Ghilarovus robisoni n. sp., first record of Zetomotrichidae (Acari, Oribatida) from North America
Behan-Pelletier, Valerie M.1 and Knee, Wayne2
1✉ Canadian National Collection of Insects, Arachnids, and Nematodes, Agriculture and Agri-Food Canada, 960 Carling Avenue, K.W. Neatby Building, Ottawa, Ontario, K1A 0C6, Canada.
2Canadian National Collection of Insects, Arachnids, and Nematodes, Agriculture and Agri-Food Canada, 960 Carling Avenue, K.W. Neatby Building, Ottawa, Ontario, K1A 0C6, Canada.
2019 - Volume: 59 Issue: 2 pages: 226-241
https://doi.org/10.24349/acarologia/20194327ZooBank LSID: 70C869DC-D9CF-4C21-A400-FEF69DC0F022
Original research
Keywords
Abstract
Introduction
Representatives of the Zetomotrichidae are unknown from North America, north of Mexico (Marshall et al. 1987). They have been found in warm regions across the Palaearctic from southern Europe to India, China, Japan and Vietnam (Krivolutsky 1966, Krivolutsky & Karppinen 2006, Krivolutsky & Smelyansky 1996; Bayartogtokh & Smelyansky 2007), and are also known from Australia (Lee & Pajak 1987), Mexico (Mahunka 1983) and South America (Hammer 1958). First described from North Africa (Grandjean 1934), a diverse fauna is known in South Africa (Coetzee 1993, 1995). We describe a new zetomotrichid species, Ghilarovus robisoni n. sp., on the basis of adults collected from dry, usually rocky, vertical microhabitats in forests of Arkansas, New Mexico and Texas, USA. It represents the first record of this genus and family from temperate North America.
Species of Ghilarovus Krivolutsky, 1966 are found in southern Mexico, Spain, across central Asia, in China and Japan. The original short diagnosis of Krivolutsky (1966) was slightly modified by Bayartogtokh and Smelyansky (2007), who also described 3 new species, provided a key to world species and summarized distributions (their Fig. 9). Our objectives are to build on this latter work by describing the North American species, providing an expanded diagnosis for Ghilarovus adults, and revising their key to include G. robisoni n. sp. Then, we discuss the family classification and characters that are unique to Zetomotrichidae.
Materials and Methods
Terminology and Conventions
Morphological terminology used in this study follows that developed by Grandjean (see Travé & Vachon 1975 for references, and Norton & Behan-Pelletier 2009 for overview). The following conventions of measurement and description are used: measurements are in micrometers; prodorsal setae measured on dissected, slide-mounted specimens (ro, rostral seta; le, lamellar seta; in, interlamellar seta; ex, exobothridial seta; bs, bothridial seta (sensillus)); total length, measured from tip of rostrum to posterior edge of notogaster on specimens in cavity slides, except when noted; notogastral width, measured at widest part of notogaster on specimens in cavity slides; leg setal formula, given as setal count per segment, with famulus included in tarsus I count, and solenidial counts given in parentheses. The inclusion of a single leg setal notation in parentheses denotes a pseudosymmetrical pair. The unideficience nomenclature for notogastral setae is used herein; probable synonymies of this nomenclature with the holotrichous nomenclature of Grandjean were outlined by R. A. Norton in Balogh and Balogh (1988).
Material Examined
Specimens examined are housed in various collections (see below).
Abbreviations for Collections
• CNC – Canadian National Collection of Insects, Arachnids and Nematodes, Agriculture and Agri-Food Canada, Ottawa, Ontario, Canada.
• RNC – Personal collection of Roy A. Norton, Syracuse, New York, USA.
• USNM – National Museum of Natural History, Washington, D.C., USA.
Imagery
Specimens for scanning electron microscopy (SEM Quanta 600 FEI Company TM, Brno, Czech Republic) were removed from alcohol and cleaned by soaking in Terg-a-zyme® solution for 6–12 h, followed by brief (1–2 s) submersion in an ultrasonic bath. Specimens were critical-point dried using the EM CPD300 (Leica Microsystems, Vienna, Austria), mounted on Al-stubs with double sided sticky tape, and gold-coated in a Hummer sputter apparatus.
Differential interference contrast images were obtained using a Nikon DS-Fi1 camera and any image stacks were merged with the aid of the Helicon Focus Pro (v. 5.3) suite.
Taxonomy
Ghilarovus Krivolutsky, 1966
Type species: Ghilarovus humeridens Krivolutsky, 1966
Expanded diagnosis. Adult. Small to medium sized mites, 312‒540. Integument. Surface of notogaster smooth. Cerotegument granular, present laterally on podosoma (Figs 5C, 5E). Prodorsum. Rostral margin denticulate (Figs 1A, 4A, 5D). Rostrum and region lateral to seta le with macropores (Fig. 4A). Lamella absent. Carina extending from seta in posteriorly to bothridium (Fig. 1A). Tutorium absent. Genal tooth absent. Pedotectum I uniformly curved; pedotectum II small, scaliform (Fig. 5D). Laterorostral carina extending dorsal to pedotectum I, following contour of pedotectum I, reaching anteriorly, midway to rostral margin (Fig. 5D). Bothridial seta filiform, barbed (Figs 1A, 5C). Bothridium cup-shaped (Fig. 6D). Dorsophragma absent; cheliceral retractor muscles inserting directly on prodorsal and notogastral cuticle (Figs 1A, 3D). Notogaster. Dorsosejugal scissure interrupted medially in region of insertions of cheliceral retractor muscles. Lenticulus absent. Octotaxic system expressed as macropores (Figs 1A, 6C). Posterior notogastral tectum divided or undivided; when divided, edges rounded, overlapping or not. 10 pairs of notogastral setae. Humeral region of notogaster with prominent process bearing seta c2 (Figs 1A, 3A, 6C, 6D); seta c2 longer, thicker and more barbed than other notogastral setae. Lyrifissure ia usually modified as humeral sac; lyrissure im usually unmodified, occasionally modified as pyriform organ. Lateral podosomal and epimeral region. Apodemes III and IV absent (Fig. 1B). Epimeral setal formula 3-1-3-3 or 3-1-3-2. Custodium present (Figs 4B, 5C). Discidium absent. Circumpedal carina absent. Porose areas Al, Am, Ah absent. Acetabula I‒IV in longitudinal alignment (Fig. 5C). Anogenital region. Four pairs of genital setae; one pair aggenital setae; one or two pairs of anal setae; two or three pairs of adanal setae (Figs 1B, 5B). Postanal porose area absent. Lyrifissure ian absent; lyrifissure iad oblique, positioned lateral to anterior margin of anal plate (Fig. 1B). Gnathosoma. Axillary saccule of subcapitulum absent. Palp setal formula 0-1-2-3-9(1), solenidion baculiform, appressed to palp surface, not forming double horn with eupathidium acm (Figs 1C, 6A). Chelicera chelate-dentate; setae of different forms: chb flattened, leaf-like (Figs 1D, 6A). Subcapitulum with seta h strongly directed anteriorly, setae m and a directed medially; adoral setae setose (Fig. 6B). Postpalpal seta ep spiniform. Trägårdh's organ present. Legs. Tridactylous. Leg IV not modified for jumping. Trochanters III and IV with dorsal ridge (Fig. 5C); trochanter III with outwardly curved ventral edge (Figs 5C, 5D). Femora III and IV with narrow ventral carina (Fig. 5C). Porose areas adaxially on femora I‒IV and trochanters III and IV and ventrally on tibia and tarsi I‒IV (Figs 2A‒E). Solenidion ω1 most proximal setiform structure on tarsus II, well separated from ω2 (Figs 2C, 4D).
Juveniles: Unknown.
Included extant species (in alphabetical order after type):
Type species: Ghilarovus humeridens Krivolutsky, 1966; Central Asia, Iran
Ghilarovus armenicus Khanbekyan, 1990; Armenia
Ghilarovus changuligensis Wen, 1990; China
Ghilarovus daliensis Yamamoto and Aoki, 2000; Japan
Ghilarovus elegans Mahunka, 1983; southern Mexico
Ghilarovus hispanicus guadarramicus Subías, 1977; Spain
Ghilarovus hispanicus hispanicus Subías and Pérez-Íñigo, 1977; Spain
Ghilarovus khentiicus Bayartogtokh and Smelyansky, 2007; Mongolia
Ghilarovus krivolutskyi Bayartogtokh and Smelyansky, 2007; Mongolia
Ghilarovus kvavadzei Murvanidze, 2014; Caucasus
Ghilarovus mongolicus Bayartogtokh and Smelyansky, 2007; Mongolia
Ghilarovus robisoni n. sp.; USA
Ghilarovus sanukiensis Fujikawa, 2005; Japan
Ghilarovus saxicola Aoki and Hirauchi, 2000; Japan
Ghilarovus stipatus Krivolutsky and Smelyansky, 1997; Central Asia
Ghilarovus turcmenicus Krivolutsky, 1974; Turkmenistan
Remarks
• 1 Coetzee (1993) included Ghilarovus in the subfamily Zetomotrichinae Grandjean, 1954 (see below). Ghilarovus is distinct from other genera in the subfamily by the combination of: leg IV not modified for jumping, notogastral seta c2 expressed as long, thick seta, 4 pairs of genital setae, notogastral surface smooth (Coetzee 1993).
• 2 In Zetomotrichidae lyrifissures ia and im can be modified as either a large or small sac-like structure. Grandjean (1934, 1954b) named the sac in the position of lyrissure ia a `humeral sac' because of its shape and position in Zetomotrichus lacrimans Grandjean, 1934, the type species; and the structure in the position of lyrifissure im a `pyriform organ' because of its pear-like shape. However, in the zetomotrichid genus Mikizetes, lyrifissure ia is modified as a pyriform shaped structure, which Covarrubias (1969) named `humeral pyriform organ', `pyriform organ' and `humeral organ' in the same publication.
In Ghilarovus lyrifissure ia is modified as a humeral sac (hs), but this modification appears variable among Ghilarovus species, as indicated by Murvanidze (2014). The humeral sac is present in G. elegans, G. khentiicus, G. krivolutskyi, G. mongolicus, G. sanukensis, G. saxicola and G. robisoni n. sp. It is not mentioned or illustrated in the descriptions of G. armenicus, G. humeridens, G. stipatus and G. turcmenicus but may have been overlooked, especially as it could collapse in older specimens. In the descriptions of G. changliensis, G. daliensis, G. kvavadzei, G. hispanicus hispanicus and G. hispanicus guadarramicus it is noted as not visible.
• 3 There has been confusion in descriptions of Ghilarovus species on the presence or absence of the pyriform organ found in the position of lyrifissure im. The pyriform organ is illustrated as present in G. elegans (Mahunka 1983, his Fig. 58), but lyrifissure im is also illustrated. Yamamoto and Aoki (2000) described both im and the presence of the pyriform organ ``near im, inside the body'' in G. daliensis. Similarly, Bayartogtokh and Smelyansky (2007) described both im and the pyriform organ as present in G. krivolutskyi, as did Murvanidze (2014) in G. kvavadzei. We consider these as possible misinterpretations, with the illustrated structure possibly the darkened contents of the opisthonotal gland rather than the pyriform organ described by Grandjean (1954b). In Figs 1A and 3A of G. robisoni n. sp., im is shown as anterior to the opisthonotal gland, which has a dark, vase-like shape, which could be misinterpreted (Figs 3A, 3F).
Hypertrophy of lyrifissures is not unique to Zetomotrichidae. Grandjean (1957) noted a similar hypertrophy or transformation of either lyrifissure ih or ips in the galumnid Cryptogalumna cryptodonta Grandjean, 1957, where the hypertrophy is in the form of an interal mass positioned lateral to the opening of the opisthonotal gland. A modification of lyrifissure im as a tubercle-like structure was described in Scapheremaeus argentinensis Travé and Fernandez (1986). However, in all examples of lyrifissure hypertrophication the function of each modification and the contents of the humeral sac and pyriform organ are unknown.
• 4 The notogaster of some Ghilarovus species has a complete posterior tectum (G. armenicus, G. changlingensis, G. elegans, G. hispanicus hispanicus, G. hispanicus guadarramicus, G. humeridens, G. kvavadzei, G. sanukiensis, G. stipatus), while in others it is medially divided, with lobes either overlapping (G. krivolutskyi, G. mongolicus, G. robisoni n. sp., G. saxicola) or separate (G. khentiicus). The medial division can be difficult to detect and could have been overlooked in some species, e.g., G. daliensis and G. turcmenicus, where it is neither illustrated nor described.
Description



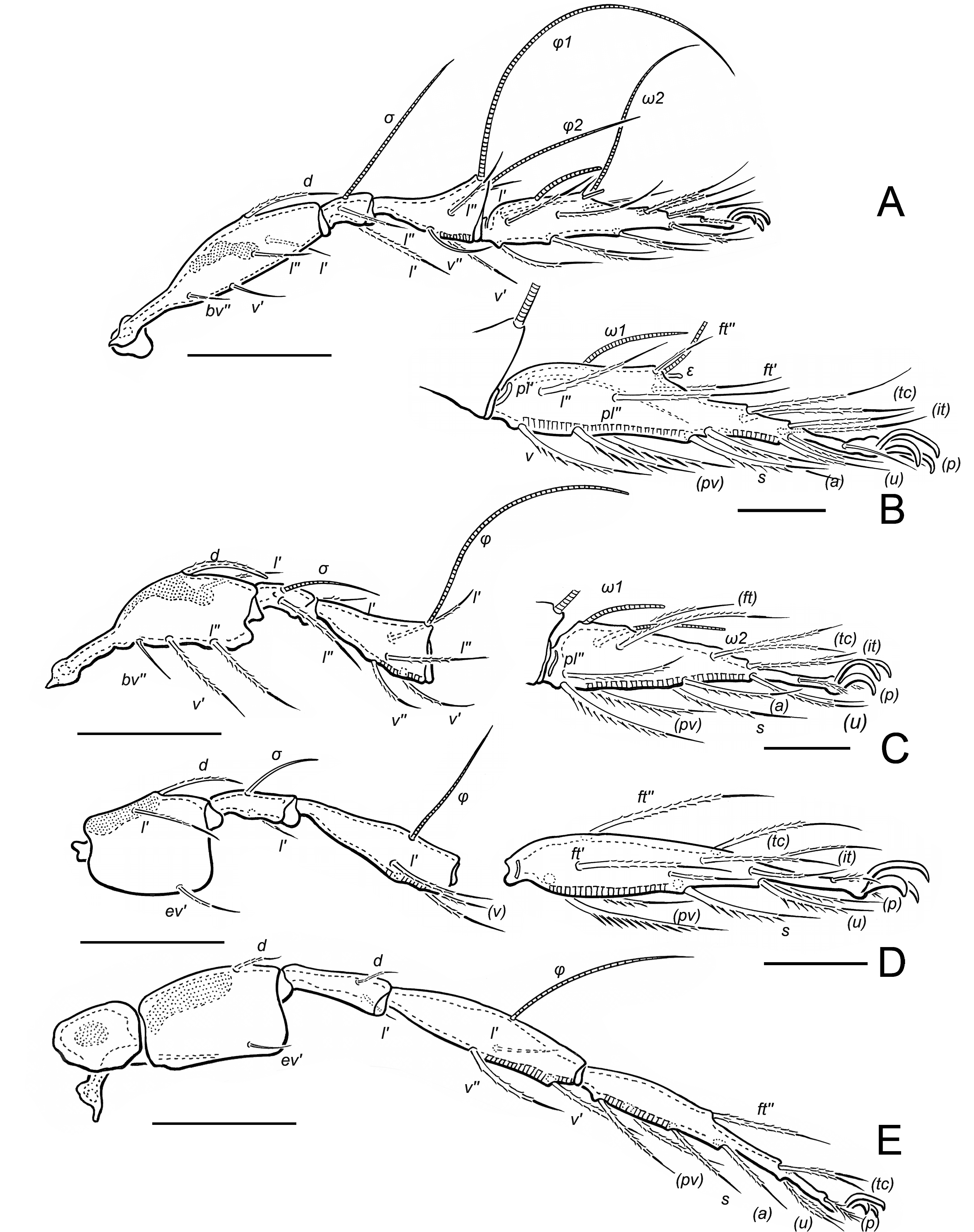


Ghilarovus robisoni n. sp.
ZOOBANK: 8C2690BD-DDFB-4C9F-8A49-9548F60CF136 ![]()
Figures 1‒6
Material Examined
Holotype: adult female (CNC1097998), Arkansas, Montgomery Co., Ouachita National Park, Crystal Vista Train, 34°5.06' N 93°6.00' W, 18.x.2009 (V. Behan-Pelletier) from lichens on rocks.
Paratypes: 10 with same data as holotype; Texas, Uvalde Co., Garner State Park, Big Cedar Trail, 29°34.090' N 99°44.876' W, 27-ii-2007 (V. Behan-Pelletier) 6 from cedar, oak, madrone litter; Guadeloupe State Park, 29°52.443'N 98°29.139'W, 3.iii.2007 (V. Behan-Pelletier) 7 from moss, maidenhair fern on ledge in floodplain; Bandera Co., Lost Maples State Natural Area, Can Creek Trail, 29°48.984' N 99°34.599' W, 28.ii.2007 (V. Behan-Pelletier) 2 males from moss and lichens on overhanging N-facing rockwall; Madera Canyon Camp, 20 mi NW Fort Davis, 6000', 21.vii.1973, (E. E. Lindquist) 1 male from high water litter by stream edge; New Mexico, Eddy Co., 17.vi.1992 (J. Cokendolpher) 2 females from entrance of Hidden cave. Paratypes deposited in the CNC (CNC1097999‒1098005), USNM, and RNC.
Other material examined: Additional individuals from the type locality were studied with scanning electron microscopy.
Diagnosis
Adult. Total length 288‒365. Rostrum with 15‒18 short dentes, subequal in size; medial dentes more closely positioned than lateral ones. Seta ro 60‒65 long, barbed, acuminate. Lamellar seta 103‒107 long, heavily barbed, tapered distally. Seta in 62‒70 long, barbed (subequally to le). Bothridial seta filiform, 76‒82 long. Five to 7 macropores present laterally between bothridium and seta le. Posterior notogastral tectum divided, with overlapping lobes. Humeral sac (hs) present, extending posteriorly almost to level of lyrifissure im. Notogastral setae smooth, acuminate, 13‒17 long, except c2 52‒55 long, strongly barbed, tapered. Pyriform organ absent. Prodorsum with 3 pores in longitudinal alignment lateral and posterior to seta in. Notogaster with 3 pores closely adjacent posterior to seta c2. Custodium 23‒25 long, strongly tapered, extending to base of pedotectum I. Adanal setation 3 pairs.






Description
Adults
Dimensions — Total length: females (n = 7) 344 (322‒365); males (n = 4) 300 (range 288–326). Notogastral width: females (n = 2) 228 (216, 240); males (n = 2) 211 (211, 211).
Integument — Cerotegument present laterally on podosoma, finely and densely granulate (Fig. 5E). Integument with dark spots laterally and posteriorly on notogaster (Figs. 3B, 3G). Longitudinal, fine ridges present laterally on epimeres, podosoma and anterior notogaster (Fig. 5E). System of macropores positioned on prodorsum and notogaster as indicated in Fig. 1A; pores often covered by small patch of cerotegument (Fig. 5D). On prodorsum 3 pores in longitudinal alignment lateral and posterior to seta in (Fig. 4A), others on rostral tectum. On notogaster 3 pores closely adjacent posterior to seta c2 (Fig. 3C, arrow).
Prodorsum — Rostrum with 15‒18 short dentes, subequal in size, about 2 in length; medially dentes more closely adjacent than lateral ones. Seta ro 60‒65 long, barbed along length, acuminate, curved anteromedially; mutual distance about 38 (Fig. 1A). Seta le 103‒107 long, heavily barbed, tapered distally; mutual distance about 47 (Fig. 5A). Seta in 62‒70 long, barbed (subequally to le), tapered; mutual distance about 61 (Fig. 1A). Bothridial seta setiform, 76‒82 long, directed posterolaterally (Figs 1A, 6C). Seta ex thin, weakly barbed, 11‒14 (Fig. 6D). Three to 5 pairs of overlapping muscle sigilla in dorsosejugal region (Fig. 3D).
Lateral region of podosoma — Laterorostral ridge dorsal of pedotectum I 55‒60 long (Fig. 4C). Custodium 23‒25 long, strongly tapered, extending to base of pedotectum I (Figs 1B, 4B arrow).
Notogaster — Humeral region rounded dorsally, with cleft (Fig. 6D) and pointed to rounded lobe ventrally (Figs 3A, 3C, 6C, 6D). Setae smooth, acuminate, 13‒17 long, except c2 52‒55 long, strongly barbed, tapered, positioned dorsally on humeral tubercle (Figs 1A, 3E, 5A, 5E). Seta lm positioned medially, in longitudinal alignment with bothridial seta (Fig. 1A). Lyrifissure ia not evident (Fig. 1A). Humeral sac about 50 long (Fig. 3A), difficult to see in preserved specimens. Lyrifissure im positioned lateral of seta lm (Figs 1A, 3A). Opening of opisthonotal gland (gla) between im and seta lp (Figs 1A, 3A).
Ventral Region — Epimeral setae barbed, acuminate, with 1a and 1b strongly directed anteriorly (Fig. 5B); setae 1a, 1b, 1c thicker than other epimeral setae; measurements: 1a, 25‒36; 1b, 25‒28, 1c, 30‒35; 2a, 12‒19; 3a, 10‒19; 3b, 38; 3c, 28‒30; 4a, 14, 4b, 11; 4c, 22‒26. Genital, aggenital, anal and adanal setae smooth; genital setae 10‒16, other setae about 9. Lyrifissure iad anterolateral of anal plate. Narrow band (about 2 wide) of continuously porose integument extending along edge of ventral plate; discrete marginoventral series of porose areas absent.
Gnathosoma — Cheliceral length 95‒110; digits each with 5 teeth (Figs 1D, 6A). Cheliceral seta cha thick, setiform, acuminate, about 43, with distal barbs splayed and longer than proximal barbs; chb strongly barbed, penicillate, tapered, 23‒25 (Figs 1D, 6A). Length of seta h > m > a seta h strongly barbed, directed anteriorly, 39 long; m barbed, directed almost transversely, 36 long; a strongly barbed, curving anteriorly, 12 long (Fig. 6A). Adoral setae strongly barbed, about 17 (Figs 6A, 6B). Solenidion on palptarsus about 6 long, tightly adpressed to segment; acm, (ul) and su about 4; other palptarsal setae setiform. Trägårdh's organ as illustrated by Grandjean (1934).






Legs (Figs 2A‒E, 4D, 5B, 5E) — Setal and solenidial formula (I to IV): trochanters: 1-1-2-0; femora: 5-5-3-2; genua: 2(1)-2(1)-1(1)-2; tibiae: 4(2)-4(1)-3(1)-3(1); tarsi: 20(2)-16(2)-15-12; leg setation given in Table 1. Only setae (p) on tarsus I eupathidial. Tarsal lyrifissure positioned antiaxially. Dorsolateral porose areas present on femora I‒IV and trochanters III, IV (Fig. 5E); with anteroventral porose area on tibiae I‒IV and posteroventral porose area on tarsi I‒IV (Figs 2A‒E). On tarsi I and II, ventral porose area extending to base of seta s (Figs 2B, 2C). Solenidion φ1 of tibia I borne on anterodorsal tubercle. Solenidion ω1 of tarsus II proximal to seta ft' (Figs 2C, 4D).



Gender differences
No sexual dimorphism exists in external morphology, except for males being slightly smaller than females, their genital plates being slightly smaller proportionally than in females, and in the typical genitalic differences.
Etymology
This species is named for Henry W. Robison, Emeritus Professor at Southern Arkansas University, who in addition to a long history of distinguished research in ichthyology and herpetology has collected Acari for the CNC throughout Arkansas.
Ecology
This species is a saxicole, found associated with mosses and lichens among rocks and rock faces in forest and prairie habitats. This is generally similar to the habitat where Subías and Pérez-Íñigo (1977) found G. hispanicus hispanicus and Subías (1977) found G. hispanicus guadarramicus, species these authors also called saxicoles. Gut contents indicate that adults feed on fungi, and decomposed plant material (Fig. 4F).
Remarks
• 1 The expression of the palptarsal solenidion and eupathidium acm in Ghilarovus robisoni n. sp. is identical to that in Zetomotrichus lacrimans, in Mikizetes as described by Covarrubias (1969) and in G. hispanicus hispanicus as described by Subías and Pérez-Iñigo (1977). The solenidion is short and recumbent and is positioned half way between acm and setae (lt); acm is closely adjacent to the terminal eupathidia (ul) and sul. Such an expression of the solenidion with relationship to acm is unknown in poronotic Brachypylina (Grandjean 1954a) and is more similar to that found in some pycnonotic Brachypyline, e.g., Anderemaeus (Norton & Ermilov 2019).
• 2 Solenidion ω1 is positioned proximally on tarsus II and is proximal to seta ft'. The usual position in poronotic Brachypylina is distal to seta ft' and almost aligned transversely with ft'' (Grandjean 1940). We found a scattered distribution of this trait in Ceratozetidae, Punctoribatidae and the tectoribatid Tectoribates (Behan-Pelletier & Walter 2013).
• 3 Grandjean (1954b) considered the prodorsal and notogastral macropores in Zetomotrichidae as possibly the result of fragmentation of the octotaxic system and porose areas Al, Am, Ah, and this hypothesis was supported by Covarrubias (1969) and tentatively for notogastral macropores by Norton and Alberti (1997). This hypothesis is supported by SEM studies of macropores in early derivative Brachypylina, Hermanniella punctulata and Poroliodes farinosus where pores were associated with small saccules (Alberti et al. 1997). In G. robisoni n. sp. there are patches of either cerotegument or secretion visible on the surface of some macropores (Fig. 5D).
• 4 The dark spots on the lateral and posterior of the notogaster in G. robisoni n. sp. and also found in Zetomothricus lacrimans, are not tubercles or depressions; they are within the integument, as described by Grandjean (1954b); these have also been noted in G. hispanicus hispanicus by Subías and Pérez‒Iñigo (1977).
World key to adults of Ghilarovus
1. Adanal setation 2 pairs
...... 2
— Adanal setation 3 pairs
...... 6
2. Posterior tectum of notogaster medially divided
...... 3
— Posterior tectum of notogaster not divided
...... 5
3. Borders of divided posterior tectum not overlapping
...... G. khentiicus Bayartogtokh & Smelyansky, 2007
— Borders of divided posterior tectum with overlapping lobes
...... 4
4. Humeral sac absent
...... G. daliensis Yamamoto & Aoki, 2000
— Humeral sac present
...... G. saxicola Aoki & Hirauchi, 2000
5. Humeral sac absent
...... G. kvavadzei Murvanidze, 2014
— Humeral sac present
...... G. sanukiensis Fujikawa, 2005
6. Posterior tectum of notogaster medially divided
...... 7
— Posterior tectum of notogaster undivided
...... 9
7. Bothridial seta subequal in length to rostral seta and seta c2
...... G. mongolicus Bayartogtokh & Smelyansky, 2007
— Bothridial seta longer than rostral seta and seta c2
...... 8
8. Seta le 103‒107, 2x length of seta c2 (52‒55), > 1.5x seta ro
...... G. robisoni n. sp.
— Seta le < 2x seta c2 and seta ro
...... G. krivolutskyi Bayartogtokh & Smelyansky, 2007
9. Both humeral sac and pyriform organ present
...... G. elegans Mahunka, 1983
— Humeral sac and pyriform organ absent
...... 10
10. Rostral dentes regular in size, small to medium
...... 11
— Rostral dentes irregular in size; medial six much longer than lateral dentes
...... G. turcmenicus Krivolutsky, 1974
11. Rostral dentes medium in length
...... 12
— Rostral dentes short
...... 13
12. Rostrum with about 12 dentes of similar spacing; medially ones slightly longer than lateral ones; body length 430‒434
...... G. armenicus Khanbekyan, 1990
— Anterior four dentes of rostrum slightly larger than lateral teeth, with deep incision posterior to fourth anterior tooth; prodorsal setae smooth; body length 482
...... G. humeridens Krivolutsky, 1966
13. Prodorsal and epimeral setae smooth
...... G. stipatus Krivolutsky & Smelyansky, 1997
— Prodorsal setae and epimeral setae 1a, 1b, 1c, 3c and 4c conspicuously barbed
...... 14
14. One pair of anal setae present
...... G. hispanicus guadarramicus Subías, 1977
— Two pairs of anal setae present
...... G. hispanicus hispanicus Subías & Perez-Iñigo, 1977 and G. changlingensis Wen, 1990 (we are unable to separate these species based on their descriptions)
Discussion
Without known immatures, Grandjean (1954a, b) was unable to place Zetomotrichidae in a superfamily, but Covarrubias (1969) described the protonymph and deutonymph of Mikizetes diamantensis Hammer, 1958, and placed Zetomotrichidae in the unranked taxon Excentrosclerosae, based on the presence of excentrosclerites at the base of gastronotal setae la, lp and h2. The deutonymph of the zetomotrichid Desertozetes metsamoricus Khanbekyan, 1990 was also illustrated, although as noted by Norton and Ermilov (2014) (Appendix, 2017), the association with the adult is doubtful. Zetomotrichidae were formally placed in Oripodoidea (as the junior synonym Oribatuloidea) by Balogh (1961). Subsequently, Subías (2004) recognized the monobasic superfamily Zetomotrichoidea, but his classification generally has not been followed, e.g., Norton and Behan-Pelletier (2009), Schatz et al. (2011).
There is a suite of character states that contribute to the uniqueness of Zetomotrichidae (Grandjean 1954a, b). Species of Zetomotrichus (and subsequently described Keralotrichus Mahunka, 1985, Demisalto Coetzee, 1993, Saltatrichus, Coetzee 1993) can possibly jump in a manner thought to be similar to that found in Zetorchestidae, using modified legs IV which are displaced dorsally and have robust spine-like genu seta l' and tarsal setae ft'' and tc'', though jumping has not been observed for any species. Other genera in the family, Mikizetes Hammer, 1958, Ghilarovus Krivolutsky, 1966, Pallidacarus Krivolutsky, 1975, Rohria Balogh and Mahunka, 1977, Oglasascarus Bernini, 1978, Anoplozetes Lee and Pajak, 1987, Desertozetes Khanbekyan, 1990, Mabulatrichus Coetzee, 1993, Floritrichus Coetzee, 2003, Turkmenitrichus Krivolutsky and Karppinen, 2006, lack these modifications of leg IV. The notogaster has a posterior tectum with or without medially overlapping lobes; a character state found in Licneremaeoidea (Adhaesozetidae) and Ceratozetoidea (Chamobatidae, Humerobatidae, Maudheimiidae, Punctoribatidae, Ramsayellidae, Zetomimidae), but unknown elsewhere in Oripodoidea (Behan-Pelletier 2001). The usual octotaxic system is absent but the notogaster carries macropores which are possible fragments of this system. On the palptarsus the solenidion is independent, not associated with eupathidium acm to form the ``double horn'', unknown elsewhere in Oripodoidea. Members of Zetomotrichidae also lack the circumpedal carina.
Presently, 2 subfamilies are recognized (Coetzee 2003), Zetomotrichinae Grandjean, 1954, including Zetomotrichus, Keralotrichus, Demisalto, Saltatrichus, Mikizetes, Desertozetes, Ghilarovus, Oglasascarus, Anoplozetes, Mabulatrichus, Turkmenitrichus, and Rohriinae Balogh and Balogh, 1984, including Rohria, Pallidacarus, Floritrichus. Subías (2004) considered Saltatrichus a subgenus of Demisalto, Oglasascarus a subgenus of Mikizetes and Keralotrichus a subgenus of Zetomotrichus. Clearly, a family revision is needed.
Acknowledgments
For his many helpful suggestions on this manuscript, we thank Roy. A. Norton, Emeritus Professor, State University of New York, Syracuse, NY. We thank Barry Flahey, retired from the Research Branch, Agriculture and Agri-Food Canada who inked the figures, and Inna Nei of the Canadian Food Inspection Agency for translation of texts in Russian. We thank Keith Hubbard of the EM Center from Ottawa RDC for assistance with scanning electron microscopy.
References
Alberti G., Norton R.A., Adis J., Fernandez N.A., Franklin E., Kratzmann M., Moreno A.I., Weigmann G., Woas S. 1997. Porose integumental organs of oribatid mites (Acari, Oribatida). 2. Fine structure. Zoologica, Stuttgart, 146: 33-114.
Aoki J., Hirauchi Y. 2000. Two new species of the family Zetomotrichidae (Acari, Oribatida) from Japan. Spec. Div., 5(4): 351-359. doi:10.12782/specdiv.5.351 ![]()
Balogh J. 1961. Identification keys of world oribatid (Acari) families and genera. Acta Zool. Acad. Sci. Hung., 7(3-4): 243-344.
Balogh J. Balogh P. 1984. A review of the Oribatuloidea Thor, 1929 (Acari: Oribatei). Acta Zool. Acad. Sci. Hung., 30(3-4): 257-313.
Balogh J., Balogh P. 1988. Oribatid Mites of the Neotropical Region I. In: Balogh J.,Mahunka S. (Eds.). The soil mites of the world. Akad. Kiadó, Budapest, vol. 2: 335 pp.
Balogh J., Mahunka S. 1977. New data to the knowledge of the oribatid fauna of Neogaea (Acari) II. Acta Zool. Acad. Sci. Hung., 23(3-4): 247-265. doi:10.5962/bhl.part.91385 ![]()
Bayartogtokh B., Smelyansky I.E. 2007. Oribatid mites of the genus Ghilarovus (Acari: Oribatida: Zetomotrichidae) from Russia and Mongolia with remarks on ecology and biogeography of known species. Acarologia 47(1-2) (2006): 79-97.
Behan-Pelletier V.M. 2001. Phylogenetic relationships of Hypozetes (Acari: Tegoribatidae). In: Halliday R.B., Walter D.E., Proctor H.C., Norton R.A., Colloff M.J. (Eds.). Acarology: Proceedings of the 10th International Congress. CSIRO Publishing, Melbourne, Australia: 50-57.
Behan-Pelletier V.M., Walter D.E. 2013. Phylogenetic relationships of Tectoribates: nymphal characters of new North American species place the genus in Tegoribatidae (Acari, Oribatida). Zootaxa, 3741(4): 459-489. doi:10.11646/zootaxa.3741.4.2 ![]()
Bernini F. 1978. Notulae Oribatologicae XVIII. Oglasacarus oglasae n. gen., n. sp., un nuovo Zetomotrichidae raccolto sull'isola di Montecristoforo (Acarida, Oribatida). Redia, 61: 273-289.
Coetzee L. 1993. New genera and species of the family Zetomotrichidae Grandjean, 1954 (Acari, Oribatida, Oripodoidea) from South Africa. Navors. Nas. Mus. Bloemfontein, 9(5): 133-179.
Coetzee L. 1995. A new species of the genus Saltatrichus Coetzee, 1993 (Acari, Oribatida, Zetomotrichidae) from South Africa. Navors. Nas. Mus. Bloemfontein, 11(1): 1-12.
Coetzee L. 2003. A new genus and species Floritrichus louisbothai (Acari, Oribatida, Oripodoidea, Zetomotrichidae) from South Africa. Navors. Nas. Mus. Bloemfontein, 19(5): 93-100.
Covarrubias R. 1969. Observations sur le genre Mikizetes (Oribates). Acarologia, 11(4): 828-846.
Fujikawa T. 2005. A new species of Zetomotrichidae from Shikoku Island in Nippon (Acari, Oribatida). Acarologia, 46(4) (2004): 341-347.
Grandjean F. 1934. Oribates de l'Afrique du Nord (2me série). Bull. Soc. Hist. Nat. Afrique N., 25: 235-252.
Grandjean F. 1940. Les poils et les organes sensitifs portes par les pattes et le palpe chez les Oribates. Deuxième partie. Bull. Soc. zool. Fr., 45, 32-44.
Grandjean F. 1954a. Essai de classification des Oribates (Acariens). Bull. Soc. zool. Fr., 78: 421-446.
Grandjean F. 1954b. Zetomotrichus lacrimans, Acarien sauteur (Oribate) (Acar. Zetomotrichidae). Ann. Soc. entomol. Fr., 153: 1-16.
Grandjean F. 1957. Galumnidae sans carènes lamellaires (Acariens, Oribates), 2e série. Bull. Soc. zool. Fr., 82: 57-71.
Hammer, M. 1958. Investigations on the Oribatid Fauna of the Andes Mountains I. The Argentine and Bolivia. Biol. Skr. Dan. vid. Selsk. 10, 1-262.
Khanbekyan Yu. R. 1990. On representatives of the family Zetomotrichidae from Armenia: Desertozetes metsamoricus gen. nov., sp. nov. and Ghilarovus armenicus sp. nov. (Acariformes, Oribatei). Dokl. Akad. Nauk Armenii, 91(2): 86-90. [in Russian]
Krivolutsky D.A. 1966. On the oribatid mites (Oribatei, Acariformes) of the soils of Central Asia. Zool. Zh., 45: 1628-1639.
Krivolutsky D.A. 1974. New oribatid mites of the USSR. Zool. Zh., 53(12): 1880-1885. [in Russian]
Krivolutsky DA. 1975. Zetomotrichidae. In: Ghilarov, M.S. & Krivolutsky, D.A. (Eds.). A key to Soil-Inhabitinng Mites. Sarcoptiformes. Nauka, Moskva. P. 260-262. [in Russian].
Krivolutsky D.A., Smelyansky I.E. 1997. Zetomotrichidae from the southern Urals, a family of Oribatei (Acarina: Acariformes) newly found in Russia. Dokl. Biol. Sci., 355: 395-398.
Krivolutsky D.A., Karppinen E. 2006. Oribatid mites of Zetomotrichidae family in arid zone of Palaearctic. Arid Ecosyst., 12(29): 59-62. [in Russian]
Lee, D.C., Pajak GA. 1987. Anoplozetes, a new genus of Zetomotrichidae (Acarida; Cryptostigmata) from South Australia. Trans. R. Soc. S. Aust., 111(2): 99-103.
Mahunka S. 1983. Neue und interessante Milben aus dem Genfer Museum. 45. Oribatida Americana 6: Mexico II (Acari). Revue Suisse Zool., 90(2): 269-298. doi:10.5962/bhl.part.81976 ![]()
Mahunka S. 1985. Neue und interessante Milben aus dem Genfer Museum. LIV. Oribatids from South India I (Acari: Oribatida). Rev. suisse Zool., 92(2): 367-383. doi:10.5962/bhl.part.81605 ![]()
Marshall, V.G., Reeves, R.M., Norton, R.A. 1987. Catalogue of the Oribatida (Acari) of continental United States and Canada. Mem. Entomol. Soc. Can. , 139, 1-418. doi:10.4039/entm119139fv ![]()
Murvanidze M. 2014. Oribatid mites of Georgian (Caucasus) caves including the description of a new species of Ghilarovus Krivolutsky, 1966. Int. J. Acarol. , 40(6): 463-472. doi:10.1080/01647954.2014.950604 ![]()
Norton R.A., Alberti G. 1997. Porose integumental organs of oribatid mites (Acari, Oribatida). 3. Evolutionary and ecological aspects. Zoologica, Stuttgart, 146: 115-143.
Norton R.A., Behan-Pelletier V.M. 2009. Chapter 15, Oribatida. In: Krantz G. W., Walter D. E. (Eds). A Manual of Acarology. Lubbock, Texas: Texas Tech University Press. p. 430-564.
Norton R.A., Ermilov S.G. 2014. Catalogue and historical overview of juvenile instars of oribatid mites (Acari: Oribatida). Zootaxa, 3833: 1-132. doi:10.11646/zootaxa.3833.1.1 ![]()
Norton R.A., Ermilov S.G. 2019. Anderemaeus (Acari, Oribatida) - overview, three new species from South America and reassessment of Anderemaeidae supported by ontogeny. Zootaxa, In press.
Schatz H., Behan-Pelletier V.M., OConnor B.M., Norton R.A. 2011. Suborder Oribatida van der Hammen, 1968. In: Zhang Z.-Q. (ed.): Animal biodiversity: An outline of higher-level classification and survey of taxonomic richness. Zootaxa, 3148: 141-148. doi:10.11646/zootaxa.3148.1.26 ![]()
Subías L.S. 1977. Taxonomía y ecología de los oribátidos saxícolas y arborícolas de la Sierra del Guadarrama (Acarida, Oribatida). Trabajos de la Cátedra de Artrópodos, Facultad de Biología, Universidad Complutense de Madrid, 24, 379 pp. (Dissertation)
Subías L.S. 2004. Listado sistemático, sinonímico y biogeográfico de los Ácaros Oribátidos (Acariformes, Oribatida) del mundo (1758-2002). Graellsia, 60, 3-305. doi:10.3989/graellsia.2004.v60.iExtra.218 doi:10.3989/graellsia.2004.v60.iExtra.218 ![]()
Subías L.S., Pérez-Iñigo C. 1977. Notes sur les Oribates d'Espagne I. Description de Ghilarovus hispanicus n. sp. et quelques considérations sur les Zetomotrichidae (Acari, Oribatei). Acarologia, 18(4): 729-739.
Travé J., Fernandez N.A. 1986. Contribution a la connaissance du genre Scapheremaeus: S. argentinensis, n. sp. (Oribate). Acarologia, 27(4): 349-359.
Travé J., Vachon M. 1975. François Grandjean 1882-1975 (Notice biographique et bibliographique). Acarologia, 17: 1-19.
Wen Z. 1990. Description of new and unrecorded oribatid mites from Jilin Province, China (Acari: Oribatida). J. N. E. Norm. Univ., 1: 125-131.
Yamamoto Y., Aoki J. 2000. Six new species of oribatid mites from Mt. Jizushan and Mt. Xuerefeng, Yunnan Province in China (Acari: Oribatida). In: Aoki J., Yin W., Imadate G. (Eds.). Taxonomical Studies on the Soil Fauna of Yunnan Province in Southwest China. Tokai University Press, Tokyo: 13-22.



2019-05-07
Date accepted:
2019-05-29
Date published:
2019-06-11
Edited by:
Baumann, Julia

This work is licensed under a Creative Commons Attribution 4.0 International License
2019 Behan-Pelletier, Valerie M. and Knee, Wayne
Download the citation
RIS with abstract
(Zotero, Endnote, Reference Manager, ProCite, RefWorks, Mendeley)
RIS without abstract
BIB
(Zotero, BibTeX)
TXT
(PubMed, Txt)




