaxy des pattes chez les Acaridiae. Bull. Soc. Zool. France, 64: 50-60.
Griffiths D. A., Atyeo W. T., Norton R. A. , Lynch C. A. 1990. The idiosomal chaetotaxy of astigmatid mites. J. Zool., 220: 1-32. doi:10.1111/j.1469-7998.1990.tb04291.x ![]()
Krantz G., Walter D. (Eds.) 2009. A manual of Acarology. Third Edition. Texas University Press, Lubbock. 807 p.
Mironov S.V., Kivganov D.A. 2010. Descriptions of adult stages of new and little known mite species of the family Hypoderatidae (Acari: Astigmata) from nests of aquatic birds. Acarina, 18: 37-59.
Mironov S.V., OConnor B.M. 2013. Two new genera of mites of the family Hypoderatidae (Acari: Astigmata) from swifts and their nests (Apodiformes: Apodidae) from the Philippines. Int. J. Acarol., 39: 209-234. doi:10.1080/01647954.2012.758654 ![]()
Norton R.A. 1998. Morphological evidence for the evolutionary origin of Astigmata (Acari: Acariformes). Exp. Appl. Acarol., 22: 559-594.
OConnor B.M. 1985. Hypoderatid mites (Acari) associated with cormorants (Aves: Phalacrocoracidae), with description of a new species. J. Med. Entomol., 22: 324-331. doi:10.1093/jmedent/22.3.324 ![]()
Pence D.B. 1971. The hypopi (Acarina: Hypoderidae) from the subcutaneous tissues of the white ibis Eudocimus albus L. J. Parasitol., 67: 1321-1323. doi:10.2307/3277992 ![]()
Pence D.B. 1973. Hypopi (Acarina: Hypoderidae) from the subcutaneous tissues of the wood ibis, Mycteria americana L. J. Med. Entomol., 10: 240-243. doi:10.1093/jmedent/10.3.240 ![]()
Pence D.B., Courtney C.H. 1973. The hypopi (Acarina: Hypoderidae) from the subcutaneous tissues of the brown pelican, Pelecanus occidentalis carolinensis Gmelin. J. Parasitol., 59: 711-718. doi:10.2307/3278870 ![]()
Pence D.B., Duncan M. 1995. Hypopi (Acari: Hypoderatidae) from subcutaneous tissues of the African spoonbill (Aves: Ciconiiformes: Threskiornithidae). J. Med. Entomol., 32: 166-173. doi:10.1093/jmedent/32.2.166 ![]()
Pence D.B., Newman S. 1997. Neottialges neopelagicus new species (Acari: Hypoderatidae) from the pelagic cormorant (Aves: Phalacrocoracidae: Pelecaniformes). J. Med. Entomol., 34: 32-37. doi:10.1093/jmedent/34.1.33 ![]()
Pence D.B., Spalding M.G., Bergan J. F., Cole R. A., Newman S., Gray P.N. 1997. New records of subcutaneous mites (Acari: Hypoderatidae) in birds, with examples of potential host colonization events. J. Med. Entomol., 34: 411-416. doi:10.1093/jmedent/34.4.411 ![]()
OConnor B.M. 1982. Acariformes. Astigmata. In: Parker S.B. (Ed.). Synopsis and classification of living organisms. Vol. 2. NY: McGraw-Hill Book Company. p. 146-169.
Wurst E., Havelka P. 1997. Redescription and life history of Tytodectes strigis (Acari: Hypoderatidae), a parasite of the barn owl Tyto alba (Aves: Strigidae). Stuttgarter Beiträge zur Naturkunde, Serie A (Biologie), 554: 1-39.
Young V.E., Pence D.B. 1979. Neottialges (Pelecanectes) ibisicola sp.n. (Acari: Hypoderidae) from the subcutaneous tissues of the white-faced ibis, Plegadis chihi (Ciconiiformes: Threskiornithidae). J. Parasitol., 65: 659-661. doi:10.2307/3280337 ![]()



2019-05-13
Date accepted:
2019-07-09
Date published:
2019-07-15
Edited by:
Mąkol, Joanna

This work is licensed under a Creative Commons Attribution 4.0 International License
2019 Mironov, Sergey V. and Ramilo, David W.R.
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




